Abstract
Disrupted striatal functional connectivity is proposed to play a critical role in the development of psychotic symptoms. Previous resting-state functional magnetic resonance imaging (rs-fMRI) studies typically reported disrupted striatal connectivity in patients with psychosis and in individuals at clinical and genetic high risk of the disorder relative to healthy controls. This has not been widely studied in healthy individuals with subclinical psychotic-like experiences (schizotypy). Here we applied the emerging technology of multi-echo rs-fMRI to examine corticostriatal connectivity in this group, which is thought to drastically maximize physiological noise removal and increase BOLD contrast-to-noise ratio. Multi-echo rs-fMRI data (echo times, 12, 28, 44, 60 ms) were acquired from healthy individuals with low (LS, n = 20) and high (HS, n = 19) positive schizotypy as determined with the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE). After preprocessing to ensure optimal contrast and removal of non-BOLD signal components, whole-brain functional connectivity from six striatal seeds was compared between the HS and LS groups. Effects were considered significant at cluster-level p < .05 family-wise error correction. Compared to LS, HS subjects showed lower rs-fMRI connectivity between ventromedial prefrontal regions and ventral striatal regions. Lower connectivity was also observed between the dorsal putamen and the hippocampus, occipital regions, as well as the cerebellum. These results demonstrate that subclinical positive psychotic-like experiences in healthy individuals are associated with striatal hypoconnectivity as detected using multi-echo rs-fMRI. Further application of this approach may aid in characterizing functional connectivity abnormalities across the extended psychosis phenotype.
Keywords: Multi-echo fMRI, Schizotypy, Psychosis, Functional connectivity, Striatum, Resting-state
Highlights
-
•
Multi-echo fMRI was used to investigate resting-state connectivity in subjects with low and high psychometric schizotypy (HS).
-
•
High schizotypy subjects showed hypoconnectivity between ventral striatum and ventromedial prefrontal cortex.
-
•
These subjects also showed hypoconnectivity between dorsal striatum and temporooccipital regions.
1. Introduction
Over the last two decades, the dysconnection hypothesis of schizophrenia (Friston et al., 2016; Friston and Frith, 1995; McGuire and Frith, 1996) has gained growing neurobiological support due to technical advances in structural and functional magnetic resonance imaging (Pettersson-Yeo et al., 2011). The dysconnection hypothesis suggests that the hallmark symptoms of schizophrenia arise from abnormal functional integration between distributed brain regions due to altered neuromodulation of synaptic plasticity, particularly in regions with dopaminergic afferents (Adams et al., 2013; Friston et al., 2016). A key area is the striatum, which receives prominent innervations from dopaminergic neurons in the midbrain, and is central to the orchestration of activity of limbic, associative and motor brain regions through interconnected cortico-striatal loops, thereby supporting a range of neural computations necessary for normal cognitive function (Haber, 2016). Striatal dysregulation may therefore be involved in widespread disruption of these circuits and the emergence of positive symptoms. Indeed, a number of studies in patients with schizophrenia and individuals at clinical high risk (CHR) of psychosis reported increased presynaptic dopamine synthesis capacity and release in the striatum (see Howes et al., 2012 for a meta-analysis), and a direct correlation between the extent of striatal dysfunction and the severity of positive psychotic symptoms in patients (Allen et al., 2016; Kindler et al., 2017; Sorg et al., 2013). Moreover, there is evidence to suggest that positive symptoms may be associated with disrupted task-related striatal activation and connectivity during the attribution of aberrant salience to otherwise irrelevant stimuli in healthy individuals, CHR subjects, and patients with a full-blown psychotic disorder (Boehme et al., 2015; Diaconescu et al., 2010; Pankow et al., 2016; Roiser et al., 2013; Schlagenhauf et al., 2009; Winton-Brown et al., 2017, Winton-Brown et al., 2014). Such evidence aligns well with predictions based on animal models of psychosis (Lodge and Grace, 2011) which show that striatal dysfunction may result from increased hippocampal activity, which may in turn be related to prefrontal cortex (PFC) abnormalities (Gomes and Grace, 2017), and propose that disrupted interactions within this corticostriatal circuit contribute to the development of aberrant salience processing and positive symptoms (Grace, 2016).
In this context, resting-state functional magnetic resonance imaging (rs-fMRI) provides a powerful tool to examine patterns of altered functional connectivity and their relationship to symptomatology in patients with established psychosis as well as in individuals at genetic or CHR of psychosis (e.g. Dandash et al., 2014; Fornito et al., 2013; Peters et al., 2017). rs-fMRI studies focusing on striatal connectivity in patients with schizophrenia and in their relatives have reported altered functional integration of this region with a number of cortical areas, including mainly the prefrontal and temporal cortices (Horga et al., 2016; Lin et al., 2018; Peters et al., 2017; Sole-Padulles et al., 2016; Tu et al., 2012). Dimensional views of psychosis postulate that there is continuity between subclinical psychotic-like experiences which can be detected in healthy people using validated self-report questionnaires (that is, schizotypy) and psychotic symptoms in patients with schizophrenia (Linscott and van Os, 2013; Nelson et al., 2013). Consistent with this psychosis continuum view, a recent rs-fMRI study reported that scores on the positive dimension of schizotypy (relating to cognitive-perceptual experiences) were positively associated with ventral striatal–PFC connectivity, and negatively associated with dorsal striatal–posterior cingulate connectivity (Wang et al., 2018). Similarly, another recent study by Rössler and colleagues reported ventral striatal dysconnectivity in a schizotypy sample and provided preliminary evidence that this might indeed result from dopaminergic alterations, supporting the dysconnection hypothesis (Rössler et al., 2018). In particular, the authors found lower ventral striatal connectivity in participants who scored higher on a schizotypy scale regardless of whether they had received an L-Dopa or placebo challenge, whereas participants with lower schizotypy scores showed striatal dysconnectivity following L-Dopa administration (Rössler et al., 2018). However, the samples used in both studies above were largely composed of individuals with scores in the low to moderate range. It thus remains unclear whether corticostriatal dysconnectivity extends to individuals with high positive schizotypy scores. This is an important question as previous studies in schizophrenia and CHR subjects indicate that the greater the rs-fMRI dysconnectivity, the higher the severity of positive symptoms (Dandash et al., 2014; Fornito et al., 2013), and that high scores in positive schizotypy scales are associated with higher severity of positive symptoms in patients with schizophrenia (Barrantes-Vidal et al., 2015; Cochrane et al., 2010).
The high schizotypy paradigm is a widely used strategy to examine neurobiological factors related to the expression of psychotic symptoms in the absence of possibly confounding disease-related effects such as antipsychotic medication exposure and illness chronicity which can affect rs-fMRI data (Sarpal et al., 2017, 2015). However, additional confounders in imaging studies may arise from technical limitations, as for example, rs-fMRI data tends to be noisy and may result in indeterminacy of the sources of blood oxygenation level dependent (BOLD) signals, particularly within subcortical regions (Power et al., 2012; Shmueli et al., 2007). Previous studies in psychosis and schizotypy used standard rs-fMRI, which is based on single-echo echo-planar imaging (EPI) sequences employing echo times (TEs) designed to roughly correspond to the average tissue T2*, in order to optimize contrast (Kundu et al., 2012). However, because T2* varies regionally, so does the signal-to-contrast ratio, resulting in signal loss in parts of the brain where T2* is particularly short or long (e.g. Posse et al., 1999). This compromises the quality of the data, especially in low T2* regions such as the inferior temporal cortices, or indeed the orbitofrontal cortex and ventral striatum (Kundu et al., 2017). Adding to this issue, rs-fMRI connectivity findings are highly vulnerable to spurious effects: because they are often based on correlational analyses, any factor that simultaneously influences signal in more than one region of the brain will increase observed connectivity, while factors that influence signal in a single region will decrease observed connectivity, such as head motion, cardiac and respiratory rates, arterial CO₂ concentration and blood pressure (see e.g. Murphy et al., 2013 for a detailed discussion). Typically, this type of so called physiological noise is dealt with using band-pass-filtering for the BOLD signal frequency band (0.01 Hz–0.08 Hz) and removal of the variance explained by separately acquired physiological nuisance recordings using linear regression (Murphy et al., 2013). However, significant noise remains even after data clean-up (Power et al., 2012), and nuisance variation that has not been modelled will inevitably remain. These limitations can be addressed by using an fMRI sequence that collects multiple echoes after each pulse. Firstly, the collection of multiple echoes allows for the relaxometric estimation of region specific T2* values, and hence for the voxel-wise computation of a contrast optimized signal from appropriately weighted echoes, which drastically improves overall contrast-to-noise ratio (Kundu et al., 2017; Posse et al., 1999). Secondly, the collection of multiple echoes allows for blind separation of BOLD-like from non-BOLD-like signal components: while the observed percent signal change ΔS always depends on both changes in the initial signal intensity (S0) and changes in T2*, BOLD effects modulate T2* much more than S0, and non-BOLD effects (e.g. head motion, cerebrovascular pulsatility, hardware related fluctuation etc.) modulate S0 much more than T2*. Since T2* scales linearly with TE but S0 does not, regression of signal components identified using independent component analysis against TE and S0 can be used to differentiate between BOLD- and non-BOLD-like components (Kundu et al., 2017, Kundu et al., 2013, Kundu et al., 2012). Thus, nuisance contributions can be reliably removed even if their source is unknown.
To date, these technical limitations have not been addressed in investigations of rs-fMRI connectivity in the psychosis spectrum. Hence, this study sought to investigate rs-fMRI corticostriatal connectivity in a sample of healthy adults with high scores on a psychometric measure of positive schizotypy using contrast-optimized, and independent components analysis (ICA)-denoised, multi-echo (ME) EPI data. Based on findings implicating corticostriatal dysconnectivity in the emergence of positive psychotic symptoms (Dandash et al., 2014; Fornito et al., 2013; Rössler et al., 2018; Wang et al., 2018), we hypothesized that individuals with high positive schizotypy (HS) would show altered corticostriatal functional connectivity compared to a group of similar individuals with low positive schizotypy (LS) scores as control group.
2. Methods and materials
2.1. Participants
Two hundred and fifty potential participants who had responded to online advertising via the Research Volunteer Recruitment Webpage of King's College London were pre-screened using the short version of the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) (Mason and Claridge, 2006). Subjects were invited to participate in the study if they scored <2 (low positive schizotypy, LS) or >7 (high positive schizotypy, HS) on the Unusual Experiences subscale of the O-LIFE, as in a previous imaging study in our center (Premkumar et al., 2012). The UE subscale of the O-LIFE questionnaire reflects positive schizotypy and is associated with positive symptoms in schizophrenia patients (Cochrane et al., 2010).
Participants were excluded if they had a history of neurologic/psychiatric disorders as assessed using the Mini International Neuropsychiatric Inventory (Sheehan et al., 1998) and the Psychosis Screening Questionnaire (Bebbington and Nayani, 1995) (administered by a trained interviewer and reviewed by an experienced neuropsychologist, GM). Other exclusion criteria included contraindications to MRI scanning, having a first-degree relative with present/past history of psychotic disorder, present/past history of use of psychotropic medications, and use of recreational drugs in the two weeks prior to scanning or meeting criteria for substance abuse/dependence by self-report. The final sample included 20 participants in both the HS (10 males; age range 18–39 years, M = 26.35, SD = 5.47) and LS groups (11 males; age range 18–44 years, M = 26.70, SD = 7.06). Three studies have reported previous findings from overlapping sub-samples of this cohort with other imaging modalities (Modinos et al., 2018, Modinos et al., 2017a, Modinos et al., 2017b).
Ethical approval for the study was obtained from the KCL College Research Ethics Committee (CREC) and all participants provided written informed consent before initiating any study procedures.
2.2. Behavioral assessments
On the day of scanning, before scanning commenced, participants completed a semi-structured interview adapted from the Early Psychosis Prevention and Intervention Centre (EPPIC) Drug and Alcohol Assessment Schedule (http://www.eppic.org.au) to assess current/past medication use and current/past use of alcohol, tobacco and cannabis; the Social Function Questionnaire (SFQ) (Tyrer et al., 2005) to measure social functioning; and a validated short version of the Wechsler Adult Intelligence Scale-III (WAIS- III) (Velthorst et al., 2013) to measure intellectual ability. Analysis of demographic and questionnaire data was performed in SPSS 24 (https://www.ibm.com/analytics/us/en/technology/spss/), with the effect of group being tested using independent sample t-tests for parametric data and χ2-tests for non-parametric data (significance threshold p < .05).
2.3. Imaging acquisition
Scanning was performed on a General Electric Discovery MR750 3 T system (Milwaukee, WI, USA) at the Institute of Psychiatry, Psychology & Neuroscience, King's College London. For the rs-fMRI, participants were asked to lie still with their eyes open, and to think of nothing in particular while a fixation cross was displayed in the center of a screen which they viewed through a mirror system. Scanning time for the rs-fMRI was 12 min. During this time, ME-EPI images sensitive to BOLD contrast were acquired to measure hemodynamic responses (repetition time: 2500 ms; echo times, 12, 28, 44, 60 ms; flip angle, 80°; 4.0 × 4.0 × 3.0-mm voxels; field of view, 240; 32 axial sections collected with sequential (top down) acquisition and 1-mm interslice gap). A structural scan was acquired for co-registration of the ME-EPI data by means of a three-dimensional T1-weighted inversion recovery-prepared gradient echo sequence (voxel size: 1.05 × 1.05 × 1.2 mm, field of view: 270 mm, 196 slices, repetition time: 7.3 ms, echo time: 3.0 ms, inversion time: 400 ms).
2.4. Imaging preprocessing
After resetting of the origins for both T1-weighted and ME-EP images, a study specific template was created using Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/) for later normalization, in order to reduce localization error and improve sensitivity (Huang et al., 2010). One subject from the HS group had to be excluded because of atypical anatomy which undermined the template quality, resulting in a final sample of 20 LS and 19 HS subjects. The ME-EPI echoes were separated into four distinct time series (corresponding to the four individual echoes), which were then de-spiked using 3dDespike in the Analysis of Functional NeuroImages (AFNI) framework (https://afni.nimh.nih.gov), and slice time corrected using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Parameters for motion correction were estimated from the first echo, and applied to all four echoes using FSL's mcFLIRT (Greve and Fischl, 2009; Jenkinson et al., 2002; Jenkinson and Smith, 2001). Subjects' ME-EP images were then co-registered to the T1 scan using boundary-based registration as implemented in FLIRT. Again, parameters were estimated for the first echo, and subsequently applied to all four echoes. All echoes were spatially normalized to the study-specific template, and from there to Montreal Neurological Institute (MNI) space. Finally, the images from all echoes were z-concatenated for further processing, i.e. the space-by-time matrices from each echo were appended to one another in the z-direction to form a single matrix using the 3dZcat function in AFNI. TEDANA, a python script that forms part of the Multi Echo Independent Component Analysis (MEICA) package (https://afni.nimh.nih.gov/pub/dist/src/pkundu/meica.py) (Kundu et al., 2017, Kundu et al., 2013, Kundu et al., 2012) was called to perform TE dependent ICA-based denoising and T2* weighted averaging (optimal combination) of echoes as described above. The denoised, optimally combined images were subsequently taken forward for motion correction, removal of white matter (WM) and cerebrospinal fluid (CSF) signal via regression, and band-pass-filtering (frequency range 0.08–0.01 Hz). A comparison of the mean framewise displacement (FD) (Power et al., 2012) between HS and LS subjects revealed no significant difference in head motion between groups (t = 0.358, p = .72). No individual subject showed mean FD in excess of 0.12 mm.
2.5. Imaging analysis
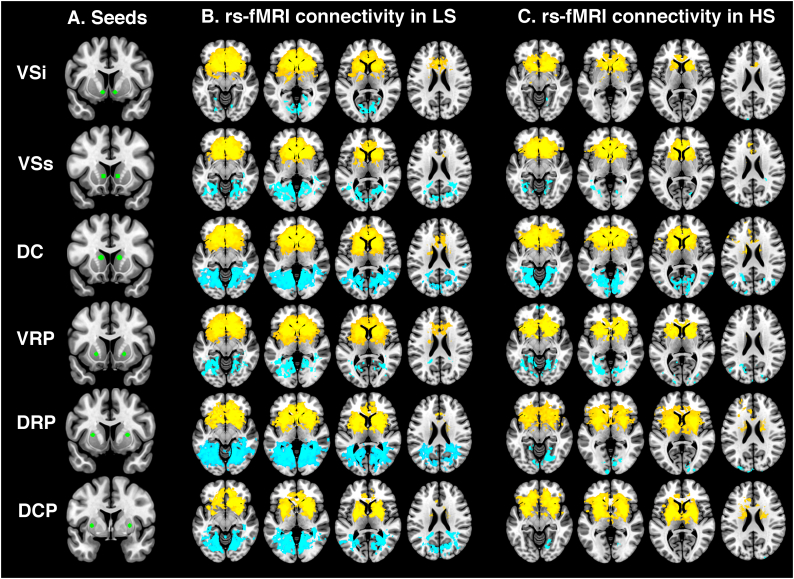
We defined six bilateral striatal seeds based on previous validated work on striatal connectivity (Dandash et al., 2015, Dandash et al., 2014; Di Martino et al., 2008; Fornito et al., 2013; Rössler et al., 2018; Wang et al., 2018, Wang et al., 2016): ventral striatum inferior/nucleus accumbens (VSi; ±9, 9, −8); ventral striatum superior (VSs; ±10, 15, 0); dorsal caudate (DC; ±13, 15, 9); dorsal caudal putamen (DCP; ±28, 1, 3); dorsal rostral putamen (DRP; ±25, 8, 6); and ventral rostral putamen (VRP; ±20, 12,–3) (Fig. 2A), with the radius set at 3.5 mm (Di Martino et al., 2008). The mean signal was extracted from the seed regions (voxel-wise) using the REST toolbox (Song et al., 2011) to perform Pearson's correlation coefficients between these regressors and the rest of the brain (voxel-wise), which were subsequently Fisher's Z transformed.
Fig. 2.
(A) Location of striatal seed regions (green). Within-group functional connectivity maps between each striatal seed region and the whole brain in (B) LS subjects and (C) HS subjects. Blue refers to negative coupling, yellow refers to positive coupling. Maps thresholded at p < .001 uncorrected for display purposes. DC, dorsal caudate; DCP, dorsocaudal putamen; DRP, dorsorostral putamen; VRP, ventrorostral putamen; VSi, ventral caudate inferior; VSs, ventral caudate superior.
The resulting Z-maps were then taken to group-level whole-brain analysis using the General Linear Model as implemented in SPM12. Connectivity differences between groups were examined using t-contrasts. We used a cluster forming threshold of p < .001 uncorrected, to then enforce cluster-wise correction for multiple testing at p < .05 family-wise error (FWE) rate, based on previous studies (Dandash et al., 2014; Di Martino et al., 2008; Fornito et al., 2013; Wang et al., 2018). Potential effects of age or substance use (alcohol, cigarettes and cannabis) on areas showing significant group differences in connectivity were examined with an additional ANCOVA in SPM.
Finally, associations between symptom scores (O-LIFE UE) in HS subjects and Z-scores averaged across clusters showing group differences were analyzed using linear regression in SPSS.
3. Results
3.1. Demographic and questionnaire results
Table 1 summarizes the sociodemographic characteristics of each group. HS and LS differed, by design, only on the schizotypy measures. Specifically, HS had higher scores on the O-LIFE subscales measuring unusual experiences, cognitive disorganization, and impulsive non-conformity (all p < .001).
Table 1.
Demographic and questionnaire data.
| Characteristic | Low Schizotypy (n = 20) | High Schizotypy (n = 19) | t/χ2 | P |
|---|---|---|---|---|
| Gender (male/female) | 10/10 | 10/09 | 0.027 | 0.869 |
| Age (years) | 26.35 ± 5.47 | 26.37 ± 7.09 | −0.009 | 0.993 |
| Education (years) | 17.41 ± 3.75 | 18.25 ± 5.12 | −0.532 | 0.599 |
| Urbanicity (% urban) | 94.1 | 64.3 | 5.723 | 0.057 |
| ESeC social class (% salariat) | 63.2 | 82.4 | 3.195 | 0.202 |
| SFQ | 4.20 ± 3.22 | 5.84 ± 2.93 | −1.662 | 0.105 |
| WAIS III | 121.85 ± 12.42 | 119.79 ± 17.49 | 0.422 | 0.676 |
| O-LIFE Total | 16.16 ± 9.34 | 39.0 ± 11.82 | −6.388 | < 0.001 |
| O-LIFE Unusual Experiences | 0.75 ± 0.97 | 11.42 ± 4.31 | −9.838 | < 0.001 |
| O-LIFE Cognitive Disorganization | 5.45 ± 4.56 | 11.72 ± 6.33 | −3.530 | 0.001 |
| O-LIFE Impulsive Nonconformity | 4.05 ± 2.76 | 8.71 ± 1.99 | −5.783 | < 0.001 |
| O-LIFE Introvertive Anhedonia | 6.11 ± 4.70 | 6.44 ± 4.03 | −0.235 | 0.816 |
| Daily tobacco use | 0.82 ± 3.44 | 0.35 ± 0.81 | 0.581 | 0.565 |
| Daily caffeine use | 1.67 ± 1.48 | 2.82 ± 2.48 | −1.777 | 0.084 |
| Alcohol use (median(range)) | 2 (0–5) | 1 (0–4) | 0.648 | 0.421 |
| Marijuana use (median(range)) | 1 (0–3) (n = 19) | 0 (0–3) | 1.727 | 0.189 |
Abbreviations: ESeC, European Socio-economic Classification; CTQ, Childhood Trauma Questionnaire; SFQ, Social Function Questionnaire; WAIS III, Wechsler Adult Intelligence Scale III; O-LIFE, Oxford–Liverpool Inventory of Feelings and Experiences; SPQ, Schizotypal Personality Questionnaire. For the Social Functioning Questionnaire, higher scores indicate greater social impairment.
3.2. Imaging results
3.2.1. Resting-state fMRI connectivity
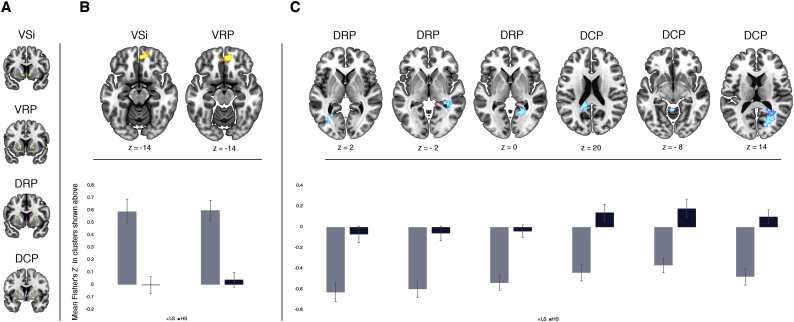
To test our hypothesis that individuals with HS would show altered functional connectivity of the striatum relative to subjects with LS, we compared Fisher's Z-values of whole-brain connectivity for each striatal seed between groups. This analysis revealed hypoconnectivity in HS compared to LS individuals between ventral striatal regions and the ventromedial PFC. Specifically, hypoconnectivity was observed between the VSi and a cluster including the bilateral gyrus rectus and right medial orbital gyrus (cluster-wise pFWE = 0.037), and between the VRP a cluster including the right medial orbital gyrus, left gyrus rectus and right anterior cingulate cortex (cluster-wise pFWE < 0.001) (Table 2, Fig. 1B).
Table 2.
Differences in Fisher's Z-values for resting-state fMRI striatal connectivity between high and low schizotypy.
| Seed |
Area |
Side |
MNI coordinates |
Z |
KE |
pFWE |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Cluster | |||||
| LS > HS | ||||||||
| VSi | Gyrus rectus | R | 10 | 50 | −18 | 4.01 | 64 | 0.037 |
| Medial Orbital Gyrus | R | 2 | 52 | −14 | 3.54 | |||
| Frontopolar cortex | R | 18 | 58 | −14 | 3.27 | |||
| VRP | Medial Orbital Gyrus | R | 8 | 48 | −14 | 4.85 | 172 | 0.000 |
| Gyrus rectus | L | −10 | 54 | −18 | 4.23 | |||
| Anterior Cingulate Cortex | R | 6 | 42 | −2 | 3.89 | |||
| LS < HS | ||||||||
| DRP | Hippocampus | R | 32 | −38 | −2 | 3.98 | 98 | 0.003 |
| Middle Temporal Gyrus | R | 40 | −48 | 4 | 3.79 | |||
| Hippocampus | R | 40 | −34 | −6 | 3.70 | |||
| Calcarine Sulcus | R | 34 | −56 | 8 | 3.97 | 151 | 0.000 | |
| Middle Occipital Gyrus | L | −28 | −60 | 2 | 3.91 | 90 | 0.005 | |
| Middle Occipital Gyrus | L | −32 | −70 | 4 | 3.71 | |||
| Calcarine Sulcus | L | −32 | −58 | 10 | 3.56 | |||
| DCP | Middle Occipital Gyrus | R | 30 | −60 | 20 | 4.35 | 488 | 0.000 |
| Calcarine Sulcus | R | 28 | −60 | 4 | 4.38 | |||
| Calcarine Sulcus | R | 28 | −50 | 8 | 4.26 | |||
| Posterior Cingulate | L | −18 | −46 | 20 | 4.25 | 293 | 0.000 | |
| Hippocampus | L | −30 | −40 | 2 | 4.16 | |||
| Calcarine Sulcus | L | −28 | −56 | 6 | 3.93 | |||
| Cerebellum | L | −2 | −50 | −8 | 4.01 | 64 | 0.038 | |
| Culmen | L | −6 | −60 | −2 | 3.72 | |||
Results are considered significant at cluster-wise pFWE < 0.05.
DC, dorsal caudate; DCP, dorsocaudal putamen; DRP, dorsorostral putamen; FWE, family-wise error; HS, high schizotypy; kE, cluster extent. L, left; LS, low schizotypy; R, right; VRP, ventrorostral putamen; VSi, ventral caudate inferior; VSs, ventral caudate superior.
Fig. 1.
Statistical parametric maps showing (A) relevant seed regions, as well as (B) decreased positive and (C) decreased negative functional connectivity in high (HS) vs low (LS) schizotypy subjects, by seed, and corresponding mean Fisher's Z-values by group. Error bars in graphs represent standard error of the mean.
Furthermore, we found hypoconnectivity between dorsal striatal regions and temporo-occipital areas in HS compared to LS subjects. More specifically, HS subjects showed hypoconnectivity between the DRP and a cluster centered on the right hippocampus (cluster-wise pFWE < 0.001) extending into occipital regions, left middle occipital gyrus (cluster-wise pFWE = 0.005), and calcarine sulcus (cluster-wise pFWE = 0.001); and between the DCP and the right middle occipital gyrus/calcarine sulcus (cluster-wise pFWE < 0.001), the left hippocampus (cluster-wise pFWE < 0.001), and cerebellar areas (cluster-wise pFWE = 0.038) (Table 2, Fig. 1C).
There were no other regions showing significant differences in rs-fMRI. For completeness, within-group rs-fMRI connectivity results for each striatal seed and the rest of the brain are shown in Fig. 2B and C. For a full list of significant clusters within each group, see Supplementary Table 1.
3.2.2. Effect of potential confounders
Groups were matched in demographic variables and including age as a covariate of no interest in the imaging analysis did not change the results. The hypoconnectivity between between DRP – calcarine sulcus, DCP – hippocampus, and DCP – middle occipital gyrus remained apparent at cluster-wise pFWE < 0.05 when alcohol, cigarette and cannabis use were added to the statistical model as covariates of no interest. However, adding these variables as covariates of no interest rendered the reductions in VSi – vmPFC, VRP – vmPFC, DCP-cerebellum and DRP-middle occipital gyrus connectivity no longer significant at cluster-wise pFWE < 0.05.
3.2.3. Associations with schizotypy scores
Within HS subjects, linear regression of Z-scores averaged across significant clusters against O-LIFE UE scores revealed no significant associations with either ventral or dorsal striatal connectivity (all p > .05). However, there were trend-level positive associations between positive schizotypy scores as assessed by O-LIFE UE and VSi-vmPFC and VRP-vmPFC connectivity, respectively (F(17,1) = 4.046, p = .061, R2 = 0.152; and F(17,1) = 4.403, p = .052, R2 = 0.167).
In an exploratory analysis, we further assessed the association between significant clusters and the O-LIFE subscores Impulsive Non-conformity and Cognitive Disorganization in HS subjects. Linear regression revealed no significant associations with either ventral or dorsal striatal connectivity (all p > .05). Because the O-LIFE subscore Introvertive Anhedonia did not differ between groups, we tested the association between this subscore and connectivity indices across both HS and LS. Again, linear regression revealed no significant associations with either ventral or dorsal striatal connectivity (all p > .05).
4. Discussion
Using novel multi-echo rs-fMRI methodology, the present study found lower corticostriatal resting-state functional connectivity in healthy individuals with high levels of psychotic-like experiences compared to those without such experiences (LS). These findings support the notion that corticostriatal dysconnectivity is involved in the expression of psychotic-like experiences across the extended psychosis phenotype, including healthy individuals, people at CHR of psychosis, and patients with an established psychotic disorder (Dandash et al., 2014; Fornito et al., 2013; Rössler et al., 2018; Wang et al., 2018). Interestingly, rs-fMRI striatal connectivity in HS subjects was characterized by both a lower level of positive coupling and a lower level of negative coupling compared to LS subjects. Specifically, distinct functional connectivity patterns were detected along the ventral-dorsal striatal axis; HS subjects showed lower positive coupling between the ventral striatum and vmPFC, and lower negative coupling between the dorsal striatum and temporo-occipital regions including the hippocampus, middle occipital gyrus and calcarine sulcus.
Previous studies have shown that striatal dysconnectivity in patients with schizophrenia (Fornito et al., 2013), CHR subjects (Dandash et al., 2014) and individuals with low-to-moderate levels of positive schizotypy (Rössler et al., 2018; Wang et al., 2018) may be characterized by both higher and lower connectivity of different striatal regions. This pattern has been proposed to indicate a potential risk biomarker for psychosis onset in vulnerable individuals (Dandash et al., 2014). However, the directionality of the changes in previous studies has shown some inconsistency; the above studies reported a dorsal-to-ventral gradient of hypoconnectivity to hyperconnectivity with frontal regions (Dandash et al., 2014; Fornito et al., 2013; Wang et al., 2018). In contrast, reduced connectivity between ventral striatal and ventral prefrontal areas has been reported in unmedicated patients with schizophrenia (Lin et al., 2018), in line with the results of the present study in high positive schizotypy. To our knowledge, there are no previous reports of hypoconnectivity between dorsal striatal areas and temporo-parietal regions in patients that would mirror our findings in high schizotypes, suggesting a lack of continuity of this phenotype across the psychosis spectrum (e.g., Fornito et al., 2013; Lin et al., 2018). Further, we found no evidence of an association between dorsal system alterations and symptom scores in our data. We are therefore cautious to interpret our finding as a reflection of psychotic symptoms. A possible explanation is that, rather than a pathological mechanism, the group difference might reflect a resilience mechanism that protects healthy individuals with high psychometric schizotypy from their psychotic experiences to become clinically relevant.
Previous work on schizotypy has reported striatal hypoconnectivity with posterior regions (albeit with ventral striatal regions) (Rössler et al., 2018), but lower connectivity with the vmPFC has not been reported (Rössler et al., 2018; Wang et al., 2018). These discrepancies may relate to the nature of the sample, as we used a group of subjects comprising high scorers in positive schizotypy as identified using a measure of subclinical psychotic experiences such as the O-LIFE, while Rössler et al. (2018) and Wang et al. (2018) studied low-to-moderate scorers as identified using the Schizotypal Personality Disorder Questionnaire (SPQ). The differences may also relate to the application of a multi-echo rs-fMRI sequence in our study but not in Rössler et al.'s (2018) and Wang et al.'s (2018) studies, such that our finding of lower vmPFC-ventral striatal connectivity in the HS group may have been masked in those studies since these regions have low CNR in standard EPI-sequences (Kundu et al., 2017).
While we did not detect significant associations between connectivity differences and positive schizotypy scores in the HS group, there was trend level evidence for a positive relationship between ventral striatum – vmPFC connectivity and the Unusual Experiences scale of the O-LIFE. Due to the limited size (N = 19) and low variability along the schizotypy dimension within the HS group, the possibility that there was insufficient power to detect significant associations with UE scores cannot be ruled out. Nevertheless, our findings highlight the promising applicability of multi-echo rs-fMRI methods for the detection of dysconnectivity patterns in a cross-sectional study design. Further research assessing functional connectivity across different groups across the psychosis spectrum (healthy individuals with low and high schizotypy, CHR subjects and unmedicated first-episode psychosis patients) with larger sample sizes will help clarify whether rs-fMRI connectivity changes vary according to the degree of vulnerability and of severity of psychotic experiences.
Mechanistically, the dysconnection hypothesis of psychosis proposes that the likely neurobiological basis for dysconnectivity would be aberrant neuromodulation (Friston et al., 2016). Animal work suggests that altered striatal dopaminergic signaling may disrupt the ventral aspects of frontostriatal connectivity in relation to psychosis phenotypes as, for example, in the rodent nucleus accumbens, mPFC afferents are modulated by dopamine via D2 receptors, such that increases in tonic dopamine levels attenuate mPFC inputs (Grace et al., 2007). In this context, ventral striatal-vmPFC hypoconnectivity in positive schizotypy could be driven by increased tonic striatal dopamine (Howes et al., 2012). Indeed, there is some evidence of an association between schizotypy scores, disrupted dopaminergic neurotransmission, and striatal dysconnectivity (Rössler et al., 2018; Woodward et al., 2011), although findings have been less consistent than in frank psychosis, possibly due to high heterogeneity in the experimental designs and methods used (Mohr and Ettinger, 2014).
An interesting corollary of the hypothesis that a dopaminergic dysfunction of the striatum in the psychosis spectrum compromises the functional integrity of the limbic cortico-striatal loop is that it might also account for reduced vmPFC connectivity with the default mode network (DMN) in psychosis patients (Camchong et al., 2011; Hu et al., 2017). Striatal dopamine function has been directly associated with vmPFC-DMN connectivity in a study of the effects of antipsychotics on the DMN (Sambataro et al., 2010), as well as in an investigation of a single nucleotide polymorphism of the D2 receptor gene (Sambataro et al., 2013). Consistent with a disruption of coordinated activity of the vmPFC driven by putatively dopaminergic striatal abnormalities, Wang et al. report a breakdown of the reciprocal interaction between the striatum and DMN nodes, including the vmPFC, in schizophrenia (Wang et al., 2015a, Wang et al., 2015b).
An alternative mechanistic explanation could be that connectivity alterations relate to changes in GABA- or glutamatergic alterations in the striatum, or indeed that primary vmPFC dysfunction may lead to dysconnectivity, as preclinical evidence suggests that striatal hyperdopaminergia may be a downstream effect of a failure of the mPFC to regulate hippocampal hyperresponsivity (Gomes and Grace, 2017; Grace, 2016; Patton et al., 2013; Zimmerman and Grace, 2016). Consistent with this notion, our recent positive schizotypy work found increased resting-state perfusion of the hippocampus in a largely overlapping sample (Modinos et al., 2018), in line with previous CHR studies (Allen et al., 2017, Allen et al., 2016; Schobel et al., 2013).
In this study, we extend the existing knowledge by investigating resting-state connectivity in otherwise healthy individuals on the high end of the schizotypy spectrum using multi-echo fMRI data. Our approach is advantageous for two reasons: First, the acquisition of multiple echoes is thought to afford superior noise removal and contrast optimization compared to traditional techniques, yielding better quality data. Second, variation due to illness chronicity, medication status, impaired global function and other illness associated factors are curtailed as in other studies on schizotypy, but high schizotypes are arguably phenotypically closer to psychosis patients that those in the low to moderate range that were previously examined (Rössler et al., 2018; Wang et al., 2018). Thus, our results are an important addition to the literature on the role of striatal dysconnectivity in the development and maintenance of psychotic traits.
One limitation of the present study is that the cross-sectional nature of the study prevents elucidating whether the observed findings reflect a risk or a resilience phenotype, in particular given reports that high schizotypes have a lower likelihood of developing psychosis than a CHR group (Kwapil et al., 2013). Further, while participants were asked to refrain from taking recreational drugs for two weeks prior to scanning, obtaining a biological measure of drug use on the day of scanning would have helped further confirm this issue (e.g., urine sample). The ventral striatal – vmPFC and dorsal striatal-occipital hypoconnectivity were no longer significant after including substance use (alcohol, tobacco and cannabis) in the statistical analysis. Finally, our study aimed at elucidating the role of striatal connectivity in the expression of psychotic-like experiences of the positive dimension based on previous literature in patients with psychosis and animal models, but further studies including schizotypal individuals based on negative and disorganized dimensions would clarify whether differences in functional connectivity are also related to negative and disorganized traits.
Future work with multi-echo rs-fMRI should directly investigate how striatal connectivity differences relate to markers of striatal dopaminergic function across the extended psychosis phenotype in patients, high-risk individuals, and high schizotypes. This would provide crucial evidence regarding the similarities and differences in striatal connectivity between individuals across the spectrum, yielding clues as to the potential determinants of psychosis risk, the occurrence of symptoms, illness-status and severity. Additionally, longitudinal studies should investigate which connectivity changes accompany transition to frank psychosis, as well as potential protective factors. Further, the functional and behavioral consequences of aberrant connectivity in psychosis spectrum disorders should be ascertained in studies combining resting state and functional measures of e.g. salience attribution.
4.1. Conclusion
Using a multi echo rs-fMRI sequence and independent component analysis, we found that high compared to low positive schizotypy was associated with lower functional connectivity between ventral aspects of the striatum and the vmPFC and between dorsal striatal regions and temporo-occipital areas. Given that aberrant functional integration has been implicated in the pathophysiology of psychosis, the present results offer some support to the notion of a central role of striatal dysconnectivity in the extended psychosis spectrum.
The following is the supplementary data related to this article.
Resting-state fMRI striatal connectivity in high and low schizotypy.
Declaration of interest
GJB received honoraria for teaching from General Electric Healthcare, and acted as a consultant for IXICO, at the time of this study. The other authors declare no competing financial interests.
Acknowledgements
This work was supported by a Brain & Behavior Research Foundation NARSAD Young Investigator Grant to G.M. (#21200, Lieber Investigator). G.M. is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (#202397/Z/16/Z). The authors wish to thank the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and KCL for their on-going support of our neuroimaging research, and gratefully acknowledge the MRI radiographers for their expert assistance in this work. We also thank Meghan O'Sullivan for her help with subject recruitment, Anna McLaughlin, Kurtis Stewart and Yun He for their help with data entry, and our study participants.
References
- Adams R.A., Stephan K.E., Brown H.R., Frith C.D., Friston K.J. The computational anatomy of psychosis. Front. Psychiatry. 2013;4(47) doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P., Chaddock C.A., Egerton A., Howes O.D., Bonoldi I., Zelaya F., Bhattacharyya S., Murray R., McGuire P. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am. J. Psychiatry. 2016;173:392–399. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- Allen P., Azis M., Modinos G., Bossong M.G., Bonoldi I., Samson C., Quinn B., Kempton M.J., Howes O.D., Stone J.M., Calem M., Perez J., Bhattacharayya S., Broome M.R., Grace A.A., Zelaya F., McGuire P. Increased resting hippocampal and basal ganglia perfusion in people at ultra high risk for psychosis: replication in a second cohort. Schizophr. Bull. 2017 doi: 10.1093/schbul/sbx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes-Vidal N., Grant P., Kwapil T.R. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr. Bull. 2015;41:S408–S416. doi: 10.1093/schbul/sbu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebbington P., Nayani T. The psychosis screening questionnaire. Int. J. Methods Psychiatr. Res. 1995;5(1):11–19. [Google Scholar]
- Boehme R., Deserno L., Gleich T., Katthagen T., Pankow A., Behr J., Buchert R., Roiser J.P., Heinz A., Schlagenhauf F. Aberrant salience is related to reduced reinforcement learning signals and elevated dopamine synthesis capacity in healthy adults. J. Neurosci. 2015;35:10103–10111. doi: 10.1523/JNEUROSCI.0805-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., MacDonald Angus W.I.I.I., Bell C., Mueller B.A., Lim K.O. Altered functional and anatomical connectivity in schizophrenia. Schizophr. Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane M., Petch I., Pickering A.D. Do measures of schizotypal personality provide non-clinical analogues of schizophrenic symptomatology? Psychiatry Res. 2010;176:150–154. doi: 10.1016/j.psychres.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dandash O., Fornito A., Lee J., Keefe R.S.E., Chee M.W.L., Adcock R.A., Pantelis C., Wood S.J., Harrison B.J. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr. Bull. 2014;40:904–913. doi: 10.1093/schbul/sbt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandash O., Harrison B.J., Adapa R., Gaillard R., Giorlando F., Wood S.J., Fletcher P.C., Fornito A. Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: implications for psychosis. Neuropsychopharmacology. 2015;40:622–631. doi: 10.1038/npp.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M.C., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state fMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Diaconescu A.O., Jensen J., Wang H., Willeit M., Menon M., Kapur S., McIntosh A.R. Aberrant effective connectivity in schizophrenia patients during appetitive conditioning. Front. Hum. Neurosci. 2010;4:239. doi: 10.3389/fnhum.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Goodby E., Dean A., Ooi C., Nathan P.J., Lennox B.R., Jones P.B., Suckling J., Bullmore E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston K., Brown H.R., Siemerkus J., Stephan K.E. The dysconnection hypothesis (2016) Schizophr. Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes F.V., Grace A.A. Prefrontal cortex dysfunction increases susceptibility to schizophrenia-like changes induced by adolescent stress exposure. Schizophr. Bull. 2017;43:592–600. doi: 10.1093/schbul/sbw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A., Floresco S.B., Goto Y., Lodge D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G., Cassidy C.M., Xu X., Moore H., Slifstein M., Van Snellenberg J.X., Abi-Dargham A. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 2016;73:862–870. doi: 10.1001/jamapsychiatry.2016.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.-L., Zong X.-F., Mann J.J., Zheng J.-J., Liao Y.-H., Li Z.-C., He Y., Chen X.-G., Tang J.-S. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-M., Lee S.-H., Hsiao I.-T., Kuan W.-C., Wai Y.-Y., Ko H.-J., Wan Y.-L., Hsu Y.-Y., Liu H.-L. Study-specific EPI template improves group analysis in functional MRI of young and older adults. J. Neurosci. Methods. 2010;189:257–266. doi: 10.1016/j.jneumeth.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kindler J., Schultze-Lutter F., Hauf M., Dierks T., Federspiel A., Walther S., Schimmelmann B.G., Hubl D. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr. Bull. 2017 doi: 10.1093/schbul/sbx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.-M., Bandettini P.A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. 2012;60:1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Brenowitz N.D., Voon V., Worbe Y., Vértes P.E., Inati S.J., Saad Z.S., Bandettini P.A., Bullmore E.T. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl. Acad. Sci. 2013;110:16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Voon V., Balchandani P., Lombardo M.V., Poser B.A., Bandettini P.A. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage. 2017;154:59–80. doi: 10.1016/j.neuroimage.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Kwapil T.R., Gross G.M., Silvia P.J., Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans' ten-year longitudinal study. J. Abnorm. Psychol. 2013;122:807–815. doi: 10.1037/a0033759. [DOI] [PubMed] [Google Scholar]
- Lin P., Wang X., Zhang B., Kirkpatrick B., Ongur D., Levitt J.J., Jovicich J., Yao S., Wang X. Functional dysconnectivity of the limbic loop of frontostriatal circuits in first-episode, treatment-naive schizophrenia. Hum. Brain Mapp. 2018;39:747–757. doi: 10.1002/hbm.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscott R.J., van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol. Med. 2013;43:1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- Lodge D.J., Grace A.A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol. Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason O., Claridge G. The Oxford-liverpool inventory of feelings and experiences (O-LIFE): further description and extended norms. Schizophr. Res. 2006;82:203–211. doi: 10.1016/j.schres.2005.12.845. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Frith C.D. Disordered functional connectivity in schizophrenia. Psychol. Med. 1996;26(4):663–667. doi: 10.1017/s0033291700037673. [DOI] [PubMed] [Google Scholar]
- Modinos G., McLaughlin A., Egerton A., McMullen K., Kumari V., Barker G.J., Keysers C., Williams S.C.R. Corticolimbic hyper-response to emotion and glutamatergic function in people with high schizotypy: a multimodal fMRI-MRS study. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., McLaughlin A., Egerton A., McMullen K., Kumari V., Barker G.J., Keysers C., Williams S.C.R. Relationship between prefrontal glutamate levels and functional activation during emotional processing in individuals with high schizotypy. Schizophr. Bull. 2017;43:S102. [Google Scholar]
- Modinos G., Egerton A., McMullen K., McLaughlin A.P., Kumari V., Barker G.J., Williams S.C.R., Zelaya F.O. Increased resting perfusion of the hippocampus in high positive schizotypy: a pseudo-continuous arterial spin labeling study. Hum. Brain Mapp. 2018;39(10):4055–4064. doi: 10.1002/hbm.24231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C., Ettinger U. An overview of the association between schizotypy and dopamine. Front. Psychiatry. 2014;5(184) doi: 10.3389/fpsyt.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Bandettini P.A. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.T., Seal M.L., Pantelis C., Phillips L.J. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci. Biobehav. Rev. 2013;37:317–327. doi: 10.1016/j.neubiorev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Pankow A., Katthagen T., Diner S., Deserno L., Boehme R., Kathmann N., Gleich T., Gaebler M., Walter H., Heinz A., Schlagenhauf F. Aberrant salience is related to dysfunctional self-referential processing in psychosis. Schizophr. Bull. 2016;42:67–76. doi: 10.1093/schbul/sbv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton M.H., Bizup B.T., Grace A.A. The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J. Neurosci. 2013;33:16865–16873. doi: 10.1523/JNEUROSCI.2449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H., Riedl V., Manoliu A., Scherr M., Schwerthoffer D., Zimmer C., Forstl H., Bauml J., Sorg C., Koch K. Changes in extra-striatal functional connectivity in patients with schizophrenia in a psychotic episode. Br. J. Psychiatry. 2017;210:75–82. doi: 10.1192/bjp.bp.114.151928. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Posse S., Wiese S., Gembris D., Mathiak K., Kessler C., Grosse-Ruyken M.L., Elghahwagi B., Richards T., Dager S.R., Kiselev V.G. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med. 1999;42:87–97. doi: 10.1002/(sici)1522-2594(199907)42:1<87::aid-mrm13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P., Ettinger U., Inchley-Mort S., Sumich A., Williams S.C.R., Kuipers E., Kumari V. Neural processing of social rejection: the role of schizotypal personality traits. Hum. Brain Mapp. 2012;33:695–706. doi: 10.1002/hbm.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser J.P., Howes O.D., Chaddock C.A., Joyce E.M., McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr. Bull. 2013;39:1328–1336. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler J., Unterassner L., Wyss T., Haker H., Brugger P., Rössler W., Wotruba D. Schizotypal traits are linked to dopamine-induced striato-cortical decoupling: a randomized double-blind placebo-controlled study. Schizophr. Bull. 2018 doi: 10.1093/schbul/sby079. sby079-sby079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Blasi G., Fazio L., Caforio G., Taurisano P., Romano R., Di Giorgio A., Gelao B., Lo Bianco L., Papazacharias A., Popolizio T., Nardini M., Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Fazio L., Taurisano P., Gelao B., Porcelli A., Mancini M., Sinibaldi L., Ursini G., Masellis R., Caforio G., Di Giorgio A., Niccoli-Asabella A., Popolizio T., Blasi G., Bertolino A. DRD2 genotype-based variation of default mode network activity and of its relationship with striatal DAT binding. Schizophr. Bull. 2013;39:206–216. doi: 10.1093/schbul/sbr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal D.K., Robinson D.G., Fales C., Lencz T., Argyelan M., Karlsgodt K.H., Gallego J.A., John M., Kane J.M., Szeszko P.R., Malhotra A.K. Relationship between duration of untreated psychosis and intrinsic corticostriatal connectivity in patients with early phase schizophrenia. Neuropsychopharmacology. 2017;42:2214–2221. doi: 10.1038/npp.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F., Sterzer P., Schmack K., Ballmaier M., Rapp M., Wrase J., Juckel G., Gallinat J., Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol. Psychiatry. 2009;65:1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Schobel S.A., Chaudhury N.H., Khan U.A., Paniagua B., Styner M.A., Asllani I., Inbar B.P., Corcoran C.M., Lieberman J.A., Moore H., Small S.A. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 2):22–57. [PubMed] [Google Scholar]
- Shmueli K., van Gelderen P., de Zwart J.A., Horovitz S.G., Fukunaga M., Jansma J.M., Duyn J.H. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C., Castro-Fornieles J., de la Serna E., Romero S., Calvo A., Sanchez-Gistau V., Padros-Fornieles M., Baeza I., Bargallo N., Frangou S., Sugranyes G. Altered cortico-striatal connectivity in offspring of schizophrenia patients relative to offspring of bipolar patients and controls. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., He Y., Yan C.-G., Zang Y.-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C., Manoliu A., Neufang S., Myers N., Peters H., Schwerthoffer D., Scherr M., Muhlau M., Zimmer C., Drzezga A., Forstl H., Bauml J., Eichele T., Wohlschlager A.M., Riedl V. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr. Bull. 2013;39:387–395. doi: 10.1093/schbul/sbr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P.-C., Hsieh J.-C., Li C.-T., Bai Y.-M., Su T.-P. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. NeuroImage. 2012;59:238–247. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- Tyrer P., Nur U., Crawford M., Karlsen S., McLean C., Rao B., Johnson T. The social functioning questionnaire: a rapid and robust measure of perceived functioning. Int. J. Soc. Psychiatry. 2005;51:265–275. [PubMed] [Google Scholar]
- Velthorst E., Levine S.Z., Henquet C., de Haan L., van Os J., Myin-Germeys I., Reichenberg A. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in schizophrenia—a control-case family study. Cogn. Neuropsychiatry. 2013;18:574–593. doi: 10.1080/13546805.2012.731390. [DOI] [PubMed] [Google Scholar]
- Wang X., Li F., Zheng H., Wang W., Zhang W., Liu Z., Sun Y., Chan R.C.K., Chen A. Breakdown of the striatal-default mode network loop in schizophrenia. Schizophr. Res. 2015;168:366–372. doi: 10.1016/j.schres.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yan C., Yin D., Fan M., Cheung E.F.C., Pantelis C., Chan R.C.K. Neurobiological changes of schizotypy: evidence from both volume-based morphometric analysis and resting-state functional connectivity. Schizophr. Bull. 2015;41(Suppl. 2):S444–S454. doi: 10.1093/schbul/sbu178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu W.-H., Li Z., Wei X.-H., Jiang X.-Q., Geng F.-L., Zou L.-Q., Lui S.S.Y., Cheung E.F.C., Pantelis C., Chan R.C.K. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol. Med. 2016;46:125–135. doi: 10.1017/S0033291715001592. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ettinger U., Meindl T., Chan R.C.K. Association of schizotypy with striatocortical functional connectivity and its asymmetry in healthy adults. Hum. Brain Mapp. 2018;39:288–299. doi: 10.1002/hbm.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton-Brown T.T., Fusar-Poli P., Ungless M.A., Howes O.D. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85–94. doi: 10.1016/j.tins.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Winton-Brown T., Schmidt A., Roiser J.P., Howes O.D., Egerton A., Fusar-Poli P., Bunzeck N., Grace A.A., Duzel E., Kapur S., McGuire P. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.D., Cowan R.L., Park S., Ansari M.S., Baldwin R.M., Li R., Doop M., Kessler R.M., Zald D.H. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am. J. Psychiatry. 2011;168:418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E.C., Grace A.A. The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J. Neurosci. 2016;36:8977–8984. doi: 10.1523/JNEUROSCI.1402-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Resting-state fMRI striatal connectivity in high and low schizotypy.