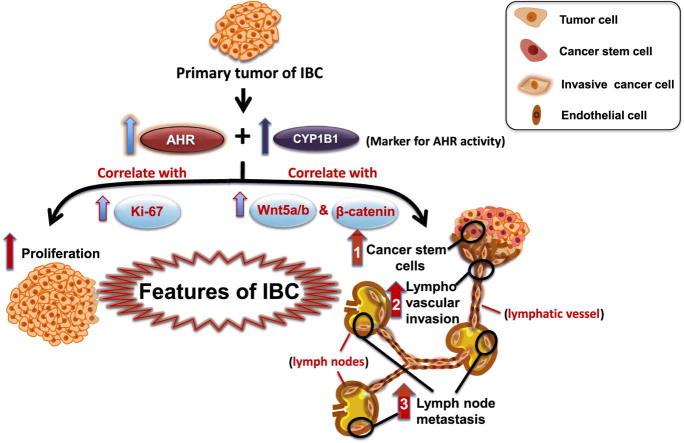
Graphical abstract
Keywords: Inflammatory breast cancer, Aryl hydrocarbon receptor, CYP1B1, Wnt5a/b and β-catenin, CD44+/CD24(−/low) subset and lymphovascular invasion
Highlights
-
•
AHR is over-expressed and hyperactivated in carcinoma tissues of IBC patients.
-
•
AHR knockdown inhibits expression of CYP1B1 and Wnt5a in IBC cells.
-
•
AHR and CYP1B1 expression correlates with Wnt5 a/b and b-catenin expression levels.
-
•
AHR and CYP1B1 expression correlates with percentage of CD44(+)/CD24(−/low) subset in IBC.
-
•
AHR and its surrogate molecules correlate with IBC poor prognosis.
Abstract
The aim of the present study was to evaluate the expression levels of the aryl hydrocarbon receptor (AHR) and its target gene CYP1B1 and to correlate their expression with Wnt5a/b-β-catenin, the CD44+/CD24(−/low) cancer stem cell (CSC) subset and factors associated with poor prognosis in inflammatory breast cancer (IBC) and non-IBC patients. The methods of analysis used were quantitative real-time PCR, western blotting, immunohistochemistry and flow cytometry. Compared to non-IBC tissues, IBC tissues exhibited the overexpression of AHR and its target gene/protein CYP1B1. AHR and CYP1B1 mRNA levels were associated with the poor clinical prognosis markers tumour grade, lymphovascular invasion, cell proliferation and lymph node metastasis. Furthermore, AHR expression correlated with the expression of Wnt5a/b and β-catenin signalling molecules, and Wnt5a mRNA expression was downregulated in the SUM149 human IBC cell line and the MDA-MB-231 non-IBC cell line upon inhibition of AHR. AHR gene knockout (CRISPR-Cas9) inhibits CYP1B1 and Wnt5a expression in the IBC cell line. The CD44+/CD24(−/low) subset was significantly correlated with the expression of AHR, CYP1B1, Wnt5a/b and β-catenin in IBC tissues. The overexpression of AHR and its target CYP1B1 correlated with the expression of Wnt5a/b and β-catenin, CSCs, and poor clinical prognostic factors of IBC. Thus, targeting AHR and/or its downstream target molecules CYP1B1 and Wnt5a/b may represent a therapeutic approach for IBC.
Introduction
Inflammatory breast cancer (IBC) is the most deadly form of breast cancer, with a higher incidence among younger women, with a mean age of 55 years in the USA [1] and 50.3 years in Egypt [2]. Compared with patients with non-IBC tumours, patients diagnosed with IBC have low survival rates [3]. Follow-up at 60 months showed a 59% survival rate for IBC patients who underwent mastectomy and a 57% survival rate for IBC patients who underwent a partial mastectomy [4]. Relative to the low incidence of IBC (1–6% of all breast cancers) in the USA [4], a much higher incidence has been reported in North African countries, such as Egypt, Tunisia, and Morocco (15–20% of all breast cancers) [5], with correspondingly increased mortality compared to that of non-IBC [6]. Of note, international collaboration in IBC research and treatment has introduced different options for clinical management of the disease [7].
The aryl hydrocarbon receptor (AHR), an environmental carcinogen receptor, is a cytosolic ligand-activated transcription factor belonging to the basic-helix-loop-helix-Per-ARNT-Sim (bHLH-PAS) family that regulates the transcription of several genes involved in xenobiotic metabolism and tumour aggression [8], [9], [10], [11]. The cytosolic AHR translocates to the nucleus after binding to its ligand and forming a heterodimer with the AHR nuclear translocator (ARNT) protein. The AHR-ARNT heterodimer then binds to dioxin/xenobiotic responsive elements (DREs/XREs) on the promoters of several AHR target genes, including genes encoding cytochrome P450 enzymes CYP1A1, CYP1A2 and CYP1B1, stimulating transcription [12]. CYP1B1 is an extra-hepatic CYP in the CYP1 family that plays an essential role in the metabolism of several therapeutic drugs and in the bioactivation and detoxification of numerous polycyclic hydrocarbons. Compared to that of normal epithelial cells, AHR expression in DMBA-induced rat mammary carcinoma is extremely high and is associated with higher CYP1B1 expression, a validated marker of AHR activity [13], [14]. Similarly, higher AHR expression, constitutive AHR activation, and elevated levels of CYP1B1 in breast carcinoma cells induce the expression of stem properties and the formation of a mammosphere [15]. Indeed, constitutive AHR activation, enforced by the production of tumour-derived endogenous AHR ligand activity, drives high levels of CYP1B1 expression in breast cancer [16], [17], [18].
Elevated AHR expression and activity is also a characteristic of other cancer types. In gastric cancer, AHR expression is associated with a poor clinical pathological pattern, including lymph node and distant metastasis [19]. In upper urinary tract urothelial cancer, the expression of AHR induces invasion of carcinoma cells via the activation of matrix-metalloproteinase-9 (MMP-9) [20]. In breast cancer, AHR expression correlates with p53 levels and is associated with malignant progression of breast cancer [17], [18]. AHR induces cell proliferation and migration [21], a hallmark of IBC. Suppression of AHR inhibits the proliferation of head and neck tumour cells [22].
IBC is characterized by aggressive local invasion into the lymphatic vessels of the skin and the formation of emboli and distant metastases. Tumour emboli in lymphatic vessels are characterized by the expression of CSC markers and distinguish IBC from other forms of breast cancer. Tumour cells within the lymphatic emboli are highly adherent and metastasize to different organs as a clump of cells [23].
Previously, it was demonstrated that AHR ligands induce breast cancer stem-like cells in human triple-negative breast cancer (TNBC) and IBC [11] cells. Furthermore, correlations were found between the AHR signalling pathway and markers of cancer stem-like cells and the Wnt signalling pathway [24]. A higher incidence of CD44+/CD24(−/low) cells was found to be significantly associated with axillary lymph node metastasis and is a presumptive predictor of metastatic disease in breast cancer patients [25].
Several studies have reported that IBC aggressiveness may be attributed to relatively high levels of radio- and chemoresistant CSCs [26], [27], [28]. CSCs are characterized by self-renewal, resistance to apoptosis, an efficient DNA repair capacity, and the expression of multidrug-resistance proteins [29], [30]. CSCs can be distinguished from the cells making up the bulk of the tumour by the surface expression of CD44 and CD24 (as a CD44+/CD24(−/low) subpopulation) and the presence of elevated aldehyde dehydrogenase-1 (ALDH1) activity [26], [27], [28]. Given their central role in tumour metastasis and resistance to treatment, it is critical to better understand the mechanisms regulating CSC development, particularly in highly aggressive cancers, such as IBC, and to devise rational strategies for targeting CSCs in IBC [31], [32]. CSC development and maintenance are modulated by several signalling pathways, including the Wnt pathway [15]. Cross talk between AHR and Wnt signaling pathways suggested to play a crucial role in the mechanisms of carcinogenesis including tumour initiation, promotion and progression (for review see [33]). The Wnt5a is one of the Wnt family members’ found to regulate cancer stem cell properties, cancer proliferation and migration via canonical Wnt/β-catenin signalling [34]. Expression and intracellular localization of β-catenin may exist associated with E-cadherin at the plasma membrane or intracellular, however, β-catenin- associated with Wnt signaling found to be localized cytoplasmic and/or nuclear [35].
Signalling pathways involved in CSC development are regulated by multiple transcription factors, including the AHR [11], [33]. For example, Al-Dhfyan and colleagues showed that the induction of AHR/CYP1A1 in MCF-7 cells induced the overexpression of β-catenin and its downstream target Cyclin D1 at the mRNA and protein levels. Moreover, the AHR/CYP1A1 signalling pathway mediates breast CSC proliferation and chemoresistance via inhibition of PTEN and activation of the β-catenin and Akt pathways [15].
Because hyper-expressed AHR is associated with increased aggressiveness in vivo, the hypothesis in this study is that AHR expression and activity, as measured by CYP1B1 target gene/protein expression, would be elevated in IBC compared with normal or non-IBC breast cancers and would correlate with Wnt expression and poor prognosis. Furthermore, this study predicted that AHR expression and/or activity would correlate with elevated CSC levels and Wnt signalling. To test these predictions, the expression levels of AHR and CYP1B1, as a marker of AHR activity, were determined in carcinoma samples from non-IBC and IBC patients. Additionally, we assessed whether AHR and CYP1B1 expression correlates with Wnt/β-catenin levels, the relative abundance of CD44+/CD24(−/low) cells and poor prognostic factors, such as tumour grade, the involvement of metastatic lymph nodes, and lymphovascular invasion in non-IBC and IBC patients. Finally, to demonstrate a causal relationship between AHR activity and at least one driver of CSC formation, the ability of an AHR inhibitor to downregulate Wnt5a in non-IBC and IBC cell lines was evaluated. In addition, Knockout of AHR gene inhibited the expression of CYP1B1 and Wnt5a in IBC cells.
Patient and methods
Patient samples
A total of 14 healthy volunteers undergoing breast reduction mammoplasty and 61 breast cancer patients (33 non-IBC and 28 IBC) were enrolled in these studies. The study protocol was approved by the Institutional Review Board of the Faculty of Medicine of Ain Shams University (IRB#00006379), Egypt. IBC patients were diagnosed clinically and pathologically, as described previously [36].
Immunohistochemistry (IHC)
Protein staining using IHC was assessed using 4-μm-thick paraffin-embedded tissue sections, as described previously [28], using antibodies against AHR (dilution 1:500) (Santa Cruz, Texas, USA), CYP1B1 (dilution 1:500) (Abcam, MA, USA), CD44 (clone 156-3-c11) (dilution 1:800) (Cell Signaling Technology, MA, USA) and Ki-67 (SP6) (Cell Marque Corporation, CA, USA). IHC staining was carried out by adding 100 µL of the chromogen DAB + diluted 1:50 in substrate buffer [EnVision + Dual Link System-HRP (DAB + )] for 10 min. Finally, tissue specimens were washed in phosphate-buffered saline (PBS), the nuclei were counterstained with haematoxylin, and the specimens were mounted using Permount® for examination by light microscopy. The stained area fractions were evaluated using ImageJ software (National Institutes of Health, MD, USA).
Quantitative real-time PCR
Total RNA was extracted from fresh breast tissue and cells using a GeneJETTM RNA Purification Kit (Thermo Scientific, ON, Canada). The total RNA concentration was assessed by Infinite®200 PRO NanoQuant (Tecan, Zürich, Switzerland). One microgram of RNA was reverse transcribed into complementary DNA (cDNA) using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, ON, Canada). Quantitative PCR was conducted as described previously by Ibrahim and colleagues [28] via the StepOnePlus 96-well device (Applied Biosystems, CA, USA) in a 25 μL total reaction volume using 12.5 μL of SYBR green master mix (Applied Biosystems, Brumath, France), 1 μL of each primer (AHR, CYP1B1, 10 pmol\μL (Qiagen, CA, USA); Wnt5a, 10 pmol\μL (a gift from Prof. Dr. Martin Götte, Department of Gynecology and Obstetrics, Münster University, Münster, Germany), Forward 5′-TCGTTAGCAGCATCAGTCCACA-3′ and reverse 5′-GACCTGTGCCTTCGTGCCTA-3′), 2.5 μL of cDNA and 8 μL of RNase free water. The thermal profile of PCR started with an initial denaturation at 95 °C for 10 min, followed by 45 cycles of 94 °C for 15 sec, 55 °C for 30 sec and 60 °C for 30 sec. The data presented as relative gene expression of AHR and CYP1B1 were evaluated using the 2−ΔΔCt method after normalization to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Qiagen, CA, USA) (10 pmol\μL).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting
Tissue lysates were prepared from fresh breast tissues obtained during surgery, as described by El-Shinawi and colleagues [37]. A Bradford assay was used to determine the protein concentration (BioRad Laboratories, CA, USA) using Infinite®200 PRO NanoQuant (Tecan, Zürich, Switzerland). Protein samples with equal concentrations (30 μg/well) were separated by 10% SDS-PAGE, using Mini-PROTEAN® II Electrophoresis Cell (BioRad Laboratories, CA, USA) apparatus that handle two gels. The two gels one gel non-IBC patient samples and one gel IBC samples run in parallel in the same Gel System chamber connected to one electrophoresis apparatus and the run was performed according to manufacturer’s instruction (BioRad Laboratories, CA, USA). Proteins were transferred from gel to polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA) using Mini Trans-Blot® Electrophoretic Transfer Cell apparatus (BioRad Laboratories, CA, USA). Western blots were performed by blotting each gel onto PVDF membrane and the 2 gels (non-IBC and IBC) runs in the same holder cassettes according to manufacturer’s instruction (BioRad Laboratories, CA, USA). SDS-PAGE and Western Blot were conducted several times to detect the candidate markers in each patient sample non-IBC (n = 33) and IBC (n = 28). The PVDF membranes were blocked for 1 h with 5% non-fat dry milk in TBS-0.5% Tween 20 followed by incubation overnight at 4 °C with antibodies specific for the AHR (Santa Cruz, Texas, USA), CYP1B1 (Abcam, Cambridge, United Kingdom), β-catenin (Cell Signaling, MA, USA) or Wnt5a/b (Cell Signaling, MA, USA) and then washed and incubated for 1 h with a 1:10,000 dilution of a peroxidase-labelled goat anti-rabbit secondary antibody. After washing, protein bands were visualized by enhanced chemiluminescence (ECL) (Thermo Scientific, ON, Canada). Luminescence was measured digitally by using an iBox Scientia 615 Imaging System (UVP Upland, CA, USA) analyzed and measured by with ImageJ software (National Institutes of Health, MA, USA) using β-tubulin as a loading control [38]. It should be noted that the membranes were stripped using a mild stripping buffer (15 g glycine, 1 g SDS, 10 mL Tween 20, dissolve in 1 L distilled water, pH 2.2) and re-stained with β-tubulin antibody using same method as described above.
Flow cytometry
Single-cell suspensions were prepared by mincing 1 mm3 slices of breast carcinoma tissues with paired scalpels, followed by digestion with 5 mg/mL collagenase (SERVA, Heidelberg, Germany) and dispase (Sigma Aldrich, MO, USA) [28]. Enzymatic dissociation was continued at 37 °C until a predominantly single-cell suspension was obtained (2–4 h). Undigested cell clumps were removed by filtering through a 50 µm pore size cell strainer. Breast cancer cells were separated from leukocytes and debris by Ficoll-Hypaque density gradient centrifugation (Lonza, Amboise, France). Pellets of single-cell suspensions were centrifuged at 800 rpm for 5 min and washed twice with staining buffer (PBS/1% BSA). Staining was achieved by incubating 7 × 105 cells in 100 µL of staining buffer with 10 µL of a FITC-conjugated CD44-specific antibody (Immunotools, Friesoythe, Germany) and 10 µL of a PE-conjugated CD24-specific antibody (Immunotools, Friesoythe, Germany). FITC and PE isotype control antibodies were used as negative controls. Incubation was carried out at room temperature in the dark for 30 min. The stained cells were then washed twice, re-suspended in 1 mL of staining buffer, and acquired by a Partec Cube 8 Flow Cytometer (Sysmex Partec, Görlitz, Germany). Gating to exclude cell debris by forward and side scatter and subsequent data analysis were performed using FSC Express 4 (De Novo software, CA, USA).
Human breast cancer cell lines and AHR inhibition
MDA-MB-231 cells, representing ER−PR−Her2− non-IBC, and ER−PR−Her2− SUM149 cells, representing ER−PR−Her2− IBC (a gift from Prof. Dr. Martin Götte, Department of Gynecology and Obstetrics, Münster University, Münster, Germany) were used in the present study. MDA-MB-231 and SUM149 cells were cultured in 6-well plates (2 × 105 and 3 × 105 cells/well, respectively). MDA-MB-231 cells were seeded in 2 mL of Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS). SUM149 cells were seeded in Ham's F-12 supplemented with 10% FBS and appropriate nutrients. The cells were incubated overnight at 37 °C in a humidified CO2 incubator. MDA-MB-231 and SUM149 cells were then treated with the AHR inhibitor CH223191 (10 µM) (Sigma Aldrich, Missouri, USA) for 48 h in a 5% CO2 humidified incubator at 37 °C. The cells were washed twice with PBS, seeded in culture medium without FBS, and collected for quantitative real-time PCR, as described previously [11].
AHR knockout (KO) in SUM149 cells
As previously described by Rothhammer and colleagues [39], SUM149 cells (Prof. Dr. David Sherr, Department of Environmental Health, School of Public Health, Boston University) in which the AHR was knocked out (AHRKO) were generated as follows: the vector lentiCRISPR v2 (Addgene, MA, USA) contains Cas9 and a guide RNA cloning site (BsmBI). The two target sequences (5′-CCTACGCCAGTCGCAAGCGG-3′ and 5′-CCGAGCGCGTCCTCATCGCG-3′, NM_001621), selected by CRISPR designer (http://crispr.mit.edu), located in the first exon of the AHR. The construct was confirmed by DNA sequencing. SUM149 cells were infected with AHR guide RNA/lentiCRISPRv2 lentivirus according to the standard protocol [40]. Cells were selected for 10 days with 2.0 μg/mL puromycin. Controls consisted of cells transfected with Cas9 without guide RNA and selected as above. AHR knockout was confirmed by Western blotting using AHR-specific antibody, dilution 1:1000 (Cell Signaling Technology, MA, USA) using β-actin-dilution 1:2000 as control (Sigma-Aldrich, MO, USA) as published before by the authors [39].
Total RNA from AHRKO and control cells was extracted using the RNeasy Mini Kit (Qiagen, CA, USA) according to the manufacturer’s instructions. cDNA was generated using the High Capacity cDNA reverse Transcription Kit (Applied Biosystems, CA, USA) following manufacturer instructions. Quantitative PCR analysis was conducted on StepOnePlus™ Real-Time PCR System. Relative mRNA expression was quantified using the 2−ΔΔCt method as described before by the authors [41]. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used in each reaction as an internal reference gene. Assays were performed in quadruplicate. TaqMan probes were used for the human WNT5A (Hs00998537_m1), CYP1B1 (Hs00164383_m1), and GAPDH (Hs99999905_m1) from the TaqMan®Gene Expression Assays (Applied Biosystems, CA, USA).
Statistical analysis
SPSS 22.0 software was used in all statistical analyses performed in this study. The significance of differences among patient groups were assessed by Student’s t test, Wilcoxon test and the Chi square test, while associations with patient clinical data were assessed by Pearson’s correlation coefficient. All obtained data are represented as the mean ± standard deviation (SD).
Results
Clinical and pathological characterization of non-IBC and IBC patients
The clinical and pathological characteristics of the patients are described in Table 1. Statistical analysis revealed that compared with non-IBC patients, IBC patients were characterized by a significantly larger tumour size and a higher tumour grade (P = 0.007 and 0.046, respectively). Lymph node metastasis and the presence of lymphovascular invasion were significantly more frequent (P = 0.002 and 0.001, respectively) in IBC patients than in non-IBC patients.
Table 1.
Clinical and pathological characterization of non-IBC versus IBC patients.
| Characteristic | Non-IBC | IBC | P value |
|---|---|---|---|
| (N = 33) | (N = 28) | ||
| Age [year] | |||
| Range | 34–72 | 29–56 | 0.253a |
| Mean ± SD | 53 ± 10.1 | 50.2 ± 10.4 | |
| Tumour size [cm] | |||
| Mean ± SD | 4.3 ± 2.5 | 5.32 ± 2.3 | 0.007*,b |
| ≤4 | 22 (66.7%) | 9 (32.1%) | |
| >4 | 11 (33.3%) | 19 (67.9%) | |
| Tumour grade | |||
| G1 | 4 (12.1%) | 0 (0%) | 0.046*,b |
| G2 | 26 (78.8%) | 19 (67.8%) | |
| G3 | 3 (9.1%) | 8 (28.6%) | |
| G4 | 0 (0%) | 1 (3.6%) | |
| Axillary lymph node metastasis | |||
| ≤4 | 24 (72.7%) | 6 (21.4%) | 0.002*,b |
| >4 | 9 (27.3%) | 22 (78.6%) | |
| Lymphovascular invasion | |||
| Negative | 28 (84.8%) | 8 (28.6%) | 0.001**,b |
| Positive | 5 (15.2%) | 20 (71.4%) | |
| ER | |||
| Negative | 17 (51.5%) | 16 (57.1%) | 0.428b |
| Positive | 16 (48.5%) | 12 (42.9%) | |
| PR | |||
| Negative | 19 (57.6%) | 16 (57.1%) | 0.589b |
| Positive | 14 (42.4%) | 12 (42.9%) | |
| Her-2 | |||
| Negative | 27 (81.8%) | 23 (82.1%) | 0.621b |
| Positive | 6 (18.2%) | 5 (17.9%) | |
Data are reported as the mean ± SD, numbers and percentage.
Statistical significance.
Student’s t test.
Chi square test.
P ≤ 0.05.
P ≤ 0.001.
AHR and CYP1B1 mRNA and protein levels are higher in IBC than in non-IBC tissues
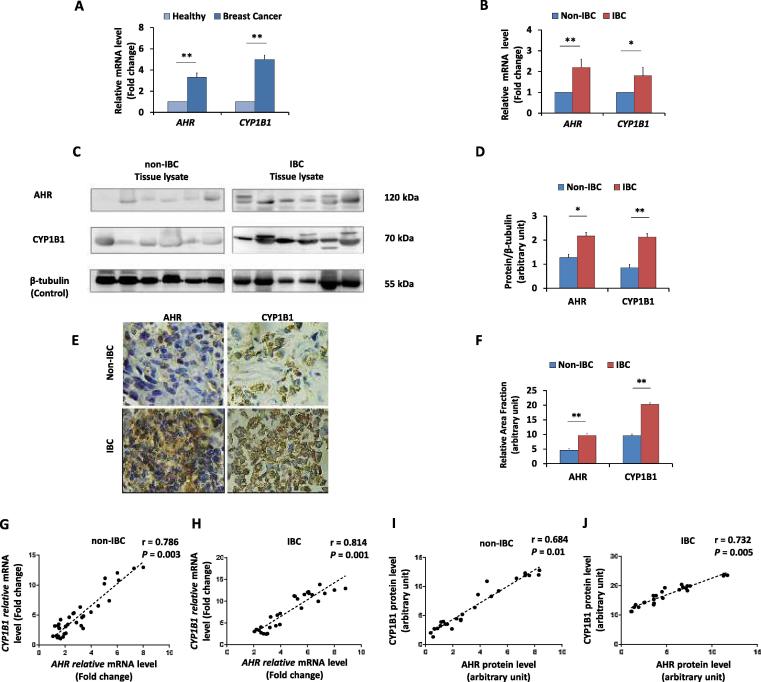
The expression of AHR and its target CYP1B1 was assessed in carcinoma tissue from IBC and non-IBC patients at the mRNA and protein levels relative to their expression in normal breast tissues obtained from mammoplasties. The expression of AHR and CYP1B1 mRNA was significantly higher in breast carcinoma tissues (P = 0.01 and 0.003, respectively) than in normal breast tissues (Fig. 1A). Moreover, the mRNA expression of AHR and CYP1B1 was significantly higher in IBC (P = 0.02 and 0.01, respectively) than in non-IBC tissues (Fig. 1B). Western blot analysis (Fig. 1C & D) showed that the expression of the AHR and CYP1B1 proteins was significantly higher in IBC than in non-IBC tissue samples (P = 0.02 and 0.005, respectively). Using IHC (Fig. 1E & F), significantly stronger immunostaining of AHR and CYP1B1 proteins was detected in IBC than in non-IBC tissues (P = 0.01 and 0.004, respectively). Since the expression of CYP1B1 is a surrogate marker for AHR activity [14], [18], the correlation between the expression of AHR and CYP1B1 at the mRNA and protein levels was tested in carcinoma tissues from IBC versus non-IBC patient groups. As predicted from previous work [10], a significant positive correlation was detected between the expression levels of AHR and CYP1B1 mRNA in non-IBC tissues (r = 0.786 and P = 0.003) and in IBC tissues (r = 0.814 and P = 0.001) (Fig. 1G and H). Similarly, a positive correlation between AHR and CYP1B1 protein expression was detected in non-IBC samples (r = 0.684 and P = 0.01), which was significantly stronger in IBC (r = 0.732 and P = 0.005) samples (Fig. 1I and J).
Fig. 1.
mRNA and protein expression levels of AHR and CYP1B1 in non-IBC and IBC carcinoma tissues. Bars represent the fold change (RQ = 2−ΔΔCt) of mRNA expression of AHR and CYP1B1 in (A) breast cancer tissues (n = 61) after normalization to values of healthy tissues (n = 14), and in (B) IBC carcinoma tissues (n = 28) after normalization to values of non-IBC carcinoma tissues (n = 33). Data represent mean ± SD, *P ≤ 0.05 and **P ≤ 0.001 as determined by Student’s t-test. (C) Representative of immunoblots membranes showing the protein expression of AHR and CYP1B1 in non-IBC and IBC, tissue lysates of non-IBC and IBC were analyzed by SDS-PAGE, transferred into PVDF membranes and immunoblotted with antibodies specific for AHR and CYP1B1. (D) Bars represent the relative density values of detected protein bands assessed by ImageJ software and normalized against the loading control β-tubulin, showing significantly higher expression of AHR and CYP1B1 in IBC (n = 28) than in non-IBC (n = 33) carcinoma tissues. (E) Representative fields of immunostaining (brown colour stain) of AHR and CYP1B1 in paraffin embedded breast carcinoma tissue sections showing high density of cancer cells positive for AHR and CYP1B1 in IBC (n = 20) compared to non-IBC patients (n = 25) (magnification 40X). (F) Bars represent the relative area fraction to healthy breast tissues calculated by using ImageJ software. Data represent the mean ± SD, *P ≤ 0.05 and **P ≤ 0.001 as determined by Student’s t-test. (G and H) Represent scatter charts showing the positive correlations between the mRNA expression of AHR and CYP1B1 in non-IBC and IBC carcinoma tissues. (I and J) Represent scatter charts showing the positive correlations between the protein expression of AHR and CYP1B1 in non-IBC tissues and IBC tissues. Correlation coefficients (r values) were calculated by Pearson’s correlation test.
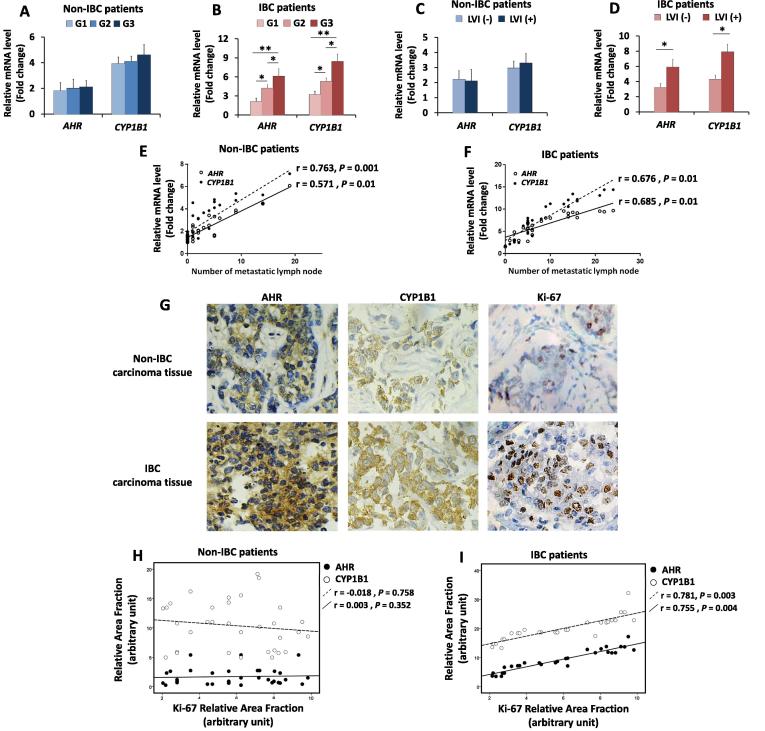
AHR and CYP1b1 mRNA expression positively correlates with the number of metastatic lymph nodes and with tumour grade, lymphovascular invasion and the expression of Ki-67 in IBC
The AHR pathway is associated with poor prognostic markers, such as tumour grade, tumour stage, lymph node metastasis and lymphovascular invasion and MMP expression, in upper urinary tract urothelial cancer [20]. Therefore, an evaluation of whether there was a similar correlation between AHR and CYP1B1 expression was performed in non-IBC and IBC patients. Statistical analysis revealed that AHR and CYP1B1 expression was associated with tumour grade in IBC patients (P = 0.006 and 0.002, respectively) but not in non-IBC patients (Fig. 2A and B). In addition, AHR and CYP1B1 expression correlated with the presence of lymphovascular invasion (r = 0.589. P = 0.01 and r = 0.536, P = 0.02, respectively) in IBC but not in non-IBC (Fig. 2C and D). The expression of AHR and CYP1B1 mRNA was positively correlated with the number of metastatic lymph nodes in non-IBC (r = 0.571, P = 0.01 and r = 0.763, P = 0.001, respectively) (Fig. 2E) and IBC patients (r = 0.685, P = 0.01 and r = 0.676, P = 0.01, respectively) (Fig. 2F).
Fig. 2.
Correlation between the expression of AHR and CYP1B1 and patient clinical and pathological properties. Bars represent the fold change (RQ = 2−ΔΔCt) of mRNA expression non-IBC and IBC patients sub-grouped into different tumour grade (G1, G2, G3). (A) mRNA expression of AHR and CYP1B1 in non-IBC patients show no statistical correlation with tumour grade. (B) mRNA expression of AHR and CYP1B1 significantly correlates with high tumour grade in IBC patients. (C and D) Bars represent mRNA expression of AHR and CYP1B1 in non-IBC and IBC patients sub-grouped with negative and positive lymphovascular invasion (LVI) (C) mRNA expression of AHR and CYP1B1 in non-IBC patients, with no statistical correlation with LVI. (D) mRNA expression of AHR and CYP1B1 significantly correlates with LVI in IBC patients. Data represent mean + SD, *P ≤ 0.05 and **P ≤ 0.001 as determined by one-way ANOVA followed by Tukey’s multiple comparison test. (E and F) Scatter charts showing the positive correlation between the mRNA expression of AHR and CYP1B1 and the number of metastatic lymph nodes in non-IBC and IBC patients, respectively. (G) Representative fields of immunostaining (brown colour) of highly expressed AHR, CYP1B1 and Ki-67 in in paraffin embedded IBC and non-IBC carcinoma tissues. (H and I) Scatter charts showing a strong positive correlation between AHR and CYP1B1 expression and Ki-67 expression in IBC but not non-IBC tissues. Correlation coefficient (r values) were calculated by Pearson’s correlation test.
The proliferation marker Ki-67 plays a crucial role in treatment decisions for breast cancer patients [42]. AHR was found to augment cell proliferation and survival [43]. In the present study, the expression of Ki-67 (Fig. 2G) was assessed by IHC, and whether AHR and CYP1B1 protein expression was correlated with Ki-67 expression in carcinoma tissues from non-IBC and IBC patients was tested. IHC revealed that both AHR and CYP1B1 protein expression positively correlated with the expression of Ki-67 in IBC patients (r = 0.781, P = 0.003 and r = 0.755, P = 0.004, respectively) but not in non-IBC patients (Fig. 2H and I).
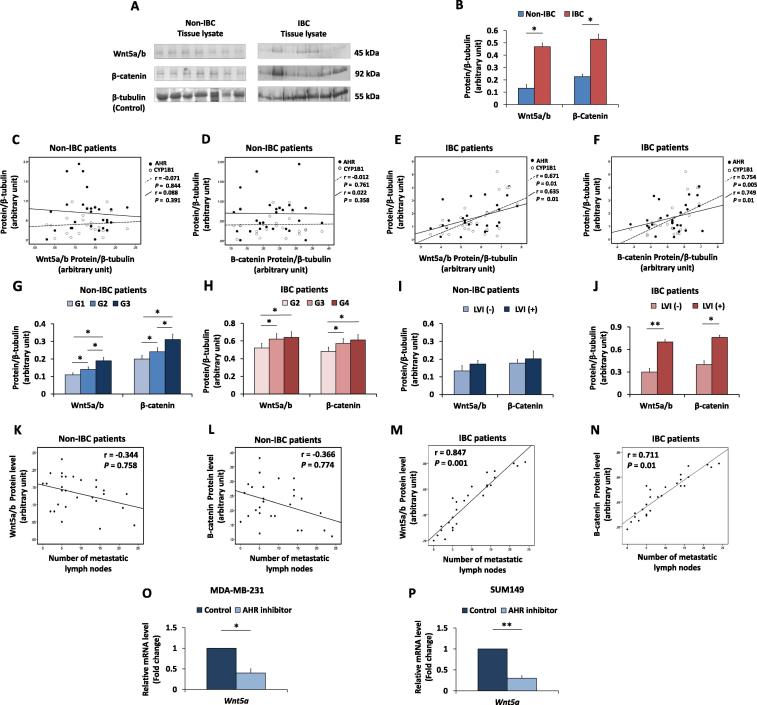
Wnt5a/b and β-catenin expression are significantly higher in IBC than in non-IBC carcinoma tissues and positively correlated with AHR and CYP1B1 protein expression, tumour grade and lymphovascular invasion
Different cellular molecules are able to control Wnt/β-catenin signalling pathways involved in the regulation of CSC properties (reviewed in [44]). In prostate cancer, the association between the upregulation of AHR and activation of the Wnt/beta-catenin pathway was found to play a significant role in disease progression [45]. Previous studies showed that treatment with the prototypical polycyclic aromatic hydrocarbon (PAH) 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary tumours characterized by AHR overexpression and subsequent activation of downstream regulatory signalling pathways, including Wnt signalling [24].
Herein, western blot analysis revealed a significant increase in the expression of Wnt5a/b and β-catenin in IBC tissue compared to that in non-IBC tissue (P = 0.03 and 0.02, respectively) (Fig. 3A & B). In non-IBC carcinoma tissues, AHR and CYP1B1 protein expression failed to significantly correlate with the expression of Wnt5a/b (Fig. 3C) or β-catenin (Fig. 3D). However, in IBC carcinoma tissues, AHR and CYP1B1 protein expression was positively correlated with the expression of Wnt5a/b (r = 0.671, P = 0.01 and r = 0.635, P = 0.01, respectively) (Fig. 3E) and with the expression of β-catenin (r = 0.754, P = 0.005 and r = 0.749, P = 0.01, respectively) (Fig. 3F). Moreover, the expression of Wnt5a/b and β-catenin positively correlated with a high tumour grade in both non-IBC (P = 0.03 and 0.02, respectively) (Fig. 3G) and IBC (P = 0.048 and 0.041, respectively) (Fig. 3H) patients. The expression of Wnt5a/b and β-catenin positively correlated with the presence of lymphovascular invasion (r = 0.735, P = 0.01 and r = 0.620, P = 0.03, respectively) (Fig. 3I and J) in IBC but not in non-IBC. Next, a possible correlation between the expression of Wnt5a/b and β-catenin and the number of metastatic lymph nodes in non-IBC and IBC patients was evaluated. Statistical analysis revealed no correlation between Wnt5a/b or β-catenin and lymph node metastases in non-IBC breast cancer patients (Fig. 3K and L). In contrast, a significant correlation between Wnt5a/b and β-catenin and the number of metastatic lymph nodes (r = 0.847, P = 0.001 and r = 0.711, P = 0.01, respectively) was detected in IBC patients (Fig. 3M and N). Indeed, Wnt5a is an activator of canonical and non-canonical Wnt signalling cascades (23).
Fig. 3.
Wnt5a/b and β-catenin expression in carcinoma tissues from non-IBC and IBC patients and their correlations with AHR/CYP1B1 and patents clinical pathological properties. (A) Representatives of protein expression of Wnt5a/b and β-catenin in non-IBC and IBC carcinoma tissue lysates analyzed by SDS-PAGE, transferred into PVDF membranes showing high expression of Wnt5a/b and β-catenin in breast carcinoma tissues of IBC patients. (B) Bars represent the relative density values of protein bands assessed by ImageJ software and normalized against the loading control β-tubulin and showing significant expression of Wnt5a/b and β-catenin in IBC tissues (n = 28) compared to non-IBC tissues (n = 33). Data represent the mean ± SD, *P ≤ 0.05 and **P ≤ 0.001 as determined by Student’s t-test. (C and D) Scatter charts showing no correlation between AHR and CYP1B1 expression and Wnt5a/b and β-catenin expression in non-IBC carcinoma tissues. (E and F) Scatter charts showing a linear positive correlation between AHR/CYP1B1 expression and Wnt5a/b and β-catenin expression in IBC carcinoma tissues. (G and H) Bars represent expression of Wnt5a/b and β-catenin in non-IBC and IBC patients sub-grouped according to the tumour grade, both correlates with tumour grade in the non-IBC (G) and IBC (H) patient groups. (I and J) Bars represent the relative density values of Wnt5a/b and β-catenin proteins sub-grouped into negative and positive LVI, results showed no statistical correlation with LVI in non-IBC patients and a significant correlation with lymphovascular invasion in IBC patients. Data represent mean ± SD, *P ≤ 0.05 and **P ≤ 0.001 as determined by one-way ANOVA followed by Tukey’s multiple comparison test. (K – N) Scatter charts showing a weak correlation between the number of metastatic lymph nodes and the relative density values of Wnt5a/b (K) and β-catenin (L) proteins in non-IBC tissues and a linear positive correlation between the number of metastatic lymph nodes and the relative density values of Wnt5a/b (M) and β-catenin (N) proteins in IBC tissues. Correlation coefficient (r values) were calculated by Pearson’s correlation test. (O and P) Bars represent the fold change (RQ = 2−ΔΔCt) of mRNA expression of Wnt5a in MDA-MB-231 (O) and SUM149 (P) cells after treatment with an AHR inhibitor. The results are representative of at least three independent experiments. Data represent the mean ± SD. *P ≤ 0.05 and **P ≤ 0.001 as determined by Student’s t test.
Previously, it was shown that Wnt5a is a downstream effector of AHR activation [24] in mammary tumour cells. Given the correlation between AHR and Wnt5a expression and the implication of both of these proteins in CSC development, our hypothesis predicted that activation of AHR stimulates activation of Wnt5a pathways and the development of CSCs in IBC. To determine if there was a causal relationship between the AHR and Wnt5a, the effect of an AHR inhibitor on Wnt5a levels was quantified in the triple-negative IBC SUM149 cell line and the non-IBC MDA-MB-231 cell line. Relative to control cells seeded in culture medium, quantitative real-time PCR results showed that there was a significant downregulation of Wnt5a (P = 0.01 and 0.02, respectively) upon AHR inhibition (Fig. 3O and P), demonstrating the potential for AHR regulation of the Wnt signalling pathway in both tumour types.
AHR and CYP1B1 mRNA positively correlate with the percentage of CD44(+)/CD24(−) cells in IBC
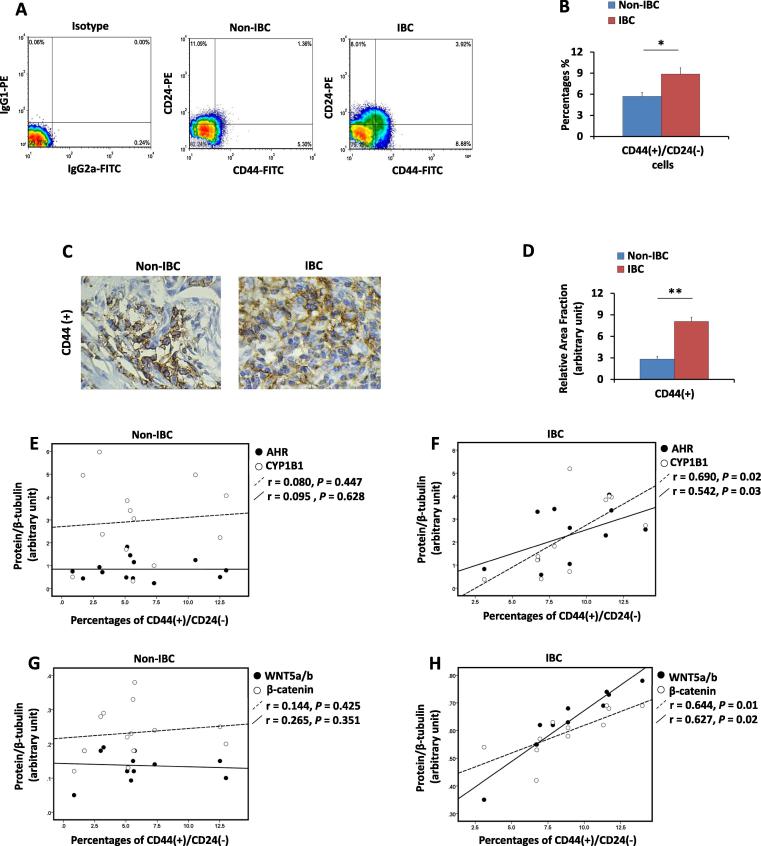
IBCs are characterized by the presence of tumour emboli enriched with CD44(+)/CD24(−/low) CSCs that enhance tumourigenic and metastatic properties [46]. Therefore, the percentage of CD44(+)/CD24(−/low) cells in IBC and non-IBC tumours was quantified and correlated with AHR and CYP1B1 levels. Flow cytometry analysis revealed a significant enrichment of the CD44(+)/CD24(−/low) subpopulation in IBC tissue compared with that of non-IBC tissue (P = 0.04) (Fig. 4A & B). Similarly, IHC for CD44 staining showed that IBC tissues are characterized by a significantly higher level of CD44 immunostaining (P = 0.001) than non-IBC tissues (Fig. 4C & D). A previous study demonstrated that AHR and CYP1B1 protein expression is elevated in CSC-like breast cancer cells in which AHR controls the expression of important self-renewal- and chemo-resistance-associated genes [11]. To extend this notion to the cohort of tissue specimens, the proportion of CD44(+)/CD24(−/low) cells was compared with AHR and CYP1B1 expression in IBC and non-IBC tissues. The percentage of CD44(+)/CD24(−/low) cells in the carcinoma tissues of non-IBC did not show a correlation with AHR or CYP1B1 expression (r = 0.08, P = 0.477 and r = 0.09, P = 0.628) (Fig. 4E). However, the percentage of CD44(+)/CD24(−/low) cells positively correlated with AHR and CYP1B1 expression (r = 0.690, P = 0.02 and r = 0.542, P = 0.03, respectively) (Fig. 4F).
Fig. 4.
Percentage of CD44(+)/CD24(−/low) cells in non-IBC and IBC carcinoma tissues. (A) A representative flow cytometric bivariate plots showing the percentages of CD44(+)/CD24(−/low) cells in non-IBC and IBC tissues. (B) Bars represent the percentages of CD44(+)/CD24(−/low) cells in non-IBC and IBC single-cell suspensions prepared from carcinoma tissues. Data shown are a single experiment of different independent experiments and represent mean ± SD. *P ≤ 0.05 and **P ≤ 0.001 as determined by Wilcoxon test. (C) Representative fields of immunostaining (brown colour stain) of CD44(+) in non-IBC and IBC tissues (magnification 40X). (D) Bars represent the relative area fraction of CD44(+) IHC staining using the ImageJ program in tissue sections with a significant increase in the percentages of CD44(+) cells in IBC compared to non-IBC patients. Data represent mean + SD. *P ≤ 0.05 and **P ≤ 0.001 as determined by Student’s t test. (E and F) Scatter chart showing correlations between the protein expression of AHR and CYP1B1 and the percentage of CD44(+)/CD24(−/low) cells. A weak correlation was detected in non-IBC patients (E), and a linear positive correlation was detected in IBC patients (F). (G and H) Scatter chart showing the correlations between the protein expression of Wnt5a/b and β-catenin and the percentage of CD44(+)/CD24(−/low) cells. A weak correlation was detected in non-IBC patients (G), and a linear positive correlation was detected in IBC patients (H). Correlation coefficient (r values) were calculated by Pearson’s correlation test.
a correlation between the proportion of CD44(+)/CD24(−/low) cells and the expression of Wnt5a/b or β-catenin in non-IBC samples was not detected (r = 0.144, P = 0.425 and r = 0.265, P = 0.351, respectively) (Fig. 4G) On the other hand, the proportion of CD44(+)/CD24(−/low) cells positively correlated with the expression of Wnt5a/b and β-catenin in IBC patients (r = 0.644, P = 0.01 and r = 0.627, P = 0.02, respectively) (Fig. 4H).
Down regulation of Wnt5a expression after FICZ hyper-activation of AHR and after AHR knock down in SUM149 IBC cells
Since AHR and CYP1B1 levels correlated with Wnt5a/b and β-catenin in IBC but not in non-IBC (Fig. 3E and F). In addition, AHR and CYP1B1 correlated with an increase in the proportion of CD44(+)/CD24(−/low)cells, and Wnt/β-catenin signalling that drives breast cancer metastasis and stem cell properties [47], [48],
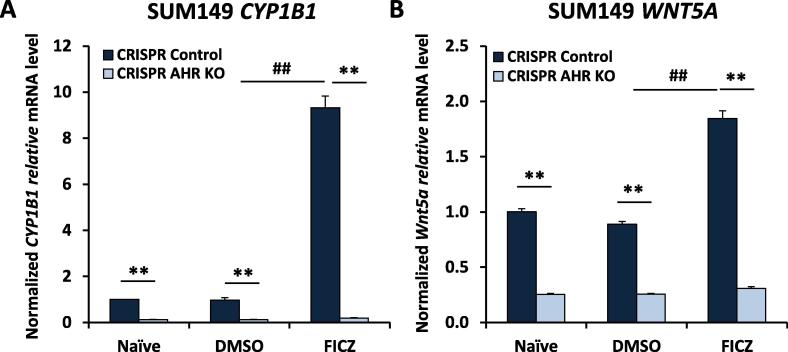
To further demonstrate AHR control of Wnt5a in IBC, the AHR was deleted from SUM149 cells by CRISPR-Cas9 gene editing (AHRKO cells) and evaluated for CYP1B1 (as a positive control for AHR knockout) and Wnt5a expression by quantitative PCR. Consistent with the Dr. Sherr’s lab recent and previous studies [11], [39], SUM149 cells exhibited a significant baseline of CYP1B1 expression that was decreased by AHR knockout and increased by treatment with an AHR agonist, FICZ (Fig. 5A). The FICZ-induced increase in CYP1B1 also was prevented by AHR knockout indicating AHR control of CYP1B1. Similarly, AHR knockout significantly decreased baseline and FICZ-induced Wnt5a expression (Fig. 5B) supporting the hypothesis that the AHR strongly influences Wnt5a expression. No significant changes in other members of the Wnt signaling pathway such as Wnt5b or Wnt11 were seen (data not shown).
Fig. 5.
WNT5a expression after FICZ hyper-activation of the AHR and after AHR knock down from SUM149 IBC cells. Human SUM149 cells stably transduced only with Cas 9 (no guide RNA) (“CRISPR Control”) or SUM149 cells in which AHR was deleted using Cas9 and guide RNA (AHR KO) were evaluated for CYP1B1 (A), WNT5A (B) or GAPDH (for normalization) mRNAs by qPCR following a 24-h treatment with nothing (naïve), DMSO (vehicle) or 0.5 uM of the AHR agonist FICZ. Data are presented as averages (normalized GAPDH mRNA) ± SE from four independent experiments. **P < 0.01 relative to the respective CRISPR controls. ##P < 0.01 relative to DMSO-treated, CRISPR control groups.
Discussion
Previous studies demonstrated that the AHR contributes to mammary carcinogenesis [14]. For example, AHR inhibition in IBC cells reduces the irregular colony formation that is characteristic of invasive cells, the migration and metastasis of human carcinoma cells in vivo in a zebrafish model [41], the induction of cancer stem-like cells, and tumour growth in vivo [11]. IBC patients display aggressive pathological properties, such as involvement of lymph node metastasis at the time of diagnosis, rapid progression to metastasis and a high incidence of chemo- and radio-resistant CSCs [3], [28]. The approach in the present study was to investigate whether the expression of AHR and its downstream target CYP1B1 drives cellular mechanisms associated with poor IBC prognosis and the stem cell-like phenotype. Herein, AHR and its downstream target CYP1B1 were overexpressed in IBC carcinoma tissues compared with healthy breast tissues obtained from mammoplasty or with non-IBC tissues. In IBC, co-expression of AHR and CYP1B1 was found to be associated with tumour grade and lymphovascular invasion. In the present study, the Nottingham grading system was used for histopathological grading to describe cell morphology, abnormalities and differentiation. The expression of AHR and CYP1B1 was associated with a high tumour grade score in IBC but not non-IBC patients. Previous studies also failed to show a correlation between AHR and tumour grade in non-IBC patients [17]. However, the recent results showed that AHR correlates with tumour grade in the IBC aggressive phenotype and suggested that AHR may contribute to the differentiation of tumour stages during IBC disease progression.
In addition, the results showed that the expression of AHR and CYP1B1 mRNA was associated with the presence of lymphovascular invasion and the number of metastatic lymph nodes in IBC. The strong ability of IBC carcinoma cells to degrade extracellular matrix proteins, migrate and invade lymphatic vessels, and form dermal and parenchymal lymphovascular tumour emboli, are hallmarks of IBC diagnosis. Indeed, IBC patients are characterized by a higher number of metastatic lymph nodes than non-IBC patients [49]. Recent results are consistent with the results of other studies showing that AHR is associated with lymph node metastasis and the dissemination of carcinoma cells in gastric cancer patients [19].
Importantly, AHR and CYP1B1 protein expression was positively correlated with the proliferation marker Ki-67 in IBC but not in non-IBC tissues. These findings suggest that AHR/CYP1B1 may play a role in the poor prognosis of IBC disease. This finding is in line with the role of the AHR pathway in the progression of different tumours, including upper urinary tract urothelial cancer [20], glioblastoma [50] and meningioma [51].
The canonical Wnt/β-catenin signalling pathway plays an important role in the regeneration of many tissues by enhancing the growth of CSCs and multipotent progenitors [52]. The Wnt/β-catenin signalling pathway regulates cell proliferation, migration, and differentiation [53]. Furthermore, Wnt signalling “defines” CSCs in different types of malignancies [54]. In breast cancer, Wnt/β-catenin signalling was found to be more highly activated in triple-negative cell lines [53]. Targeting Wnt/β-catenin inhibits breast cancer metastasis via suppression of the expression of CSC-related genes in animal models and in vitro culture [47].
The results showed that carcinoma tissues from IBC patients are characterized by high expression of Wnt5a/b and β-catenin. In addition, the expression of Wnt5a/b and β-catenin was associated with the presence of lymphovascular invasion and lymph node metastasis in IBC compared with non-IBC patients. The pharmacological inhibition of AHR downregulates Wnt5a in MDA-MB-231 and SUM149 cells. Furthermore, in the present study we also showed that knockdown of AHR gene leads to down-regulation of CYP1B1 and Wnt5a in IBC cells. This finding is consistent with the notion that activation of AHR induces upregulation of Wnt5a together with genes involved in cancer cell plasticity [55]. Taken together, these results suggest that AHR signalling regulates Wnt5a/b and β-catenin expression in IBC.
Importantly, the AHR signalling pathway was found to regulate the development and proliferation of CSCs via activation of the β-catenin and Akt pathways and via the inhibition of PTEN in different breast cancer cell lines [15]. Furthermore, AHR hyper-activation significantly enhances CSC properties and accelerates cell migration, and AHR knockout inhibits tumour sphere formation, blocks the rapid migration of cells expressing high ALDHhigh and reduces ALDHhigh cell chemoresistance [11].
Several studies have demonstrated a high incidence of CSC phenotypes in cancer specimens from IBC patients [23], [28], [56]. Indeed, dissemination, metastatic properties and treatment resistance in IBC are “associated with the cancer stem cell hypothesis” [57]. In the present study, AHR/CYP1B1 was associated with a high incidence of CSCs in IBC compared to non-IBC tissue. In addition, the proportion of CSCs was correlated with the expression of Wnt5a/b and β-catenin signalling in IBC tissue but not in non-IBC tissue. Based on our recent findings and previous studies, this study suggests that the co-expression of AHR/CYP1B1 augments the expression of Wnt5a/b and β-catenin in IBC breast cancer patients, which may in turn induce the CSC properties and therapeutic resistance of the aggressive IBC phenotype.
Conclusions
In conclusion, the expression of AHR and its target gene/protein CYP1B1 in IBC carcinoma tissues is associated with poor prognostic markers, such as tumour grade, lymphovascular invasion, cellular proliferation and the number of lymph node metastases. Furthermore, AHR may contribute to CSCs and therapeutic resistance of the IBC aggressive phenotype by stimulating the Wnt5a/β-catenin signalling pathway. Therefore, the present study has implications for developing therapeutic approaches for IBC based on AHR and its downstream effectors CYP1B1 and Wnt5a/β-catenin.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
The authors are supported and funded by the Avon Foundation-USA (MMM and DHS) (Grant ID 61855-15) and the Cairo University Scientific Research Sector (MMM, SAI). The authors would like to thank Dr. Eslam A. El-Ghonaimy (Cancer Biology Research Lab, Zoology Department, Faculty of Science, Cairo University, Egypt) for his help with western blotting experiments.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Goldner B., Behrendt C.E., Schoellhammer H.F., Lee B., Chen S.L. Incidence of inflammatory breast cancer in women, 1992–2009, United States. Ann Surg Oncol. 2014;21(4):1267–1270. doi: 10.1245/s10434-013-3439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schairer C., Soliman A.S., Omar S., Khaled H., Eissa S., Ayed F.B. Assessment of diagnosis of inflammatory breast cancer cases at two cancer centers in Egypt and Tunisia. Cancer Med. 2013;2(2):178–184. doi: 10.1002/cam4.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamed M.M., Al-Raawi D., Sabet S.F., El-Shinawi M. Inflammatory breast cancer: new factors contribute to disease etiology: a review. J Adv Res. 2014;5(5):525–536. doi: 10.1016/j.jare.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonev V., Evangelista M., Chen J.H., Su M.Y., Lane K., Mehta R. Long-term follow-up of breast-conserving therapy in patients with inflammatory breast cancer treated with neoadjuvant chemotherapy. Am Surg. 2014;80(10):940–943. [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman A.S., Kleer C.G., Mrad K., Karkouri M., Omar S., Khaled H.M. Inflammatory breast cancer in north Africa: comparison of clinical and molecular epidemiologic characteristics of patients from Egypt, Tunisia, and Morocco. Breast Dis. 2011;33(4):159–169. doi: 10.3233/BD-2012-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer B., Banerjee M., Omar S., Khaled H., Anwar N., Zaghloul M.S. Survival of inflammatory breast cancer patients compared to non-inflammatory breast cancer patients in Egypt. Breast J. 2011;17(5):545–547. doi: 10.1111/j.1524-4741.2011.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno N.T., Espinosa Fernandez J.R., Cristofanilli M., Overmoyer B., Rea D., Berdichevski F. International consensus on the clinical management of inflammatory breast cancer from the morgan welch inflammatory breast cancer research program 10th anniversary conference. J Cancer. 2018;9(8):1437–1447. doi: 10.7150/jca.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtake F., Fujii-Kuriyama Y., Kato S. AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem Pharmacol. 2009;77(4):474–484. doi: 10.1016/j.bcp.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Powell J.B., Goode G.D., Eltom S.E. The aryl hydrocarbon receptor: a target for breast cancer therapy. J Cancer Ther. 2013;4(7):1177–1186. doi: 10.4236/jct.2013.47137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novikov O., Wang Z., Stanford E.A., Parks A.J., Ramirez-Cardenas A., Landesman E. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2-human breast cancer cells. Mol Pharmacol. 2016;90(5):674–688. doi: 10.1124/mol.116.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanford E.A., Wang Z., Novikov O., Mulas F., Landesman-Bollag E., Monti S. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016;14:20. doi: 10.1186/s12915-016-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahia Z.A., Adam A.A., Elgizouli M., Hussein A., Masri M.A., Kamal M. Epstein Barr virus: a prime candidate of breast cancer aetiology in Sudanese patients. Infect Agent Cancer. 2014;9(1):9. doi: 10.1186/1750-9378-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dialyna I.A., Arvanitis D.A., Spandidos D.A. Genetic polymorphisms and transcriptional pattern analysis of CYP1A1, AhR, GSTM1, GSTP1 and GSTT1 genes in breast cancer. Int J Mol Med. 2001;8(1):79–87. doi: 10.3892/ijmm.8.1.79. [DOI] [PubMed] [Google Scholar]
- 14.Trombino A.F., Near R.I., Matulka R.A., Yang S., Hafer L.J., Toselli P.A. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1 gene transcripts in a rat model of mammary tumorigenesis. Breast Cancer Res Treat. 2000;63(2):117–131. doi: 10.1023/a:1006443104670. [DOI] [PubMed] [Google Scholar]
- 15.Al-Dhfyan A., Alhoshani A., Korashy H.M. Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and beta-Catenin and Akt activation. Mol Cancer. 2017;16(1):14. doi: 10.1186/s12943-016-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen M.C., Angus W.G., Brake P.B., Eltom S.E., Sukow K.A., Jefcoate C.R. Characterization of CYP1B1 and CYP1A1 expression in human mammary epithelial cells: role of the aryl hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism. Cancer Res. 1998;58(11):2366–2374. [PubMed] [Google Scholar]
- 17.Li Z.D., Wang K., Yang X.W., Zhuang Z.G., Wang J.J., Tong X.W. Expression of aryl hydrocarbon receptor in relation to p53 status and clinicopathological parameters in breast cancer. Int J Clin Exp Pathol. 2014;7(11):7931–7937. [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X., Solomon S., Fraser L.R., Trombino A.F., Liu D., Sonenshein G.E. Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J Cell Biochem. 2008;104(2):402–417. doi: 10.1002/jcb.21630. [DOI] [PubMed] [Google Scholar]
- 19.Lai D.W., Liu S.H., Karlsson A.I., Lee W.J., Wang K.B., Chen Y.C. The novel Aryl hydrocarbon receptor inhibitor biseugenol inhibits gastric tumor growth and peritoneal dissemination. Oncotarget. 2014;5(17):7788–7804. doi: 10.18632/oncotarget.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida M., Mikami S., Kikuchi E., Kosaka T., Miyajima A., Nakagawa K. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 2010;31(2):287–295. doi: 10.1093/carcin/bgp222. [DOI] [PubMed] [Google Scholar]
- 21.Barouki R., Aggerbeck M., Aggerbeck L., Coumoul X. The aryl hydrocarbon receptor system. Drug Metabol Drug Interact. 2012;27(1):3–8. doi: 10.1515/dmdi-2011-0035. [DOI] [PubMed] [Google Scholar]
- 22.DiNatale B.C., Smith K., John K., Krishnegowda G., Amin S.G., Perdew G.H. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol Cancer Res. 2012;10(10):1369–1379. doi: 10.1158/1541-7786.MCR-12-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y., Ye Y., Zou X., Jones S., Yearsley K., Shetuni B. The lymphovascular embolus of inflammatory breast cancer exhibits a Notch 3 addiction. Oncogene. 2011;30(3):287–300. doi: 10.1038/onc.2010.405. [DOI] [PubMed] [Google Scholar]
- 24.Currier N., Solomon S.E., Demicco E.G., Chang D.L., Farago M., Ying H. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33(6):726–737. doi: 10.1080/01926230500352226. [DOI] [PubMed] [Google Scholar]
- 25.Wei W., Hu H., Tan H., Chow L.W., Yip A.Y., Loo W.T. Relationship of CD44+CD24-/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med. 2012;10(Suppl 1):S6. doi: 10.1186/1479-5876-10-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y., Wang J., Huo L., Wei W., Ueno N.T., Woodward W.A. Aldehyde dehydrogenase 1 expression in inflammatory breast cancer as measured by immunohistochemical staining. Clin Breast Cancer. 2014;14(3):e81–e88. doi: 10.1016/j.clbc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe A.R., Trenton N.J., Debeb B.G., Larson R., Ruffell B., Chu K. Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget. 2016;7(50):82482–82492. doi: 10.18632/oncotarget.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim S.A., Gadalla R., El-Ghonaimy E.A., Samir O., Mohamed H.T., Hassan H. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. 2017;16(1):57. doi: 10.1186/s12943-017-0621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardal R., Clarke M.F., Morrison S.J. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim S.A., Hassan H., Vilardo L., Kumar S.K., Kumar A.V., Kelsch R. Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS One. 2013;8(12):e85737. doi: 10.1371/journal.pone.0085737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charafe-Jauffret E., Ginestier C., Iovino F., Tarpin C., Diebel M., Esterni B. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris E.E., Schultz D., Bertsch H., Fox K., Glick J., Solin L.J. Ten-year outcome after combined modality therapy for inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1200–1208. doi: 10.1016/s0360-3016(02)04201-3. [DOI] [PubMed] [Google Scholar]
- 33.Schneider A.J., Branam A.M., Peterson R.E. Intersection of AHR and Wnt signaling in development, health, and disease. Int J Mol Sci. 2014;15(10):17852–17885. doi: 10.3390/ijms151017852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y., Kipps T.J., Zhang S. Wnt5a Signaling in Normal and Cancer Stem Cells. Stem Cells Int. 2017;2017:5295286. doi: 10.1155/2017/5295286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misztal K., Wisniewska M.B., Ambrozkiewicz M., Nagalski A., Kuznicki J. WNT protein-independent constitutive nuclear localization of beta-catenin protein and its low degradation rate in thalamic neurons. J Biol Chem. 2011;286(36):31781–31788. doi: 10.1074/jbc.M111.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouh M.A., Mohamed M.M., El-Shinawi M., Shaalan M.A., Cavallo-Medved D., Khaled H.M. Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Shinawi M., Mohamed H.T., El-Ghonaimy E.A., Tantawy M., Younis A., Schneider R.J. Human cytomegalovirus infection enhances NF-kappaB/p65 signaling in inflammatory breast cancer patients. PLoS One. 2013;8(2):e55755. doi: 10.1371/journal.pone.0055755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed M.M., El-Ghonaimy E.A., Nouh M.A., Schneider R.J., Sloane B.F., El-Shinawi M. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int J Biochem Cell Biol. 2014;46:138–147. doi: 10.1016/j.biocel.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothhammer V., Borucki D.M., Kenison J.E., Hewson P., Wang Z., Bakshi R. Detection of aryl hydrocarbon receptor agonists in human samples. Sci Rep. 2018;8(1):4970. doi: 10.1038/s41598-018-23323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalem O., Sanjana N.E., Zhang F. High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet. 2015;16(5):299–311. doi: 10.1038/nrg3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narasimhan S., Stanford Zulick E., Novikov O., Parks A.J., Schlezinger J.J., Wang Z. Towards resolving the pro- and anti-tumor effects of the aryl hydrocarbon receptor. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inwald E.C., Klinkhammer-Schalke M., Hofstadter F., Zeman F., Koller M., Gerstenhauer M. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J., Sheng B., Qiu Y., Yang K., Xiao W., Yang H. Role of AhR in positive regulation of cell proliferation and survival. CellProlif. 2016;49(5):554–560. doi: 10.1111/cpr.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holland J.D., Klaus A., Garratt A.N., Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25(2):254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Chesire D.R., Dunn T.A., Ewing C.M., Luo J., Isaacs W.B. Identification of aryl hydrocarbon receptor as a putative Wnt/beta-catenin pathway target gene in prostate cancer cells. Cancer Res. 2004;64(7):2523–2533. doi: 10.1158/0008-5472.can-03-3309. [DOI] [PubMed] [Google Scholar]
- 46.Xiao Y., Ye Y., Yearsley K., Jones S., Barsky S.H. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol. 2008;173(2):561–574. doi: 10.2353/ajpath.2008.071214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang G.B., Kim J.Y., Cho S.D., Park K.S., Jung J.Y., Lee H.Y. Blockade of Wnt/beta-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci Rep. 2015;5:12465. doi: 10.1038/srep12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kretzschmar K., Clevers H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev Biol. 2017 doi: 10.1016/j.ydbio.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Robertson F.M., Bondy M., Yang W., Yamauchi H., Wiggins S., Kamrudin S. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60(6):351–375. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 50.Guastella A.R., Michelhaugh S.K., Klinger N.V., Fadel H.A., Kiousis S., Ali-Fehmi R. Investigation of the aryl hydrocarbon receptor and the intrinsic tumoral component of the kynurenine pathway of tryptophan metabolism in primary brain tumors. J Neurooncol. 2018 doi: 10.1007/s11060-018-2869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talari N.K., Panigrahi M.K., Madigubba S., Phanithi P.B. Overexpression of aryl hydrocarbon receptor (AHR) signalling pathway in human meningioma. J Neurooncol. 2018;137(2):241–248. doi: 10.1007/s11060-017-2730-3. [DOI] [PubMed] [Google Scholar]
- 52.MacDonald Bryan T., Tamai K., He Xi. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):18. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King T.D., Suto M.J., Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113(1):13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Sousa E.M.F., Vermeulen L. Wnt signaling in cancer stem cell biology. Cancers (Basel) 2016;8(7) doi: 10.3390/cancers8070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hruba E., Vondracek J., Libalova H., Topinka J., Bryja V., Soucek K. Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol Lett. 2011;206(2):178–188. doi: 10.1016/j.toxlet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Abraham B.K., Fritz P., McClellan M., Hauptvogel P., Athelogou M., Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11(3):1154–1159. [PubMed] [Google Scholar]
- 57.Lacerda L., Reddy J.P., Liu D., Larson R., Li L., Masuda H. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Transl Med. 2014;3(7):849–856. doi: 10.5966/sctm.2013-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]