Abstract
Sporadic multiple endocrine neoplasia type 1 (MEN1) is defined as the occurrence of tumours in two of three main endocrine tissue types: parathyroid, pituitary and pancreaticoduodenal. A prolactinoma variant or Burin variant of MEN1 was found to occur in three large kindreds, with more prolactinomas and fewer gastrinomas than typical MEN1. MEN1 tumours differ from common tumours by showing features from the MEN1 gene (e.g. larger pituitary tumours). They also show various expressions of tumour multiplicity; however, pituitary tumour in MEN1 is usually solitary. Diagnosis in MEN1 carriers during childhood is not directed at cancers but at benign morbid tumours. Morbid prolactinoma occurred at the age of 5 years in one MEN1 individual; hence, this is the earliest age at which to recommend tumour surveillance in carriers. The MEN1 gene shows biallelic inactivation in 30% of some types of common variety endocrine tumours (e.g. parathyroid adenoma, gastrinoma, insulinoma and bronchial carcinoid), but in only 1–5% of common pituitary tumours. Heterozygous knockout of MEN1 in mice provides a robust model of MEN1 and has been found to support further research on anti-angiogenesis therapy for pituitary tumours. The rarity of MEN1 mutations in some MEN1-like states aids the identification of other mutated genes, such as AIP, HRPT2 and p27Kip1. We present recent clinical and basic findings about the MEN1 gene, particularly concerning hereditary vs. common variety pituitary tumours.
Keywords: Angiogenesis, MEN1, Mouse, p27Kip1, Pituitary tumour, Prolactinoma, Tumour suppressor
MEN1 and MEN1-Like States
MEN1
Multiple endocrine neoplasia type 1 (MEN1) is defined as tumours in two of its three main endocrine tissues: parathyroid, pituitary and pancreaticoduodenal [1], with familial MEN1 defined as MEN1 plus one first-degree relative with a tumour in one of these three tissues. MEN1 is the most heterogeneous of tumour syndromes, causing tumours among 25 mainly endocrine tissues (table 1) [2]. Its prevalence is only 1 in 30,000, but the gene is central to many common tumours. ‘MEN1-like’ is a much broader term than MEN1 for sporadic or hereditary states with a tumour in as few as one of the three main tissues. Several such states are discussed in this article.
Table 1.
Tissue selectivity of tumours in probands and families with MEN1
| Hormone-secreting tumours | |
| Parathyroid adenoma (90%) | Anterior pituitary tumour |
| Pancreaticoduodenal tumour | Prolactinoma (20%) |
| Gastrinoma (40%) | Other growth hormone + prolactin (5%) |
| Insulinoma (10%) | Growth hormone (5%), NF (5%) |
| NF, also pancreatic polypeptide (20%) | ACTH (2%) or TSH (rare) |
| Glucagon, vasoactive intestinal polypeptide, | Adrenal |
| somatostatin, etc. (2%) | Cortex NF (25%) |
| Foregut carcinoid | Medulla (1%) |
| Thymic carcinoid NF (4%) | |
| Bronchial carcinoid NF (2%) | |
| Gastric enterochromaffin-like NF (10%) | |
| Hormone non-secreting tumours | |
| Facial angiofibroma (85%) | Leiomyoma |
| Truncal collagenoma (70%) | Uterus in female (30%?) |
| Lipoma (30%) | Oesophagus (5%) |
| Meningioma (5%) | Ependymoma (1%) |
| Barrett’s oesophagus (5%) |
NF = Non-functioning; TSH = thyroid-stimulating hormone. Percentages in parentheses/brackets indicate penetrance at age 40 years. Terms in bold font indicate tumour type with malignant potential for 25% or more of cases. Modified from Gagel and Marx [2].
Typically, each MEN1 tissue with a tumour or at risk of a tumour must be managed independently. Anterior pituitary tumour in MEN1 has an age and sex distribution and hormone profile similar to those of common variety pituitary tumours [3]. Typical onset is from the ages of 30–35 years, with nearly maximal penetrance of 50% by age 50 years among carriers. Management is similar to that for sporadic pituitary tumour, but treatments in MEN1 seem less effective [3].
Prolactinoma Variant of MEN1
A high prevalence of prolactinoma was found in four large MEN1 families located near the Burin Peninsula of Newfoundland, Canada [4]. This has been termed the prolactinoma variant or the Burin variant of MEN1. These four Newfoundland families share an identical mutation in the MEN1 gene and have an identical 11q13 haplotype about the gene [5, 6], implying that they must be descended from a common founder. The prolactinoma variant of MEN1 should be diagnosed only in a large family; such features in a small family cannot be distinguished from random expression of typical MEN1. Three very large families (scoring the Newfoundland cluster as one huge family) have this phenotype (fig. 1) [7]. Unlike typical MEN1, they show a lower prevalence of gastrinoma (10 vs. 42%, p < 0.01) and higher prevalence of prolactinoma (40 vs. 22%, p < 0.01). The three families do not share a common MEN1 mutation pattern or a common environmental feature. We speculate that each family has a similar, tightly linked polymorphism in or near the MEN1 gene at 11q13 [8] that affects expression of the MEN1 syndrome. Although the seeming proximity of the AIP gene (also at 11q13) is intriguing, this cannot be the explanation because the substantial subchromosomal distance of AIP from MEN1 (elaborated below) indicates that any polymorphism within the AIP gene must segregate away from the MEN1 gene in these large families [9].
Fig. 1.
Prevalence of gastrinoma and prolactinoma in seven reviews of typical MEN1 (♦) versus in three large kindreds with the prolactinoma or Burin variant of MEN1 [7]. The low prevalence of gastrinoma in the so-called prolactinoma variant kindreds is particularly striking.
Familial Isolated Pituitary Tumour
Isolated pituitary tumour is a MEN1-like state that can occur in families and that is linked to 11q13 [10]. In this state, growth hormone (GH) is often oversecreted. In theory, such a syndrome could be caused by the MEN1 gene also at 11q13, but MEN1 mutation has not been observed in over 100 families [10]. Indeed, the cause of isolated pituitary tumour in 15% of these families is the AIP gene [9]. Although in this gene the subchromosomal locus is close to the MEN1 gene and is separated by 2.7 million bases, this proximity seems to be a random coincidence. Familial isolated pituitary tumour and its gene are covered separately in detail [11].
Familial Isolated Parathyroid Tumour
Isolated parathyroid tumour is another MEN1-like state that can occur in families. MEN1 mutation accounts for some 5% of these families, and there is no specific MEN1 genotype [12, 13]. Other similarly uncommon causes are mutation of the CASR or HRPT2 genes [12–14]. Each of these three genes causes a characteristic syndrome in and beyond the parathyroids; however, each can also present in an incomplete form as familial isolated hyperparathyroidism. In most affected families, isolated hyperparathyroidism is probably caused by unidentified genes, although genetic linkage suggests the involvement of a locus at chromosome 2p [15].
Familial Isolated Carcinoid Tumour
Carcinoid of the foregut occurs in 16% of MEN1 cases (table 1) [2]. A few small MEN1 families have shown a modest clustering of these carcinoids without a specific MEN1 genotype [16].
Isolated carcinoid tumour has been reported in a few kindreds, mainly with only two to three affected members [17–19]. The carcinoids in these families have been located mainly in the duodenum, lung and colon, with a repetition of organ location evident in few families [17, 19]. Epidemiological studies of carcinoid suggest that familial clustering is more frequent than reported [20]. No germline MEN1 mutation has been reported in a family with isolated carcinoid.
MEN1-Like State from p27 Mutation (MEN4)
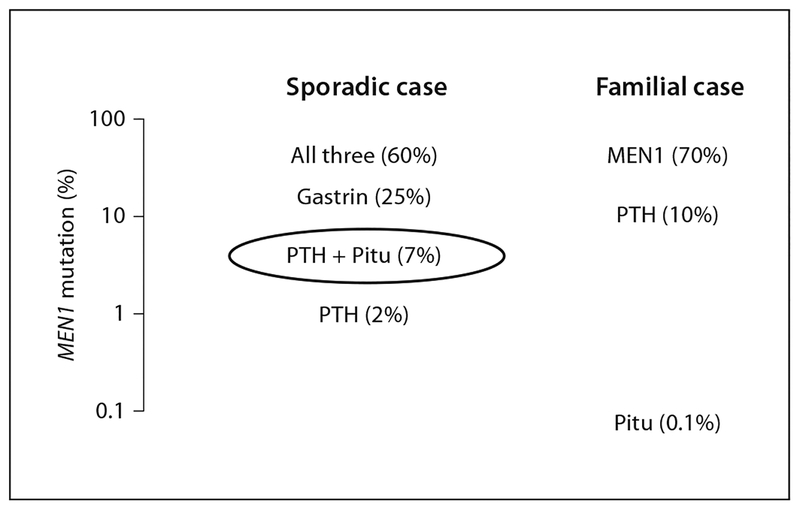
Mice with combined knockouts of the p18INK4c (CDKN2C) and p27Kip1 (CDKN1B) cyclin-dependent kinase inhibitor (CDKI) genes express combinations of the tumours of MEN1 and MEN2 [21]. A strain of rat, termed MENX, was discovered with similar combined tumours. MENX is caused by homozygous inactivating mutation of p27 [22]. The same group reported one human index case and relatives with MEN1, caused by heterozygous mutation of p27 [22]. Subsequently, another MEN1 case with mutation of p27 was reported [23]. Considering the few cases identified, the full spectrum of this p27 gene-related syndrome remains to be established. Notably, three of the four affected cases in these two families had a pituitary tumour (two GH, one adrenocorticotropic hormone [ACTH]). Because of this preponderance of pituitary tumours and because sporadic MEN1 cases with both parathyroid and pituitary tumour have had a particularly low finding (7%) of MEN1 mutation (fig. 2), we screened 34 such index cases, each lacking MEN1 mutation, but found no p27 mutation [24]. Thus, p27 mutation is likely to cause 1% or less in this MEN1 category. In OMIM (Online Mendelian Inheritance of Man), this rare p27 syndrome is termed MEN4 [25]. Subsequently, three more of the remaining six CDKI genes have been similarly implicated [26].
Fig. 2.
Estimates of the prevalences of MEN1 mutation in selected clinical groups. Each group fits the broad definition of MEN1 or MEN1-like, but mutation prevalences differ dramatically, justifying the logarithmic scale. The group of sporadic cases with both parathyroid (PTH) and pituitary (Pitu) tumour is circled because of its meeting the formal definition of MEN1 but having a particularly low prevalence (7%) of identified MEN1 mutation.
Endocrine Tumours Differ in MEN1 vs. in Other States
Single Tumour Reflects Its Main Gene
Pituitary Tumours
Tumours, and particularly cancers, can have mutations in several genes [27], and sometimes one gene, such as MEN1, dominates the clinical expression.
Size.
Pituitary tumours in MEN1 are larger than common variety pituitary tumours, with macroadenoma in 85% of the MEN1 cases compared with 42% of the common variety cases [3].
Hormone Profile.
MEN1 pituitary tumours show the following hormone profile: prolactin (62%), GH (9%), ACTH (4%), co-secreting mostly with prolactin (10%) and non-secreting (15%) [3]. This profile is similar to that of common variety pituitary tumours, but different from the profiles of other hereditary pituitary tumours (from GNAS, AIP or PRKAR1A) that secrete mainly GH.
Tumours Other than Pituitary
Location.
Carcinoid tumour in MEN1 has unusual locations in the foregut (table 1), unlike common variety carcinoid, which is mainly in the mid- or hindgut.
Pathology.
Hereditary parathyroid tumours have a wide spectrum of pathologies with syndrome specificity [27]. The extremes of the spectrum are near-normal parathyroid pathology in familial hypocalciuric hypercalcaemia and parathyroid cancer in 20% of cases with hyper-parathyroid jaw tumour syndrome [28]. The parathyroid tumour of MEN1 is in the middle of this spectrum, resembling common variety adenoma.
Tumour Multiplicity
Tumour multiplicity is a feature of most hereditary tumours. It reflects the germline mutation in all cells of a tumour target tissue, making each cell susceptible to hyperfunction. In MEN1, this multiplicity is seen at four levels:
Hyperplasia as tumour precursor. Hyperplasia is a histological diagnosis, well recognized in some hereditary tumours [29]. It is not well recognized as a tumour precursor state in most MEN1 tumours, including the pituitary. However, it is a striking feature of the pancreatic islets of mice with MEN1 [30–32], and is a less-evident feature in the gastrin cells of MEN1 in man [33].
Tumour in multiple tissues. MEN1 has tumours in about 25 endocrine and even non-endocrine tissue types (table 1).
Tumour in a dispersed tissue. The normal gastrin cells are dispersed in the submucosa of the duodenum. MEN1 patients show multiple microgastrinomas in this tissue [33].
Tumour in a continuous tissue. The parathyroid gland is quite homogeneous. Multiple tumour nodules, presumably from distinct clones, sometimes arise there in MEN1 [2].
Tumour treatments in MEN1 must be inclusive (i.e. developed to address all of the multiple tumours in an affected organ). These treatments vary from total resection (for thymus) and subtotal resection (for parathyroids), to no resection (drug treatment with acid-pump blockers for gastrin cells).
Pituitary Breaks the Rule of Tumour Multiplicity
Multiple tumours of the pituitary are rare in common variety disease [34] and surprisingly rare even in MEN1 [35, 36]. This is particularly notable since pituitary tumours have a very high penetrance in MEN1 (table 1) and multiple pituitary tumours could easily be imaged by magnetic resonance imaging. We speculate that a dominant pituitary tumour in MEN1 releases factors that inhibit the growth of nearby pituitary tissues. This could be related to the process of oncogene-induced cell senescence [37].
Preventing Morbidity in MEN1
Preventing or Treating Cancer
Prevention or cure of C-cell cancer has long been possible in MEN2, based on early diagnosis using calcitonin assays and, more recently, using RET sequencing [2]. This treatment is mainly based on a favourable location (inside the thyroid gland) of the tumour target tissue.
Twenty-five percent of MEN1 cases will die from a MEN1-related cancer [2]. Although diagnosis by MEN1 gene testing during childhood is widely available, cancer prevention at any age has not been practical in MEN1 because of the problematic foregut locations of duodenopancreatic and carcinoid tumours.
Preventing and Treating Benign but Morbid Disease
Once a patient is diagnosed as a likely MEN1 carrier, rather complex protocols for periodic tumour surveil-lance can help to recognize tumour emergence (table 2). The age at which to begin such periodic monitoring should be determined, in part, by the age at which morbid disease might be treated successfully. In MEN1, there have been two cases of insulinoma at age 6 years [38, 39] and one with pituitary macroadenoma, secreting prolactin and GH, at age 5 years or earlier [40].
Table 2.
Protocol for periodic surveys for donor emergence in a known or highly likely carrier of MEN1. A positive result should lead to further considerations, regarding false-positives or additional tests for confirmations
| Tumour | Age to begin (year) | Biochemical tests (annually) | Imaging tests (every 3–5 years) |
|---|---|---|---|
| Anterior pituitary | 5 | Prolactin, IGF-I | MRI |
| Parathyroid | 5 | Calcium, PTH | None |
| Insulinoma | 5 | Fasting glucose | None |
| Gastrinoma | 20 | Gastrin | None |
| Other gastrointestinal | 20 | Nonea | CAT or MRI, Octreoscanb |
| Foregut carcinoid | 20 | Nonea | CATb during gastroscopy |
CAT = Computerized axial tomography; IGF-I, insulin-like growth factor I; MRI = magnetic resonance imaging; PTH = parathyroid hormone.
Chromogranin A has some utility in following a large endocrine tumour burden. It has not been shown to be useful in screening for small tumours.
New tests such as 18F-fluorodopa positron emission tomography or endoscopic ultrasound are promising. Modified from Gagel and Marx [2].
Families and physicians face a difficult decision: should a young child at 50% risk of the mutation undergo MEN1 sequencing and then periodic monitoring for benign but morbid tumours that are very rare in early childhood? Is it preferable to accept the possibility that the rare tumour might not occur or might be recognized early by sensitivity to its symptoms? We generally recommend periodic surveillance for tumours in known MEN1 carriers, beginning at age 5 years (table 2).
Exploiting MEN1 and Related Genes
Rodent Models and Cell-Cycle Genes
Mice and rats with combined knockouts of p18 and p27 CDKIs provide a model for MEN1 and MEN2 (see above [21, 22]). Mice have also been engineered to express inactivation of one copy of the MEN1 gene [30–32]; these provide a robust model of MEN1 with pituitary tumour, parathyroid tumour, insulinoma and other tumours. Unlike the pituitary tumours of the intermediate lobe of mice with Rb1 inactivation, the pituitary tumours from MEN1 mutation are mainly prolactinomas. In mice, knockout of MEN1 synergizes with knockout of p18 in pituitary and other tumorigenesis, but not with knockout of p27 or Rb1 [41–43]. MEN1 mice have been used to test the efficacy of a monoclonal antibody to vascular endothelial growth factor A, which was subsequently found to inhibit pituitary tumour growth, inhibit growth of pituitary tumour as implants and cause less elevation in serum prolactin concentration. There was similar efficacy against islet tumours [44]. Such studies suggest the importance of the vascular component of some or even all tumours in MEN1 [45].
Role in Common Tumours
Often the gene for a rare hereditary disorder also has important roles in common tumours of similar types. Thus, the RET gene is implicated in common phaeochromocytoma, common C-cell cancer and papillary thyroid cancer (the latter as the RET-PTC rearrangements) [2].
The MEN1 gene is the initiator in about 30% of all common parathyroid adenomas, gastrinomas, insulinomas and bronchial carcinoid tumours (fig. 3) [2]. Surprisingly, it initiates only 1–5% of common pituitary tumours [46]. The finding of MEN1 mutation in many common endocrine tumours suggests that identifying the menin pathway could lead to the development of drugs for treating many tumours.
Fig. 3.
Contributions from inactivation of the MEN1 gene in groups with common variety endocrine tumour (i.e. no DNA or kindred evidence of MEN1). Inactivation of the MEN1 gene is found as small mutations in the MEN1 gene sequence and as large subchromosomal losses, the latter determined by loss of heterozygosity at 11q13. Modified from Gagel and Marx [2].
MEN1 Mutation in Probands
The germline MEN1 sequence test is the gold standard for identifying MEN1 carriers among probands with MEN1-like features [2, 47]. If a mutation is identified, then simplified DNA testing can be discussed with relatives.
Recognizing MEN1-Like States Caused by Other Genes
MEN1 sequence testing has led to the recognition of states in which MEN1 mutation seems deficient (fig. 2) [2, 47]. For example, 30% of patients with typical MEN1 do not have an identifiable MEN1 mutation. This could reflect a MEN1 mutation invisible to current methods of detection or a mutation in other gene(s) [2, 26].
Menin Molecular Pathway
The MEN1 gene encodes menin, a protein of 610 amino acids. Other than several nuclear localization sequences, menin has no homologies or recognizable domains. Efforts to understand its molecular pathway have begun with protein partnering methods. Some 25 menin partners have been identified [48]; none have been shown conclusively to be important in pathophysiology. MLL and junD have received the most attention. A tumorigenic mechanism has been proposed for each, independent of the other. Each mechanism must begin with the inactivation of both copies of the MEN1 gene. Menin inactivation can switch junD from growth suppressor to growth promoter [49, 50]. Alternately, menin inactivation can destabilize the MLL-COMPASS complex and thereby stop its transcriptional activation of p18 and p27, each of which is a growth suppressor [51–53].
Conclusion
Germline heterozygous loss of function of the MEN1 gene gives a high penetrance for development of tumour of the anterior pituitary, mainly prolactinoma. This is also expressed in mouse models. Among all pituitary tumour syndromes, only MEN1 in man or mouse shows a hormonal profile similar to that of common pituitary tumours. However, biallelic somatic mutation of MEN1 is rare in common variety pituitary tumours. Future research must explore other mechanisms of MEN1 gene inactivation in common variety tumours of the pituitary. This would include epigenetic changes, such as promoter methylation, dysregulation of menin protein [54] and other changes in menin pathways. The principal clinical and therapeutic implications are summarized in table 3.
Table 3.
Clinical and therapeutic implications
| Clinical |
|
| Therapeutic |
|
Acknowledgement
This work was supported by the intramural programme of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ: Guidelines for diagnosis and therapy of multiple endocrine neoplasia type 1 and type 2. J Clin Endocrinol Metab 2001; 86:5658–5671. [DOI] [PubMed] [Google Scholar]
- 2.Gagel RF, Marx SJ: Multiple endocrine neoplasia; in Kronenberg HM, Melmed S, Polonsky KS, Reed Larsen P (eds): Williams Textbook of Endocrinology, ed 11 Philadelphia, Saunders, 2007, pp 1705–1746. [Google Scholar]
- 3.Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A: Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 2002; 87:457–465. [DOI] [PubMed] [Google Scholar]
- 4.Farid NR, Buehler A, Russell NA, Maroun FB, Allerdice P, Smyth HS: Prolactinomas in familial multiple endocrine neoplasia syndrome type 1. Am J Med 1980; 69:874–880. [DOI] [PubMed] [Google Scholar]
- 5.Petty EM, Green JS, Marx SJ, Taggart RT, Farid N, Bale AE: Mapping the gene for hereditary hyperparathyroidism and prolactinoma (MEN1-Burin) to chromosome 11q: evidence for a founder effect in patients from Newfoundland. Am J Hum Genet 1994; 54:1060–1066. [PMC free article] [PubMed] [Google Scholar]
- 6.Olufemi SE, Green JS, Manickam P, Guru SC, Agarwal SK, Kester MB, Dong Q, Burns AL, Spiegel AM, Marx SJ, Collins FS, Chandrasekharappa SC: Common ancestral mutation in the MEN1 gene is likely responsible for the prolactinoma variant of MEN1 (MEN1Burin) in four kindreds from Newfoundland. Hum Mutat 1998; 11:264–269. [DOI] [PubMed] [Google Scholar]
- 7.Hao W, Skarulis MC, Simonds WF, Weinstein LS, Agarwal SK, Mateo C, James-Newton L, Hobbs GR, Gibril F, Jensen RT, Marx SJ: MEN1 variant with frequent prolactinoma and rare gastrinoma. J Clin Endocrinol Metab 2004; 89:3776–3784. [DOI] [PubMed] [Google Scholar]
- 8.Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjöld M: Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 1988; 332:85–87. [DOI] [PubMed] [Google Scholar]
- 9.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA: Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 2006; 312:1228–1230. [DOI] [PubMed] [Google Scholar]
- 10.Beckers A, Daly AF: The clinical, pathological, and genetic features of familial isolated pituitary adenomas. Eur J Endocrinol 2007; 157: 371–382. [DOI] [PubMed] [Google Scholar]
- 11.Daly AF, Tichomirowa MA, Beckers A: Genetic, molecular and clinical features of familial isolated pituitary adenomas. Horm Res 2009;71(suppl 2): 116–122. [DOI] [PubMed] [Google Scholar]
- 12.Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, Marx SJ: Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002; 81: 1–26. [DOI] [PubMed] [Google Scholar]
- 13.Warner J, Epstein M, Sweet A, Singh D, Burgess J, Stranks S, Hill P, Perry-Keene D, Lear-oyd D, Robinson B, Birdsey P, Mackenzie E, Teh BT, Prins JB, Cardinal J: Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J Med Genet 2004; 41: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonds WF, Robbins CM, Agarwal SK, Hendy GN, Carpten JD, Marx SJ: Familial isolated hyperparathyroidism is rarely caused by germline mutation in HRPT2, the gene for the hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab 2004; 89: 96–102. [DOI] [PubMed] [Google Scholar]
- 15.Warner J, Nyholt DR, Busfield F, Epstein M, Burgess J, Stranks S, Hill P, Perry-Keene D, Learoyd D, Robinson B, Teh BT, Prins JB, Cardinal JW: Familial isolated hyperparathyroidism is linked to a 1.7 Mb region on chromosome 2p13.3–14. J Med Genet 2006; 43:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferolla P, Falchetti A, Filosso P, Tomassetti P, Tamburrano G, Avenia N, Daddi G, Puma F, Ribacchi R, Santeusanio F, Angeletti G, Brandi ML: Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab 2005; 90: 2603–2609. [DOI] [PubMed] [Google Scholar]
- 17.Yeatman TJ, Sharp JV, Kimura AK: Can susceptibility to carcinoid tumors be inherited? Cancer 1989; 3: 390–393. [DOI] [PubMed] [Google Scholar]
- 18.Babovic-Vuksanovic D, Constantinou CL, Rubin J, Rowland CM, Schaid DJ, Karnes PS: Familial occurrence of carcinoid tumors and association with other malignant neoplasms. Cancer Epidemiol Biomarkers Prev 1999; 8: 715–719. [PubMed] [Google Scholar]
- 19.Oliveira AM, Tazelaar HD, Wentzlaff KA, Kosugi NS, Hai N, Benson A, Miller DL, Yang P: Familial pulmonary carcinoid tumors. Cancer 2001; 91: 2104–2109. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K, Li X: Familial carcinoid tumors and subsequent cancers: a nation-wide epidemiologic study from Sweden. Int J Cancer 2001; 94: 444–448. [DOI] [PubMed] [Google Scholar]
- 21.Franklin DS, Godfrey VL, O’Brien DA, Deng C, Xiong Y: Functional collaboration between different cyclin-dependent kinase inhibitors suppress tumor growth with distinct tissue specificity. Mol Cell Biol 2000; 20: 6147–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ: Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 2006; 103: 15558–15563 [erratum in Proc Natl Acad Sci USA 2006; 103: 19213]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA: Germline CDKN1B/p27KIP1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab 2007; 92: 3321–3325. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa A, Agarwal SK, Mateo CM, Burns AL, Rice TS, Kennedy PA, Quigley CM, Simonds WF, Weinstein LS, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ: The parathyroid/pituitary variant of MEN1 usually has causes other than p27 Kip1 mutations. J Clin Endocrinol Metab 2007; 92: 1948–1951. [DOI] [PubMed] [Google Scholar]
- 25.Online Mendelian Inheritance of Man (OMIM): Multiple Endocrine Neoplasia, Type IV; MEN4; #610755. Available at: http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=610755. Creation date 2/12/2007, updated 5/3/2007. Last accessed 27 November 2008.
- 26.Agarwal SK, Mateo C, Marx SJ: Rare germ-line mutations in cyclin-dependent kinase inhibitor genes in MEN1 and related states. J Clin Endocrinol Metab DOI 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bach-man KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE: The consensus coding sequences of human breast and colorectal cancers. Science 2006; 314: 268–274. [DOI] [PubMed] [Google Scholar]
- 28.Arnold A, Marx SJ: Familial hyperparathyroidism; in Rosen C (ed): Primer on the Metabolic Bone Diseases and Mineral Metabolism, ed 7 Philadelphia, Lippincott Williams and Wilkins, 2008. [Google Scholar]
- 29.Marx SJ, Simonds WF: Hereditary hormone excess: genes, molecular pathways, and syndromes. Endocr Rev 2005; 26: 615–661. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS: A mouse model of MEN1 develops multiple endocrine tumors. Proc Natl Acad Sci USA 2001; 98: 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, Hager JH, Hanahan D, Edlund H, Magnuson MA, Garrett-Beal L, Burns AL, Ried T, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS: Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol Cell Biol 2003; 23: 6075–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loffler KA, Biondi CA, Gartside M, Waring P, Stark M, Serewko-Auret MM, Muller HK, Hayward NK, Kay GF: Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer 2006; 120: 259–267. [DOI] [PubMed] [Google Scholar]
- 33.Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Klöppel G: Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 2005; 128: 1187–1198. [DOI] [PubMed] [Google Scholar]
- 34.Ratliff JK, Oldfield EH: Multiple pituitary adenomas in Cushing’s disease. J Neurosurg 2000; 93: 753–761. [DOI] [PubMed] [Google Scholar]
- 35.Jastania RA, Alsaad KO, Al-Shraim M, Kovacs K, Asa SL: Double adenomas of the pituitary: transcription factors Pit-1, T-pit, and SF-1 identify cytogenesis and differentiation. Endocr Pathol 2005; 16: 187–194. [DOI] [PubMed] [Google Scholar]
- 36.Al Brahim NY, Rambaldini G, Ezzat S, Asa SL: Complex endocrinopathies in MEN-1: diagnostic dilemmas in endocrine oncology. Endocr Pathol 2007; 18: 37–41. [DOI] [PubMed] [Google Scholar]
- 37.Mooi WJ, Peeper DS: Oncogene-induced cell senescence – halting on the road to cancer. N Engl J Med 2006; 355: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 38.Lamers CB, Froeling PG: Clinical significance of hyperparathyroidism in familial multiple endocrine adenomatosis type I (MEA I). Am J Med 1979; 66: 422–424. [DOI] [PubMed] [Google Scholar]
- 39.Giraud S, Zhang CX, Serova-Sinilnikova O, Wautot V, Salandre J, Buisson N, Waterlot C, Bauters C, Porchet N, Aubert JP, Emy P, Cadiot G, Delemer B, Chabre O, Niccoli P, Leprat F, Duron F, Emperauger B, Cougard P, Goudet P, Sarfati E, Riou JP, Guichard S, Rodier M, Meyrier A, Caron P, Vantyghem MC, Assayag M, Peix JL, Pugeat M, Rohmer V, Vallotton M, Lenoir G, Gaudray P, Proye C, Conte-Devolx B, Chanson P, Shugart YY, Goldgar D, Murat A, Calender A: Germ-line mutation analysis in patients with multiple endocrine neoplasia type 1 and related disorders. Am J Hum Genet 1997; 63: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, Skarulis MC, Weil RJ, Lubensky IA, Zhuang Z, Old-field EH, Marx SJ: Pituitary macroadenoma in a 5 year old: an early expression of MEN1. J Clin Endocrinol Metab 2000; 85: 4776–4780. [DOI] [PubMed] [Google Scholar]
- 41.Bai F, Pei X-H, Nishikawa T, Smith MD, Xiong Y: p18Ink-4c, but not p27Kip1, collaborates with Men1 to suppress neuroendocrine organ tumors. Mol Cell Biol 2007; 27: 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loffler KA, Biondi CA, Gartside MG, Serewko-Auret MM, Duncan R, Tonks ID, Mould AW, Waring P, Muller HK, Kay GF, Hayward NK: Lack of augmentation of tumor spectrum or severity in dual heterozygous Men1 and Rb1 knockout mice. Oncogene 2007; 26: 4009–4017. [DOI] [PubMed] [Google Scholar]
- 43.Matoso A, Zhou Z, Hayama R, Flesken-Nikitin A, Nikitin AY: Cell lineage-specific interactions between Men1 and Rb in neuroendocrine neoplasia. Carcinogenesis 2008; 29: 620–628. [DOI] [PubMed] [Google Scholar]
- 44.Korsisaari N, Ross J, Wu X, Kowanetz M, Pal N, Hall L, Eastham-Anderson J, Forrest WF, Van Bruggen N, Peale FV, Ferrara N: Blocking vascular endothelial growth factor-A inhibits the growth of pituitary adenomas and lowers serum prolactin levels in a mouse model of multiple endocrine neoplasia type 1. Cancer Res 2008; 14: 249–258. [DOI] [PubMed] [Google Scholar]
- 45.Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, Wang ZQ: Pancreatic beta cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res 2003; 63: 4836–4841. [PubMed] [Google Scholar]
- 46.Zhuang Z, Ezzat SZ, Vortmeyer AO, Weil R, Oldfield EH, Park WS, Pack S, Huang S, Agarwal SK, Guru SC, Manickam P, Debelenko LV, Kester MB, Olufemi SE, Heppner C, Crabtree JS, Burns AL, Spiegel AM, Marx SJ, Chandrasekharappa SC, Collins FS, Emmert-Buck MR, Liotta LA, Asa SL, Lubensky IA: Mutations of the MEN1 tumor suppressor gene in pituitary tumors. Cancer Res 1997; 57: 5446–5451. [PubMed] [Google Scholar]
- 47.Lemos MC, Thakker RV: Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat 2008; 29: 22–32. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal SK, Scacheri PC, Rice TS, Kennedy PA, Ozawa A, Burgess-Hickman A, Popson SA, Burns AL, Mateo C, Simonds WF, Oliver B, Libutti SK, Novotny EA, Halawi MJ, Ji Y, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ: MEN1 gene: mutation and pathophysiology. Ann Endocrinol (Paris) 2006; 67(suppl 4):1S12–1S13. [Google Scholar]
- 49.Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ: Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci USA 2003; 100: 10770–10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozanne BW, Spence HJ, McGarry LC, Hennigan RF: Transcription factors control invasion: AP-1 the first among equals. Onco-gene 2007; 26: 1–10. [DOI] [PubMed] [Google Scholar]
- 51.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs D, Hua X, Roeder RG, Meyerson M, Hess JL: Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci USA 2005; 102: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karnik SK, Hughes CM, Gu XY, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK: Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27(Kip1) and p18(INK4c). Proc Natl Acad Sci USA 2005; 102: 14659–14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoyama A, Somervaille TCP, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML: The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005; 123: 207–218. [DOI] [PubMed] [Google Scholar]
- 54.Karnik SK, Chen HN, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK: Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007; 318: 806–809. [DOI] [PubMed] [Google Scholar]