Abstract
Children born very preterm (VPT; <32 weeks gestational age [GA]) are at greater risk for a range of cognitive deficits that typically manifest at school age. Here we examine the hypothesis that these children have altered myelin maturational that can be detected by myelin sensitive MRI measures prior to school age. We included 33 four-year old children born VPT (mean GA; 28.7 weeks) and 23 four-year old full term (FT) children and completed magnetization transfer (MT), T1-weighted (T1-w) and T2-weighted (T1-w) magnetic resonance imaging as well as developmental assessments. Both MT ratio (MTR) and T1-w/T2-w ratio images were calculated, and group differences were probed using tract-based spatial statistics (TBSS) in white matter, and region of interest (ROI) analysis in white, subcortical gray and cortical gray matter. The relations between MTR and T1-w/T2-w ratio, as well as with developmental assessments, were investigated in all three brain divisions. In children born VPT, TBSS and ROI analysis revealed that both MTR and T1-w/T2-w ratio were significantly reduced in white matter compared to children born FT. ROI analysis showed reductions in T1-w/T2-w ratio in VPT children compared to FT children in the thalamus, putamen and amygdala, as well as in the occipital and temporal lobes. Across the VPT and FT children, T1-w/T2-w ratio and MTR were highly correlated across white, subcortical gray and cortical gray matter. Both measures correlated positively with developmental assessments in individual white matter tracts and cortical and subcortical ROIs, suggesting that higher MTR and T1-w/T2-w ratio is related to better cognitive performance. Together these findings are consistent with delayed myelination in VPT born children.
Keywords: Magnetic resonance imaging, Preterm, T1-weighted/T2-weighted ratio, Magnetization transfer ratio, Myelin enhanced contrast
Abbreviations: VPT, Very preterm; GA, gestational age; FT, full term; MT, magnetization transfer; T1-w, T1-weighted; T2-w, T2-weighted; MTR, magnetization transfer ratio; TBSS, tract-based spatial statistics; ROI, region of interest; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging; FSIQ, Full Scale Intelligence Quotients; CL, Core Language; VMI, Visual-Motor Integration; DK, Desikan-Killiany; FA, fractional anisotropy; TFCE, threshold-free cluster enhancement; ANCOVA, analysis of covariance; FDR, false discovery rate; Ant.TR, anterior thalamic radiation; CS, corticospinal tract; C.CG, cingulum cingulate gyrus; C.H, cingulum hippocampus; F.MAJ, forceps major; F.MIN, forceps minor; Inf.FOF, inferior fronto-occipital fasciculus; Inf.LF, inferior longitudinal fasciculus; Sup.LF, superior longitudinal fasciculus; UF, uncinate fasciculus; Sup.LFT, superior longitudinal fasciculus temporal part
Highlights
-
•
Very preterm children have widespread decreased MTR in white matter.
-
•
T1-w/T2-w ratio measures showed consistent white matter alterations.
-
•
T1-w/T2-w ratio was also reduced in subcortical, occipital and temporal regions.
-
•
MTR and T1-w/T2-w were correlated throughout the brain.
-
•
MTR and T1-w/T2-w correlated with developmental assessments.
1. Introduction
Approximately 10% of babies worldwide are born preterm (<37 weeks gestational age (GA)) with rates rising globally, resulting in a significant burden on both families and the healthcare systems (Beck et al., 2010). Despite a significant decrease in mortality and neonatal morbidity rates over the past few decades due to improved medical care, children born very preterm (VPT; <32 weeks GA) are at significant risk of developing a wide range of cognitive difficulties throughout childhood (Anderson, 2014). By four years of age, children born VPT are more likely to exhibit cognitive delays, language delays and/or emotional and behavioural adjustment problems, and are three times more likely to experience difficulties in more than one of these areas (Woodward et al., 2009). Between four and 12 years of age, those born VPT on average significantly underperform those born at full term (FT) in terms of general intellectual and cognitive functioning, and trends based on current evidence indicate it is unlikely that they will recover from these early delays (Mangin et al., 2017), producing deficits into adulthood (e.g., Eryigit Madzwamuse et al., 2015; Kroll et al., 2017).
Magnetic resonance imaging (MRI) has been widely used to study brain development in children born VPT and elucidate differences in structural gray and white matter. MRI studies have focused primarily on neuroanatomical volumetric differences between children born VPT and children born FT (Ball et al., 2012; Boardman et al., 2006; Nosarti et al., 2008), and as well as differences in white matter organization revealed using diffusion tensor imaging (DTI; Anjari et al., 2007; Counsell et al., 2008; Duerden et al., 2015; Young et al., 2018). Studies have also investigated the association between MRI structural measures and cognitive outcomes in children born VPT, and whether there is a relation between gestational age and these measures (Lax et al., 2013; Lemola et al., 2017; Nosarti and Froudist-Walsh, 2016; Woo Nam et al., 2015).
Magnetization transfer (MT) imaging is an MRI contrast based in the interaction between immobile macromolecular protons and mobile water protons (Wolff and Balaban, 1994). It can be used as a quantitative measure of brain tissue structure, and is most easily acquired as an MT ratio (MTR) contrast, which is the percentage change between images with and without MT weighting. Whereas diffusion MR imaging offers insight into the organization of tissue through restriction of water motion by membranes or cellular structures (Soares et al., 2013), MT detects the macromolecules that make up these membranes and other cellular components. MT contrast is sensitive to myelin and has been widely used to study white matter disease (Filippi and Rocca, 2004). While MT is defined in terms of atomic-scale physical interactions, a growing body of supporting data from disease pathophysiology, animal models and histopathology enables its interpretation on a biological level (Sled, 2017).
MT imaging has been used to study structural changes of brain development in healthy children and adults. Studies have shown marked increases in MTR from birth until early childhood, with dominant changes occurring before two years of age, consistent with expected myelination changes (Engelbrecht et al., 1998; Rademacher et al., 1999; Van Buchem et al., 2001). MTR has been shown to continue to increase into early adulthood, at which point a slow decline commences that accelerates at about 50 years of age (Ge et al., 2002).
Few studies have used MT imaging in children born VPT, and, to our knowledge, MT imaging has not yet been used to study early childhood in children born VPT. Xydis et al., (2006b) examined the developmental changes in MTR in white matter and subcortical structures in preterm children up to two years of age, and used MTR to show that preterms with periventricular leukomalacia had an abnormal and accelerated myelination process compared to preterms without (Xydis et al., 2006a). Nossin-Manor et al. (2012) reported that VPT neonates scanned within two weeks of birth showed increasing MTR with age in the basal ganglia, thalami and pons. A follow-up analysis was performed when the neonates were at term-equivalent age (Nossin-Manor et al., 2013), and the spatiotemporal maturation was characterized using MTR and DTI parameters throughout the brain (Nossin-Manor et al., 2015).
Recently, it has been proposed that the ratio of T1-weighted (T1-w) and T2-weighted (T2-w) MRI signal intensity can be used as a measure of the degree of myelination in the human brain by simultaneously reducing the inter-subject signal intensity bias while enhancing the sensitivity to myelin signal intensity (Glasser and Van Essen, 2011). The T1-w/T2-w ratio has been used to examine cortical myelin maps (Glasser et al., 2013, Glasser et al., 2014; Van Essen et al., 2013) and evaluate their changes with age (Grydeland et al., 2013; Shafee et al., 2015). T1-w/T2-w ratios have also been applied to the study white matter myelin in neonates (Chen et al., 2017; Lee et al., 2015; Soun et al., 2017), in healthy adults (Chopra et al., 2017; Yasuno et al., 2017) and in aging adults (Teubner-Rhodes et al., 2016). Furthermore, the ratio has been useful in determining myelin differences in white matter, cortical gray matter and subcortical gray matter between healthy adults and clinical populations such as schizophrenia (Ganzetti et al., 2015; Iwatani et al., 2015), post-traumatic stress disorder (Chao et al., 2015), multiple sclerosis (Beer et al., 2016; Granberg et al., 2017; Nakamura et al., 2017; Righart et al., 2017) and bipolar disorder (Ishida et al., 2017). Improved calibration techniques to account for inconsistencies across datasets have been proposed (Ganzetti et al., 2014; Lee et al., 2015; Shafee et al., 2015), although not all studies have used these techniques (Chao et al., 2015; Ishida et al., 2017; Iwatani et al., 2015).
Compared to MTR, T1-w/T2-w ratios have the advantage of being easily computed from T1-w and T2-w scans that typically would be acquired for neuroanatomical assessment. Moreover, while MTR is sensitive to myelin, its properties cannot be mapped directly to myelin content, and it has been shown also to be sensitive to the macromolecular density of axonal cytoskeleton components such as microtubules and neurofilaments (Laule et al., 2007; Nossin-Manor et al., 2013).
The primary aim of the present study was to investigate maturation and structural differences of white matter, subcortical gray matter and cortical gray matter in children born VPT compared to FT children at four years of age. Simultaneously, the relations between T1-w/T2-w ratio and MTR contrasts within this cohort were investigated as a means to validate T1-w/T2-w ratio as a potential alternative when MTR contrasts are not available. We employed two types of analyses: a whole brain approach using tract-based spatial statistics to characterize white matter differences in VPT and FT children in both MTR and T1-w/T2-w ratio; and region of interest (ROI) analysis to investigate white matter, subcortical gray matter and cortical gray matter differences in both MTR and T1-w/T2-w ratio, as well as their correlations within the ROIs across groups. In both sets of analyses, the relations between MTR and T1-w/T2-w ratio with developmental assessments were tested across both cohorts, and the relation with GA was tested in the children born VPT.
2. Material methods
2.1. Participants
As part of a larger longitudinal study, 53 very preterm (VPT) participants (born at <32 weeks gestational age (GA)) were studied at four years of age. VPT participants were excluded from initial recruitment on the basis of any known chromosomal or major congenital abnormalities. Twenty-eight full term-born (FT) children (born at >37 weeks GA) were recruited at four years of age as part of the same study. FT participants were excluded from recruitment on the basis of prematurity, learning, language, neurological or developmental disabilities, and MRI incompatibility. All families agreed to MRI scans, access to medical records and developmental assessments with signed informed consent; the children gave informed assent at four years of age. The study protocol was approved by the research ethics board at the Hospital for Sick Children (SickKids) in Toronto.
Developmental assessments were performed on the VPT and FT participants. Full Scale Intelligence Quotients (FSIQ) were obtained via the Wechsler Preschool and Primary Scales of Intelligence – Third Edition (Weshcler, 2002) using Canadian norms to measure cognitive abilities. Core Language (CL) was obtained via the Clinical Evaluation of Language Fundamentals – Preschool, Second Edition (Semel et al., 2004) to summarize expressive and receptive language ability. Visual-Motor Integration (VMI) was measured via the Beery-Buktenica Test of Visual Motor Integration (Beery et al., 2010). Raw scores were converted into standardized scores with a population mean of 100 (50th percentile of typical development) and a standard deviation of 15.
2.2. MRI protocol
All MRI scans were acquired on a 3T Siemens Trio scanner with a 12-channel head coil at SickKids. T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (repetition time (TR): 2300 ms; echo time (TE): 2.96 ms; inversion time (TI): 900 ms; flip angle: 9°; field of view (FOV): 250 mm; matrix: 240 × 256; 192 slices of 1.0 mm; scan time: 5 min). T2-weighted images were acquired using a turbo spin echo (TSE) sequence (TR: 9000 ms; TE: 104 ms; FA: 120°; FOV: 230 mm; matrix: 191 × 230; 128 slices of 1.2 mm; scan time 3 min). MT images were acquired using a proton-density weighted 3D spoiled gradient recalled (SPGR) sequence (TR/TE: 51/3.81 ms; flip angle: 10°; 1.2 kHz off-resonance 500° Gaussian shaped MT saturation pulses of 10 ms duration and 100 Hz bandwidth, equivalent continuous wave nutation rate 75 Hz; FOV: 192 mm; matrix: 128 × 128; 104 slices of 1.5 mm; scan time: 8 min) by acquiring the sequence twice – once with an off-resonance MT transfer pulse (MTOFF) and once without (MTON).
2.3. Preprocessing
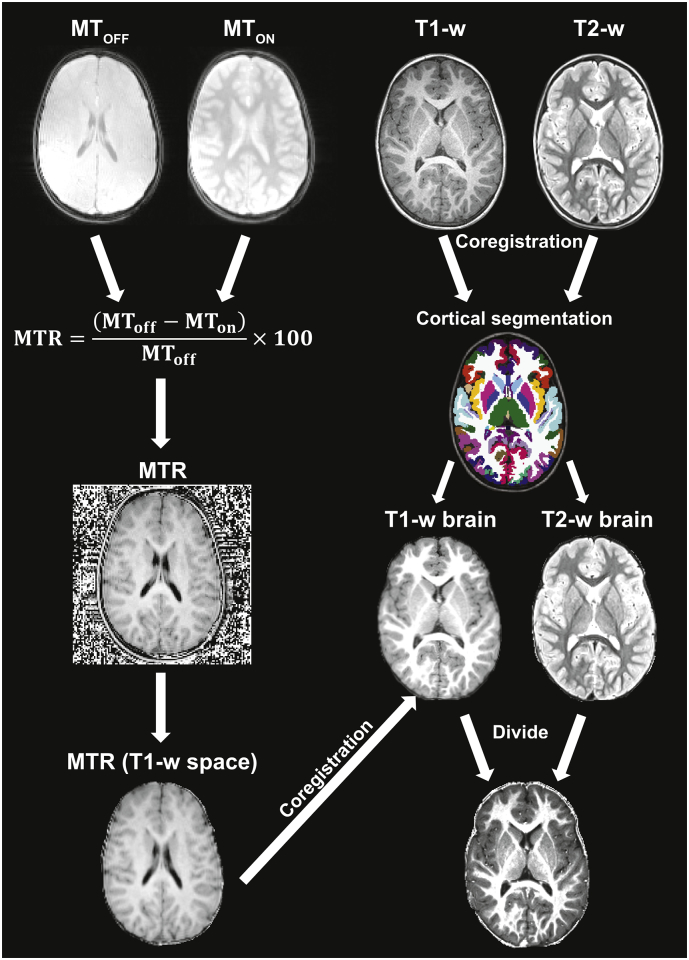
For each subject, the T1-weighted image was skull-stripped and parcellated into 34 cortical ROIs per hemisphere and 7 subcortical ROIs per hemisphere according to the Desikan-Killiany (DK) atlas (Desikan et al., 2006) using the Freesurfer image analysis suite, which is documented and freely available for download (http://surfer.nmr.mgh.harvard.edu/). The T2-weighted image was used to improve the skull-stripping and surface reconstruction. Quality assessment was performed visually, and brain mask and parcellation errors were corrected with manual editing and control points. The T2-weighted image was then linearly registered to the T1-weighted image using FSL FLIRT (Andersson et al., 2007; Jenkinson et al., 2012), and skull-stripped using the skull-stripped T1-w image. The T1-w/T2-w ratio image was obtained by dividing the T1-w image by the T2-w image.
The MTON image was linearly registered to the MTOFF image using FSL FLIRT (Jenkinson et al., 2002), and the MTR maps were obtained by calculating the percent difference of these two images. Each child's resulting MTR image was then registered to his/her skull-stripped T1-weighted image using FSL FLIRT (Jenkinson et al., 2002) and masked to produce a skull-stripped MTR image. See Fig. 1 for a visual representation of the preprocessing pipeline.
Fig. 1.
Preprocessing pipeline.
2.4. TBSS analysis
White matter differences in MTR and T1-w/T2-w ratio were investigated using Tract Based Spatial Statistics (TBSS; Smith et al., 2006). Although TBSS was developed to analyze fractional anisotropy (FA) images from diffusion tensor imaging, the contrast in T1-w/T2-w ratio, where white matter tracts appear bright, is also suitable for analysis by this procedure. In this approach, a group of subjects' T1-w/T2-w ratio images are projected onto a tract skeleton to reduce partial volume effects and registration inaccuracies when performing voxelwise statistics. The voxels from the T1-w/T2-w ratio image that are projected onto the skeleton are identified by searching for the maximum T1-w/T2-w ratio value perpendicular to local tangent of the skeleton.
In preparation for this projection procedure, the children's T1-w/T2-w ratio images were first aligned to a common cohort-specific space using FSL's nonlinear registration tool, FNIRT (Andersson et al., 2007). Next, the mean T1-w/T2-w ratio image was calculated and thinned to create a mean T1-w/T2-w ratio skeleton, representing the centres of all tracts common to the group. The skeleton was thresholded at 0.5 (determined empirically to include the most data while minimizing the cross-subject variability, as is done in the standard FA TBSS) to reduce across-subject variability and eliminate tracts with low T1-w/T2-w ratio. Then, the skeleton was applied to each subject's aligned T1-w/T2-w ratio. The subjects' MTR images were aligned to the same space, and the skeleton mask was also applied to these images.
2.5. ROI analysis
To gain further insight into the significant tracts from the TBSS analysis, the JHU DTI-based tractography atlas (Mori et al., 2005) was used to identify defined white matter tracts. The atlas was aligned to the common cohort-specific space using FSL's nonlinear registration tool, FNIRT (Andersson et al., 2007), the atlas was masked by the tract skeleton to reduce partial volume effects and registration inaccuracies. For each subject, the average MTR and T1-w/T2-w ratio values were calculated in each tract, and averaged over hemispheres (11 tracts in total). MTR and T1-w/T2-w ratio values were correlated with each other in each individual tract, as well as across all tracts, to establish a relationship between the two metrics. MTR and T1-w/T2-w ratio in each tract and the average of all tracts were correlated with the measures of FSIQ, CL and VMI to determine their impact on outcomes.
For each subject, the average MTR and T1-w/T2-w ratio was also calculated for each of the 7 subcortical and 34 cortical regions, after eroding each ROI by 1 mm to mitigate partial volume effects. Resulting values were averaged across hemisphere, and were further averaged over the frontal, occipital, parietal and temporal lobes. The MTR and T1-w/T2-w ratio values were correlated with each other, and with the measures of FSIQ, CL and VMI.
2.6. Statistical analysis
Differences in age, sex and behavioural measures between the VPT and FT groups were tested using two-tailed t-tests and chi-squared tests. Tests were carried out using MATLAB (The Mathworks Inc., 2016) software, and significance values were held at p < .05.
For TBSS, group differences in MTR and T1-w/T2-w ratio and correlations with FSIQ, CL and VMI were tested using voxel-wise analyses based on the skeleton with FSL randomise (Winkler et al., 2014) using 5000 permutations, threshold-free cluster enhancement (TFCE), and age and sex as covariates. For the VPT group, correlation with GA was also investigated.
For the white matter, cortical and subcortical ROI analyses, VPT and FT differences in MTR and T1-w/T2-w ratio were investigated using an analysis of covariance (ANCOVA), with age and sex as covariates. Non-parametric permutation testing was used to establish statistical significance. The ANCOVA was run with randomly permuted group labels 5000 times, obtaining a null distribution of t-statistics. The observed t-statistic was compared to the null distribution to establish a p-value. Correlations between MTR and T1-w/T2-w ratio and with FSIQ, CL and VMI were determined using linear regression modelling. MTR and T1-w/T2-w ratio correlations across all ROIs and correlations between the developmental assessments and average MTR and T1-w/T2-w ratio are presented in the Results section, while within-ROI correlations can be found in supplemental materials. All analyses were implemented using MATLAB. Significance was determined to be q < 0.05 following false discovery rate (FDR) correction to control for multiple comparisons across sets of ROIs (Benjamini and Hochberg, 1995).
3. Results
3.1. Participant characteristics
After excluding children whose MTOFF, MTON, T1-w, or T2-w images were corrupted by motion (reviewed on a case-by-case basis), 33 VPT and 23 FT children remained (out of 39 VPT and 25 FT children who had completed the full imaging protocol). Table 1 shows a summary of study demographics. Full developmental assessments were not available for all participants; however, data were collected in over 80% of both VPT and FT children for each measure (FSIQ, CL and VMI). There were significant group differences in age, FSIQ, CL and VMI (p < .05). Although there was a significant difference in age, the difference in group mean was less than four months, and all subjects were within the four-to-five-year-old range. As a precaution, age and sex were included as covariates in all group difference analyses.
Table 1.
Demographic and developmental assessment information for MT and DT image participants (mean ± standard deviation, with the number of participants (N) for whom the developmental assessments were obtained where appropriate).
| N | Sex (M:F) | Age | FSIQ | CL | VMI | GA | |
|---|---|---|---|---|---|---|---|
| VPT | 33 | 20:13 | 4.2 ± 0.2 | 96.1 ± 14.2 N = 31 | 96.1 ± 17.1 N = 28 | 99.5 ± 11.2 N = 32 | 28.7 ± 1.6 |
| FT | 23 | 10:13 | 4.5 ± 0.3 | 107.6 ± 13.9 N = 21 | 109.8 ± 12.8 N = 18 | 108.9 ± 9.0 N = 21 | 39.1 ± 1.8 |
| χ2/t-statistic | 2.00 | 5.54 | 2.89 | 2.90 | 3.24 | ||
| p-value | 0.16 | 9.715(10-07) | 0.006 | 0.006 | 0.002 | ||
FSIQ full scale IQ, CL core language, VMI visual motor integration, GA gestational age.
3.2. TBSS analysis
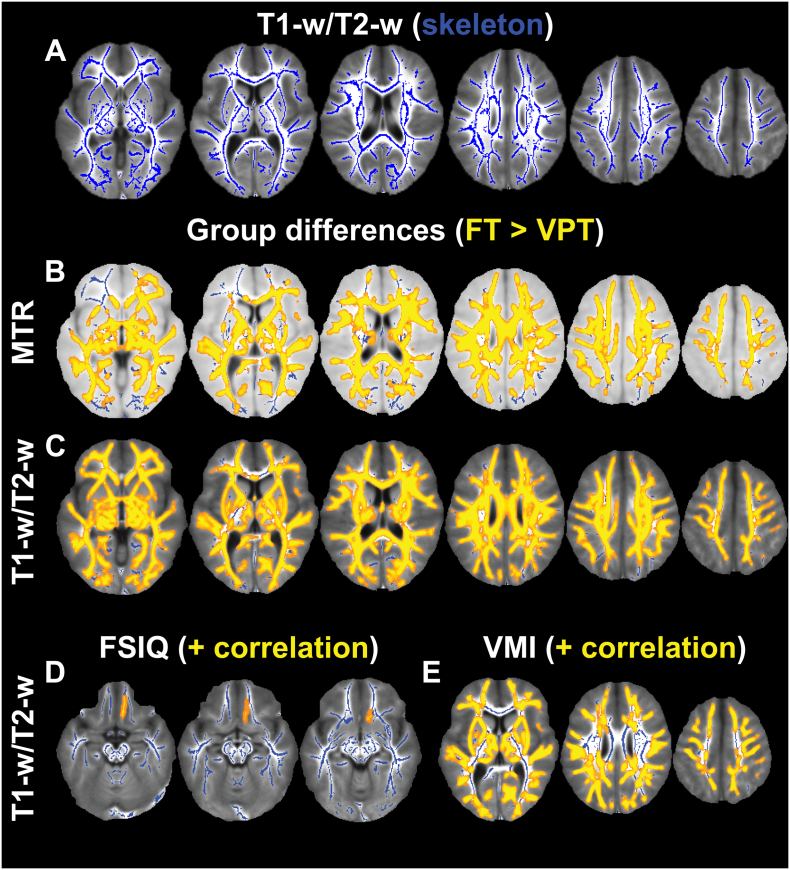
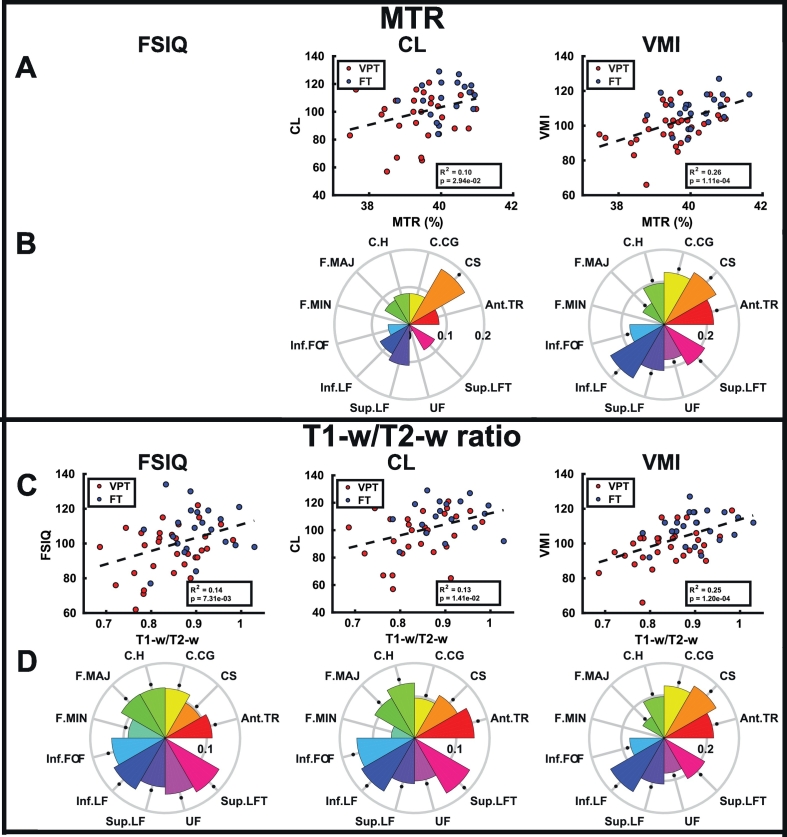
A skeleton was derived from the T1-w/T2-w ratio (Fig. 2A), and TBSS analyses showed that children born VPT exhibited a significant reduction in both MTR (Fig. 2B) and T1-w/T2-w ratio (Fig. 2C) in almost all tracts within the white matter skeleton. Tracts where differences were less extensive in MTR included the inferior fronto-occipital fasciculus and the forceps minor and major, and for T1-w/T2-w, differences were less widespread in the corticospinal tract and forceps minor. Significant positive correlations were found between T1-w/T2-w ratio and FSIQ in the right uncinate fasciculus (Fig. 2D) and VMI in tracts throughout the brain, excluding the forceps major and minor and less extensively in the inferior longitudinal fasciculus (Fig. 2E), while no correlations between MTR and the developmental assessments were significant.
Fig. 2.
T1-w/T2-w ratio derived white matter skeleton (A, blue), and voxels (yellow) with significant reductions in MTR (B) and T1-w/T2-w ratio (C) in VPTs along the white matter tracts (blue) displayed on the mean MTR and T1-w/T2-w ratio images. T1-w/T2-w ratio positively correlates with FSIQ (D) and VMI (E) in the corresponding yellow voxels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. ROI analysis
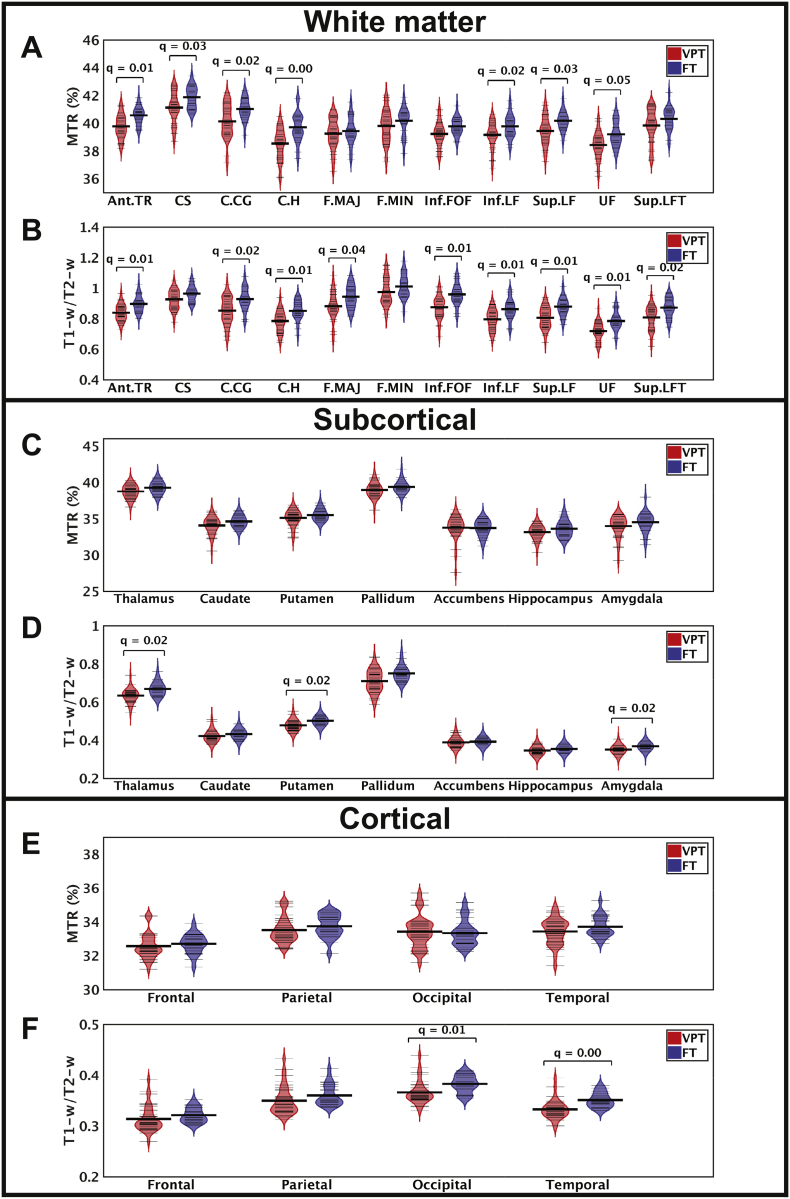
ROI analyses using the JHU-tractography atlas revealed that the FT children had significantly higher MTR (Fig. 3A; Table 2) and T1-w/T2-w ratio (Fig. 3B; Table 2) than the children born VPT in the anterior thalamic radiation (q = 0.0044; q = 0.0077), cingulum (cingulate gyral (q = 0.0220; q = 0.0101) and hippocampal (q = 0.0022; q = 0.0057) parts, inferior (q = 0.0281; q = 0.0072) and superior (q = 0.0337; q = 0.0077) longitudinal fasciculus. FT children also had significantly higher MTR in the corticospinal tract (q = 0.0337), while they exhibited significantly higher T1-w/T2-w ratio than children born VPT in the forceps major (q = 0.0445), inferior fronto-occipital fasciculus (q = 0.0044), the uncinate fasciculus (q = 0.0066) and the temporal part of the superior longitudinal fasciculus (q = 0.0202). Across all ROIs, there was a trend of FT children having higher MTR and T1-w/T2-w ratio than children born VPT.
Fig. 3.
Violin plots of the MTR and T1-w/T2-w ratio data for VPTs (red) and FTs (blue) in the white matter (A, B), subcortical (C, D), and cortical (E, F) ROIs. Thick black lines show group means, while thin black lines indicate individual participant ratios. White matter tracts: anterior thalamic radiation (Ant.TR), corticospinal tract (CS), cingulum (cingulate gyrus, C.CG), cingulum (hippocampus, C·H), forceps major (F.MAJ), forceps minor (F.MIN), inferior fronto-occipital fasciculus (Inf.FOF), inferior longitudinal fasciculus (Inf.LF), superior longitudinal fasciculus (Sup.LF), uncinate fasciculus (UF), superior longitudinal fasciculus (temporal part, Sup.LFT).
Table 2.
White matter ROI group difference results.
| MTR |
T1-w/T2-w ratio |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± Std. dev. |
t-Statistic | q-Value | Mean ± Std. dev. |
t-Statistic | q-Value | |||
| PT (%) | FT (%) | PT | FT | |||||
| Ant.TR | 39.78 ± 0.84 | 40.58 ± 0.63 | 12.29 | 0.01 | 0.84 ± 0.05 | 0.90 ± 0.05 | 9.30 | 0.01 |
| CS | 41.13 ± 1.11 | 41.89 ± 0.79 | 5.74 | 0.03 | 0.93 ± 0.06 | 0.96 ± 0.05 | 3.91 | 0.05 |
| C.CG | 40.13 ± 1.23 | 41.03 ± 0.78 | 8.15 | 0.02 | 0.85 ± 0.09 | 0.93 ± 0.07 | 7.66 | 0.02 |
| C·H | 38.54 ± 1.12 | 39.71 ± 1.07 | 16.35 | 0.00 | 0.79 ± 0.07 | 0.85 ± 0.07 | 11.14 | 0.01 |
| F.MAJ | 39.26 ± 1.12 | 39.43 ± 0.99 | 0.45 | 0.51 | 0.88 ± 0.09 | 0.94 ± 0.08 | 4.56 | 0.04 |
| F.MIN | 39.81 ± 1.19 | 40.17 ± 0.99 | 0.54 | 0.51 | 0.97 ± 0.09 | 1.01 ± 0.08 | 1.23 | 0.28 |
| Inf.FOF | 39.24 ± 0.86 | 39.78 ± 0.62 | 4.57 | 0.05 | 0.88 ± 0.07 | 0.96 ± 0.07 | 12.11 | 0.01 |
| Inf.LF | 39.16 ± 0.94 | 39.78 ± 0.81 | 7.25 | 0.02 | 0.80 ± 0.07 | 0.86 ± 0.07 | 9.93 | 0.01 |
| Sup.LF | 39.45 ± 1.03 | 40.17 ± 0.66 | 6.02 | 0.03 | 0.81 ± 0.07 | 0.88 ± 0.06 | 9.05 | 0.01 |
| UF | 38.43 ± 0.93 | 39.21 ± 0.91 | 4.94 | 0.05 | 0.72 ± 0.06 | 0.79 ± 0.06 | 10.02 | 0.01 |
| Sup.LFT | 39.84 ± 1.21 | 40.32 ± 0.84 | 2.52 | 0.15 | 0.81 ± 0.08 | 0.87 ± 0.06 | 6.49 | 0.02 |
Ant.TR anterior thalamic radiation, CS corticospinal tract, C.CG cingulum (cingulate gyrus), C.H cingulum (hippocampus), F.MAJ forceps major, F.MIN forceps minor, Inf.FOF inferior fronto-occipital fasciculus, Inf.LF inferior longitudinal fasciculus, Sup.LF superior longitudinal fasciculus, UF uncinate fasciculus, Sup.LFT superior longitudinal fasciculus (temporal part).
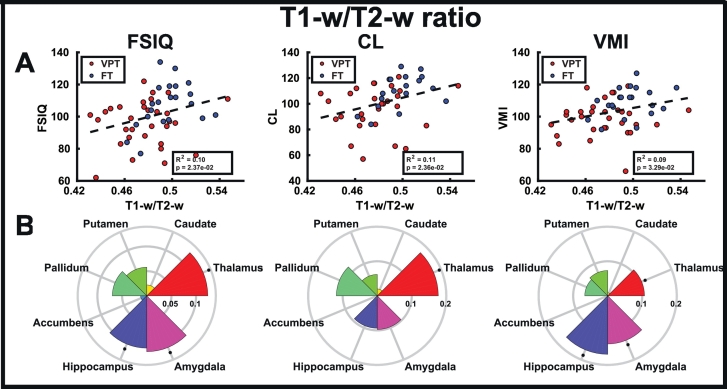
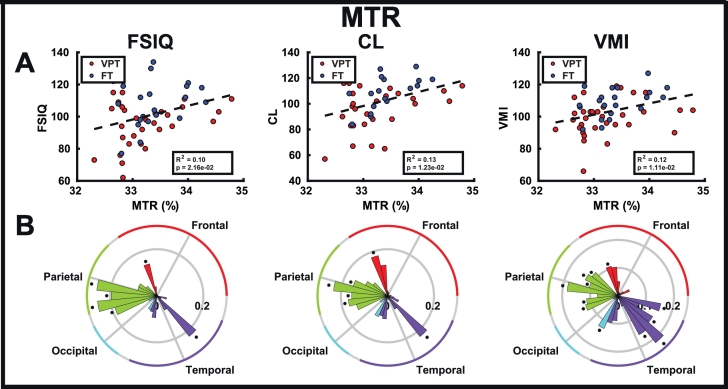
In subcortical structures, there were no significant group differences in MTR (Fig. 3C; Table 3); however, FT children had significantly higher T1-w/T2-w ratio (Fig. 3D; Table 3) than children born VPT in the thalamus (q = 0.0182), putamen (q = 0.0280) and amygdala (q = 0.0182). Similarly in the cortical lobes, significant group differences were only found in T1-w/T2-w ratio, where FT children showed significantly higher ratios than children born VPT (Fig. 3E, F; Table 4) in the occipital (q = 0.0432) and temporal (q = 0.0048) lobes (see Supplemental Table 1 for individual cortical ROI results).
Table 3.
Subcortical ROI group difference results.
| MTR |
T1-w/T2-w ratio |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± Std. dev. |
t-Statistic | q-Value | Mean ± Std. dev. |
t-Statistic | q-Value | |||
| PT (%) | FT (%) | PT | FT | |||||
| Thalamus | 38.72 ± 0.86 | 39.27 ± 0.79 | 5.01 | 0.24 | 0.63 ± 0.03 | 0.67 ± 0.04 | 8.03 | 0.02 |
| Caudate | 34.07 ± 1.14 | 34.62 ± 0.76 | 2.04 | 0.48 | 0.42 ± 0.03 | 0.43 ± 0.02 | 0.12 | 0.74 |
| Putamen | 35.10 ± 1.07 | 35.51 ± 0.74 | 1.21 | 0.48 | 0.48 ± 0.03 | 0.50 ± 0.02 | 7.26 | 0.02 |
| Pallidum | 38.93 ± 0.97 | 39.37 ± 0.82 | 0.70 | 0.48 | 0.71 ± 0.06 | 0.75 ± 0.04 | 0.57 | 0.58 |
| Accumbens | 33.75 ± 1.72 | 33.71 ± 1.07 | 0.05 | 0.82 | 0.39 ± 0.02 | 0.39 ± 0.02 | 0.47 | 0.58 |
| Hippocampus | 33.14 ± 0.95 | 33.61 ± 1.11 | 1.11 | 0.48 | 0.35 ± 0.02 | 0.35 ± 0.01 | 2.54 | 0.21 |
| Amygdala | 33.97 ± 1.49 | 34.53 ± 1.37 | 0.71 | 0.48 | 0.35 ± 0.02 | 0.37 ± 0.01 | 8.29 | 0.02 |
Table 4.
Cortical lobe group difference results.
| MTR |
T1-w/T2-w ratio |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± Std. dev. |
t-Statistic | q-Value | Mean ± Std. dev. |
t-Statistic | q-Value | |||
| PT (%) | FT (%) | PT | FT | |||||
| Frontal | 32.57 ± 0.76 | 32.70 ± 0.58 | 0.15 | 0.73 | 0.31 ± 0.03 | 0.32 ± 0.01 | 1.24 | 0.27 |
| Parietal | 33.51 ± 0.77 | 33.74 ± 0.68 | 1.27 | 0.51 | 0.35 ± 0.03 | 0.36 ± 0.02 | 2.13 | 0.20 |
| Occipital | 33.42 ± 0.94 | 33.33 ± 0.76 | 0.13 | 0.73 | 0.37 ± 0.02 | 0.38 ± 0.02 | 8.41 | 0.01 |
| Temporal | 33.42 ± 0.79 | 33.70 ± 0.62 | 1.50 | 0.51 | 0.33 ± 0.02 | 0.35 ± 0.01 | 11.28 | 0.00 |
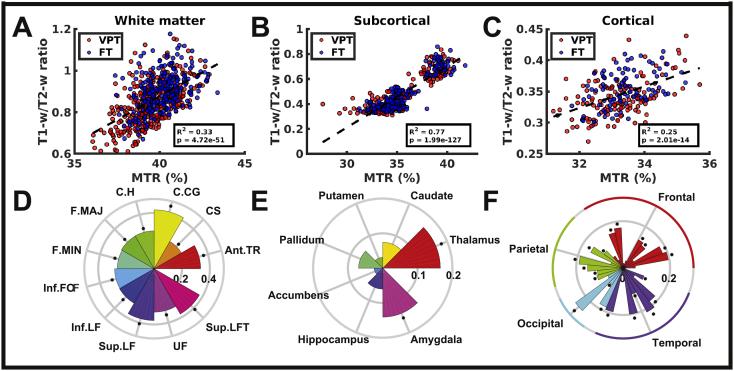
Across both groups, MTR and T1-w/T2-w ratio were significantly correlated across all white matter tracts (Fig. 4A; Supplemental Table 2; p = 4.72(10−51); R2 = 0.33), subcortical structures (Fig. 4B; Supplemental Table 3; p = 2(10−127); R2 = 0.77) and cortical lobes (Fig. 4C; Supplemental Table 4; p = 2(10−14); R2 = 0.25; see Supplemental Table 5 for individual cortical ROI results). Within individual ROIs, MTR and T1-w/T2-w ratio were significantly correlated in all white matter tracts (Fig. 4D) and cortical structures (Fig. 4F), as well as within the thalamus and amygdala (Fig. 4E).
Fig. 4.
Correlation between MTR and T1-w/T2-w ratio in the white matter (A), subcortical (B), and cortical (C) ROIs. Scatter plots (top) show correlation across all ROIs in both VPTs (red) and FTs (blue), while circular bar charts (bottom) show individual ROI correlations, where bar height indicates the R2 value, and asterisks indicate significant correlations (q < 0.05). White matter tracts: anterior thalamic radiation (Ant.TR), corticospinal tract (CS), cingulum (cingulate gyrus, C.CG), cingulum (hippocampus, C.H), forceps major (F.MAJ), forceps minor (F.MIN), inferior fronto-occipital fasciculus (Inf.FOF), inferior longitudinal fasciculus (Inf.LF), superior longitudinal fasciculus (Sup.LF), uncinate fasciculus (UF), superior longitudinal fasciculus (temporal part, Sup.LFT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In white matter (Fig. 5; Supplemental Tables 6–7) and subcortical structures (Fig. 6; Supplemental Tables 8–9), T1-w/T2-w ratios showed more correlations with FSIQ, CL and VMI than MTR. When averaged over all white matter tracts, the T1-w/T2-w ratio significantly correlated with FSIQ (p = 0.0073), while this relation did not exist with MTR. Mean white matter MTR and T1-w/T2-w ratio both correlated with CL (p =0.0294; R2 = 0.10; p =0.0141; R2 = 0.13) and VMI (p =0.0001; R2 = 0.26; p =0.0001; R2 = 0.25). In the subcortical structures, only mean T1-w/T2-w ratio correlated with FSIQ (p =0.0237; R2 = 0.10), CL (p =0.0236; R2 = 0.11) and VMI (p =0.0329; R2 = 0.09). However, in the cortical structures (Fig. 7; Supplemental Tables 10–13), MTR correlated more with FSIQ, CL and VMI than T1-w/T2-w ratio. Only MTR correlated with FSIQ (p =0.0216; R2 = 0.10), CL (Fig. 5C; p =0.0123; R2 = 0.13) and VMI (Fig. 5C; p =0.0111; R2 = 0.12) when averaged across all lobes. There were no correlations between GA in either MTR and T1-w/T2-w ratio in white matter, subcortical structures or cortical lobes.
Fig. 5.
Correlations of the developmental assessments (FSIQ, CL, and VMI) with both MTR and T1-w/T2-w ratio averaged across white matter (A, C, respectively), and in each white matter ROI (B, D, respectively), where bar height indicates the R2 value, and asterisks indicate significant correlations (q < 0.05). White matter tracts: anterior thalamic radiation (Ant.TR), corticospinal tract (CS), cingulum (cingulate gyrus, C.CG), cingulum (hippocampus, C.H), forceps major (F.MAJ), forceps minor (F.MIN), inferior fronto-occipital fasciculus (Inf.FOF), inferior longitudinal fasciculus (Inf.LF), superior longitudinal fasciculus (Sup.LF), uncinate fasciculus (UF), superior longitudinal fasciculus (temporal part, Sup.LFT).
Fig. 6.
Correlations of the developmental assessments (FSIQ, CL and VMI) with T1-w/T2-w ratio averaged across subcortical gray matter (A), and in each subcortical gray matter ROI (B), where bar height indicates the R2 value, and asterisks indicate significant correlations (q < 0.05).
Fig. 7.
Correlations of the developmental assessments (FSIQ, CL, and VMI) with MTR averaged across cortical gray matter (A), and in each cortical gray matter ROI (B), where bar height indicates the R2 value, and asterisks indicate significant correlations (q < 0.05).
4. Discussion
VPT birth results in widespread structural differences in white matter, subcortical and cortical gray matter that is robust to imaging modality. In the present study, we demonstrated that measures of white matter microstructure including those sensitive to myelin are extensively reduced in VPT children compared to FT children. The reductions are widespread, occurring in white matter tracts throughout the brain, in the thalamus, putamen and amygdala, and in the occipital and temporal lobes. Our results suggest a whole-brain atypical tissue profile in children born VPT.
Across the two groups of VPT and FT children, high T1-w/T2-w ratio in white matter and subcortical structures and high MTR in cortical structures corresponded to better performance in measures of intelligence, language and visual-motor abilities. By combining the two groups, increased power and a broader distribution of the measures revealed the relations between the T1-w/T2-w ratio and MTR measures with outcomes. Based on Fig. 5, it was evident that the VPT children contributed to this association by demonstrating lower T1-w/T2-w ratio, MTR and cognitive measures while the FT children tended to have higher measures. This suggests a link between reduced myelination and other cellular components of white matter as measured by T1-w/T2-w ratio and MTR in the VPT children with lower cognitive outcomes. The correlations from the ROI analysis were more robust in detecting relations with outcomes than the voxel-wise TBSS analyses. We also found that as the T1-w/T2-w ratio measures were more sensitive in detecting group differences, they were also more sensitive in detecting associations with outcomes in white matter as well as the subcortical structures.
To our knowledge, this is the first study to investigate structural differences between VPT children at four years of age and term-born controls using MTR and T1-w/T2-w ratio. Previous studies of VPT children using diffusion tensor imaging have reported widespread structural organizational differences, which usually present as reduced fractional anisotropy (Constable et al., 2008; Lax et al., 2013; Loe et al., 2013; Mullen et al., 2011; Travis et al., 2015; Vangberg et al., 2006; Young et al., 2018). Given reports that these differences may be due to reduced myelination (Anjari et al., 2007; Ball et al., 2013; Song et al., 2005; Young et al., 2018), the present analysis contributes to our understanding by providing observations for two MRI measures with proposed specificity for myelin.
Across all white matter, subcortical gray matter and cortical gray matter, there was a very high degree of correlation between T1-w/T2-w ratio and MTR. At the individual structure level, this relationship was maintained in white matter and cortical gray matter, but was less strong in subcortical gray matter. That T1-w/T2-w ratio and MTR are related is supported at a molecular level, as macromolecules such as membrane layers decrease the T1 and T2 of surrounding water molecules in addition to providing magnetization transfer contrast. While myelin is an important factor in T1-w, T2-w and MTR contrast, all three are affected by a range of cellular and molecular factors. For example, axonal cytoskeleton components such as microtubules and neurofilaments are proposed as important influences affecting these contrasts (Laule et al., 2007; Nossin-Manor et al., 2013). There has been some controversy regarding the validity of using T1-w/T2-w ratio as a method to assess myelin content in white matter (Arshad et al., 2017; Uddin et al., 2018), with some authors arguing that it should only be used as an overall measure of myelination, inflammation and general white matter microstructure. Our results showing the strong correlations with MTR, however, suggest that T1-w/T2-w ratio can index myelin in white matter.
Another important factor in MRI tissue contrast is the presence of non-heme forms of iron such as ferritin (Vymazal et al., 1995) that act to reduce signal intensity in T2-w and to a lesser degree increase signal intensity in T1-w scans. The observed weakening of the relation between MTR and T1-w/T2-w ratio in subcortical gray matter may in part reflect the contributions of iron to the contrast in these regions. The observation that more regions that differed between VPT and FT children were detected when comparing T1-w/T2-w ratio than MTR likely reflects of a combination of the aforementioned factors as well as the greater signal-to-noise ratio efficiency of the T1-w/T2-w ratio scan protocol. A similar increase in sensitivity has been reported in a study comparing multiple sclerosis and healthy subjects (Nakamura et al., 2017) and points to an important technical advantage when studying paediatric populations, where resolution and scan time are important considerations.
Although MTR is a more direct measure of myelin, these results demonstrate that in situations where MT imaging is not available, T1-w/T2-w can be used in substitution. Since T1-w, and even T2-w images, are often included in standard imaging acquisition protocols, while MTOFF and MTON images are less common, the T1-w/T2-w ratio analysis allows for retrospective analysis of brain structure in already existing datasets, as well as reduces the imaging acquisition time by reducing the number of required images. This is particularly valuable when imaging children or clinical populations, when time constraints are often present.
There are some limitations to consider with this study. Although more complex T1-w/T2-w ratio preprocessing methods have been proposed (Ganzetti et al., 2014; Shafee et al., 2015), they were not used in the present study. However, the high degree of correlation between T1-w/T2-w ratio and MTR suggest that the corrections may not necessary in this case, and the methods in this paper are simplistic enough to be clinically feasible to assess brain structure. Secondly, both T1-w/T2-w ratio and MTR measures are not quantitative, and thus cannot be compared across scanners or protocols; further work is necessary to investigate the reproducibility across different conditions. Additionally, there were more males in the VPT than FT group; hence, sex was added as a covariate in all statistical analyses. Finally, more studies are necessary to fully characterize the tissue characteristics measured by T1-w/T2-w ratio and its relationship to other imaging modalities.
In conclusion, compared to FT children, four year old children born VPT show widespread structural differences throughout the brain consistent with delayed myelination. Both the MTR and T1-w/T2-w ratio were found to positively correlate with cognitive scores. The greater strength of association and greater number of regions showing an association suggests that the T1-w/T2-w ratio may have an important sensitivity advantage when used to predict subsequent school-age cognitive performance in this population.
Acknowledgements
We thank all of the families who participated in the study. We also thank Tammy Rayner, Ruth Weiss, Dr. Steven Miller, Dr. Charles Raybaud, Dr. Aideen Moore, Dr. Hilary Whyte, Tamara Powell and Wayne Lee for their valuable support and contributions. Funding was provided by the Canadian Institutes of Health Research (CIHR - MOP-84399 and MOP-137115). The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101635.
Appendix A. Supplementary data
Supplementary material
References
- Anderson P.J. Elsevier Ltd.; 2014. Neuropsychological Outcomes of Children Born Very Preterm, 19 Seminars in Fetal and Neonatal Medicine. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Department of Clinical Neurology, Oxford University; Oxford, UK: 2007. Non-Linear Registration, Aka Spatial Normalisation. FMRIB Technial Report TR07JA2. [Google Scholar]
- Anjari M., Srinivasan L., Allsop J.M., Hajnal J.V., Rutherford M.A., Edwards A.D., Counsell S.J. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35(3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Arshad M., Stanley J.A., Raz N. Test–retest reliability and concurrent validity of in vivo myelin content indices: myelin water fraction and calibrated T1w/T2w image ratio. Hum. Brain Mapp. 2017;38(4):1780–1790. doi: 10.1002/hbm.23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G., Boardman J.P., Rueckert D., Aljabar P., Arichi T., Merchant N.…Counsell S.J. The effect of preterm birth on thalamic and cortical development. Cereb. Cortex. 2012;22(5):1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G., Srinivasan L., Aljabar P., Counsell S.J., Durighel G., Hajnal J.V.…Edwards A.D. Development of cortical microstructure in the preterm human brain. Proc. Natl. Acad. Sci. U. S. A. 2013;110(23):9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Wojdyla D., Say L., Betran A.P., Merialdi M., Requejo J.H.…Van Look P.F.A. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A., Biberacher V., Schmidt P., Righart R., Buck D., Berthele A.…Mühlau M. Tissue damage within normal appearing white matter in early multiple sclerosis: assessment by the ratio of T1- and T2-weighted MR image intensity. J. Neurol. 2016;263(8):1495–1502. doi: 10.1007/s00415-016-8156-6. [DOI] [PubMed] [Google Scholar]
- Beery K., Buktenica N., Beery N. The Psychological Corporation; San Antonio, TX: 2010. Beery-Buktenica Test of Visual Motor Integration. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. 1995;57(1):289–300. [Google Scholar]
- Boardman J.P., Counsell S.J., Rueckert D., Kapellou O., Bhatia K.K., Aljabar P.…Edwardsa A.D. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. NeuroImage. 2006;32(1):70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Chao L.L., Tosun D., Woodward S.H., Kaufer D., Neylan T.C. Preliminary evidence of increased Hippocampal Myelin Content in Veterans with Posttraumatic stress Disorder. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Budin, F., Noel, J., Prieto, J. C., Gilmore, J., Rasmussen, J., … Styner, M. (2017). White matter fiber-based analysis of T1w/T2w ratio map (Vol. 10133, p. 101330P–10133–7). [DOI] [PMC free article] [PubMed]
- Chopra S., Shaw M., Shaw T., Sachdev P.S., Anstey K.J., Cherbuin N. More highly myelinated white matter tracts are associated with faster processing speed in healthy adults. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.12.069. [DOI] [PubMed] [Google Scholar]
- Constable R.T., Ment L.R., Vohr B.R., Kesler S.R., Fulbright R.K., Lacadie C.…Reiss A.R. Prematurely born Children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Edwards A.D., Chew A.T.M.M., Anjari M., Dyet L.E., Srinivasan L.…Cowan F.M. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131(12):3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Se F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duerden E.G., Foong J., Chau V., Branson H., Poskitt K.J., Grunau R.E.…Miller S.P. Tract-based spatial statistics in preterm-born neonates predicts cognitive and motor outcomes at 18 months. Am. J. Neuroradiol. 2015;36(8):1565–1571. doi: 10.3174/ajnr.A4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht V., Rassek M., Preiss S., Wald C., Mödder U. Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. Am. J. Neuroradiol. 1998;19(10):1923–1929. [PMC free article] [PubMed] [Google Scholar]
- Eryigit Madzwamuse S., Baumann N., Jaekel J., Bartmann P., Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J. Child Psychol. Psych. Allied Discipl. 2015;56(8):857–864. doi: 10.1111/jcpp.12358. [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.a. Magnetization transfer magnetic resonance imaging in the assessment of neurological diseases. J. Neuroimaging. 2004;14(4):303–313. doi: 10.1177/1051228404265708. [DOI] [PubMed] [Google Scholar]
- Ganzetti M., Wenderoth N., Mantini D. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzetti M., Wenderoth N., Mantini D. Mapping pathological changes in brain structure by combining T1- and T2-weighted MR imaging data. Neuroradiology. 2015;57(9):917–928. doi: 10.1007/s00234-015-1550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y., Grossman R.I., Babb J.S., Rabin M.L., Mannon L.J., Kolson D.L. Age-related total gray matter and white matter changes in normal adult brain. Part II: Quantitative magnetization transfer ratio histogram analysis. Am. J. Neuroradiol. 2002;23(8):1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Van Essen D.C. Mapping Human Cortical areas in Vivo based on Myelin Content as Revealed by T1- and T2-Weighted MRI. J. Neurosci. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L.…Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser, M. F., Goyal, M. S., Preuss, T. M., Raichle, M. E., & Van Essen, D. C. (2014). Trends and properties of human cerebral cortex: Correlations with cortical myelin content. NeuroImage 10.1016/j.neuroimage.2013.03.060. [DOI] [PMC free article] [PubMed]
- Granberg T., Fan Q., Treaba C.A., Ouellette R., Herranz E., Mangeat G.…Mainero C. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain. 2017;140(11):2912–2926. doi: 10.1093/brain/awx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H., Walhovd K.B., Tamnes C.K., Westlye L.T., Fjell A.M. Intracortical Myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J. Neurosci. 2013;33(47):18618–18630. doi: 10.1523/JNEUROSCI.2811-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Donishi T., Iwatani J., Yamada S., Takahashi S., Ukai S.…Kaneoke Y. Elucidating the aberrant brain regions in bipolar disorder using T1-weighted/T2-weighted magnetic resonance ratio images. Psych. Res. – Neuroimag. 2017;263:76–84. doi: 10.1016/j.pscychresns.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Iwatani J., Ishida T., Donishi T., Ukai S., Shinosaki K., Terada M., Kaneoke Y. Use of T1-weighted/T2-weighted magnetic resonance ratio images to elucidate changes in the schizophrenic brain. Brain Behav. 2015;5(10) doi: 10.1002/brb3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kroll J., Karolis V., Brittain P.J., Tseng C.E.J., Froudist-Walsh S., Murray R.M., Nosarti C. Real-life impact of executive function impairments in adults who were born very preterm. J. Int. Neuropsychol. Soc. 2017;23(5):381–389. doi: 10.1017/S1355617717000169. [DOI] [PubMed] [Google Scholar]
- Laule C., Vavasour I.M., Kolind S.H., Li D.K.B., Traboulsee T.L., Moore G.R.W., MacKay A.L. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4(3):460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I.D., Duerden E.G., Lin S.Y., Mallar Chakravarty M., Donner E.J., Lerch J.P., Taylor M.J. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct. Funct. 2013;218(2):575–585. doi: 10.1007/s00429-012-0417-2. [DOI] [PubMed] [Google Scholar]
- Lee, K., Cherel, M., Budin, F., Gilmore, J., Consing, K. Z., Rasmussen, J., … Styner, M. (2015). Early postnatal myelin content estimate of white matter via T1w/T2w ratio (Vol. 9417, p. 94171R–9417–7). [DOI] [PMC free article] [PubMed]
- Lemola S., Oser N., Urfer-Maurer N., Brand S., Holsboer-Trachsler E., Bechtel N.…Datta A.N. Effects of gestational age on brain volume and cognitive functions in generally healthy very preterm born children during school-age: a voxel-based morphometry study. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe I.M., Lee E.S., Feldman H.M. Attention and internalizing behaviors in relation to white matter in children born preterm. J. Dev. Behav. Pediatr. 2013;34(3):156–164. doi: 10.1097/DBP.0b013e3182842122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin K.S., Horwood L.J., Woodward L.J. Cognitive development trajectories of very preterm and typically developing children. Child Dev. 2017;88(1):282–298. doi: 10.1111/cdev.12585. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C.M., Nagai-Poetscher L.M. Elsevier; Amsterdam, The Netherlands: 2005. MRI Atlas of Human White Matter. [Google Scholar]
- Mullen K.M., Vohr B.R., Katz K.H., Schneider K.C., Lacadie C., Hampson M.…Ment L.R. Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Chen J.T., Ontaneda D., Fox R.J., Trapp B.D. T1−/T2-weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann. Neurol. 2017;82(4):635–639. doi: 10.1002/ana.25019. [DOI] [PubMed] [Google Scholar]
- Nosarti C., Froudist-Walsh S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev. Med. Child Neurol. 2016;58:35–45. doi: 10.1111/dmcn.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C., Giouroukou E., Healy E., Rifkin L., Walshe M., Reichenberg A.…Murray R.M. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(1):205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R., Chung a.D., Whyte H.E.a., Shroff M.M., Taylor M.J., Sled J.G. Deep gray matter maturation in very preterm neonates: regional variations and pathology-related age-dependent changes in magnetization transfer ratio. Radiology. 2012;263(2):510–517. doi: 10.1148/radiol.12110367. [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R., Card D., Morris D., Noormohamed S., Shroff M.M., Whyte H.E., Sled J.G. Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T1 imaging. NeuroImage. 2013;64(1):505–516. doi: 10.1016/j.neuroimage.2012.08.086. [DOI] [PubMed] [Google Scholar]
- Nossin-Manor R., Card D., Raybaud C., Taylor M.J., Sled J.G. Cerebral maturation in the early preterm period-a magnetization transfer and diffusion tensor imaging study using voxel-based analysis. NeuroImage. 2015;112:30–42. doi: 10.1016/j.neuroimage.2015.02.051. [DOI] [PubMed] [Google Scholar]
- Rademacher J., Engelbrecht V., Bürgel U., Freund H.J., Zilles K. Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. NeuroImage. 1999;9(4):393–406. doi: 10.1006/nimg.1998.0416. [DOI] [PubMed] [Google Scholar]
- Righart R., Biberacher V., Jonkman L.E., Klaver R., Schmidt P., Buck D.…Mühlau M. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann. Neurol. 2017;82(4):519–529. doi: 10.1002/ana.25020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E., Wiig E., Secord W. 2nd Edn. The Psychological Corporation; San Antonio, TX: 2004. Clinical Evaluation of Language Fundamentals - Preschool. [Google Scholar]
- Shafee R., Buckner R.L., Fischl B. Gray matter myelination of 1555 human brains using partial volume corrected MRI images. NeuroImage. 2015;105:473–485. doi: 10.1016/j.neuroimage.2014.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G. Modelling and interpretation of magnetization transfer imaging in the brain. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.11.065. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., MacKay C.E.…Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Soares J.M., Marques P., Alves V., Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;(7 Mar) doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Soun J.E., Liu M.Z., Cauley K.A., Grinband J. Evaluation of neonatal brain myelination using the T1- and T2-weighted MRI ratio. J. Magn. Reson. Imaging. 2017;46(3):690–696. doi: 10.1002/jmri.25570. [DOI] [PubMed] [Google Scholar]
- Teubner-Rhodes S., Vaden K.I., Cute S.L., Yeatman J.D., Dougherty R.F., Eckert M.A. Aging-resilient associations between the arcuate fasciculus and vocabulary knowledge: microstructure or morphology? J. Neurosci. 2016;36(27):7210–7222. doi: 10.1523/JNEUROSCI.4342-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Mathworks Inc MATLAB. 2016. www.Mathworks.Com/Products/Matlab Natick, Massachusetts, USA.
- Travis K.E., Adams J.N., Ben-Shachar M., Feldman H.M. Decreased and increased anisotropy along major cerebral white matter tracts in preterm children and adolescents. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.N., Figley T.D., Marrie R.A., Figley C.R. Can T1w/T2w ratio be used as a myelin-specific measure in subcortical structures? Comparisons between FSE-based T1w/T2w ratios, GRASE-based T1w/T2w ratios and multi-echo GRASE-based myelin water fractions. NMR Biomed. 2018 doi: 10.1002/nbm.3868. [DOI] [PubMed] [Google Scholar]
- Van Buchem M.A., Steens S.C.A., Vrooman H.A., Zwinderman A.H., McGowan J.C., Rassek M., Engelbrecht V. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. Am. J. Neuroradiol. 2001;22(4):762–766. [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Smith S.M., Barch D.M., Behrens T.E.J., Yacoub E., Ugurbil K. The WU-Minn human connectome project: an overview. NeuroImage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangberg T.R., Skranes J., Dale A.M., Martinussen M., Brubakk A.M., Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32(4):1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Vymazal J., Brooks R. a, Patronas N., Hajek M., Bulte J.W., Di Chiro G. Magnetic resonance imaging of brain iron in health and disease. J. Neurol. Sci. 1995;133(Suppl):19–26. doi: 10.1016/0022-510x(95)00204-f. https://doi.org/0022510X9500204F [pii] [DOI] [PubMed] [Google Scholar]
- Weshcler D. 3rd Edition. The Psychological Corporation; San Antonio, TX: 2002. Wechsler Preschool and Primary Scales of Intelligence. [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S.D., Balaban R.S. Magnetization transfer imaging: practical aspects and clinical applications. Radiology. 1994;192(3):593–599. doi: 10.1148/radiology.192.3.8058919. [DOI] [PubMed] [Google Scholar]
- Woo Nam K., Castellanos N., Simmons A., Froudist-Walsh S., Allin M.P., Walshe M.…Nosarti C. Alterations in cortical thickness development in preterm-born individuals: implications for high-order cognitive functions. NeuroImage. 2015;115:64–75. doi: 10.1016/j.neuroimage.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L.J., Moor S., Hood K.M., Champion P.R., Foster-Cohen S., Inder T.E., Austin N.C. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94(5):F339–F344. doi: 10.1136/adc.2008.146282. [DOI] [PubMed] [Google Scholar]
- Xydis V., Astrakas L., Drougia A., Gassias D., Andronikou S., Argyropoulou M. Myelination process in preterm subjects with periventricular leucomalacia assessed by magnetization transfer ratio. Pediatr. Radiol. 2006;36(9):934–939. doi: 10.1007/s00247-006-0235-x. [DOI] [PubMed] [Google Scholar]
- Xydis V., Astrakas L., Zikou A., Pantou K., Andronikou S., Argyropoulou M.I. Magnetization transfer ratio in the brain of preterm subjects: Age-related changes during the first 2 years of life. Eur. Radiol. 2006;16(1):215–220. doi: 10.1007/s00330-005-2796-8. [DOI] [PubMed] [Google Scholar]
- Yasuno F., Kudo T., Yamamoto A., Matsuoka K., Takahashi M., Iida H.…Kishimoto T. Significant correlation between openness personality in normal subjects and brain myelin mapping with T1/T2-weighted MR imaging. Heliyon. 2017;3(9) doi: 10.1016/j.heliyon.2017.e00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.M., Vandewouw M.M., Morgan B.R., Smith M. Lou, Sled J.G., Taylor M.J. Altered white matter development in children born very preterm. Brain Struct. Funct. 2018 doi: 10.1007/s00429-018-1614-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material