Abstract
Introduction:
In Mexico, Neisseria meningitidis is considered to be a rare cause of bacterial meningitis (BM), however, one national publication using active surveillance has suggested the opposite. Group B Streptococcus (GBS) is also considered to be infrequent in young infants as a cause of BM in central Mexico. Streptococcus pneumoniae vaccination using the 13-valent conjugate vaccine (PCV13) started in our region in May 2012. We focused our research on whether N. meningitidis and GBS are important causes of BM, and to examine the effectiveness of PCV13 on pneumococcal BM.
Methods:
From October 2005 to September 2018, active/prospective surveillance looking for all patients admitted with suspected BM <16 years of age was performed at the Tijuana, Mexico, General Hospital. Tijuana, Mexico to San Diego, Unites States of America (USA), is the most transited border in the world. Isolation of pathogens was by either conventional culture or Real Time-polymerase chain reaction (RT-PCR), all patients were followed during and 3 months after discharge, and a descriptive analysis was performed. The effectiveness of PCV13 was determined by comparing the proportion of cases per month on pneumococcal BM before and after its implementation.
Results:
There were 86 confirmed BM cases. N. meningitidis was the leading cause (60.5%, and 61.5% caused by serogroup C), followed by S. pneumoniae (18.6%). PCV13 effectiveness on pneumococcal BM was of 64.3% and was associated with the disappearance of serotype 19A. A total of 22 infants <3 months old had BM; GBS was the leading cause at this age group (27.3%), followed by N. meningitidis (22.7%). The overall mortality was 24%.
Conclusions:
BM by N. meningitidis is endemic in Tijuana, Mexico, and meningococcal vaccination should be seriously considered in the region. PCV13 is currently showing high effectiveness on pneumococcal BM, and we need to continue active surveillance to see whether maternal screening/prophylaxis for GBS should also be introduced in the region.
Keywords: bacterial meningitis, Group B Streptococcus, Neisseria meningitides, pneumococcal conjugate vaccine, Streptococcus pneumonia
Introduction
Bacterial meningitis (BM) is an infectious disease associated with high lethality and morbidity, and survivors could have long-term sequelae.1–4
In Mexico, based on previous published data, BM due to Neisseria meningitidis is considered to be a rare condition, with a total number of cases in the whole country as low as two per year.5
Streptococcus pneumoniae is a highly pathogenic bacteria, associated with sepsis, pneumonia (complicated/noncomplicated), otomastoiditis, and meningitis, among problems.6,7 Before implementation of the 13-valent pneumococcal conjugate vaccine (PCV13), S. pneumoniae was the leading cause of BM in many countries;6–8 however, the use of this vaccine has drastically reduced BM by this pathogen.9–11
Group B Streptococcus (GBS) is the leading cause of BM in young infants (<3 months of age) in many parts of the world,12–15 nevertheless, publications from Mexico City have shown that GBS is uncommon, with Enterobacteriaceae reported as the primary causes of BM in this age group.16
In Mexico, vaccination against Haemophilus influenzae type b was introduced in 1999; PCV7 was introduced in May 2007; PCV13 in Tijuana, Mexico was introduced in May/June 2010; however, meningococcal vaccination, and GBS screening for pregnant women, are not part of Mexican health preventive policies.
The border between Tijuana, Mexico and San Diego, USA is the most populated on the planet, with significant bi-national health issues.17–19
We researched to see whether N. meningitidis is an important cause of BM in children <16 years of age from our hospital, as well as to determine the effectiveness of PCV13 on pneumococcal BM, and the incidence of GBS as a cause of BM in young infants.
Materials and methods
From 1 October 2005 to 30 September 2018 (13 years), active/prospective surveillance for all children <16 years old admitted with suspected BM was performed at the Tijuana, Mexico, General Hospital.
Active surveillance is defined as ‘syndromatic’ and prospective, and was performed as follows:
All patients <16 years of age with clinical signs and symptoms suggestive of acute meningitis (<10 days of fever, with cephalalgia, meningeal signs, seizures, bulging of anterior fontanelle in young infants, altered state of consciousness), clinical febrile purpura, or sepsis, were included to confirm or rule out BM. Once the patient was admitted, both blood and cerebrospinal fluid (CSF) samples were taken for cytological and chemical analysis, 1 ml was immediately placed into enriched broth media (BactecTM) and followed by rapid (<3 minutes) incubation at 37°C with 5% of CO2. Final identification was performed using a MicroScan system (Vitek2® bioMerieux,San Diego, California, USA).
Confirmation of BM was done by conventional isolation from all positive broth media cultures, and, from 2013, polymerase chain reaction (PCR) was only used for all culture-negative ‘granulocytic meningitis’ (defined as a CSF with >5 cells/mm3 and at least 50% polymorphonuclear leukocytes, and a normal CSF cytochemical analysis 3 days later). For all N. meningitidis isolates, serogroup identification was performed by the Pastorex meningitis kit (Alere, Ltd®, Stockport, UK) which detects serogroups A, B, C, Y and W only, or by PCR. S. pneumoniae serotypes were detected by either the Quellung reaction (Statens Serum Institute®) or PCR.
For RT-PCR, DNA extraction from CSF was performed using QIAGEN QIAmp DNA blood mini kit (QIAGEN, Shanghai, China) following the product instructions. An aliquot of 200 µl of CSF was processed, and the DNA eluted in 100 µl of TE buffer. A RT-PCR assay on Mx3000 QPCR Systems (Stratagene®, California USA) was used to identify seven common pathogens that cause BM (targeted genes: 16S, femA, hly, ctrA, lytA, bexA, and cfb, for Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, Neisseria meningitidis, Streptococcus pneumoniae, Haemophilus influenzae, and GBS, respectively).
For the N. meningitidis serogroup PCR identification: PCR from the 1yS rDNA was sequenced with a ThermoSequenase radiolabeled terminator cycle sequencing kit (Amersham, Piscataway, New Jersey, USA®). Sequencing reaction contained 10 ng DNA amplified DT (Directed Termination)-PCR and purified with Microspins columns (Phamacia Biotech®) and 2.5 pmol Men1/Men2 primers. To assign a serogroup, a gene cassette required for the biosynthesis of the capsule of serogroups A, C, B, and W was used.
For S. pneumoniae molecular serotyping, a sequential multiplex PCR method approved by the CDC (Center for Disease Control and Preven-tion, Atlanta, Georgia, USA) was performed. All serotype-specific primers were first tested with individual isolates of the targeted serotypes. To ensure that the primers would detect all strains within the given serotype, an additional 5 to 10 different clinical isolates within each targeted serotype were amplified with its corresponding primer sets.
Among survivors, all were discharged with a brain computed tomography scan and reviewed 3 months later to search for any neurological and other types of sequelae (these evaluations were done by the Departments of Pediatric Infectious Diseases and Pediatric Neurology). This last evaluation was merely a physical evaluation, with no other procedures performed.
Descriptive data were analyzed using Microsoft Excel®.
PCV13 effectiveness, was performed by counting pneumococcal BM per month before and after its introduction (May 2012), followed by comparing the reduction in percentage (%) of that ‘specific cases per month’ number.
The hospital’s standard of care was implemented for every admitted child, and the patient’s (parent or tutor) consent form was the same as that used in the hospital. A datasheet containing information on every patient did not show names or other forms of identification to expose a patient’s identity; this datasheet was only kept with the corresponding author of this study, and only privately showed when the hospital’s institutional review board (IRB) required information. The hospital’s IRB accepted the study based on these specifications.
Results
Demographic and clinical characteristics of all patients has partially been mentioned in the Materials and Methods section. Specific features for clinical presentations are described below, particularly for meningococcal and pneumococcal BM, and infants <3 months old.
During 13 years, 469 spinal taps were performed in children <16 years old, from which in 143, CSF cytologic and chemical analyses were normal, 221 were defined as ‘potential viral meningitis’ (CSF with at least 51% mononuclear cells, and a second spinal tap 3 days later with a cytological analysis reporting <5 leukocytes/mm3, and culture-negative), and 105 as ‘granulocytic meningitis’ (defined in the Materials and Methods section).
From all ‘granulocytic meningitis’, bacterial identification was successful in 86 (81.9%) cases. From all 86 confirmed BM cases, 76 (84.88%) were isolated by conventional cultures, and 13 (15.12%) by RT-PCR. A total of nine patients (10.4%) also had a positive blood culture; RT-PCR was not performed on blood.
The general characteristics of the overall number of confirmed BM cases are shown in Table 1.
Table 1.
General characterstics of children with confirmed bacterial meningitis (n = 86), 2005–2013.
| Bacterial etiologies, ages, and clinical outcomes. | |
| Meningococcal | 52 (60.5% |
| Pneumococcal | 18 (18.6%) |
| Group B Streptococcus | 6 (7%) |
| Streptococcus pyogenes | 4 (4.6%) |
| Enterobacteriaceae | 4 (4.6%) |
| N. gonorrhoeae | 2 (2.3%) |
| L. monocytogenes | 1 (1.2%) |
| M. catarrhalis | 1 (1.2%) |
| Median age | 3.1 years (2 days to 15 years) |
| Median hospitalization days | 15 (1–37) |
| Lethality | 23 (26.7%) |
| Sequelae among survivors | 13 (20.6%) |
The leading cause was N. meningitidis in 52 cases (60.5%), followed by S. pneumoniae in 16 cases (18.6%), GBS in 6 cases (7%), and 18 others (20.9%), shown in Figure 1.
Figure 1.
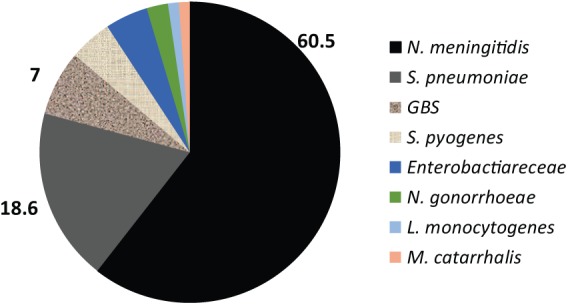
Confirmed BM IN %.
Neisseria meningitidis:52, Streptococcus pneumoniae:16, GBS: 6, Enterobacteriaceae: 4, Streptococcus pyogenes: 4, Neisseria gonorrhoeae: 2, Listeria monocytogenes: 1, Moraxella catarrhalis: 1 (n = 86).
BM, bacterial meningitis: GBS, Group B Streptococcus.
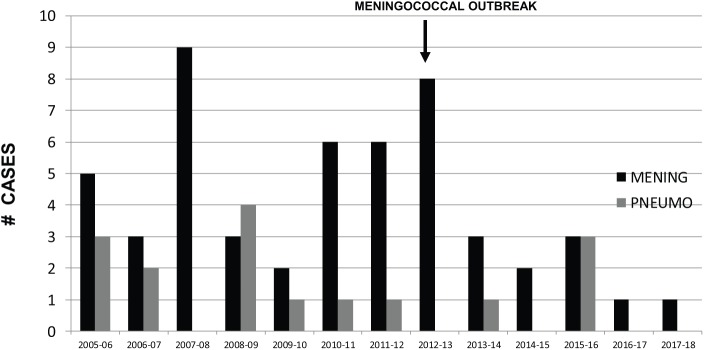
Meningococcal BM predominantly affected children <2 years old (n=22, 42.3%), with an age variation from 5 days to 15 years old. with the exception of a meningococcal outbreak during 2013 in which older children and adolescents were the predominant age group.20 A total of 28 patients (53.8%) did not develop purpura. Serogroup distribution is shown in Figure 2, with serogroup C the predominant type (61.5%), followed by Y and B. Mortality was 13 cases (25%), being higher in children who developed purpura, and, at admission, had thrombocytopenia or prolonged clotting times (46.15, 34.6, and 36.5%, respectively). Among survivors, 3 months after discharge, 13 patients (33%) had neurologic sequelae. The cases per year for both meningococcal and pneumococcal BM is shown in Figure 3, clearly confirming a consistent, but variable, number of cases, particularly by meningococcal BM.
Figure 2.
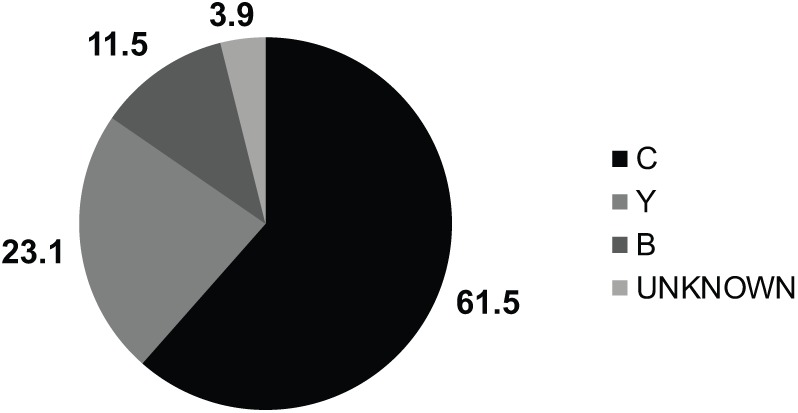
Meningococcal serogroups IN % (n = 52).
C: 32 (61.5%) Y: 12 (23.1%) B: 6 (11.5%) W: 0 ignored: 2 (3.9%).
Figure 3.
Meningococcal and pneumococcal meningitis cases per year (October 2005–2006 to September 2017–2-18), 13 years.
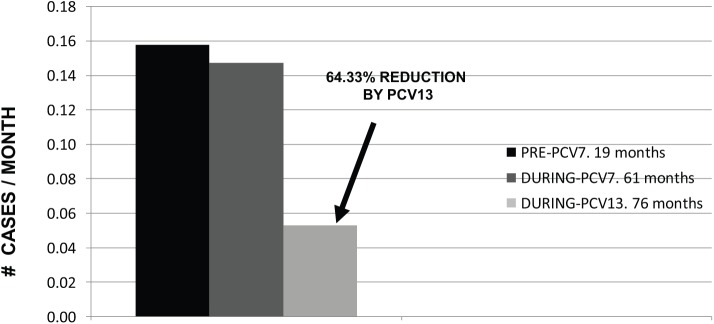
Pneumococcal BM was also more common in children <2 years of age (n=8, 50%), with an age variation from 1 month to 15 years old. All but one (a 1-month-old infant) had at least one dose of PCV13, and 13 (81.25%) had a complete PCV13 vaccination schedule according to their age. None developed purpura. Death occurred in three patients (18.75%) and two of these deaths were caused by S. pneumoniae serotype 19A, with neurologic sequelae found in six survivors (46.15%). For pneumococcal serotype distribution, before PCV13 introduction in May 2012, there were 12 cases (serotypes 19A-5, 7F-3, 6B-2, 23F-1, unknown-1), which decreased to 4 cases (serotypes 7F, 18C, 35B, 22F, one each). Patients with meningitis due to serotypes 7F and 18C (included in PCV13) were adolescents previously vaccinated only with PCV7, but none received PCV13. Furthermore, one had a splenectomy performed 6 years before admission and received one additional dose of pneumococcal polysaccharide vaccine (PPS23), which indeed we could assume is a failure to this vaccine. PCV13 reduced all pneumococcal BM from 0.1475 cases per month to 0.0526 (64.33% PCV13 impact, see Figure 4), and the disappearance of serotype 19A.
Figure 4.
Pneumococcal meningitis by ‘PCV period’: number of months before any PCV October 2005–April 2007, with PCV7 (May 2007–April/May 2012), and PCV13 universal vaccination (May/June 2012–September 2018). Cases per month (n = 16).
PCV13 impact (PCV13 implemented in May 2012) was based on the decreased % of number of cases following PCV13 introduction.
PCV, 13-valent conjugate vaccine.
A total of 22 confirmed BM cases were found in young infants (<3 months old). The leading cause at this age group was GBS (n=6, 27.27%), followed by N. meningitidis (n=5, 22.72%, serogroups: 2-C, 2-B, and 1-Y), S. pneumoniae (n=4, 18.18%, serotypes 19A, 33C, 18B, and 12, one of each), Enterobacteriaceae (n=4, 18.18%), N. gonorrheae,2 and L. monocytogenes1 (see Figure 5). In this age group, death was mostly associated with newborns (<1 month old) with 6/16 deaths (37.5%).
Figure 5.
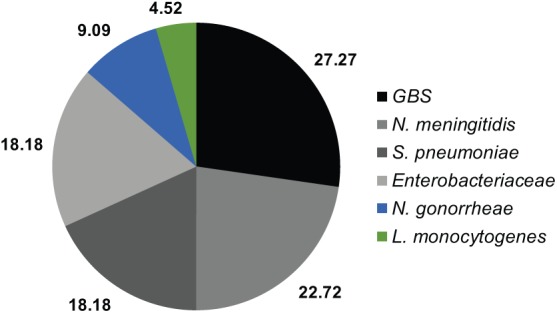
Confirmed BM IN infants <3 months old IN % (n = 22).
BM, bacterial meningitis.
Discussion
The data presented in this, long, active-surveillance-based study, confirm that, contrary to what has been reported in one publication in Mexico,5 N. meningitidis is the leading cause of BM in Tijuana, Mexico, with serogroup C being the most prevalent. This study, along with an active-surveillance publication from nine hospitals in Mexico, and an outbreak of meningococcal BM caused by serogroup C, with clonal complex ST-11 (mortality >35%) in Tijuana during 2013,20,21 confirmed that meningococcal disease is endemic in the region, and universal vaccination should be seriously considered. Meningococcal vaccination with conjugate vaccines has shown effectiveness both in developed (USA, Canada) and developing countries in the Americas.22–25
PCV13 implementation has dramatically reduced pneumococcal meningitis in all countries where this vaccine is currently used.26–30
In our region, we have published work confirming that PCV13 universal vaccination reduces overall invasive pneumococcal disease,31 and in addition, with this present study, also the disappearance of serotype 19A, globally considered to be a very important replacement serotype following PCV7 implementation, as well as associated with high morbidity.32,33
In addition, in many countries, GBS in young infants is considered to be the leading cause of BM in this age group;12–15 however, published data from Mexico City emphasize that GBS is uncommon, with Enterobacteriaceae as predominant agents causing BM in young infants.16 Our study shows the opposite, with GBS as the leading cause, with Enterobacteriaceae being present, but in smaller numbers.
The main limitation of our study is the lack of information based on active surveillance both in other hospitals at Tijuana, as in other cities in Mexico; however, our data are solid, and surveillance should never be interrupted.
The reason why our epidemiology of BM in our region differs from what has been published in Mexico City is unknown, but we speculate that this is caused by the distance between the two cities being long (more than 2000 kilometers). Tijuana shares, with San Diego, USA, the largest frontier in the planet, with potential similarities in epidemiology.17–19 Furthermore, perhaps a problem exists with the epidemiological reporting system in Mexico, which is based on passive surveillance.
Conclusion
N. meningitidis is the leading cause of BM in Tijuana, Mexico, General Hospital. Vaccination should be seriously considered in the region as part of the mandatory immunization schedule.
PCV13 universal implementation has decreased all cases of pneumococcal BM, with the disappearance of serotype 19A.
GBS is the main cause of BM in young infants. Further studies and longer surveillance should be done in order to evaluate prophylactic measures, such as routine maternal screening and antibacterial prophylaxis.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Enrique Chacon-Cruz  https://orcid.org/0000-0003-2466-4920
https://orcid.org/0000-0003-2466-4920
Contributor Information
Enrique Chacon-Cruz, Hospital General de Tijuana, Paseo Centenario S/N, Zona Rio, Tijuana, Baja-California, 22010, Mexico.
Christopher Roberts, Department of Pediatrics, Mexican Institute of Social Security Hospital, Tijuana, Baja-California, Mexico.
Rosa Maria Rivas-Landeros, Hospital General de Tijuana, Tijuana, Mexico.
Erika Zoe Lopatynsky-Reyes, University of California in San Diego, San Diego, CA, USA.
Lucila Alejandra Almada-Salazar, Autonomous University of Baja-California, Tijuana, Baja-California, Mexico.
Jorge Arturo Alvelais-Palacios, Autonomous University of Baja-California, Campus ECISALUD, Tijuana, Baja-California, Mexico.
References
- 1. Suthar R, Sankhyan N. Bacterial infections of the central nervous system. Indian J Pediatr. 2019; 86(1):60–69. doi: 10.1007/s12098-017-2477-z [DOI] [PubMed] [Google Scholar]
- 2. Lundbo LF, Benfield T. Risk factors for community-acquired bacterial meningitis. Infect Dis 2017; 49: 433–444. [DOI] [PubMed] [Google Scholar]
- 3. Brouwer MC, van de, Beek D. Management of bacterial central nervous system infections. Handb Clin Neurol 2017; 140: 349–364. [DOI] [PubMed] [Google Scholar]
- 4. van Ettekoven CN, van de, Beek D, Brouwer MC. Update on community-acquired bacterial meningitis: guidance and challenges. Clin Microbiol Infect 2017; 23: 601–606. [DOI] [PubMed] [Google Scholar]
- 5. Almeida-González L, Franco-Paredes C, Pérez LF, et al. Meningococcal disease caused by Neisseria meningitidis: epidemiological, clinical, and preventive perspectives. Salud Publica Mex 2004; 46: 438–450. [DOI] [PubMed] [Google Scholar]
- 6. Asche V. Streptococcal update. A microbiology perspective. Aust Fam Physician 1993; 22: 1763–1768. [PubMed] [Google Scholar]
- 7. Butler JC, Dowell SF, Breiman RF. Epidemiology of emerging pneumococcal drug resistance: implications for treatment and prevention. Vaccine 1998; 16: 1693–1697. [DOI] [PubMed] [Google Scholar]
- 8. Swanson D. Meningitis. Pediatr Rev 2015; 36: 514–524. [DOI] [PubMed] [Google Scholar]
- 9. de Oliveira LH, Camacho LA, Coutinho ES, et al. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: a systematic review. PLoS One 2016; 12 :e0166736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yildirim I, Shea KM, Pelton SI. pneumococcal disease in the era of pneumococcal conjugate vaccine. Infect Dis Clin North Am 2015; 29: 679–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e51–e59. [DOI] [PubMed] [Google Scholar]
- 12. Kohli-Lynch M, Russell NJ, Seale AC, et al. Neurodevelopmental impairment in children after group B Streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017; 65(Suppl. 2): S190–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-onset neonatal sepsis. Clin Microbiol Rev 2014; 27: 21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine 2013; 31(Suppl. 4): D7–D12. [DOI] [PubMed] [Google Scholar]
- 15. Johri AK, Lata H, Yadav P, et al. Epidemiology of Group B Streptococcus in developing countries. Vaccine 2013; 31(Suppl 4): D43–D45. [DOI] [PubMed] [Google Scholar]
- 16. Solórzano-Santos F, Díaz-Ramos RD, Arredondo-García JL. Diseases caused by group B Streptococcus in Mexico. Pediatr Infect Dis J 1990; 9: 66. [DOI] [PubMed] [Google Scholar]
- 17. Chacon-Cruz E, Sugerman DE, Ginsberg MM, et al. Surveillance for invasive meningococcal disease in children, US-Mexico border, 2005–2008. Emerg Infect Dis 2011; 17: 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viani RM, Lopez G, Chacón-Cruz E, Hubbard P, et al. Poor outcome is associated with delayed tuberculosis diagnosis in HIV-infected children in Baja California, Mexico. Int J Tuberc Lung Dis 2008; 12: 411–416. [PubMed] [Google Scholar]
- 19. Rafful C, Jain S, Sun X, et al. Identification of a syndemic of blood-borne disease transmission and injection drug use initiation at the US-Mexico border. J Acquir Immune Defic Syndr. 2018; 15; 79(5): 559–565. doi: 10.1097/QAI.0000000000001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chacon-Cruz E, Espinosa-De Los Monteros LE, Navarro-Alvarez S, et al. An outbreak of serogroup C (ST-11) meningococcal disease in Tijuana, Mexico. Ther Adv Vaccines 2014; 2: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chacon-Cruz E, Martinez-Longoria CA, Llausas-Magana E, et al. Neisseria meningitidis and Streptococcus pneumoniae as leading causes of pediatric bacterial meningitis in nine Mexican hospitals following 3 years of active surveillance. Ther Adv Vaccines 2016; 4: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baccarini C, Ternouth A, Wieffer H, et al. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother 2013; 9: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borrow R, Alarcón P, Carlos J, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines 2017; 16: 313–328. [DOI] [PubMed] [Google Scholar]
- 24. Tauil Mde C, Carvalho CS, Vieira AC, et al. Meningococcal disease before and after the introduction of meningococcal serogroup C conjugate vaccine. Federal District, Brazil. Braz J Infect Dis 2014; 18: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sáfadi MA, O’Ryan M, Valenzuela Bravo MT, et al. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine 2015; 33: 6529–6536. [DOI] [PubMed] [Google Scholar]
- 26. Cohen R, Biscardi S, Levy C. The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014. Hum Vaccin Immunother 2016; 12: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobs DM, Yung F, Hart E, Nguyen MNH, et al. Trends in pneumococcal meningitis hospitalizations following the introduction of the 13-valent pneumococcal conjugate vaccine in the United States. Vaccine 2017; 35: 6160–6165. [DOI] [PubMed] [Google Scholar]
- 28. Ladhani SN, Slack MP, Andrews NJ, et al. Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales. Emerg Infect Dis 2013; 19: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ben-Shimol S, Greenberg D, Givon-Lavi N, et al. Impact of PCV7/PCV13 introduction on invasive pneumococcal disease (IPD) in young children: Comparison between meningitis and non-meningitis IPD. Vaccine 2016; 34: 4543–4550. [DOI] [PubMed] [Google Scholar]
- 30. Ali M, Chang BA, Johnson KW, et al. Incidence and aetiology of bacterial meningitis among children aged 1–59 months in South Asia: systematic review and meta-analysis. Vaccine 2018; 36: 5846–5857. [DOI] [PubMed] [Google Scholar]
- 31. Chacon-Cruz E, Rivas-Landeros RM, Volker-Soberanes ML. Early trends in invasive pneumococcal disease in children following the introduction of 13-valent pneumococcal conjugate vaccine: results from eight years of active surveillance in a Mexican hospital. Ther Adv Vaccines 2014; 2: 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song JH, Dagan R, Klugman KP, et al. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine 2012; 30: 2728–2737. [DOI] [PubMed] [Google Scholar]
- 33. Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine 2010; 28: 4249–4259. [DOI] [PubMed] [Google Scholar]