Abstract
Objectives:
Paclitaxel is a highly effective antitumor agent with notable adverse events, including hypersensitivity reactions, peripheral neuropathy, arthralgia, myalgias, and neutropenia. Solvent-based paclitaxel causes severe allergic, hypersensitivity, and anaphylactic reactions. Nanoparticle albumin-bound paclitaxel was recently developed and provides an advantage over solvent-based paclitaxel in avoiding solvent/surfactant-related adverse events. The aim of this study was to assess the adverse event profiles of solvent-based paclitaxel and nanoparticle albumin-bound paclitaxel formulations using data from the spontaneous adverse event reporting system of the US Food and Drug Administration Adverse Event Reporting System database.
Methods:
This study relied on Medical Dictionary for Regulatory Activities preferred terms and standardized queries, and calculated the reporting ratio and reporting odds ratios of paclitaxel formulations.
Results:
Of 8,867,135 reports recorded in the US Food and Drug Administration Adverse Event Reporting System database from January 2004 to December 2016, 3469 and 4447 adverse events corresponded to solvent-based paclitaxel and nanoparticle albumin-bound paclitaxel, respectively. Reporting odds ratios (95% confidence interval) for anaphylactic reaction (standardized MedDRA query code: 20000021) associated with the use of solvent-based paclitaxel and nanoparticle albumin-bound paclitaxel were 1.69 (1.56–1.84) and 0.75 (0.68–0.83), respectively. Reporting odds ratio signal for anaphylactic reaction was not detected for nanoparticle albumin-bound paclitaxel. Reporting odds ratios (95% confidence interval) for acute renal failure (standardized MedDRA query code: 20000003) associated with the use of solvent-based paclitaxel and nanoparticle albumin-bound paclitaxel were 0.75 (0.58–0.98) and 1.60 (1.37–1.89), respectively.
Conclusion:
This is the first study to evaluate the adverse event profile of nanoparticle albumin-bound paclitaxel using US Food and Drug Administration Adverse Event Reporting System data. Considering that the US Food and Drug Administration Adverse Event Reporting System database does not allow to infer causality or risk ranking, the different reporting frequencies of anaphylactic reaction and acute renal failure between solvent-based paclitaxel and nanoparticle albumin-bound paclitaxel must be further investigated via analytical observational research.
Keywords: Paclitaxel, Abraxane, Taxol, Food and Drug Administration Adverse Event Reporting System
Introduction
Paclitaxel (PTX) is an antitumor agent used for the treatment of breast cancer, ovarian cancer, non-small-cell lung cancer, bladder cancer, prostate cancer, melanoma, and esophageal cancer.1,2 PTX received Food and Drug Administration (FDA) approval in 1992 and has been formulated and marketed as solvent-based (sb)-PTX (Taxol®, Bristol-Myers Squibb, New York, NY, USA) since. Although highly effective, PTX is associated with several adverse events (AEs), including hypersensitivity reactions, peripheral neuropathy, arthralgia, myalgias, and neutropenia.3,4
Albumin is an attractive drug delivery vehicle in oncology, allowing reversible, non-covalent binding of drugs,5,6 and it has offered a breakthrough in the treatment of numerous cancers.7,8 The solvent-free, human albumin-stabilized PTX formulation Abraxane® (nanoparticle albumin-bound PTX (nab-PTX), Celgene corporation, Summit, NJ, USA) is characterized by rapid as well as preferential delivery and accumulation of PTX at tumor sites, enhancing the therapeutic effects of PTX.9–11 nab-PTX was developed as a solvent-free PTX formulation because Cremophor EL (CrEL, a synthetic, nonionic surfactant used as a solubilizer for PTX) and ethanol (co-solvent) conventionally used to prepare sb-PTX were associated with high incidence of hypersensitivity reactions. nab-PTX displays a reasonable toxicity profile, avoiding solvent/surfactant-related AEs and the need for premedication.9,12 Use of nab-PTX resulted in a lower incidence of grade 4 neutropenia than that of sb-PTX.13 However, the detailed AE profile of PTX products in clinical setting is uncertain.
The US FDA Adverse Event Reporting System (FAERS) database is a spontaneous reporting system (SRS) covering several million case reports on AEs and is used for pharmacovigilance, reflecting the realities of clinical practice.14–16 The aim of this study was to assess the AE profiles of sb-PTX and nab-PTX formulations using the FAERS database. To the best of our knowledge, this is the first study to evaluate nab-PTX using the FAERS database.
Materials and methods
FAERS data from January 2004 to December 2016 are publicly available and can be downloaded from the FDA website (www.fda.gov). All data from the FAERS database were fully anonymized by the FDA before we used them. The FAERS database comprises seven data tables: patient demographic and administrative information (DEMO); drug/biologic information (DRUG); AEs (REAC); patient outcomes (OUTC); report sources (RPSR); drug therapy start and end dates (THER); and indications for use/diagnosis (INDI). FAERS database structure complies with the international safety reporting guidelines.17 Data obtained were integrated into a relational database using FileMaker Pro 13 software (FileMaker, Inc., Santa Clara, CA, USA). Next, we followed the FDA recommendation for excluding duplicate reports for patients and used the most recent case numbers to identify and exclude such records from the analysis.18
For the extraction of cases from the FAERS database, we used two brand names—Taxol® for sb-PTX and Abraxane® for nab-PTX. MedWatch is the FDA Safety Information and AE Reporting Program. The MedWatch form is sent to the FDA for inclusion in FAERS. It is a two-page document that contains basic patient information, a description of the AE, suspected product (s), and concomitant product (s). If multiple products are reported on a MedWatch form, the reporter can identify a primary suspect (PS) drug that they feel is most responsible for the AE(s).19 Only reports with the drug code of PS were included in this analysis.
AEs in the REAC table are coded using preferred terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA, www.meddra.org), which is the terminology dictionary.20 MedDRA version 19.0 was used in this study. Standardized MedDRA queries (SMQs) are widely used to analyze SRS reports.21–23 SMQs, which are built by the Maintenance and Support Services Organization, are groupings of PTs according to the level that relates to a defined medical condition. The included terms may relate to signs, symptoms, diagnoses, syndromes, physical findings, and laboratory and other physiologic test data.21 The grouping of SMQs allows for useful data retrieval and presentation of relevant individual case safety reports. We used the following SMQs: anaphylactic reaction (SMQ code: 20000021, containing 86 related PTs), peripheral neuropathy (SMQ code: 20000034, containing 75 related PTs), acute renal failure (SMQ code: 20000003, containing 47 related PTs), hematopoietic erythropenia (SMQ code: 20000029, containing 27 related PTs), hematopoietic leucopenia (SMQ code: 20000030, containing 60 related PTs), hematopoietic thrombocytopenia (SMQ code: 20000031, containing 13 related PTs), hematopoietic cytopenias affecting more than one type of blood cell (SMQ code: 20000028, containing 27 related PTs), interstitial lung disease (SMQ code: 20000042, containing 60 related PTs), and taste and smell disorders (SMQ code: 20000046, containing 12 related PTs).
We calculated the reporting ratio and the reporting odds ratio (ROR) to study the influence of sb-PTX and nab-PTX on AEs. The strength of the association between drug of interest and reported AE compared with other drugs in the FAERS database is calculated as ROR.14–16,24,25 In our study, ROR provides an estimate for the extent to which AEs are reported in association with the use of sb-PTX or nab-PTX as suspected medication relative to the use of other drugs. To compare one of the index groups with the reference group, we calculated ROR values as (a × d)/(b × c) and expressed the data as point estimates with a 95% confidence interval (CI). Safety signals were considered significant when ROR estimates and the lower limits of the corresponding 95% CI were >1.25 Two or more cases were required to define the signal.14
Results
FAERS data used in this study consisted of 8,867,135 reports from January 2004 to December 2016. After excluding duplicate reports according to the FDA recommendations, 7,348,357 reports were analyzed, of which 3469 and 4447 AE cases corresponded to sb-PTX and nab-PTX, respectively (Table S1). Demographic information of sb-PTX and nab-PTX in the FAERS database is summarized in Table 1. The 50 most frequently reported AEs for PTX formulations are listed in Table S1. For sb-PTX, the most commonly reported AEs were dyspnea, nausea, pyrexia, and vomiting, whereas for nab-PTX, the most commonly reported AEs were death, neutropenia, nausea, and anemia.
Table 1.
Demographic information of sb-PTX and nab-PTX in the FAERS database.
| sb-PTX | Case (n) | nab-PTX | Case (n) | |
|---|---|---|---|---|
| Age | 57.6 ± 17.0* | 58.0 ± 18.5* | ||
| Sex | Male | 940 | Male | 1636 |
| Female | 2153 | Female | 2334 | |
| Occupation | Physician | 857 | Physician | 1491 |
| Pharmacist | 212 | Pharmacist | 151 | |
| Other health professional | 1182 | Other health-professional | 2368 | |
| Lawyer | 3 | Lawyer | − | |
| Consumer | 839 | Consumer | 281 | |
| Reporter country | USA | 1148 | USA | 1535 |
| Japan | 543 | Germany | 311 | |
| France | 179 | Japan | 309 | |
| Others | 1599 | Others | 2292 | |
| Type of report | PS drug | 3469 | PS drug | 4447 |
| SS drug | 5379 | SS drug | 1478 | |
| C | 2801 | C | 527 | |
| I | 11 | I | 1 | |
| Outcome | Death | 527 | Death | 1742 |
| Life-threatening | 283 | Life-threatening | 310 | |
| Hospitalization—initial or prolonged | 1388 | Hospitalization—initial or prolonged | 1986 | |
| Disability | 147 | Disability | 114 | |
| Congenital anomaly | − | Congenital anomaly | − | |
| Required invention to prevent permanent impairment/damage | 79 | Required invention to prevent permanent impairment/damage | 11 | |
| Other serious | 977 | Other serious | 1281 | |
| Concomitant drugs† | Carboplatin | 964 | Gemcitabine | 1986 |
| Dexamethasone | 396 | Carboplatin | 507 | |
| Diphenhydramine | 314 | Ondansetron | 491 | |
| Ranitidine | 166 | Dexamethasone | 440 | |
| Ondansetron | 152 | Prochlorperazine | 258 | |
| Trastuzumab | 152 | Lorazepam | 210 |
C: concomitant; FAERS: US Food and Drug Administration Adverse Event Reporting System; I: interacting; nab-PTX: nanoparticle albumin-bound paclitaxel; PS: primary suspect; sb-PTX: solvent-based paclitaxel; SS: Secondary suspect.
Mean ± SD, †top six drugs.
The reporting ratio of spontaneous AE reports of sb-PTX transiently decreased (Figure 1(a)), whereas the number of nab-PTX AE reports and reporting ratio transiently increased (Figure 1(b)).
Figure 1.
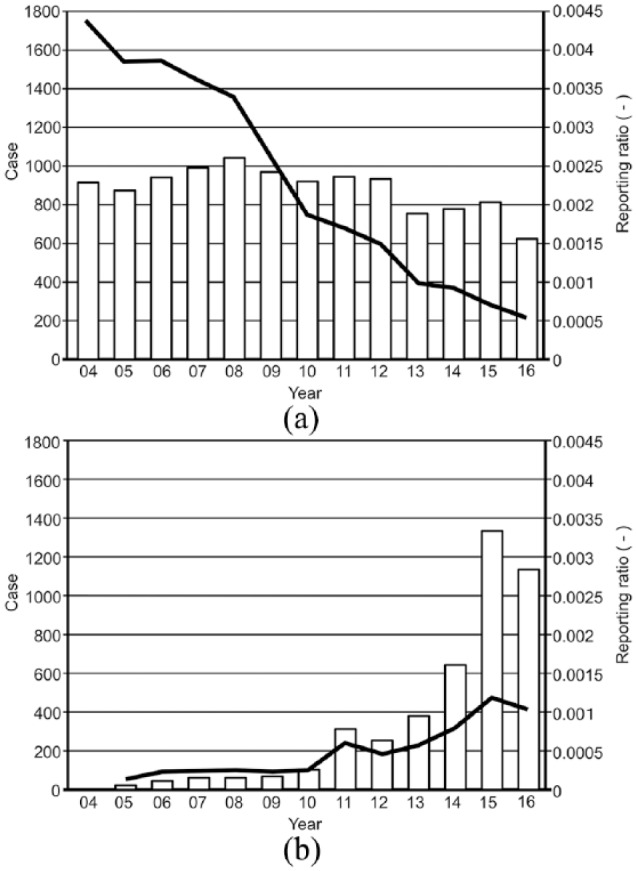
Changes in number of yearly reports of adverse events and reporting ratio of solvent-based paclitaxel (a) and nanoparticle albumin-bound paclitaxel (b).
The RORs (95% CI) for anaphylactic reaction (SMQ code: 20000021) associated with the use of sb-PTX and nab-PTX ROR were 1.69 (1.56–1.84) and 0.75 (0.68–0.83), respectively. RORs (95% CI) for hematopoietic leucopenia (SMQ code: 20000030) associated with the use of sb-PTX and nab-PTX were 5.36 (4.76–6.04) and 11.07 (10.22–11.98), respectively (Table 2). RORs (95% CI) for acute renal failure (SMQ: 20000003) associated with the use of sb-PTX and nab-PTX were 0.75 (0.58–0.98) and 1.60 (1.37–1.89), respectively. The lower limits of RORs for peripheral neuropathy, acute renal failure, hematopoietic erythropenia, hematopoietic leucopenia, hematopoietic thrombocytopenia, hematopoietic cytopenias affecting more than one type of blood cell, and interstitial lung disease associated with the use of nab-PTX were >1. The lower limits of RORs for peripheral neuropathy, hematopoietic erythropenia, hematopoietic leucopenia, hematopoietic thrombocytopenia, hematopoietic cytopenias affecting more than one type of blood cell, and interstitial lung disease associated with sb-PTX use were >1.
Table 2.
Reporting odds ratio of sb-PTX and nab-PTX in the FAERS database.
| Standardized MedDRA queries code | Total | sb-PTX |
nab-PTX |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases* | Reporting ratio (%) | ROR† | 95% CI‡ | Cases* | Reporting ratio (%) | ROR † | 95% CI‡ | |||
| 20000021 | Anaphylactic reaction | 1,025,984 | 748 | 0.07 | 1.69 | (1.56–1.84)§ | 484 | 0.05 | 0.75 | (0.68–0.83)|| |
| 20000034 | Peripheral neuropathy | 307,473 | 306 | 0.10 | 2.22 | (1.97–2.49)§ | 420 | 0.14 | 2.39 | (2.16–2.64)§ |
| 20000003 | Acute renal failure | 159,652 | 57 | 0.04 | 0.75 | (0.58–0.98)|| | 153 | 0.10 | 1.60 | (1.37–1.89)§ |
| 20000029 | Hematopoietic erythropenia | 133,889 | 118 | 0.09 | 1.90 | (1.58–2.28)§ | 347 | 0.26 | 4.57 | (4.09–5.10)§ |
| 20000030 | Hematopoietic leucopenia | 128,182 | 301 | 0.23 | 5.36 | (4.76–6.04)§ | 727 | 0.57 | 11.07 | (10.22–11.98)§ |
| 20000031 | Hematopoietic thrombocytopenia | 79,332 | 81 | 0.10 | 2.19 | (1.76–2.73)§ | 307 | 0.39 | 6.82 | (6.07–7.66)§ |
| 20000028 | Hematopoietic cytopenias affecting more than one type of blood cell | 56,782 | 76 | 0.13 | 2.88 | (2.29–3.61)§ | 154 | 0.27 | 4.62 | (3.93–5.42)§ |
| 20000042 | Interstitial lung disease | 53,531 | 215 | 0.40 | 9.04 | (7.87–10.38)§ | 155 | 0.29 | 4.93 | (4.20–5.79)§ |
| 20000046 | Taste and smell disorders | 48,835 | 13 | 0.03 | 0.56 | (0.33–0.97)|| | 38 | 0.08 | 1.29 | (0.94–1.77) |
CI: confidence interval; FAERS: US Food and Drug Administration Adverse Event Reporting System; MedDRA: Medical Dictionary for Regulatory Activities; nab-PTX: nanoparticle albumin-bound paclitaxel; ROR: reporting odds ratio; sb-PTX: solvent-based paclitaxel.
Number of patients with adverse events, †reporting odds ratio, ‡CI, §the lower limit of 95% CI was >1,||the upper limit of 95% CI was <1.
Discussion
In this study, we analyzed the AE profiles of sb-PTX and nab-PTX using FAERS data. For both PTX formulations, the lower limits of ROR 95% CI for hematopoietic erythropenia, hematopoietic leucopenia, hematopoietic thrombocytopenia, hematopoietic cytopenias affecting more than one type of blood cell, and interstitial lung disease were >1, suggesting that both sb-PTX and nab-PTX carry potential risks for these AEs. These hematopoietic AEs of PTX have been previously reported in multiple clinical trials.6,26–28
Spontaneous reporting of AEs is influenced by external factors such as duration of the drug in the market. The Weber effect is an epidemiological phenomenon describing a substantial increase in spontaneous reporting of AEs when the drug is first approved, which then plateaus and eventually declines.29–31 This effect may explain the decreased reporting ratio of AEs associated with sb-PTX (Figure 1(a)). However, the Weber effect is not always observed,31 and the number of reports generally increases over the first 2 years after launching the drug.32,33 In our study, the decline in the reporting ratio was observed after 2004. Since sb-PTX was approved by the FDA in 1992, it might be difficult to explain this decrease based on the Weber effect.
In contrast, the number of nab-PTX AE reports and the reporting ratio transiently increased (Figure 1(b)). nab-PTX was initially approved by the FDA in 200527 and its therapeutic applications were subsequently extended to include the treatment of non-small-cell lung cancer in 2012 and advanced pancreatic cancer in 2013, potentially increasing AE reporting. This increase of nab-PTX AE reports may be associated with the increased awareness of novel drugs by clinicians.
According to our findings, ROR signal for anaphylactic reaction was detected for sb-PTX and not for nab-PTX, which is in agreement with previous literature data. In a clinical trial on metastatic breast cancer, nab-PTX displayed a better safety profile for anaphylactic reaction than sb-PTX.12,34–36 Because of the high lipophilicity and poor solubility of PTX, sb-PTX employs a CrEL:ethanol vehicle. As CrEL is biologically and pharmacologically active, hypersensitivity reactions have been reported. Prolonged infusion times (3 h) and premedication with corticosteroids and antihistamines are required to reduce the risk of hypersensitivity reactions to sb-PTX.9,12,26,37,38 Corticosteroid use is inconvenient for patients and presents a drawback of this formulation.
ROR signal for acute renal failure was detected for nab-PTX. However, it was not detected for sb-PTX. Although ROR is a rough indication and does not provide sufficient evidence on causality, the risk of nab-PTX for acute renal failure might be a notable observation in the interpretation of our results. Additional studies are required to confirm this finding.
It has been reported that CrEL contributes to the development of peripheral neuropathies.13,26,39,40 sb-PTX was shown to cause neutropenia and neuropathy that may be associated with axonal degeneration.13,26,39,40 In this study, no difference was observed in ROR for peripheral neuropathy between nab-PTX and sb-PTX.
Differences in safety profiles of the studied PTX formulations may be caused by various formulation parameters. nab-PTX demonstrated a larger volume of distribution, more rapid clearance, a higher fraction of unbound drug, higher systemic exposure, and maximal concentration of unbound drug relative to sb-PTX.41,42 nab-PTX was shown to improve the pharmacokinetics and biodistribution of PTX compared to sb-PTX.41,42 However, we do not have a conclusive explanation for our data. More detailed analyses focusing on these factors are required.
The FAERS database is subject to various biases such as under-reporting, over-reporting, reporting bias favoring newer agents, notoriety bias, exclusion of healthy individuals, and confounding by comorbidities.14,25 There are several approaches to control confounding factors, such as stratification,14 multiple logistic regression,22,43 and Bayesian logistic regression.44 ROR is a well-defined and easily applicable technique that allows for adjustment of covariates through logistic regression analysis. Recently, the Breslow–Day test was introduced to compare the homogeneity of RORs.45,46 These approaches might be useful for the analysis of FAERS database.
Cases reported in the FAERS database do not always contain sufficient information regarding patient background and dose response to allow for proper evaluation. In particular, PTX dose may greatly affect AE profiles. However, FAERS database is an SRS, making it difficult to obtain and evaluate the doses and duration of drug treatments. A new therapeutic indication or regimen may influence the AE reporting profile. Close attention should be paid to investigate temporal axis when planning a pharmacovigilance analysis.47 More detailed analysis focusing on these factors is a subject for future investigation.
It should be noted that disproportionality analysis only suggests the necessity for well-organized clinical studies with respect to association. ROR differs from odds ratio commonly used in epidemiological studies. In absolute terms, ROR indicates an increased risk of AE reporting, not a risk of AE occurrence.14 ROR is not applicable to inferences of comparative degrees of causality and only offers a rough indication of signal strength. We have chosen the threshold of two cases for the calculation of ROR.14 However, efficiency of signal detection was strongly dependent on the thresholds used to define statistical significance.48 Using the proportional reporting ratio (PRR), signal is detected if the number of cases are ⩾3, and the PRR is at least 2 with an associated chi-square value of 4 or more.49 A recent article discussed a potential increase in the number of cases from 3 to 5.50 When four or more cases were present, no clear differences in disproportionality measures such as ROR and PRR were found.24 In the future, it would be necessary to reference these thresholds in order to assess how well they perform in the SRS database. These are the limitations of the methodology used in this study.
Michel et al.51 reported that disproportionality cannot be used for comparative drug safety analysis beyond basic hypothesis generation. de Boer52 emphasized that disproportionality measures used in SRS databases have important limitations, and more advanced methods might generate new and relevant information. In contrast, Montastruc et al.53 reported that none of the methods (e.g. clinical trials, case–control studies, and cohort studies) if taken alone should be considered definitive for evaluating drug risk, and disproportionality studies are, therefore, important. Despite the inherent limitations of SRS data, our findings are in agreement with those of previous studies. The FAERS database is the largest SRS in the world and can reflect real-world setting. With larger numbers of accurate reports, the FAERS database would help to optimize pharmacotherapy.
Conclusion
This is the first study to evaluate the AE profile of nab-PTX using FAERS data. Considering that the FAERS database does not allow to infer causality or risk ranking, the different reporting frequencies of anaphylactic reaction and acute renal failure between sb-PTX and nab-PTX must be further investigated via analytical observational research.
Supplemental Material
Supplemental material, 181227S1fig for Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration Adverse Event Reporting System by Misa Naganuma, Kohei Tahara, Shiori Hasegawa, Akiho Fukuda, Sayaka Sasaoka, Haruna Hatahira, Yumi Motooka, Satoshi Nakao, Ririka Mukai, Kouseki Hirade, Tomoaki Yoshimura, Takeshi Kato, Hirofumi Takeuchi and Mitsuhiro Nakamura in SAGE Open Medicine
Supplemental Material
Supplemental material, 190124S1Table for Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration Adverse Event Reporting System by Misa Naganuma, Kohei Tahara, Shiori Hasegawa, Akiho Fukuda, Sayaka Sasaoka, Haruna Hatahira, Yumi Motooka, Satoshi Nakao, Ririka Mukai, Kouseki Hirade, Tomoaki Yoshimura, Takeshi Kato, Hirofumi Takeuchi and Mitsuhiro Nakamura in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was not sought for this study because the study was an observational study without any research subjects.
Not applicable. All results were obtained from data openly available online from the FDA website (www.fda.gov). All data from the FAERS database were fully anonymized by the regulatory authority before we accessed them.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partially supported by Japan Society for the Promotion of Science KAKENHI grant number, 17K08452. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Informed consent: Not applicable. Written informed was not sought for this study because the study was an observational study without any research subjects.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Mitsuhiro Nakamura  https://orcid.org/0000-0002-5062-5522
https://orcid.org/0000-0002-5062-5522
References
- 1. Wani MC, Taylor HL, Wall ME, et al. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 1971; 93: 2325–2327. [DOI] [PubMed] [Google Scholar]
- 2. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med 1995; 332: 1004–1014. [DOI] [PubMed] [Google Scholar]
- 3. Crown J, O’Leary M. The taxanes: an update. Lancet 2000; 355: 1176–1178. [DOI] [PubMed] [Google Scholar]
- 4. Socinski MA. Single-agent paclitaxel in the treatment of advanced non-small cell lung cancer. Oncologist 1999; 4: 408–416. [PubMed] [Google Scholar]
- 5. Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev 2008; 60(8): 876–885. [DOI] [PubMed] [Google Scholar]
- 6. Miele E, Spinelli GP, Miele E, et al. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine 2009; 4: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release 2013; 170(3): 365–372. [DOI] [PubMed] [Google Scholar]
- 8. von Minckwitz G, Martin M, Wilson G, et al. Optimizing taxane use in MBC in the emerging era of targeted chemotherapy. Crit Rev Oncol Hematol 2013; 85(3): 315–331. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Chen N, Palmisano M, et al. Pharmacologic sensitivity of paclitaxel to its delivery vehicles drives distinct clinical outcomes of paclitaxel formulations. Mol Pharm 2015; 12(4): 1308–1317. [DOI] [PubMed] [Google Scholar]
- 10. Chen N, Li Y, Ye Y, et al. Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with solid tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J Clin Pharmacol 2014; 54(10): 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006; 12(4): 1317–1324. [DOI] [PubMed] [Google Scholar]
- 12. Stinchcombe TE. Nanoparticle albumin-bound paclitaxel: a novel Cremphor-EL ®-free formulation of paclitaxel. Nanomedicine 2007; 2: 415–423. [DOI] [PubMed] [Google Scholar]
- 13. Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001; 37(13): 1590–1598. [DOI] [PubMed] [Google Scholar]
- 14. Poluzzi E, Raschi E, Piccinni C, et al. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS). In: Karahoca A. (ed.) Data mining applications in engineering and medicine. London: InTech Open, 2012, pp. 265–302. [Google Scholar]
- 15. Sasaoka S, Matsui T, Hane Y, et al. Time-to-onset analysis of drug-induced long QT syndrome based on a spontaneous reporting system for adverse drug events. PLoS ONE 2016; 11(10): e0164309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuda A, Tahara K, Hane Y, et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS ONE 2017; 12(9): e0185654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Council for Harmonisation (ICH). E2B, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073090.pdf (1998, accessed 26 December 2018).
- 18. U. S. Food and Drug Administration. “README.DOC” file for the quarterly data extract (QDE) from the FDA adverse event reporting system (FAERS), http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm (accessed 26 December 2018).
- 19. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Specifications for preparing and submitting electronic ICSRs and ICSR attachments, https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/UCM601820.pdf (accessed 26 December 2018).
- 20. MedDRA Version 19.0, http://www.meddra.org/sites/default/files/guidance/file/intguide_19_0_english.pdf (accessed 26 December 2018).
- 21. MedDRA MSSO. Introductory guide for Standardized MedDRA Queries (SMQs) Version 19.0, 2016, http://www.meddra.org/sites/default/files/guidance/file/smq_intguide_19_0_english.pdf
- 22. Suzuki Y, Suzuki H, Umetsu R, et al. Analysis of the interaction between clopidogrel, aspirin, and proton pump inhibitors using the FDA adverse event reporting system database. Biol Pharm Bull 2015; 38(5): 680–686. [DOI] [PubMed] [Google Scholar]
- 23. Kato Y, Umetsu R, Abe J, et al. Hyperglycemic adverse events following antipsychotic drug administration in spontaneous adverse event reports. J Pharm Health Care Sci 2015; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf 2002; 11(1): 3–10. [DOI] [PubMed] [Google Scholar]
- 25. Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf 2009; 18(6): 427–436. [DOI] [PubMed] [Google Scholar]
- 26. Di Costanzo F, Gasperoni S, Rotella V, et al. Targeted delivery of albumin bound paclitaxel in the treatment of advanced breast cancer. Onco Targets Ther 2009; 2: 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Celgene corporation. Highlights of prescribing information. ABRAXANE® for Injectable Suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound), https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021660s040lbl.pdf (2014, accessed 28 August 2018).
- 28. Bristol-Myers Squibb Company. TAXOL® (paclitaxel) injection (patient information included), https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf (2011, accessed 28 August 2018).
- 29. Wallenstein EJ, Fife D. Temporal patterns of NSAID spontaneous adverse event reports: the Weber effect revisited. Drug Saf 2001; 24(3): 233–237. [DOI] [PubMed] [Google Scholar]
- 30. Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004; 24(6): 743–749. [DOI] [PubMed] [Google Scholar]
- 31. McAdams MA, Governale LA, Swartz L, et al. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf 2008; 17(9): 882–889. [DOI] [PubMed] [Google Scholar]
- 32. Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch Intern Med 2007; 167(16): 1752–1759. [DOI] [PubMed] [Google Scholar]
- 33. Weiss-Smith S, Deshpande G, Chung S, et al. The FDA drug safety surveillance program: adverse event reporting trends. Arch Intern Med 2011; 171(6): 591–593. [DOI] [PubMed] [Google Scholar]
- 34. Ibrahim NK, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol 2005; 23(25): 6019–6026. [DOI] [PubMed] [Google Scholar]
- 35. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23(31): 7794–7803. [DOI] [PubMed] [Google Scholar]
- 36. Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol 2005; 23(31): 7785–7793. [DOI] [PubMed] [Google Scholar]
- 37. Sofias AM, Dunne M, Storm G, et al. The battle of “nano” paclitaxel. Adv Drug Deliv Rev 2017; 122: 20–30. [DOI] [PubMed] [Google Scholar]
- 38. Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release 2008; 132(3): 171–183. [DOI] [PubMed] [Google Scholar]
- 39. Authier N, Gillet JP, Fialip J, et al. Assessment of neurotoxicity following repeated cremophor/ethanol injections in rats. Neurotox Res 2001; 3(3): 301–306. [DOI] [PubMed] [Google Scholar]
- 40. Goble S, Bear HD. Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions. Surg Clin North Am 2003; 83(4): 943–971. [DOI] [PubMed] [Google Scholar]
- 41. Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 2008; 14(13): 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin Cancer Res 2005; 11(11): 4136–4143. [DOI] [PubMed] [Google Scholar]
- 43. Abe J, Umetsu R, Uranishi H, et al. Analysis of polypharmacy effects in older patients using Japanese Adverse Drug Event Report database. PLoS ONE 2017; 12(12): e0190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 2002; 25(6): 381–392. [DOI] [PubMed] [Google Scholar]
- 45. Rahman MM, Alatawi Y, Cheng N, et al. Comparison of brand versus generic antiepileptic drug adverse event reporting rates in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS). Epilepsy Res 2017; 135: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rahman MM, Alatawi Y, Cheng N, et al. Methodological considerations for comparison of brand versus generic versus authorized generic adverse event reports in the US Food and Drug Administration Adverse Event Reporting System (FAERS). Clin Drug Investig 2017; 37(12): 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raschi E, Piccinni C, Poluzzi E, et al. The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA pharmacovigilance database. Acta Diabetol 2013; 50(4): 569–577. [DOI] [PubMed] [Google Scholar]
- 48. Candore G, Juhlin K, Manlik K, et al. Comparison of statistical signal detection methods within and across spontaneous reporting databases. Drug Saf 2015; 38(6): 577–587. [DOI] [PubMed] [Google Scholar]
- 49. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 2001; 10(6): 483–486. [DOI] [PubMed] [Google Scholar]
- 50. Slattery J, Alvarez Y, Hidalgo A. Choosing thresholds for statistical signal detection with the proportional reporting ratio. Drug Saf 2013; 36(8): 687–692. [DOI] [PubMed] [Google Scholar]
- 51. Michel C, Scosyrev E, Petrin M, et al. Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles. Clin Drug Investig 2017; 37(5): 415–422. [DOI] [PubMed] [Google Scholar]
- 52. de Boer A. When to publish measures of disproportionality derived from spontaneous reporting databases? Br J Clin Pharmacol 2011; 72(6): 909–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Montastruc JL, Sommet A, Bagheri H, et al. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 2011; 72(6): 905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 181227S1fig for Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration Adverse Event Reporting System by Misa Naganuma, Kohei Tahara, Shiori Hasegawa, Akiho Fukuda, Sayaka Sasaoka, Haruna Hatahira, Yumi Motooka, Satoshi Nakao, Ririka Mukai, Kouseki Hirade, Tomoaki Yoshimura, Takeshi Kato, Hirofumi Takeuchi and Mitsuhiro Nakamura in SAGE Open Medicine
Supplemental material, 190124S1Table for Adverse event profiles of solvent-based and nanoparticle albumin-bound paclitaxel formulations using the Food and Drug Administration Adverse Event Reporting System by Misa Naganuma, Kohei Tahara, Shiori Hasegawa, Akiho Fukuda, Sayaka Sasaoka, Haruna Hatahira, Yumi Motooka, Satoshi Nakao, Ririka Mukai, Kouseki Hirade, Tomoaki Yoshimura, Takeshi Kato, Hirofumi Takeuchi and Mitsuhiro Nakamura in SAGE Open Medicine


