Abstract
Tendinopathy is a common painful musculoskeletal disorder treated by injection of analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs), which are believed to have cytotoxicity toward tenocytes. Ascorbic acid is an antioxidant that promotes collagen biosynthesis and prevents free radical formation. It is believed to protect tenocytes from oxidative stress. The optimal concentration of ascorbic acid, especially when used in conjunction with anesthetics and NSAIDs injection, to treat different stages of tendinopathies is unknown. Human tenocytes were isolated from a torn edge of the supraspinatus tendon of a 51-year-old male patient during arthroscopic repair. We monitored real-time changes in human tenocyte proliferation upon exposure to different concentrations of ascorbic acid, bupivacaine, and ketorolac tromethamine using the xCELLigence system. No significant changes in cell index were observed between the control group and tenocytes treated with the 3 concentrations of ascorbic acid. Tenocytes exposed to 0.5% bupivacaine and 30 or 15 mg/mL ketorolac tromethamine revealed significant reduction in tenocytes proliferation. Bupivacaine 0.5% with 250 μg/mL ascorbic acid and 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid showed the least cytotoxicity against tenocytes. The optimal ascorbic acid concentration required to reduce the cytotoxic effects of bupivacaine and ketorolac tromethamine was demonstrated using this platform.
Keywords: real-time cell monitoring, cytotoxicity, tenocytes, ascorbic acid, bupivacaine, ketorolac tromethamine
Introduction
Tendons are highly specialized fibrous connective tissues connecting muscle to bone, transmitting tensile loads, and facilitating joint movement.1 They can be easily injured by sudden high strain in sports or repetitive loading in leisure activities.2 Once injured, they do not heal easily because of their limited blood supply, slow cell turnover, and low cellularity.3 Tenocytes, which are specialized mesenchymal-derived cells embedded in a 3-dimensional network of extracellular matrix (ECM), occupy only 5% of normal tendon tissue.4 Symptoms from injured tendon, defined as tendinopathy, are characterized by pain during activity, localized tenderness upon palpation, swelling of the tendon involved, and impaired performance.5 Immunohistochemical studies confirmed inflammatory cells such as mast cells, T cells, and macrophages are present in early human tendinopathies.6,7 Therefore, most treatment methods against early tendinopathy focus on anti-inflammation and analgesia. They are often empirically initiated with nonsteroidal anti-inflammatory drugs (NSAIDs) and/or anesthetic injections.8
However, when tenocytes are exposed to a certain amount NSAIDs, the differentiation of mesenchymal stem cells to tenocytic lineage gets impaired and drawn toward adipocytic lineage.9 Histologically, this leads to a marked reduction in the number of healthy tenocytes and the accumulation of “nontendon cells,” including myofibroblasts, adipocytes, chondrocytes, and osteoblasts.10 On the other hand, Scherb et al reported decreased human tenocyte proliferation and ECM production after treatment with anesthetics.11 Hence, there is a fine line between effective reduction in pain symptoms and maintenance of normal tendon homeostasis in a specific treatment. The biological and pharmacological interactions of these treatment methods are largely uncertain.12 There are currently no available data regarding maximal efficacy dosages of local anesthetics and NSAIDs in peritendinous injections.13
Ascorbic acid is a well-characterized antioxidant that can promote collagen biosynthesis and prevent free radical formation.14 Different concentrations of ascorbic acid are used in tenocyte cultures to enhance collagen synthesis.9,15 Immunofluorescence staining revealed differential localization of type I collagen in vitro, with collagen localized outside cells in the presence of ascorbic acid.16 Poulsen et al reported in 2011 that ascorbic acid can protect hamstring-derived tenocytes from oxidative stress.17 They suggested the use of ascorbic acid with dexamethasone injections to decrease its cytotoxicity. However, because of the acidity of ascorbic acid,16 it may be toxic and negatively affect cell morphology at certain concentrations. Therefore, it is important to determine the optimal concentration of ascorbic acid that can be used in conjunction with anesthetics and NSAIDs injection to treat different stages of tendinopathies.
Several in vitro studies have been conducted to achieve this goal. Carofino et al18 used radioactive thymidine assay, whereas Kraus et al19 used MTS assay (a cell proliferation colorimetric assay) to measure tenocyte proliferation. These bioassays are mostly end-point measurements and destructive analytical methods, wherein the cultured cells needed to be sacrificed. They were unable to monitor cell proliferation in real time and could provide little information on the kinetics of cellular responses when they were exposed to different kinds of stimuli.20 Recent advances in microfluidic technologies have made it possible to produce in vitro assays providing a range of stimulation capabilities as well as enabling extensive quantitative assessment of their effects on cells in a real-time manner.21 xCELLigence technology is a real-time cellular biosensor that measures the net adhesion of cells to high-density gold electrode arrays printed on custom-designed E-plates.22 Chiu et al demonstrated that human tenocytes can proliferate inside xCELLigence system, thus replacing the conventional cell culture system and end-point assays.23
Although ascorbic acid has been proposed as a tendon culture supplement to reduce the cytotoxicity of anesthetics and NSAIDs, no optimal dose or formulation has been indicated until now. We monitored the real-time changes in human tenocyte proliferation upon exposure to different concentrations of ascorbic acids, anesthetics, and NSAIDs, such as bupivacaine and ketorolac tromethamine, using the xCELLigence system. We determined the optimal ascorbic acid concentration required to reduce the cytotoxic effects of bupivacaine and ketorolac tromethamine. Our in vitro results can be useful for the clinical application of these drugs.
Materials and Methods
Level of evidence: This is a level III, controlled laboratory study.
Isolation of Human Tenocytes
Human tenocytes were isolated from a torn edge of the supraspinatus tendon of a 51-year-old male patient with Boileau stage 124 small tear during arthroscopic repair (Figure 1A and B), which was approved by the institutional review board at the hospital of the first author. The fatty infiltration of the supraspinatus muscle was grade 1 (Figure 1C and D).25 Tendon samples were digested in an enzymatic solution containing 4 mg/mL dispase (Roche, Burgess Hill, United Kingdom) and 300 U/mL collagenase type II (Gibco, Invitrogen, Paisley, United Kingdom) at 37.8°C for 16 hours. After digestion, the mixture was filtered and centrifuged at 1000 rpm (400 × g) for 5 minutes at 37°C. The cell pellet was then resuspended and maintained in culture media (minimum essential medium; α-MEM) supplemented with 10% fetal bovine serum and 1% antibiotics in standard tissue culture flasks. After the first passage, the adherent monolayer was trypsinized, and cells were seeded at 2 × 104 cells/cm2 in conventional 6-well plates and maintained in serum-free α-MEM overnight at 37.8°C prior to loading into the microfluidic system or conventional 24-well plates. Normal tenocyte morphological characteristics were confirmed by microscopy.
Figure 1.
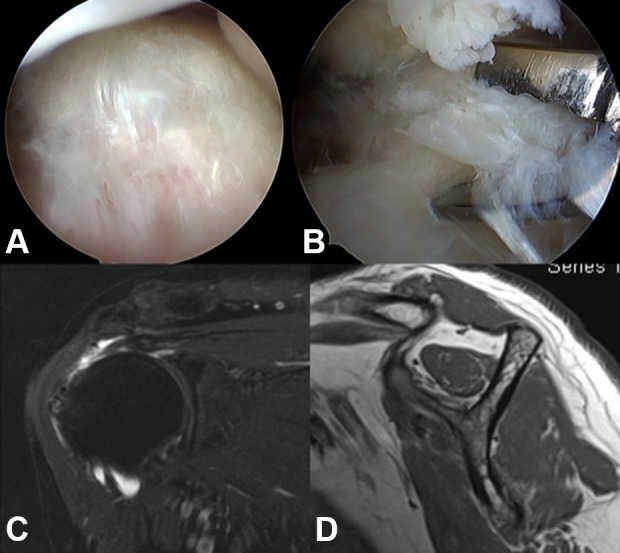
Harvest of human tenocytes. A, B, Tenocytes were isolated from a 51-year-old male patient during arthroscopic repair. C, The size of the rotator cuff tear was small. D, The fatty infiltration of the supraspinatus muscle was grade 1.
xCELLigence System
The xCELLigence system (Roche/ACEA Biosciences, San Diego, California) is a commercial microfluidic system designed to allow for continuous real-time monitoring of cellular adhesion properties in vitro in a noninvasive, label-free manner. The study was performed according to the manufacturer’s instructions.
Seeding Tenocytes Into xCELLigence E-96 Plates
First, 50 μL of complete media was added to culture wells of E-96 xCELLigence plates. After equilibration to 37°C, plates were inserted into the xCELLigence station to measure the baseline impedance. This ensured that all wells and connections were working within acceptable limits. Tenocytes were then seeded into the wells at 2 × 104 cells/cm2, as it has been previously reported as the optimal cell seeding density.23
Ascorbic Acid, Anesthetics, and NSAIDs Preparation
Three different concentrations of ascorbic acid, bupivacaine, and ketorolac tromethamine were added alone or together with tenocytes at 24 hours after seeding. For ascorbic acid (Vitacicol Inj 100mg, Taiwan Biotec Co. Ltd.) treatment, concentrations of 10, 50, and 250 μg/mL were applied in a volume of 10 μL. Recognizing that surrounding body fluids quickly dilute substances after an injection, dilutions were used to replicate conditions experienced by tenocytes in vivo.26 For bupivacaine (Myungmoon Pharmaceutical) treatment, concentrations of 0.5%, 0.25%, and 0.05% (100%, 50%, and 10% of clinical dosage) were applied in a volume of 10 μL. For ketorolac tromethamine (Yung Shin Pharmaceutical) treatment, solutions of 30, 15, and 3 mg/mL were added to wells in a volume of 10 μL. Control cultures were exposed to the saline solution under the same conditions without anesthetics, NSAIDs, or ascorbic acids.
Interactions of Ascorbic Acid Against Analgesics and NSAIDs
The interactions between the different concentration of ascorbic acid, bupivacaine, and ketorolac tromethamine were tested. To test the cytotoxicity of ascorbic acid and bupivacaine against tenocytes, 10 μL of 10, 50, or 250 μg/mL ascorbic acid was applied in the culture well with 10 μL bupivacaine at concentrations of 0.5%, 0.25%, and 0.05%. The same ascorbic acid preparation was used with 10 μL of 30, 15, or 3 mg/mL ketorolac tromethamine. All conditions are illustrated in Figure 2. The whole procedure lasted for 148 hours.
Figure 2.
Flowchart of the study.
Tenocytes Proliferation and xCELLigence Software Data Plotting
We used xCELLigence software version 1.2.1 in this experiment to provide an electronic record of the experimental details. The cell index represents the measure of cellular adhesion across each individual well. In the absence of living cells (media only) or with a suspension of dead cells, the cell index values will be close to 0. Upon attachment of tenocytes onto the electrode, the measured signal will correlate linearly with cell number throughout the experiment with sufficient accuracy as shown in many publications.22,27-31
Statistical Analysis
Each experiment was performed in at least triplicate. To compare cell indexes among different culture conditions, analysis of variance followed by Bonferroni post hoc test was used. A P value of <.05 was considered significant. All statistical analyses were performed with SPSS 21.0 for Windows (SPSS Inc).
Results
Real-Time Analysis of Tenocyte Proliferation in xCELLigence System Under Different Stimuli
In order to study the effect of various stimuli on tenocyte proliferation, we monitored dynamic changes in cell index using the xCELLigence system upon exposure to ascorbic acid, bupivacaine, and ketorolac tromethamine.
Control Group
Cell index of the control group at 24 hours before stimuli exposure was 1 ± 0.01. After 148 hours of tenocyte proliferation, the cell index progressed to 2.62 ± 0.29 at the final time point (148 hours).
Ascorbic Acid
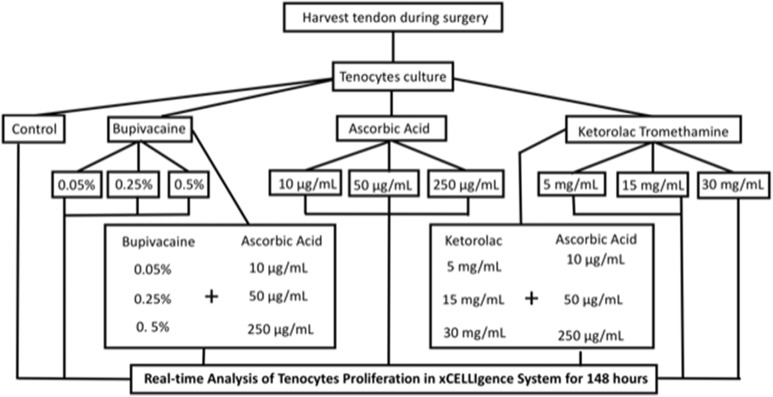
Real-time changes in cell index were observed when tenocytes were exposed to 10, 50, and 250 μg/mL ascorbic acid (Figure 3A). Cell index at 24 hours was 0.91 ± 0.1, 1.02 ± 0.2, and 1.02 ± 0.1, respectively, and 2.82 ± 0.22, 3.01 ± 0.18, and 3.11 ± 0.26, respectively, at 148 hours. There were no significant changes in cell index between the control group and tenocytes treated with ascorbic acid (Table 1).
Figure 3.
Real-time changes in cell index when tenocytes were exposed to different concentrations of stimuli. A, Cell index when tenocytes were exposed to 10, 50, or 250 μg/mL ascorbic acid. B, Cell index when tenocytes were exposed to 0.05%, 0.25%, or 0.5% bupivacaine. C, Cell index when tenocytes were exposed to 3, 15, or 30 mg/mL ketorolac tromethamine. * P < .05.
Table 1.
Cell Index of Tenocytes Treated With Ascorbic Acid.
| Ascorbic Acid 24 hours | Ascorbic Acid 148 hours | |||||
|---|---|---|---|---|---|---|
| 10 μg/mL | 50 μg/mL | 250 μg/mL | 10 μg/mL | 50 μg/mL | 250 μg/mL | |
| Tenocytes 2 × 104 cells/cm2 | 0.91 ± 0.1 | 1.02 ± 0.2 | 1.02 ± 0.1 | 2.82 ± 0.22 | 3.01 ± 0.18 | 3.11 ± 0.26 |
| P Values | 1 | 1 | 1 | 1 | 0.302 | 0.224 |
Bupivacaine
Real-time cell index at 24 hours was 0.45 ± 0.04, 0.78 ± 0.11, and 0.96 ± 0.13 when tenocytes were exposed to 0.5%, 0.25%, and 0.05% (100%, 50%, and 10% of clinical dosage) bupivacaine, respectively. At 148 hours, they were 0.45 ± 0.04, 0.78 ± 0.11, and 0.96 ± 0.13, respectively (Figure 3B). Among them, tenocytes exposed to 0.5% bupivacaine (100% clinical dosage) revealed a significant reduction in cell index (P = 0), which indicated the cytotoxicity of commonly used bupivacaine against tenocytes.
Ketorolac Tromethamine
Real-time cell index at 24 hours was 0.98 ± 0.02, 0.83 ± 0.02, and 0.99 ± 0.1 once tenocytes were exposed to 30, 15, and 3 mg/mL (100%, 50%, and 10% of clinical dosage) ketorolac tromethamine, respectively. At 148 hours, the cell index was 1.76 ± 0.17, 2 ± 0.09, and 2.12 ± 0.12 (Figure 3C), respectively. Among them, tenocytes exposed to 30 and 15 mg/mL ketorolac tromethamine (100% and 50% clinical dosage) revealed a significant reduction in cell index (P = .003 and .02), which indicated the cytotoxicity of commonly used ketorolac tromethamine against tenocytes even in its 50% dilution form.
Interaction Between Ascorbic Acid and Bupivacaine/Ketorolac Tromethamine
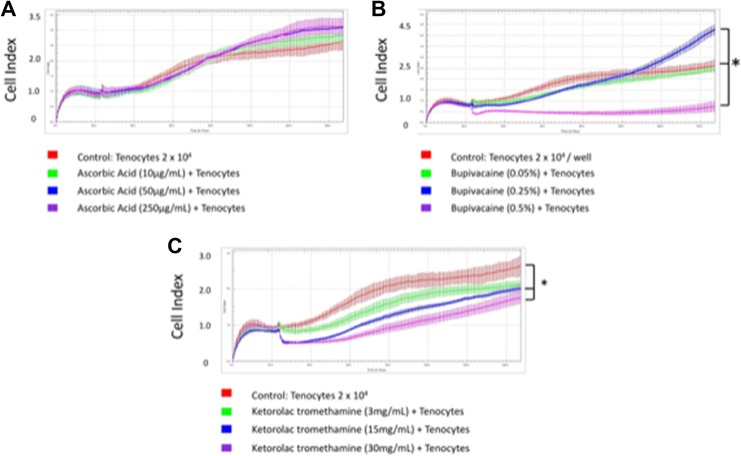
First, we exposed tenocytes to different concentrations of bupivacaine and ascorbic acid (Figure 4A-C). Readout of the cell index increased significantly when 0.5% and 0.25% bupivacaine were used in combination with any concentration of ascorbic acid (Table 2). This implied the reduced cytotoxic effects when ascorbic acid was applied along with 0.5% bupivacaine, the clinical dosage. The best tenocyte proliferation was observed in 0.5% bupivacaine and 250 μg/mL ascorbic acid group (cell index 5.14 ± 0.36).
Figure 4.
Tenocytes exposed to different concentrations of bupivacaine and ascorbic acid. A, Cell index when tenocytes were exposed to 0.05% bupivacaine and 10, 50, or 250 μg/mL ascorbic acid. B, Cell index when tenocytes were exposed to 0.25% bupivacaine and 10, 50, or 250 μg/mL ascorbic acid. C, Cell index when tenocytes were exposed to 0. 5% bupivacaine and 10, 50, or 250 μg/mL ascorbic acid. * P < .05.
Table 2.
Cell Index of Bupivacaine and Ketorolac Tromethamine Interaction With Ascorbic Acid.
| Ascorbic Acid 24 hours | Ascorbic Acid 148 hours | |||||
|---|---|---|---|---|---|---|
| 10 μg/mL | 50 μg/mL | 250 μg/mL | 10 μg/mL | 50 μg/mL | 250 μg/mL | |
| Bupivacaine | ||||||
| 0.05% | 1.12 ± 0.11 | 1.17 ± 0.19 | 1.05 ± 0.07 | 2.58 ± 0.09 | 2.8 ± 0.2 | 3.02 ± 0.13 |
| 0.25% | 1.06 ± 0.13 | 1 ± 0.13 | 0.96 ± .11 | 3.97 ± 0.24 | 3.91 ± 0.04 | 4.28 ± 0.32 |
| 0.50% | 0.84 ± 0.09 | 0.81 ± 0.02 | 0.79 ± 0.11 | 4.58 ± 0.14 | 5.06 ± 0.26 | 5.14 ± 0.36 |
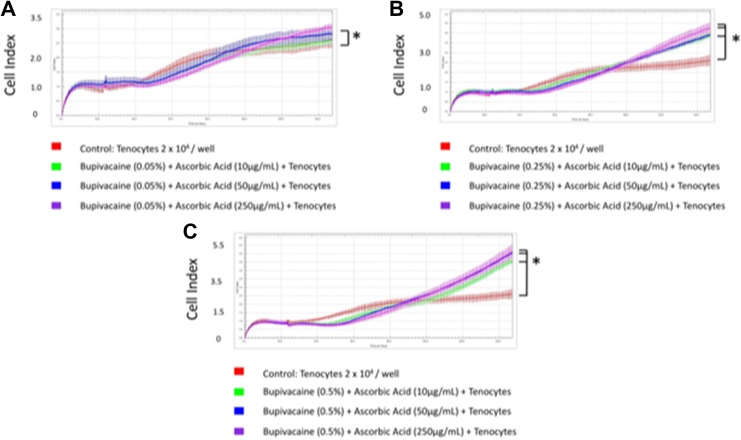
| Ketorolac tromethamine | ||||||
| 3 mg/mL | 1.15 ± 0.04 | 1.18 ± 0.08 | 1.32 ± 0.12 | 3.36 ± 0.11 | 3.5 ± 0.1 | 3.58 ± 0.14 |
| 15 mg/mL | 0.95 ± 0.08 | 1 ± 0.04 | 1.24 ± 0.11 | 2.66 ± 0.13 | 2.77 ± 0.15 | 4.35 ± 0.08 |
| 30 mg/mL | 0.88 ± 0.14 | 1.07 ± 0.11 | 0.92 ± 0.05 | 2.41 ± 0.24 | 3.47 ± 0.19 | 3.38 ± 0.24 |
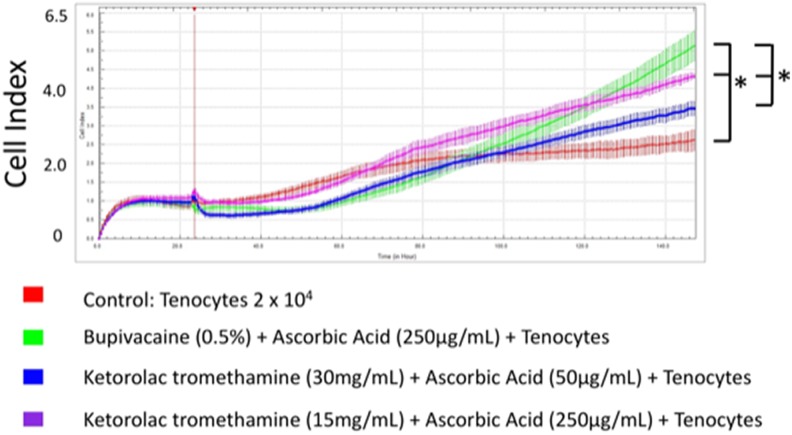
We then exposed tenocytes to different concentrations of ketorolac tromethamine and ascorbic acid (Figure 5A-C). Readout of the cell index increased significantly when 30 mg/mL ketorolac tromethamine was used along with 250 or 50 μg/mL ascorbic acid, 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid, and 3 mg/mL ketorolac tromethamine with 50 to 250 μg/mL ascorbic acid (Table 2). This indicated the reduced cytotoxicity when ascorbic acid was applied along with 30 mg/mL ketorolac tromethamine, the clinical dosage, and other diluted concentrations. The best tenocyte proliferation was observed in 15 mg/mL ketorolac tromethamine and 250 μg/mL ascorbic acid group (cell index 4.35 ± 0.08). In summary, 0.5% bupivacaine with 250 μg/mL ascorbic acid and 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid showed the least cytotoxicity against tenocytes (Figure 6). Our data could serve as an essential reference during clinical applications when bupivacaine or ketorolac tromethamine injections are considered.
Figure 5.
Tenocytes exposed to different concentrations of ketorolac tromethamine and ascorbic acid. A Cell index when tenocytes were exposed to 3 mg/mL ketorolac tromethamine and 10, 50, or 250 μg/mL ascorbic acid. B, Cell index when tenocytes were exposed to 15 mg/mL ketorolac tromethamine and 10, 50, or 250 μg/mL ascorbic acid. C, Cell index when tenocytes were exposed to 30 mg/mL ketorolac tromethamine and 10, 50, or 250 μg/mL ascorbic acid. * P < .05.
Figure 6.
Optimal tenocyte proliferation was observed in the 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid group. Bupivacaine 0.5% with 250 μg/mL ascorbic acid and 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid showed least cytotoxicity against tenocytes. * P < .05.
Discussion
This study demonstrated that tenocytes could proliferate inside xCELLigence system and their proliferation changes could be monitored in real time upon exposure to different concentrations of ascorbic acid, bupivacaine, and ketorolac tromethamine. This in vitro result provides important information because bupivacaine and ketorolac tromethamine are commonly used medications to treat tendon lesions and tendinopathies.
Tendinopathies are accompanied with subjective pain and dysfunction. Histologically, pathologic characteristics such as formation of lipids, proteoglycans, and calcified tissues in tendon lesions were identified in tendinopathies.5,32 Clinically, fatty infiltration in a musculotendinous junction is commonly observed following tendon injuries, such as in rotator cuff tears.33 Mechanisms of tendinopathy are generally believed to be “failed healing responses,” suggesting that cumulative damage and repetitive stresses are not balanced with repair responses by tenocytes.34 However, commonly used anesthetics and NSAIDs such as bupivacaine and ketorolac tromethamine that reduce pain from tendinopathy are believed to exhibit cytotoxicity toward tenocytes and affect tendon healing.35,36 Therefore, there is extensive concern regarding this therapeutic modality and a need to achieve balance between pain reduction and decreased cytotoxicity.
Ascorbic acid has been shown to improve the rate and quality of rat Achilles tendon repair.37 Concentrations from 25 to 1250 μg/mL ascorbic acid are used in tenocyte cultures to support tenocyte proliferation and collagen synthesis.9,15,38 However, Hakimi et al reported that tenocyte growth rate would decrease with the use of >100 mmol/L ascorbic acid.16 Decline in cell growth at this concentration of ascorbic acid was attributed to the pH level of the supplemented media which was highly acidic with 100 mmol/L ascorbic acid (pH 3.75).16 The authors concluded that ascorbic acid was strongly toxic to cells at concentrations above 100 mmol/L and negatively affected cell morphology at concentrations from 1 mmol/L. Clement et al also reported that ascorbic acid at concentrations above 1 mmol/L (176 μg/mL) could be toxic to various human cell lines because it generates hydrogen peroxide.39 Therefore, the best concentration of ascorbic acid for each individual’s tenocytes as well as their interaction with different concentrations of bupivacaine and ketorolac tromethamine should be determined.
Tendon repair is accomplished primarily by resident tendon fibroblasts or tenocytes, which are the principal cellular components of tendon tissue.40 Therefore, tenocyte proliferation is a good index to monitor tendon repair. However, the small amount of tissue that can be retrieved during surgery and the inherent low cellularity of tendons made screening the best regiment combination of drugs and their effects on tenocyte proliferation difficult. On the other hand, as the number of cell culture passages increase, tenocytes usually become dedifferentiated, thereby losing their specific characteristics.13 Conventional cell growth assays, such as Alamar Blue, water soluble tetrazolium salts (WSTs-1), and MTT assay (colorimetric assay for assessing cell metabolic activity), need to sacrifice the cultured cells and thus hamper the observation of subsequent cellular responses.15,41,42 They can only provide end-point measurements, and little information is known on the kinetics of real-time tenocyte interaction with different concentrations of ascorbic acid, bupivacaine, and ketorolac tromethamine. Dolkart et al and Chiu et al confirmed that rat and human tenocytes can proliferate inside an impedance-based instrument system, which made it possible to reveal the different effects of stimuli at multiple time points.23,43 In the current study, we used impedance-based xCELLigence system to see the real-time interaction of tenocytes toward different concentrations of ascorbic acid, bupivacaine, and ketorolac tromethamine. According to the cell index of the system, which corresponds to living tenocytes after exposure of stimuli, we could see that 0.5% bupivacaine with 250 μg/mL ascorbic acid and 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid had the least cytotoxicity against tenocytes. This system can be further applied using electrical, mechanical, or chemical stimuli (such as steroid or platelet-rich plasma) to determine the balance between cytotoxicity and anti-inflammatory effects before their clinical use. For example, studies have shown the protective effects of platelet-rich plasma on tenocytes against cytotoxicity of steroids.18,44,45 These in vitro results suggest that a combined treatment with a corticosteroid and platelet-rich plasma would have synergistic anti-inflammatory effects while avoiding the deleterious effects of a corticosteroid. However, the best treatment combination is still unknown.
There are certain limitations to our study. First, only tenocytes from 1 patient with grade 1 fatty infiltration were enrolled in this study. Advanced age, osteoarthritis, and other local and systemic pathological conditions could affect the quality of tendon tissue and the properties of tenocytes.46 On the other hand, Klatte-Schulz et al found reduced cell growth in the supraspinatus tenocytes of male donors above the age of 65 years when compared to those of younger patients.47 Matthew et al showed that the cuff tissue had the greatest potential for successful healing in smaller tears, whereas large to massive tears appeared to lose their ability to heal and demonstrated transition into a highly degenerative inert tissue.48 Therefore, the results of our study only represent the tenocyte interaction to different stimuli in a specific patient. The xCELLigence system provides the opportunity to use a relatively small amount of specimen to achieve this goal. Further studies should be focused on patients with different sizes of rotator cuff tear and severities of fatty infiltration. Second, different types of tendons and ligaments are clinically and biologically different. Ligaments have a higher DNA content, more cellular nuclei, greater amounts of reducible cross-links, and are composed of more type III collagen by percentage than tendons.49 The index study used only torn tendon from rotator cuff. Further studies should enroll tendon/ligaments from different body parts to evaluate their interaction between different drugs commonly used to relieve pain. Third, the in vitro effects cannot be translated to all the in vivo studies because of the many variables in a complex scenario, where interaction of tenocytes and drugs takes place in the human body. This study provided an in vitro platform to see the real-time proliferation of tenocytes under different stimuli. There was no need to sacrifice the cultured cells in the end of study. Hence, little amount of specimen was needed in this method compared to conventional ones. Fourth, the tenocyte culture was performed after and not before surgery, which made clinical preoperative application of this method impractical. For now, it is impractical that cell culture be performed before injection. However, once sufficient data are collected by the xCELLigence system in a relatively efficient way, personalized medicine in treating tendinopathies might be accomplished in determining the best drug combination for a specific condition in advance.
Conclusions
Our study utilized the ability of tenocytes to proliferate inside xCELLigence system. Using this system, we monitored the decrease in cytotoxic effects of anesthetics and NSAIDs upon ascorbic acid treatment in real time. The treatment combination with least cytotoxicity was screened using this platform. Our study can thus provide important information for clinical applications. Optimal tenocyte proliferation was observed in the 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid group. Bupivacaine 0.5% with 250 μg/mL ascorbic acid and 15 mg/mL ketorolac tromethamine with 250 μg/mL ascorbic acid showed least cytotoxicity against tenocytes.
Footnotes
Authors’ Note: CC designed the study, formulated the research goals, and wrote the article. PC performed the statistics and provided advice regarding further clinical applications. KFL provided the technical support for xCELLigence system. AC provided the study material. YC and KH provided their expertise with tenocyte proliferation. HR helped in performing the experiments. All authors have read and approved the final submitted manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully thank Taiwan Minister of Science and Technology and Linkou Chang Gung Memorial Hospital for their financial support (Grant: MOST 107-2314-B-182A-150-MY3, CORPG3G0032, CMRPG391572).
ORCID iD: Chih-Hao Chiu  https://orcid.org/0000-0001-9415-5115
https://orcid.org/0000-0001-9415-5115
References
- 1. Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61(1-2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahieu NN, Witvrouw E, Stevens V, Van Tiggelen D, Roget P. Intrinsic risk factors for the development of Achilles tendon overuse injury: a prospective study. Am J Sports Med. 2006;34(2):226–235. [DOI] [PubMed] [Google Scholar]
- 3. Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245–251. [DOI] [PubMed] [Google Scholar]
- 4. Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 5. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. [DOI] [PubMed] [Google Scholar]
- 6. Dean BJ, Gettings P, Dakin SG, Carr AJ. Are inflammatory cells increased in painful human tendinopathy? A systematic review. BMJ. 2016;50(4):216–220. [DOI] [PubMed] [Google Scholar]
- 7. Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085–2091. [DOI] [PubMed] [Google Scholar]
- 8. Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. 2013;87(7):486–490. [PubMed] [Google Scholar]
- 9. Fredriksson M, Li Y, Stalman A, Haldosen LA, Fellander-Tsai L. Diclofenac and triamcinolone acetonide impair tenocytic differentiation and promote adipocytic differentiation of mesenchymal stem cells. J Orthop Surg Res. 2013;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan KM, Maffulli N. Tendinopathy: an Achilles’ heel for athletes and clinicians. Clin J Sport Med. 1998;8(3):151–154. [PubMed] [Google Scholar]
- 11. Scherb MB, Han SH, Courneya JP, Guyton GP, Schon LC. Effect of bupivacaine on cultured tenocytes. Orthopedics. 2009;32(1):26. [DOI] [PubMed] [Google Scholar]
- 12. Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung CM, Hah YS, Kim JS, et al. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am J Sports Med. 2014;42(12):2888–2896. [DOI] [PubMed] [Google Scholar]
- 14. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietschmann MF, Wagenhauser MU, Gulecyuz MF, Ficklscherer A, Jansson V, Muller PE. The long head of the biceps tendon is a suitable cell source for tendon tissue regeneration. Arch Med Sci. 2014;10(3):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hakimi O, Poulsen R, Thakkar D, Yapp C, Carr A. Ascorbic acid is essential for significant collagen deposition by human tenocytes in vitro. Oxid Antioxid Med Sci. 2014;3(2):119–127. [Google Scholar]
- 17. Poulsen RC, Carr AJ, Hulley PA. Protection against glucocorticoid-induced damage in human tenocytes by modulation of ERK, Akt, and forkhead signaling. Endocrinology. 2011;152(2):503–514. [DOI] [PubMed] [Google Scholar]
- 18. Carofino B, Chowaniec DM, McCarthy MB, et al. Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: an in vitro study on human tendon cells. Arthroscopy. 2012;28(5):711–719. [DOI] [PubMed] [Google Scholar]
- 19. Kraus A, Woon C, Raghavan S, Megerle K, Pham H, Chang J. Co-culture of human adipose-derived stem cells with tenocytes increases proliferation and induces differentiation into a tenogenic lineage. Plast Reconstr Surg. 2013;132(5):754e–766e. [DOI] [PubMed] [Google Scholar]
- 20. Lei KF, Wu MH, Hsu CW, Chen YD. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens Bioelectron. 2014;51:16–21. [DOI] [PubMed] [Google Scholar]
- 21. Uzel SG, Pavesi A, Kamm RD. Microfabrication and microfluidics for muscle tissue models. Prog Biophys Mol Biol. 2014;115(2-3):279–293. [DOI] [PubMed] [Google Scholar]
- 22. Kho D, MacDonald C, Johnson R, et al. Application of xCELLigence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors (Basel). 2015;5(2):199–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiu CH, Lei KF, Yeh WL, et al. Comparison between xCELLigence biosensor technology and conventional cell culture system for real-time monitoring human tenocytes proliferation and drugs cytotoxicity screening. J Orthop Surg Res. 2017;12(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229–1240. [DOI] [PubMed] [Google Scholar]
- 25. Gerhardt C, Hug K, Pauly S, Marnitz T, Scheibel M. Arthroscopic single-row modified Mason-Allen repair versus double-row suture bridge reconstruction for supraspinatus tendon tears: a matched-pair analysis. Am J Sports Med. 2012;40(12):2777–2785. [DOI] [PubMed] [Google Scholar]
- 26. Ay S, Kucuk D, Gumus C, Kara MI. Distribution and absorption of local anesthetics in inferior alveolar nerve block: evaluation by magnetic resonance imaging. J Oral Maxillofac Surg. 2011;69(11):2722–2730. [DOI] [PubMed] [Google Scholar]
- 27. Diemert S, Dolga AM, Tobaben S, et al. Impedance measurement for real time detection of neuronal cell death. J Neurosci Meth. 2012;203(1):69–77. [DOI] [PubMed] [Google Scholar]
- 28. Dwane S, Durack E, Kiely PA. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res Notes. 2013;6:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Himmel HM. Drug-induced functional cardiotoxicity screening in stem cell-derived human and mouse cardiomyocytes: effects of reference compounds. J Pharmacol Toxicol Methods. 2013;68(1):97–111. [DOI] [PubMed] [Google Scholar]
- 30. Kramer AH, Joos-Vandewalle J, Edkins AL, Frost CL, Prinsloo E. Real-time monitoring of 3T3-L1 preadipocyte differentiation using a commercially available electric cell-substrate impedance sensor system. Biochem Biophys Res Commun. 2014;443(4):1245–1250. [DOI] [PubMed] [Google Scholar]
- 31. Wang T, Hu N, Cao J, Wu J, Su K, Wang P. A cardiomyocyte-based biosensor for antiarrhythmic drug evaluation by simultaneously monitoring cell growth and beating. Biosens Bioelectron. 2013;49:9–13. [DOI] [PubMed] [Google Scholar]
- 32. Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 33. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. [DOI] [PubMed] [Google Scholar]
- 34. Ker RF. The implications of the adaptable fatigue quality of tendons for their construction, repair and function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):987–1000. [DOI] [PubMed] [Google Scholar]
- 35. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35(8):1326–1333. [DOI] [PubMed] [Google Scholar]
- 36. Soreide E, Granan LP, Hjorthaug GA, Espehaug B, Dimmen S, Nordsletten L. The effect of limited perioperative nonsteroidal anti-inflammatory drugs on patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(12):3111–3118. [DOI] [PubMed] [Google Scholar]
- 37. Omeroglu S, Peker T, Turkozkan N, Omeroglu H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch Orthop Trauma Surg. 2009;129(2):281–286. [DOI] [PubMed] [Google Scholar]
- 38. Cooper JO, Bumgardner JD, Cole JA, Smith RA, Haggard WO. Co-cultured tissue-specific scaffolds for tendon/bone interface engineering. J Tissue Eng. 2014;5 doi:10.1177/2041731414542294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clement MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001;3(1):157–163. [DOI] [PubMed] [Google Scholar]
- 40. Manske PR, Lesker PA. Biochemical evidence of flexor tendon participation in the repair process – an in vitro study. J Hand Surg Br. 1984;9(2):117–120. [PubMed] [Google Scholar]
- 41. Han SH, An HJ, Song JY, et al. Effects of corticosteroid on the expressions of neuropeptide and cytokine mRNA and on tenocyte viability in lateral epicondylitis. J Inflamm (Lond). 2012;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klatte-Schulz F, Pauly S, Scheibel M, et al. Characteristics and stimulation potential with BMP-2 and BMP-7 of tenocyte-like cells isolated from the rotator cuff of female donors. PLoS One. 2013;8(6):e67209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dolkart O, Liron T, Chechik O, et al. Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway: an in vivo and in vitro study. Am J Sports Med. 2014;42(12):2869–2876. [DOI] [PubMed] [Google Scholar]
- 44. Beitzel K, McCarthy MB, Cote MP, et al. The effect of ketorolac tromethamine, methylprednisolone, and platelet-rich plasma on human chondrocyte and tenocyte viability. Arthroscopy. 2013;29(7):1164–1174. [DOI] [PubMed] [Google Scholar]
- 45. Jo CH, Lee SY, Yoon KS, Shin S. Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1beta-induced tendinopathic conditions. Am J Sports Med. 2017;45(5):1141–1150. [DOI] [PubMed] [Google Scholar]
- 46. Kothari P, Mohan N, Hunter JB, Kerslake R. Case report. Bilateral simultaneous patellar tendon ruptures associated with osteogenesis imperfecta. Ann R Coll Surg Engl. 1998;80(6):416–418. [PMC free article] [PubMed] [Google Scholar]
- 47. Klatte-Schulz F, Pauly S, Scheibel M, et al. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cell Mater. 2012;24:74–89. [DOI] [PubMed] [Google Scholar]
- 48. Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–495. [DOI] [PubMed] [Google Scholar]
- 49. Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–265. [DOI] [PubMed] [Google Scholar]