Abstract
Background
Olaratumab (LY3012207/IMC-3G3/Lartruvo™) is a fully human monoclonal antibody specific for platelet-derived growth factor receptor alpha (PDGFRα). Phase Ib/II trial results of olaratumab plus doxorubicin in adult patients with advanced soft tissue sarcoma (STS) supported accelerated FDA approval of this regimen. Radiation therapy (RT) is frequently used for high-risk localized STS. However, olaratumab has not been tested with concurrent RT. Here, we evaluate the chimeric anti-mouse PDGFRα antibody 1E10Fc as a radiosensitizer in a primary mouse model of STS.
Methods
Primary STS were initiated in mice. When tumors reached 70 mm3, mice were allocated into treatment groups: 1) isotype, 2) 1E10Fc, 3) isotype + RT, 4) 1E10Fc + RT. 1E10Fc or isotype was given biweekly. RT (25 Gy delivered in 5 daily 5 Gy fractions) was initiated on Day 0 with first drug treatment. Tumors were measured 3× per week. Upon reaching 900 mm3, tumors and lungs were harvested. A two-way ANOVA was performed to compare tumor growth delay. Primary tumors were stained for CD31 and PDGFRα and lungs were assessed for micrometastases. A Chi-square test was performed to compare the development of micrometastases in the lungs after treatment with 1E10Fc or isotype.
Findings
RT significantly delayed time to tumor quintupling compared to no RT (p < 0·0001) [two-way ANOVA], but no difference in tumor growth was seen between mice receiving isotype or 1E10Fc treatment regardless of concurrent RT. Lower microvessel density was observed in the 1E10Fc + RT group. Fewer mice treated with 1E10Fc had micrometastases, but this difference was not statistically significant (p < 0·09).
Interpretation
1E10Fc did not act as a radiosensitizer in this primary STS model.
Funding
This study was funded by a research agreement from Eli Lilly and Company.
Keywords: Olaratumab, PDGFRα, Radiation, Soft tissue sarcoma
Research in context.
Evidence before this study
We used the PubMed database to consider the existing evidence of pro-oncogenic role of the PDGFRα pathway and its involvement in the response to radiation therapy and of olaratumab as an inhibitor of the PDGFRα pathway. We included studies with the search words PDGFRα or olaratumab from 1933 to June 2018 and excluded any studies discussing the PDGFRα pathway outside of oncogenesis. We focused particularly on the phase I/IIb study by Tap and colleagues in which the combination of olaratumab and doxorubicin improved overall survival compared with doxorubicin alone and additional publications referencing this study [1],
Added value of this study
To our knowledge, this work is the first to address whether targeting PDGFRα radiosensitizes primary sarcomas in vivo.
Implications of all the available evidence
The chimeric anti-murine PDGFRα antibody 1E10Fc did not act as a radiosensitizer in this study. Although we observed decreased microvessel density with combination treatment with 1E10Fc and radiation therapy, tumor growth was not affected by this treatment combination. Metastasis was not the primary endpoint of this study; however, fewer mice treated with 1E10Fc developed micrometastases in the lung (p = 0·09) [Chi-square test], which supports future pre-clinical studies targeting PDGFRα as adjuvant therapy for soft tissue sarcomas.
Alt-text: Unlabelled Box
1. Introduction
Soft tissue sarcomas (STS) are diverse mesenchymal neoplasms that can arise from nearly any site within the body. STS include a heterogeneous group of malignancies with over 50 subtypes that differ in molecular, histological, and clinical characteristics [2]. Among high grade STS, the most common subtypes include undifferentiated polymorphic sarcoma (UPS), liposarcoma, leiomyosarcoma, and synovial sarcoma [3]. Collectively, STS are rare and account for <1% of adult cancers, although they are among the five most common causes of cancer-related deaths in the young adult and pediatric populations [3]. Most adult STS patients are diagnosed with localized disease and effective local control can be achieved in approximately 90% of patients with surgery and radiation therapy [4]. However, 10–15% of patients initially present with metastatic disease [5], and up to 50% of patients with large (>10 cm) high grade STS eventually develop metastases despite local tumor control [3]. Controversy exists regarding the value of currently available treatments, such as chemotherapy, to reduce the rate of metastasis [6]. Prognosis for metastatic STS is poor, with overall survival of approximately 18 months [7] and estimated five-year survival of 16% [8].
Management of STS requires a multidisciplinary approach which can include surgery, chemotherapy, and radiation therapy (RT). For patients with low-risk disease and relatively low probability of metastatic spread, local resection (often in conjunction with RT) is largely efficacious as reflected in the high five-year survival rate of 83% [8]. In contrast, patients with high-risk disease generally have poorer survival, despite effective local control. Doxorubicin has remained the cornerstone of chemotherapy regimens for advanced and metastatic STS both as monotherapy and in conjunction with other agents since 1975, the year in which its efficacy was first demonstrated [9], despite the heterogeneity across STS subtypes, presentations, and other patient characteristics [8]. Several other drugs have been combined with doxorubicin, including ifosfamide analogues like palifosfamide and evofosfamide and targeted agents such as bevacizumab and cixutumumab. However, these approaches have not improved overall survival over single agent doxorubicin [8].
Olaratumab (Lartruvo™) is a first-in-class fully human immunoglobulin G subclass 1 (IgG1) monoclonal antibody that binds to PDGFRα, effectively preventing PDGF-AA, PDGF-BB, and PDGF-CC from activating the receptor [10]. A phase Ib/II study comparing doxorubicin monotherapy to doxorubicin plus olaratumab followed by olaratumab monotherapy for unresectable or metastatic STS showed an overall survival benefit of 11·8 months (HR 0·46, 95% CI 0·30–0·71, p = 0·0003) with combination therapy [1]. Following this pivotal study, olaratumab in combination with doxorubicin was granted accelerated Food and Drug Administration (FDA) approval for advanced STS patients in October 2016 and conditional approval in the European Union shortly thereafter. A confirmatory phase III study (ANNOUNCE, NCT02451943) is a randomized, double-blinded, placebo-controlled, international trial evaluating the safety and efficacy of doxorubicin plus olaratumab; it has now completed accrual of over 460 STS patients with results expected in 2019.
In addition to its role in oncogenesis, the PDGF pathway is important for angiogenesis and fibrogenesis; these two processes are particularly relevant in the context of RT. Furthermore, PDGF-mediated increases in cellular proliferation, migration, and survival are crucial mechanisms for tumor resistance to RT and chemotherapy [11]. Therefore, the rationale for targeting PDGF signaling in combination with RT is multifold: potential direct anti-tumor cell effect, anti-angiogenic activity, and an anti-fibrotic mechanism that may decrease radiation-induced normal tissue toxicity. Indeed, a previous preclinical study demonstrated that combining PDGFR inhibitor with radiation reduced RT-mediated lung fibrosis [12]. However, the potential for olaratumab to act as a radiosensitizer has yet to be examined, despite the frequent use of adjuvant RT in high risk STS. The chimeric anti-mouse PDGFRα antibody 1E10Fc has been developed for use in preclinical murine studies. In this project, we use a genetically engineered and carcinogen-induced mouse model of undifferentiated pleomorphic sarcoma (UPS), the most common type of adult soft tissue sarcoma, to investigate the potential effects of 1E10Fc and RT on primary tumors.
2. Methods and materials
2.1. Mouse studies
Six to eight week-old male and female p53FRT/FRT mice [13] on a mixed 129Sv/Jae and C57BL/6J background were used in a protocol approved by the Duke University Institutional Animal Care and Use Committee (IACUC). As previously described [13], these mice were genetically engineered with FRT sites flanking exons 2–6 of the Trp53 gene so that FLP recombinase (flippase) recombines the FRT sites to delete both alleles of the Trp53 gene. Twenty-four hours after delivering FLP recombinase into the gastrocnemius muscle, mice were injected with 0·3 mg of 3-methylcholanthrene (MCA) (Sigma-Aldrich, Saint Louis, MO) at the same site, which results in temporally and spatially-controlled p53/MCA primary sarcomas at the site of injection within 6 to 10 weeks (Lee CL, Daniel AR, Mowery YM, et al., Manuscript in Preparation).
Mice were assessed twice weekly for new tumors. When tumors were detected, they were measured three times per week to assess tumor growth using the following formula:
When tumors reached ~70 mm3 (or 5 × 5 mm, Day 0), mice were divided into treatment groups of 1) isotype control, 2) 1E10Fc, 3) isotype + RT, or 4) 1E10Fc + RT. Pharmacological treatment of 1E10Fc or isotype IgG control was given biweekly at a dose of 20 mg/kg by intraperitoneal injection. Treatments of 1E10Fc or isotype continued biweekly until the tumor reached an endpoint volume of ~900 mm3 (or 12 × 12 mm). On the first day of 1E10Fc treatment (Day 0), mice in the RT groups began a five-fraction image-guided RT protocol to the tumor where clinically relevant 5 Gy fractions were administered daily for five consecutive days (25 Gy total) [14]. Mice were anesthetized using isoflurane gas and the tumor was localized within the radiation field with a source-to-subject distance of 30·76 cm using fluoroscopy at 40 kVp and 2·5 mA with a 2 mm Al filter. QC dosimetry studies confirmed that the RT dose administered during the fluoroscopy session was negligible (6–12 cGy). The target area was irradiated using opposed AP/PA beams at a dose rate of 300 cGy/min at target depth with 225 kVp and 13 mA and a 0·3 mm Cu filter. Upon reaching endpoint volume, mice were euthanized by CO2. Tumors and lungs inflated with 10% formalin were fixed in 10% formalin followed by 70% ethanol for subsequent histological evaluation.
2.2. Establishment of primary cell lines from p53/MCA tumors
Fragments of viable tumor tissue were digested in tumor dissociation buffer (PBS with Ca++/Mg++, 5 mg/mL collagenase IV, 1·3 mg/mL dispase, 0·05% trypsin) for 1 h, then washed twice with PBS (ThermoFisher) and strained through a 40 μm Corning mesh cell strainer (ThermoFisher). Cells were passaged at least five times to eliminate stromal content before FRT recombination and p53 deletion was tested via PCR genotyping of genomic DNA (primers for unrecombined p53 FRT: 5’-CAA GAG AAC TGT GCC TAA GAG -3′ and 5’-CTT TCT AAC AGC AAA GGC AAG C-3′; primers for recombined p53 FRT: 5’-CAA GAG AAC TGT GCC TAA GAG-3′ and 5’-ACT CGT GGA ACA GAA ACA GGC AGA-3′; annealing temperature 55 °C).
2.3. Western blot analysis
For in vitro analysis of 1E10Fc activity, cells were plated and incubated in 10 mL serum-free media (Gibco) overnight. Cells were then treated with 1 μM 1E10Fc or isotype control antibody for 15 min, followed by activation with PDGF-AA (1 nM, ThermoFisher) for an additional 15 min. Cells were washed with PBS and scraped in the following buffer for lysis: RIPA buffer (Sigma) containing cOmplete™ Protease Inhibitor Cocktail (Roche), PhosSTOP™ phosphatase inhibitor tablet (Roche), aprotinin (Sigma), and PMSF (Sigma). Lysates were also obtained from the homogenized tumor samples. Odyssey® Blocking Buffer (LiCor) in TBS was used for blocking and as diluent for the antibodies. Samples were run on Mini-PROTEAN® TGX™ Precast Gels (BioRad) at 100 V for 1.5 h and transferred to nitrocellulose membrane (ThermoFisher) via a wet transfer at 250 mV for 2 h. Membranes were blotted for expression of phosphorylated (1:2000, Cell Signaling #4060) and total AKT (1:1000, Cell Signaling #9272), a downstream target of PDGFRα signaling. GAPDH was used as the loading control (1:10,000, Proteintech #60004-1-Ig). IRDye® 800CW goat anti-mouse (1:10,000, LiCor # 925-32210) and IRDye® 680RD goat anti-rabbit (1,10,000, LiCor #925-68071) secondary antibodies were used. Blots were imaged and quantified on the Odyssey® CLx Imaging System (LiCor).
2.4. Histologic tumor analysis
Tumor samples were formalin-fixed and paraffin-embedded. 5 μm-thick sections were prepared. Immunohistochemical staining was used to assess for PDGFRα (1:250, Cell Signaling #3174) and CD31 (1:100, Cell Signaling #77699). Citric acid-based antigen unmasking solution (Vector Lab) was used. PBS with 0·3% Tween and 5% normal horse serum (Vector Lab) was used for blocking and as diluent for the primary and secondary antibodies. Slides were incubated in primary antibodies overnight and biotinylated secondary antibodies (1:200, Vector Lab #BA-1000) for 1 h. Expression was visualized with VECTASTAIN Elite ABC Reagent (Vector Lab) and 3,3′-Diaminobenzidine (DAB) solution (Vector Lab). Slides were counterstained with Mayer's hematoxylin (Sigma). For each mouse, four representative images were taken of the tumor if it occupied the majority of the slide (for PDGFRα staining: n = 19 for isotype, n = 19 for 1E10Fc, n = 20 for isotype + RT, and n = 18 for 1E10Fc + RT; for CD31 staining: n = 19 for isotype, n = 20 for 1E10Fc, n = 20 for isotype + RT, and n = 19 for 1E10Fc + RT). Each of the four images was quantified for fractional area positive for staining using ImageJ [15]. The four values were averaged for each mouse, and the averages were compiled for each treatment group. Experimenters were blinded to the treatment groups for staining and analysis.
2.5. Histologic lung analysis
Lungs were inflated, formalin-fixed, and paraffin-embedded. Three H&E sections were analyzed for each mouse, each taken 100 μm apart. The presence of micrometastases in each slide was determined by a sarcoma pathologist (DMC) at Duke University Hospital, who was blinded to treatment.
2.6. Statistical analysis
Statistical analysis was conducted with GraphPad Prism7. A two-way ANOVA was performed to compare tumor growth delay in the four treatment groups. A one-way ANOVA was performed to compare differences in groups (time to tumor onset, tumor size at distribution to treatment groups, time to quintupling by gender). A Tukey's multiple comparisons test was used to determine differences between each treatment group in tumor growth delay (after ANOVA). A paired t-test was used to compare the ratios of protein expression in western blotting, and one-way ANOVA was used to compare groups for immunohistochemical staining. A Chi-square test was used to determine whether the presence or absence of metastasis varied among treatment groups, and to compare the groups with and without 1E10Fc for the development of lung metastases.
2.7. Ethics
All animal protocols underwent ethical review by Duke University's Institutional Animal Care and Use Committee (IACUC). The tumor generation and radiation procedures described herein are all included on the approved protocol A053-15-03. An amendment was submitted and approved to specifically gain approval of 1E10Fc and isotype control antibodies.
3. Results
3.1. p53/MCA sarcomas resemble human UPS
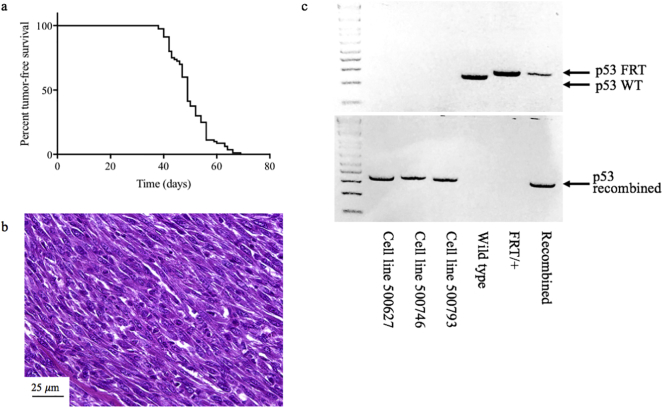
To study the combination of 1E10Fc and radiation therapy (RT) in primary STS, we first generated a cohort of mice with p53/MCA tumors. As shown in Fig. 1a, the average time to p53/MCA tumor onset was 50 days (median 49 days, range 38–69 days). Histological staining of tumors from this mouse model reveals highly pleomorphic spindle cells (Fig. 1b) similar in appearance to human UPS. Genotyping confirmed the recombination of FRT sites and deletion of p53 in each of the three cell lines derived from these tumors (Fig. 1c).
Fig. 1.
p53/MCA sarcomas arise in an average of 50 days and resemble human UPS. (a) Percent tumor-free survival (n = 80) in number of days following intramuscular delivery of flippase to delete both p53 alleles and 3-methylcholanthrene carcinogen. (b) H&E stain of a tumor from the p53/MCA model reveals highly pleomorphic spindle cells similar to human undifferentiated pleomorphic sarcoma. (c) Genotyping using primers for FRT and recombined p53 FRT sites in the cell lines generated from the primary p53/MCA sarcoma model (500627, 500746, 500693) confirms the recombination of FRT sites in all three cell lines with deletion of p53.
3.2. 1E10Fc inhibits PDGFRα signaling in primary mouse sarcoma cell lines
To test whether 1E10Fc was indeed inhibiting PDGFRα signaling in the p53/MCA sarcoma model, western blot analysis was performed on cell lines generated from three primary p53/MCA sarcomas. AKT phosphorylation was assessed as a downstream surrogate for active PDGFRα signaling (Supplemental Fig. 1). The ratio of phosphorylated to total AKT was lower in all three cell lines treated with 1E10Fc compared to isotype control (p = 0·0206) [paired t-test], suggesting that 1E10Fc is effectively inhibiting PDGFRα signaling in p53/MCA sarcoma cells in vitro.
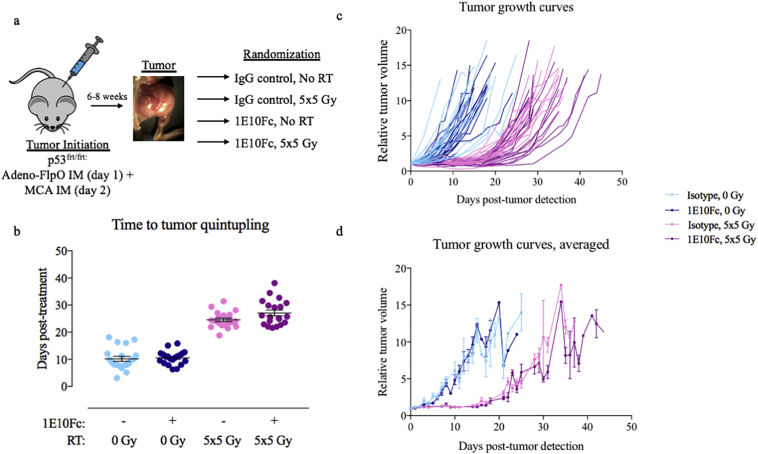
3.3. 1E10Fc does not affect tumor growth alone or in combination with RT
To determine the effects of 1E10Fc on murine primary tumor growth alone or in combination with RT, time to volume quintupling (TTQ) from initial tumor volume was compared across mice treated with isotype control, 1E10Fc, isotype + RT, or 1E10Fc + RT (Fig. 2a). While TTQ was significantly longer in mice receiving RT (p < 0·0001) compared to non-RT groups (Fig. 2b) [two-way ANOVA], no differences in TTQ were observed in mice treated with isotype and 1E10Fc groups even with the addition of RT. The delay in tumor growth achieved with RT, but not 1E10Fc, was further illustrated by comparing treatment groups throughout the duration of the study (Fig. 2c–d). To ensure that allocation to treatment groups eliminated any differences in tumor characteristics and gender that may affect outcomes, TTQ results were analyzed by time to tumor onset (Supplemental Fig. 2a), tumor size on the day of distribution to treatment group (Supplemental Fig. 2b), and gender (Supplemental Fig. 2c) with no significant differences observed in any of these groups. The two outliers seen in Supplemental Fig. 2c (shown in gray) were excluded for repeat TTQ analysis (Supplemental Fig. 2d), confirming no difference in TTQ between RT and non-RT groups.
Fig. 2.
1E10Fc treatment does not improve tumor growth delay with RT. (a) Schematic of the study design. (b) Time to tumor quintupling (TTQ) for each treatment group. Two-way ANOVA demonstrates a statistically significant difference in TTQ between the RT and non-RT groups (p < 0·0001) while no significant TTQ is seen between the isotype and 1E10Fc groups regardless of RT. A Tukey's multiple comparisons test was then used to look at differences among the four groups. The difference between groups was not statistically significant. Error bars represent SEM. (c) Individual growth curves and (d) averaged growth curves for each treatment group (error bars represent SEM) demonstrate that 1E10Fc does not affect primary tumor growth delay, but RT significantly delays time to tumor volume quintupling.
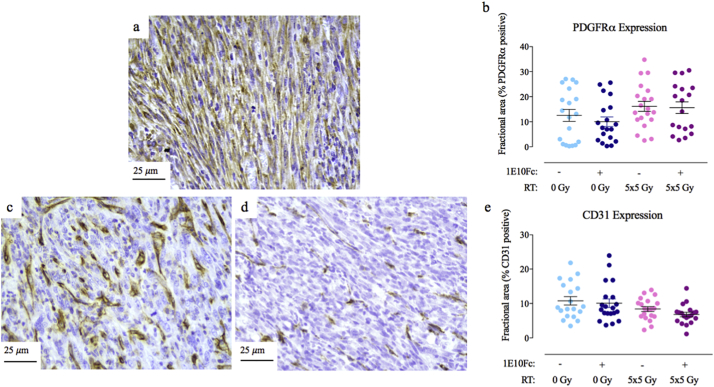
3.4. No difference in PDGFRα expression between treatment groups via immunohistochemistry
To determine whether expression levels of PDGFRα changed with treatment, immunohistochemistry for PDGFRα was performed. An example of the PDGFRα pattern of staining in primary p53/MCA tumors is shown in Fig. 3a. No difference was seen in PDGFRα expression between the treatment groups (n = 19 for isotype, 19 for 1E10Fc, 20 for isotype + RT, and 18 for 1E10Fc + RT) (Fig. 3b).
Fig. 3.
Immunohistochemistry for PDGFRα shows no differences between treatment groups, while CD31 expression is lower in tumors treated with 1E10Fc + RT. (a) Example of PDGFRα staining pattern at 40×. (b) PDGFRα expression by immunohistochemistry (as fractional area positive for staining) is not different between treatment groups. Error bars represent SEM. (c) Example of high CD31 staining pattern at 40×. (d) Example of low CD31 staining pattern at 40×. (e) CD31 expression by immunohistochemistry demonstrates slightly lower expression in tumors treated with 1E10Fc + RT compared to the isotype alone group, which suggests a lower microvessel density in these tumors. Error bars represent SEM.
3.5. CD31 expression is lower in tumors treated with 1E10Fc + RT via immunohistochemistry
As the PDGFRα pathway is involved in angiogenesis, CD31 was evaluated by immunohistochemistry to assess vasculature in tumors from mice treated with the isotype control or 1E10Fc. An example of the CD31 staining pattern in p53/MCA sarcoma tumors is shown in Fig. 3c-d. Fig. 3c demonstrates a sample from the isotype alone group, while Fig. 3d demonstrates a sample from the 1E10Fc + RT group. Lower CD31 expression was observed in tumors treated with 1E10Fc + RT as compared to isotype alone (p = 0·03) [one-way ANOVA], but not compared to RT + isotype (n = 19 for isotype, 20 for 1E10Fc, 20 for isotype + RT, and 19 for 1E10Fc + RT) (Fig. 3e). This suggests a lower microvessel density in tumors treated with 1E10Fc + RT, though this did not affect primary tumor growth compared to RT alone.
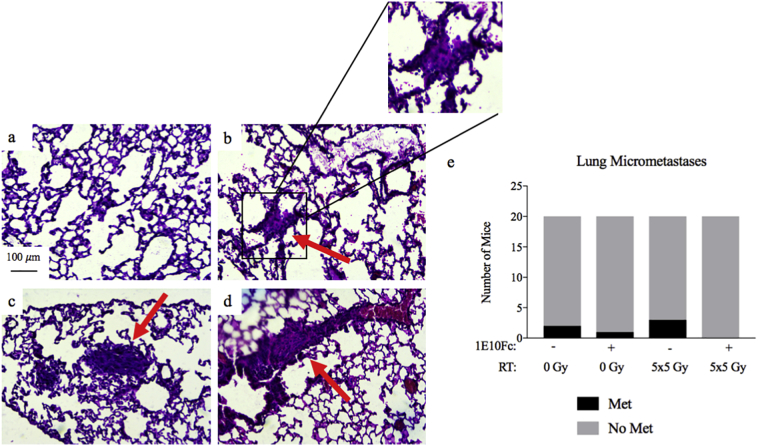
3.6. Histological analysis of lungs for micrometastases
Analysis of lung samples revealed lesions consistent with micrometastases in six mice, with examples of histology presented in Fig. 4a-d. Two of these lesions were in mice treated with isotype alone (n = 20), three in mice treated with isotype + RT (n = 20), one in mice treated with 1E10Fc alone group (n = 20), and none in mice treated with 1E10Fc + RT (n = 20) (Fig. 4e). Using a Chi-square test, there is no statistically significant difference in the presence vs. absence of micrometastatic lesions between the 4 treatment groups. When the mice are grouped by treatment with 1E10Fc (n = 40) vs isotype antibody (n = 40), more mice treated with isotype antibody developed micrometastases (5 vs. 1), but this difference did not reach statistical significance (p = 0·0895) [Chi-square test].
Fig. 4.
Histological analysis of lungs for micrometastases. (a) Example of normal lung architecture at 10×. (b–d) Examples of micrometastases identified by a sarcoma pathologist blinded to treatment group. (e) Quantification of lung metastases in each treatment group (n = 20/group). Rate of micrometastases trends lower in mice treated with 1E10Fc (p = 0·0895) [Chi-square test].
4. Discussion
This study examined the effects of the chimeric anti-mouse PDGFRα antibody 1E10Fc alone or in combination with fractionated RT in a primary mouse model of STS. While 1E10Fc appeared to inhibit PDGFRα signaling using AKT phosphorylation as a surrogate marker, blocking PDGFRα did not act as a radiosensitizer in this model of primary STS as measured by tumor growth delay. Immunohistochemical analysis of primary murine sarcomas taken at endpoint volume demonstrated a trend toward decreased microvessel density in the tumors treated with 1E10Fc + RT compared to isotype as shown by a reduction in CD31 staining, which provides evidence of 1E10Fc activity in vivo despite the lack of effect on primary sarcoma growth.
One limitation of extrapolating our results in a primary mouse model of soft tissue sarcoma to the human disease is that human sarcoma cells and stroma may behave differently to inhibition of PDGFRα. The 1E10Fc antibody specifically targets mouse PDGFRα and use of this primary mouse sarcoma model allowed us to test potential radiosensitization of sarcomas by inhibition of PDGFRα in the stroma as well as the tumor cells directly. Moreover, using a spatially- and temporally-restricted autochthonous model of soft tissue sarcoma rather than a xenograft in immunodeficient mice enabled us to evaluate 1E10Fc in an immunocompetent setting that best attempts to recapitulate the characteristics and behavior of human UPS, including tumor physiology and microenvironment and the potential for metastatic spread [16].
While p53 is the most commonly mutated gene in human UPS [17], sarcomas that retain p53 (i.e. UPS as well as other soft tissue sarcoma subtypes) may respond differently to PDGFRα inhibition. Accordingly, because p53 is so frequently mutated in UPS and in other common soft tissue sarcoma subtypes, this primary mouse model of soft tissue sarcoma is an appropriate choice for pre-clinical testing of radiation therapy with inhibition of PDGFRα [17].
Furthermore, the relatively large sample size in each treatment group (powered to detect a biologically relevant but small difference in tumor growth delay after RT), the use of clinically relevant sophisticated small animal irradiation [18], and blinding of the experimenter to treatment groups allowed for detailed and unbiased analysis of the combination of 1E10Fc and RT for treatment of primary sarcomas. Only 1 of 40 mice treated with 1E10Fc had lesions in the lungs suspicious for micrometastatic disease. In contrast, 5 of 40 mice treated with isotype control antibody developed micrometastases in the lung (p = 0·0895) [Chi-square test]. While our study was not designed to assess for metastasis specifically, our data suggest that neoadjuvant 1E10Fc may delay or prevent the development of lung metastases. A better experimental design to compare rates of metastasis between treatment groups would require achieving local control of the primary site with surgery so that mice could be followed for up to six months when the presence or absence of gross metastasis can be determined unambiguously [16,19]. Although beyond the scope of the present study, in future pre-clinical studies it would be interesting to formally test whether neoadjuvant or adjuvant 1E10Fc affects the development and growth of metastasis after surgery achieves local control.
It is noteworthy that in the phase Ib/II clinical trial of olaratumab and doxorubicin by Tap and colleagues [1], median progression-free survival (PFS) using p = 0·05 for significance was not significantly improved with olaratumab and doxorubicin compared with doxorubicin alone (HR 0·67, 95% CI 0·44–1·02, p = 0·0615) despite a significant improvement in median overall survival (OS) favoring combination therapy. Some have criticized this study because OS is a multifactorial endpoint which can be influenced by all subsequent treatment administered, and the effect of the study treatment becomes more challenging to define [9,20]. While the ANNOUNCE confirmatory phase III trial of olaratumab and doxorubicin is proceeding, it is worthwhile to consider potential mechanisms for the differences observed between the impact of combination therapy on PFS and OS. For example, the difference may be due to tumor cell autonomous effects [9] or due to alterations in the tumor-associated stroma that improve access to immune cells [21]. Indeed, the efficacy of olaratumab may not be due to targeting tumor PDGFRα expression [21] as a post-hoc analysis of the phase Ib/II clinical trial [1] found that PDGFRα expression in the tumors did not correlate with outcomes. Future studies are warranted to investigate the mechanisms by which olaratumab improves overall survival.
In conclusion, 1E10Fc did not act as a radiosensitizer in this mouse model of primary soft tissue sarcoma. Although microvessel density was decreased in tumors treated with 1E10Fc + RT, this did not affect primary tumor growth. Finally, we performed a histologic analysis of the lungs which suggested a potential decrease in micrometastases in mice treated with neoadjuvant 1E10Fc. Although this result did not reach statistical significance and our study was not powered to assess metastasis specifically, this finding provides rationale to undertake additional preclinical studies of neoadjuvant 1E10Fc to define the impact of blocking PDGFRα on lung metastasis.
Declaration of interests
Declaration of Interests: DGK reports a grant from Eli Lilly and Company, during the conduct of the study. DGK reports stocks, patents, and royalties from Lumicell, as well as grants from Lumicell, Xrad therapeutics, Merck, and Bristol Myers Squibb outside the submitted work. In addition, DGK has a patent on an imaging device licensed to Lumicell and a patent pending on radiosensitizers. CDL and LS are full-time employees of Eli Lilly and Company. LS is a Lilly shareholder. All other authors have nothing to disclose.
Authors contribution
EJS, KAA, CDL, LS, and DGK designed the study. EJS and KAA carried out the study and contributed to the statistical analysis. DGK supervised the data acquirement. LL and YM contributed to work with mice and lab support. LDSC contributed to radiation of the tumors. EJS, KAA, CDL, LS, DGK, and YMM contributed to the concept and interpretation of the data. EJS, KAA, YMM, CDL, and DGK contributed to the preparation of manuscript. All authors made a contribution in the revision of the manuscript.
Acknowledgements
We would like to thank all members of the Kirsch lab for their support, especially Amy Wisdom and Cierra Hong. This study was funded by a research agreement from Eli Lilly and Company to DGK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.046.
Appendix A. Supplementary data
Supplementary material
References
- 1.Tap W.D., Jones R.L., Van Tine B.A., Chmielowski B., Elias A.D., Adkins D. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano K., Takahashi S. Current molecular targeted therapies for bone and soft tissue sarcomas. Int J Mol Sci. 2018;19(3) doi: 10.3390/ijms19030739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.In G.K., Hu J.S., Tseng W.W. Treatment of advanced, metastatic soft tissue sarcoma: latest evidence and clinical considerations. Ther Adv Med Oncol. 2017;9(8):533–550. doi: 10.1177/1758834017712963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoski C., Suit H.D., Rosenberg A., Mankin H., Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52(4):223–230. doi: 10.1002/jso.2930520405. [DOI] [PubMed] [Google Scholar]
- 5.Vos M., Sleijfer S. EJC's biennial report on metastatic soft tissue sarcoma: State of the art and future perspectives. Eur J Cancer. 2018;88:87–91. doi: 10.1016/j.ejca.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Loong H.H., Wong K.H., Tse T. Controversies and consensus of neoadjuvant chemotherapy in soft-tissue sarcomas. ESMO Open. 2018;3(Suppl. 1) doi: 10.1136/esmoopen-2017-000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirley M. Olaratumab: first global approval. Drugs. 2017;77(1):107–112. doi: 10.1007/s40265-016-0680-2. [DOI] [PubMed] [Google Scholar]
- 8.Andrick B.J., Gandhi A. Olaratumab: a novel platelet-derived growth factor receptor alpha-inhibitor for advanced soft tissue sarcoma. Ann Pharmacother. 2017;51(12):1090–1098. doi: 10.1177/1060028017723935. [DOI] [PubMed] [Google Scholar]
- 9.Moroncini G., Maccaroni E., Fiordoliva I., Pellei C., Gabrielli A., Berardi R. Developments in the management of advanced soft-tissue sarcoma - olaratumab in context. Onco Targets Ther. 2018;11:833–842. doi: 10.2147/OTT.S127609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis E.J., Chugh R. Spotlight on olaratumab in the treatment of soft-tissue sarcoma: design, development, and place in therapy. Drug Des Devel Ther. 2017;11:3579–3587. doi: 10.2147/DDDT.S121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M., Jendrossek V., Belka C. The role of PDGF in radiation oncology. Radiat Oncol. 2007;2:5. doi: 10.1186/1748-717X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dadrich M., Nicolay N.H., Flechsig P., Bickelhaupt S., Hoeltgen L., Roeder F. Combined inhibition of TGFbeta and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. 2016;5(5) doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C.L., Moding E.J., Huang X., Li Y., Woodlief L.Z., Rodrigues R.C. Generation of primary tumors with Flp recombinase in FRT-flanked p53 mice. Dis Model Mech. 2012;5(3):397–402. doi: 10.1242/dmm.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosela-Paterczyk H., Szacht M., Morysinski T., Lugowska I., Dziewirski W., Falkowski S. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol. 2014;40(12):1641–1647. doi: 10.1016/j.ejso.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachdeva M., Mito J.K., Lee C.L., Zhang M., Li Z., Dodd R.D. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J Clin Invest. 2014;124(10):4305–4319. doi: 10.1172/JCI77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Electronic address edsc, Cancer Genome Atlas Research N Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4) doi: 10.1016/j.cell.2017.10.014. (950-65 e28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton J., Oldham M., Thomas A., Li Y., Adamovics J., Kirsch D.G. Commissioning a small-field biological irradiator using point, 2D, and 3D dosimetry techniques. Med Phys. 2011;38(12):6754–6762. doi: 10.1118/1.3663675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisinger-Mathason T.S., Zhang M., Qiu Q., Skuli N., Nakazawa M.S., Karakasheva T. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3(10):1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Graaf W.T.A. Olaratumab in soft-tissue sarcomas. Lancet. 2016;388(10043):442–444. doi: 10.1016/S0140-6736(16)30788-7. [DOI] [PubMed] [Google Scholar]
- 21.Vincenzi B., Badalamenti G., Napolitano A., Spalato Ceruso M., Pantano F., Grignani G. Olaratumab: PDGFR-alpha inhibition as a novel tool in the treatment of advanced soft tissue sarcomas. Crit Rev Oncol Hematol. 2017;118:1–6. doi: 10.1016/j.critrevonc.2017.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material