Abstract
Background
Cytosolic 5′-nucleotidase 1A (NT5C1A) dephosphorylates non-cyclic nucleoside monophosphates to produce nucleosides and inorganic phosphates. Here, we investigate NT5C1A expression in pancreatic ductal adenocarcinoma (PDAC) and its impact on gemcitabine metabolism and therapeutic efficacy.
Methods
NT5C1A expression was determined by semiquantitative immunohistochemistry using tissue microarrays. Gemcitabine metabolites and response were assessed in several human and murine PDAC cell lines using crystal violet assays, Western blot, viability assays, and liquid chromatography tandem mass-spectrometry (LC-MS/MS).
Findings
NT5C1A was strongly expressed in tumor cells of a large subgroup of resected PDAC patients in two independent patient cohorts (44–56% score 2 and 8–26% score 3, n = 414). In contrast, NT5C1A was expressed at very low levels in the tumor stroma, and neither stromal nor tumoral expression was a prognostic marker for postoperative survival. In vitro, NT5C1A overexpression increased gemcitabine resistance by reducing apoptosis levels and significantly decreased intracellular amounts of cytotoxic dFdCTP in +NT5C1A tumor cells. Co-culture experiments with conditioned media from +NT5C1A PSCs improved gemcitabine efficacy in tumor cells. In vivo, therapeutic efficacy of gemcitabine was significantly decreased and serum levels of the inactive gemcitabine metabolite dFdU significantly increased in mice bearing NT5C1A overexpressing tumors.
Interpretation
NT5C1A is robustly expressed in tumor cells of resected PDAC patients. Moreover, NT5C1A mediates gemcitabine resistance by decreasing the amount of intracellular dFdCTP, leading to reduced tumor cell apoptosis and larger pancreatic tumors in mice. Further studies should clarify the role of NT5C1A as novel predictor for gemcitabine treatment response in patients with PDAC.
Keywords: Cytosolic 5′-nucleotidase 1A, Chemotherapeutic resistance, Gemcitabine, Pancreatic cancer
Research in context.
Evidence before this study
Pancreatic ductal adenocarcinoma is highly refractory to systemic therapies. Poor therapeutic response to gemcitabine in PDAC has been attributed in part to altered drug metabolism, in particular reduced cellular uptake or rapid enzymatic inactivation. Indeed, high expression of the human equilibrative nucleoside transporter (hENT) has been shown to predict improved patient survival following gemcitabine treatment.
The 5′-nucleotidase (NT5) family of enzymes dephosphorylates non-cyclic nucleoside monophosphates to produce nucleosides and inorganic phosphates. Interestingly, NT5C1A is a previously unrecognized gemcitabine inactivating enzyme that prevents gemcitabine activation by dephosphorylating gemcitabine monophosphate (dFdCMP). However, the detailed expression pattern in PDAC patients and the role of NT5C1A in mediating resistance to gemcitabine is currently unknown.
Added value of this study
The present study provides evidence that NT5C1A is strongly expressed in tumor cells of resected PDAC patients. Mechanistically, we show that NT5C1A is implicated in gemcitabine resistance in vitro and in vivo by decreasing the amount of intracellular cytotoxic gemcitabine metabolites. Only a small fraction of PDAC patients show NT5C1A expression in the tumor stroma, and high expression in stromal cells may contribute to improved gemcitabine sensitivity in tumor cells.
Implications of all the available evidence
We have elucidated the expression pattern and function of NT5C1A in human and mouse PDAC using several in vitro and in vivo models. Thus, our study introduces NT5C1A as gemcitabine inactivating enzyme in PDAC and paves the way for the evaluation of NT5C1A for stratified treatment approaches, both for adjuvant as well as palliative therapy regimens.
Alt-text: Unlabelled Box
Abbreviations
| % | per cent |
| μm | micrometer |
| μM | micromolar |
| 5-FU | 5-fluorouracil |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| CAFs | cancer-associated fibroblasts |
| CC3 | cleaved caspase-3 |
| CDA | cytidine deaminase |
| CM | conditioned medium |
| d0 | day 0 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| dCK | deoxycytidine kinase |
| DCTD | deoxycytidylate deaminase |
| dFdC | 2′,2′-difluoro-2′-deoxycytidine |
| dFdCMP | 2′,2′-difluoro-2′-deoxycytidine monophosphate |
| dFdCDP | 2′,2′-difluoro-2′-deoxycytidine diphosphate |
| dFdCTP | 2′,2′-difluoro-2′-deoxycytidine triphosphate |
| dFdU | 2′,2′-difluoro-2′-deoxyuridine |
| DMEM | Dulbecco's Modified Eagle Medium |
| DNA | deoxyribonucleic acid |
| FBS | fetal bovine serum |
| g | gram(s) |
| h | hour(s) |
| H&E | hematoxylin and eosin |
| HA-tag | hemagglutinin tag |
| hEnt | human equilibrative nucleoside transporter |
| HRP | horseradish peroxidase |
| HSP90 | heat shock protein 90 |
| ICC | immunocytochemistry |
| IHC | immunohistochemistry |
| IgG | immunoglobulin G |
| kg | kilogram |
| KPC | LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre |
| LC-MS/MS | liquid chromatography tandem mass-spectrometry |
| MEM | Minimum Essential Media |
| mg | milligrams |
| M-MLV | Moloney Murine Leukemia Virus |
| mRNA | messenger ribonucleic acid |
| MTT | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| nM | nanomolar |
| ns | non-significant |
| NT5 | 5′-nucleotidase |
| NT5C1A | cytosolic 5′-nucleotidase 1A |
| PBS | phosphate buffered saline |
| PDAC | pancreatic ductal adenocarcinoma |
| pM | picomolar |
| PSCs | pancreatic stellate cells |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RRM1 | ribonucleotide reductase M1 |
| SEM | standard error of mean |
| TAM | tumor-associated macrophages |
| TMA | tissue microarray |
| TME | tumor microenvironment |
| U.S. | United States |
| WB | Western blot |
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive solid human tumors. Due to the lack of early symptoms, around 80% of patients are diagnosed with locally advanced or metastatic disease rendering them inoperable [[1], [2], [3]]. Current statistics project pancreatic cancer to become the second cause of cancer-related death in the U.S. by the year 2030 [4].
Systemic treatment options have been of limited success since PDAC is intrinsically highly refractory to medical therapies. Gemcitabine monotherapy has been the standard of care for palliative and adjuvant treatment for many years without providing clinically meaningful effects on overall survival [5]. More recently, intensified chemotherapy regimens have emerged that can improve overall survival. To this end, FOLFIRINOX (FOLinic acid, 5-FU, IRINotecan and OXaliplatin) and gemcitabine plus nab-paclitaxel are available in the palliative setting [6,7], and gemcitabine plus capecitabine (a 5-FU prodrug) was shown to increase long-term survival as adjuvant treatment compared to gemcitabine alone [8]. Nevertheless, therapy regimens like FOLFIRINOX require a good performance status of the patient due to increased toxicity [6]. However, despite the availability of intensified treatment protocols, the prognosis for PDAC patients remains extremely poor in comparison with other solid malignancies with median survival times below one year in patients with advanced or metastatic disease [9].
Poor therapeutic response to gemcitabine in PDAC has been attributed in part to altered drug metabolism, in particular, reduced cellular uptake or rapid enzymatic inactivation [[10], [11], [12], [13]]. Gemcitabine is a hydrophilic drug that is administered as prodrug (2′,2′-difluoro 2′-deoxycytidine; dFdC) and cellular uptake is predominantly accomplished by human equilibrative nucleoside transporters (hENT). Subsequently, the prodrug dFdC is phosphorylated to mono-, di- and tri-phosphate (dFdCMP, dFdCDP and dFdCTP), blocking de novo DNA synthesis by inhibition of ribonucleotide reductase (RRM), or terminating DNA chains through incorporation of dFdCTP into DNA [14]. The rate-limiting activation step is the phosphorylation by deoxycytidine kinase (dCK). Inactivation of gemcitabine and its metabolites is conferred by cytidine deaminase (CDA), and other enzymes such as deoxycytidylate deaminase (DCTD) [14,15]. Moreover, molecular competition through increased levels of deoxycytidine triphosphate further reduce the effective levels of gemcitabine [16].
The 5′-nucleotidase (NT5) family of enzymes dephosphorylates non-cyclic nucleoside monophosphates to produce nucleosides and inorganic phosphates. There are seven human NT5Cs of which cytosolic NT5C1A is mainly expressed in muscle and heart [17]. Under physiological conditions, NT5C1A regulates the pool of adenosine monophosphate (AMP), which is, in turn, responsible for allosterically stimulating AMPK activity [18,19]. In addition, NT5C1A is a previously unrecognized gemcitabine inactivating enzyme that dephosphorylates gemcitabine monophosphate (dFdCMP) to the prodrug dFdC, thus potentially limiting the cytotoxicity of gemcitabine by decreasing the formation of dFdCTP [[20], [21], [22]].
Notably, the role of nucleotidases that may mediate drug resistance through dephosphorylation of nucleoside analogues has not been investigated in PDAC so far. We have recently described differential expression of NT5C1A in human and murine stromal and epithelial cells for the first time in PDAC [23]. However, there are currently no data for PDAC or any other solid tumor that implicates NT5C1A in therapeutic resistance to gemcitabine. Here, we explored the expression pattern and frequency of NT5C1A in two large and independent cohorts of PDAC patients and determined the role of NT5C1A in mediating resistance to gemcitabine using several in vitro and in vivo approaches.
2. Materials and methods
2.1. Cell lines and cell culture
The LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC) mouse model is a commonly used genetically engineered mouse model for pancreatic cancer [24]. Thus, murine KPC cell lines were used for this study. These cells were derived from KPC mice as previously described [24]. KPC cells were maintained in culture according to standard procedures in high glucose DMEM with phenol red, supplemented with 10% fetal bovine serum (FBS) and 1% non-essential amino acids. MEM without phenol red, supplemented with 10% FBS and 1% l-glutamine was used for human L3.6pl cells. Immortalized murine PSCs from a previous study [23] were cultured in high glucose DMEM with phenol red and supplemented with 10% FBS.
Mycoplasma tests of KPC-BL6 cell lines and of L3.6pl cells were performed prior to orthotopic transplantation and liquid chromatography tandem mass-spectrometry (LC-MS/MS), respectively.
2.2. Establishment of cell lines stably expressing NT5C1A
KPC, L3.6pl cells, and PSCs were transfected with a pSG5-vector-derivative containing the sequence of murine NT5C1A or a control plasmid without the NT5C1A insert. We followed the protocol as described by Kari et al. [25] to generate the constructs. The pSG5-vector (a kind gift of Prof. Johnsen, University Medical Center Goettingen) containing an HA-tag upstream of a multiple cloning site, a P2A-sequence and a hygromycin resistance gene was transfected into each cell line as recently described by our group [23]. The following hygromycin concentrations (Hygromycin B Gold, InvivoGen) were used for selection: KPC1: 500 μg/ml, KPC2: 900 μg/ml, L3.6pl: 500 μg/ml, PSCs: 250 μg/ml. For maintenance, only half of these concentrations were added to the culture media.
2.3. Immunohistochemistry and immunocytochemistry
Mouse tissues were fixed in 10% neutral buffered formalin, dehydrated and embedded in paraffin. 4 μm sections were cut from the tissues and processed for H&E staining and immunohistochemistry using standard protocols as previously described [23]. Pictures were taken with 40× magnification with an Olympus DP27 camera and the Olympus cellSens Entry 1.12 software.
Immunocytochemistry was performed for KPC cell lines as previously described [23]. Briefly, cells were fixed with methanol, permeabilized using triton X-100 and incubated with primary antibodies for NT5C1A (Assay Biotech Cat# C15296, RRID:AB_10687827) and HA-tag (Cell Signaling Technology Cat# 3724, RRID:AB_1549585) at 4 °C overnight. Alexa Fluor®-conjugated secondary antibodies were used for detection and slides were mounted using DAPI-containing mounting solution (Vectashield®). Image acquisition was done utilizing a Leica DMi8 microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) and images were extracted with equal adjustments between groups using Fiji/ ImageJ (Fiji, RRID:SCR_002285) [26]. A detailed list of all antibodies and dilutions can be found in the supplementary materials.
2.4. Tissue microarrays
Tissue microarrays (TMA) were obtained from the Department of Pathology, University Medical Center Goettingen (TMA-1, n = 77 patients) and the Department of Pathology, University Medical Center Erlangen (TMA-2, n = 337 patients) from postoperative PDAC patients. Written consent was obtained from every patient prior to tissue collection and analysis. Expression levels of NT5C1A were semi-quantitatively analyzed by an experienced pathologist at each site from score 0 (no expression) to score 3 (strong expression). Immunohistochemistry was performed using standard protocols with hematoxylin counterstaining. TMA-1 staining was performed with citrate buffer pH 6.0, NT5C1A antibody (Assay Biotech Cat# C15296, RRID:AB_10687827, 1:100), and the VECTASTAIN® ABC Rabbit Kit with ImmPACT DAB Peroxidase Substrate Kit (Vector Laboratories). Pro Taqs II Antigen Enhancer pH 9.5, NT5C1A antibody (Atlas Antibodies Cat# HPA050283, RRID:AB_2681072, 1:100) and the ImmPress Reagent Kit Anti-Rabbit IgG (Vector Laboratories) with AEC-Plus Substrate-Chromogen (Dako Denmark A/S) were used for TMA-2.
2.5. Western blot analysis
Western blot analyses were performed as previously described with 35 μg of protein [23]. Membranes were incubated with chemiluminescence substrate Plus-ECL (PerkinElmer Inc.) or Ultra-ECL (for CC3-detection) and protein bands were detected at the ChemiDoc™ XRS+ imaging system (Bio-Rad Laboratories GmbH) using Image Lab Software (RRID:SCR_014210, version 5.2.1). Quantification was performed using the rectangle volume tool of the Image Lab Software. Values were normalized to HSP90 expression and to the expression of untreated protein lysates. Murine muscle lysate was used as positive control for NT5C1A expression (3 μg). A detailed list of primary and secondary antibodies is listed in supplementary materials.
2.6. Crystal violet cell proliferation assay
The commonly used crystal violet staining [27] was performed to determine the sensitivity of transfected cell lines towards gemcitabine and paclitaxel (both Sigma-Aldrich). To this end, cells were seeded at a density of 2000 cells (KPC cells, PSCs) and 7500 cells (L3.6pl) in 24-well plates and were allowed to attach for 24 h. Subsequently, cells were treated with increasing concentrations of gemcitabine-hydrochloride or paclitaxel, respectively. Cells were treated for six days and treatment media was renewed every other day. Finally, cells were washed with PBS, fixed with methanol and subsequently stained with crystal violet solution (0.1% in 20% ethanol). Regarding gemcitabine, three (tumor cell lines) and two (PSCs) independent experiments were performed, respectively. For paclitaxel treatment, two independent assays were carried out. Quantification of crystal violet assays was performed using 10% acetic acid to solubilize crystal violet stain and subsequent photometric measurements of diluted samples at 595 nm. Results were normalized to untreated control cells.
2.7. Preclinical in vivo study using a syngeneic orthotopic mouse model
All animal procedures were conducted according to institutional and national regulations. Housing conditions for the mice were at a 12 h light and 12 h dark cycle. C57BL/6-J mice (Charles River) were used for orthotopic transplantation studies. Mice were transplanted at the age of eight weeks and randomized into four groups before transplantation (n = 7 per group). Stably transfected syngeneic KPC-BL6 cells (+NT5C1A) and respective control cells were trypsinized, and 150,000 viable cells were mixed in an equal volume of matrigel prior to transplantation. A total volume of 40 μl was injected into the tail of the pancreas. Small animal high-resolution ultrasound was performed as previously described [28]. In brief, a Visual Sonics Vevo 2100 High Resolution Ultrasound System, including imaging stage and anesthesia line (FUJIFILM VisualSonics Inc., Canada) was used for the study with a Vevo 2100 MicroScan Transducer MS-550-D (22-55 MHz). Mice were anesthetized with 2% isoflurane during ultrasound examinations.
Treatment with gemcitabine or saline started seven days after surgery. Mice were then sacrificed on day 14 of the treatment, with injections on day 0, 3, 7, 10, and 13. Gemcitabine-hydrochloride was administered intraperitoneally at 100 mg/kg body weight. Comparable volumes of saline were injected for control animals. Mice were weighed three times per week. As previously described, mice were sacrificed exactly 2 h after the last gemcitabine injection [29].
2.8. RNA preparation and qRT-PCR
RNA was extracted from cells using the PeqGold Total RNA kit (Peqlab GmbH) according to the manufacturer's protocol. RNA concentration was determined using a nanophotometer (INTAS P330) and 1 μg was used for cDNA preparation. Recombinant RNasin ribonuclease inhibitor (Promega GmbH) was added to the extracted RNA samples prior to reverse transcription. M-MLV reverse transcriptase (Invitrogen) and poly(dT)15 oligo primers (Roche Diagnostics GmbH) were used for cDNA preparation. Myoblast-mRNA was kindly provided by Prof. J. Schmidt, University Medical Center Goettingen and used as a positive control for human NT5C1A expression. Murine muscle RNA served as positive control for murine NT5C1A expression. TaqMan probes for NT5C1A and for ß-actin are listed in supplementary materials.
2.9. Liquid chromatography tandem mass-spectrometry (LC-MS/MS)
For LC-MS/MS analysis of gemcitabine treated cells, 700,000 cells were seeded into 6-well plates. 24 h later, cells were exposed to 1 μM of gemcitabine-hydrochloride for 2 h. Homogenates of cell pellets were analyzed for native dFdC and its triphosphate metabolite dFdCTP by LC-MS/MS according to a previously published protocol [30,31]. Three biological replicates, with 2–3 technical replicates were analyzed for gemcitabine metabolites. Serum samples from orthotopically transplanted mice were taken 2 h after administering the last gemcitabine dose and were analyzed for 2′,2′-difluoro-2′-deoxyuridine (dFdU).
2.10. Statistical analysis
GraphPad Prism (RRID:SCR_002798, version 7.03) was used for statistical analysis. Data are shown as mean ± SEM. Statistical significance was considered for p < .05, with *<0.05, **<0.01, and ***<0.001. Two-tailed Student t-test was used for analysis, if not stated otherwise.
3. Results
3.1. NT5C1A is strongly expressed in murine and human PDAC and is not associated with overall survival
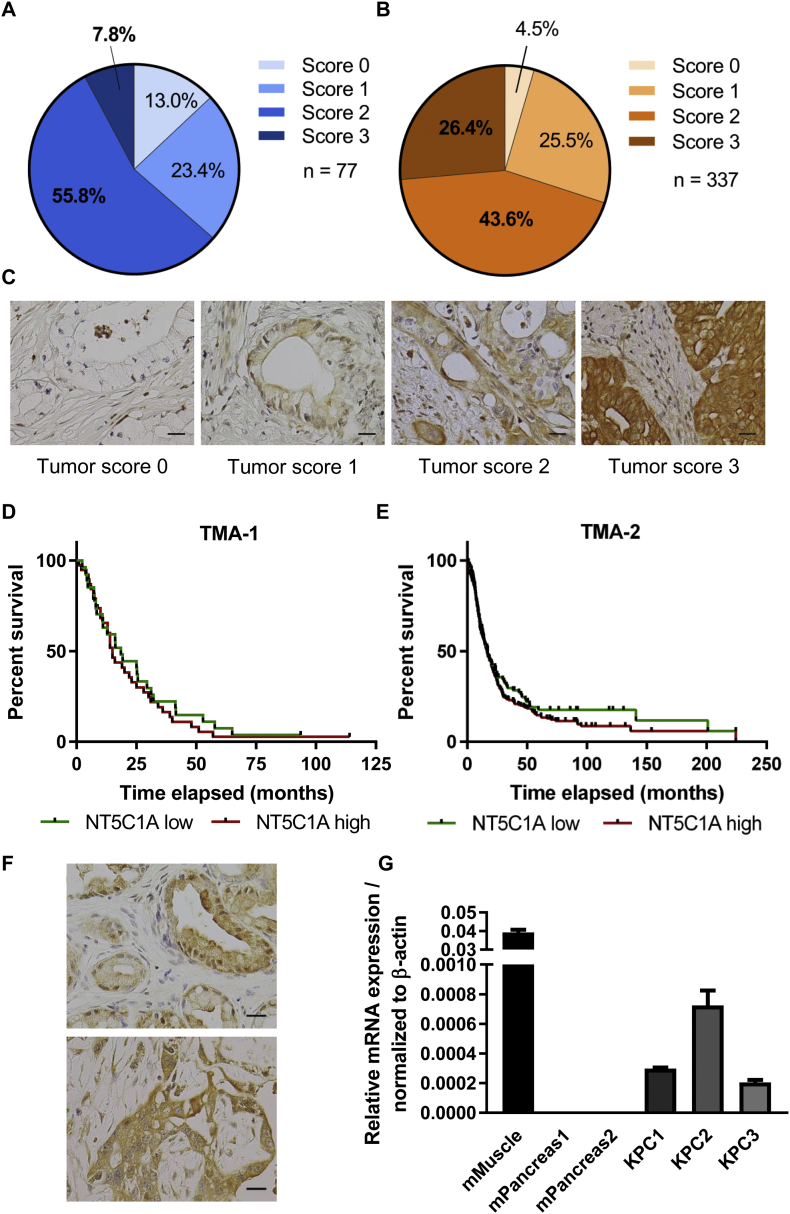
Comprehensive expression data of NT5C1A in PDAC are not available so far. To address this question, we employed two independent TMAs of resected PDAC specimens. A semi-quantitative scoring system ranging between score 0 (no expression) to score 3 (strong expression) was utilized. A relevant subgroup of patients expressed NT5C1A at high levels (score 2 and 3). 56% of all patients showed moderate expression (score 2) of NT5C1A in the epithelial compartment of the tumors and 8% expressed NT5C1A at high levels (score 3) in TMA-1 with 77 samples (Fig. 1A). A larger TMA dataset with 337 samples confirmed these findings with 44% scored with 2, and 26% of all patient samples had score 3 (Fig. 1B). Furthermore, only 13.0% (TMA-1) and 4.5% (TMA-2) of all tumors did not show immunoreactivity against NT5C1A (Fig. 1A–C). Interestingly, NT5C1A expression did not correlate with overall survival in either TMA, comparing low NT5C1A expression (score 0 and 1) with high expression levels of NT5C1A (score 2 and 3). Median survival was 18.5 vs. 15 months in TMA-1 (p = .5), and 17 vs. 16.4 months in TMA-2 (p = .3) (Fig. 1D and E). Moreover, KPC pancreatic tumors were analyzed for NT5C1A expression and robust expression could be confirmed by IHC staining in the majority of samples, and was already present in early stages of pancreatic cancer (Fig. 1F). The expression was confirmed by qRT-PCR to determine mRNA levels for NT5C1A in KPC bulk tissue. As expected no expression was found in healthy control pancreata (Fig. 1G).
Fig. 1.
Expression of cytosolic 5′-nucleotidase 1A (NT5C1A) in resected PDAC patients and in the KPC mouse model. Tissue microarrays (TMA) from A) Goettingen (TMA-1, n = 77) and B) Erlangen (TMA-2, n = 337). Score 0 = no NT5C1A expression, score 3 indicates strong intratumoral expression of NT5C1A. C) Representative images of TMA-1 for tumoral expression of NT5C1A scored with score 0, score 1, score 2, and score 3 (from left to right). Scale bars 20 μm. D) and E) Survival analysis of patients from the Goettingen TMA cohort (D) and the Erlangen TMA cohort (E). Median survival of TMA-1 with low = 18.5 months (n = 27) and high = 15 months (n = 38; p = .5, log-rank test) and of TMA-2 with low = 17 months (n = 101) and high = 16.4 months (n = 235; p = .3, log-rank test). F) Representative images of NT5C1A immunohistochemistry in KPC mice with PanINs (upper image) and invasive carcinoma (lower image). Scale bars 20 μm. G) NT5C1A-mRNA expression in KPC bulk tissue (n = 3 mice) and control normal pancreas (n = 2 mice). Mean ± SEM of two technical replicates is shown for each mouse. Murine muscle was used as positive control and values were normalized to the housekeeping gene β-actin.
3.2. NT5C1A expression in murine and human PDAC cell lines
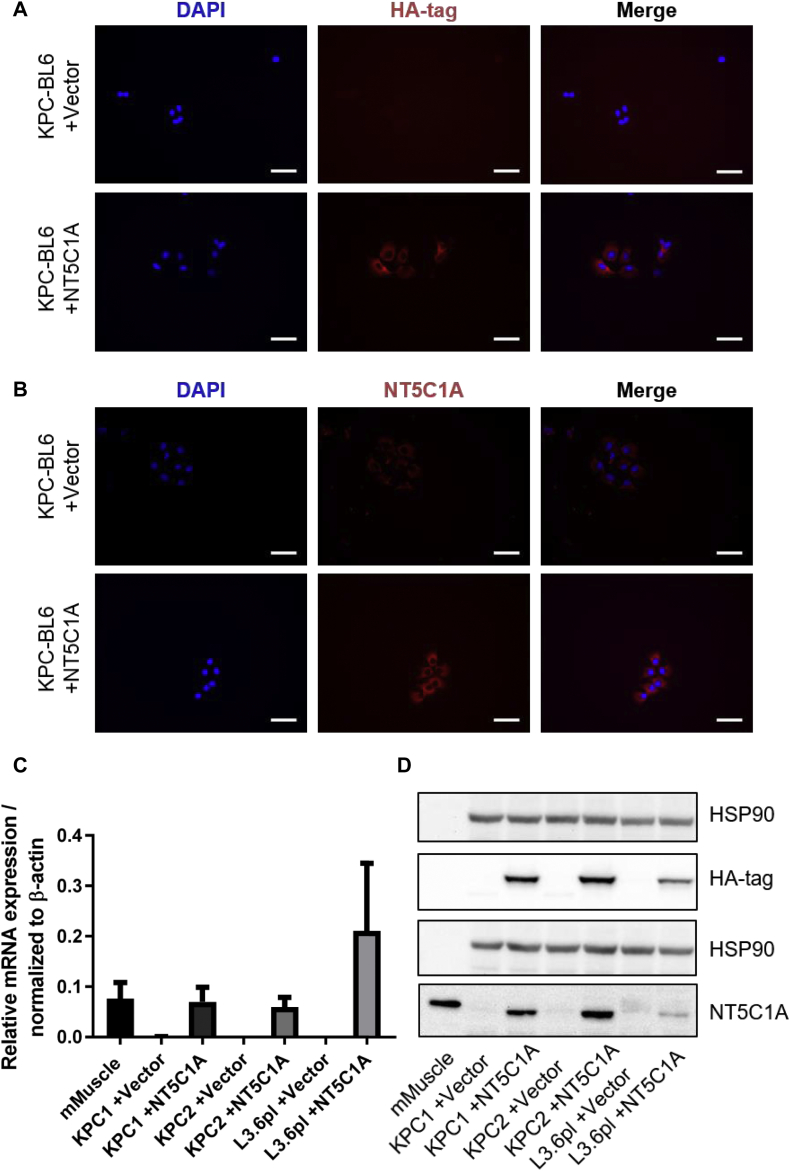
In analogy to our published data about gemcitabine drug scavenging of stromal cells [23], we hypothesized that high levels of NT5C1A within the tumor cells might be involved in chemoresistance in PDAC, in particular gemcitabine resistance. To this end, NT5C1A may affect gemcitabine metabolism by reversing the initial phosphorylation step of dFdC to dFdCMP, resulting in decreased levels of cytotoxic dFdCTP. To test this hypothesis, NT5C1A expression was investigated in murine and human pancreatic cancer cell lines. Interestingly, murine and human pancreatic cancer cells revealed only low levels of NT5C1A expression in vitro compared to robust expression levels in the tumor tissue (Fig. S1A and B). Therefore, NT5C1A was stably expressed in murine KPC cell lines (KPC1 and KPC2 = KPC-BL6) and in the human L3.6pl cell line. Stable cell lines were established using a pSG5-vector derivative with subsequent hygromycin selection. NT5C1A expression in stable cell lines was validated by Western blot analysis, immunocytochemistry, and qRT-PCR analysis (Fig. 2A–D). NT5C1A and the integrated HA-tag were robustly expressed in transfected cells. Control cells that were transfected with an empty vector, were shown to express endogenous NT5C1A at low levels and did not show HA-tag expression by immunocytochemistry (Fig. 2A and B). Additionally, qRT-PCR analysis was employed to confirm mRNA expression of NT5C1A, and Western blot analysis was used to determine NT5C1A protein levels in the overexpressing cells (Fig. 2C and D).
Fig. 2.
Recombinant expression of NT5C1A in human and murine pancreatic cancer cell lines. A) and B) Representative images of two biological replicates of immunocytochemistry staining for NT5C1A and HA-tag. Robust staining is shown for HA-tag (A, lower panel) and NT5C1A (B, lower panel) in transfected KPC-BL6 (=KPC2) cells. No staining of HA-tag (A, upper panel) and low endogenous NT5C1A expression (B, upper panel) were detected in vector control cells (n = 2), scale bars 50 μm. C) Quantitative RT-PCR analysis confirmed overexpression of NT5C1A in transfected cells. Diagram indicates mean ± SEM of three biological replicates. Murine muscle sample was used as positive control and values were normalized to the housekeeping gene β-actin. D) Western blot analysis confirmed NT5C1A protein expression in transfected tumor cells with hardly any expression in vector control cells. Robust expression of HA-tag is shown in all +NT5C1A cell lines. Representative image of three independent experiments is shown. Murine muscle lysate was used as positive control for NT5C1A expression.
3.3. Pharmacokinetics of gemcitabine upon recombinant NT5C1A expression
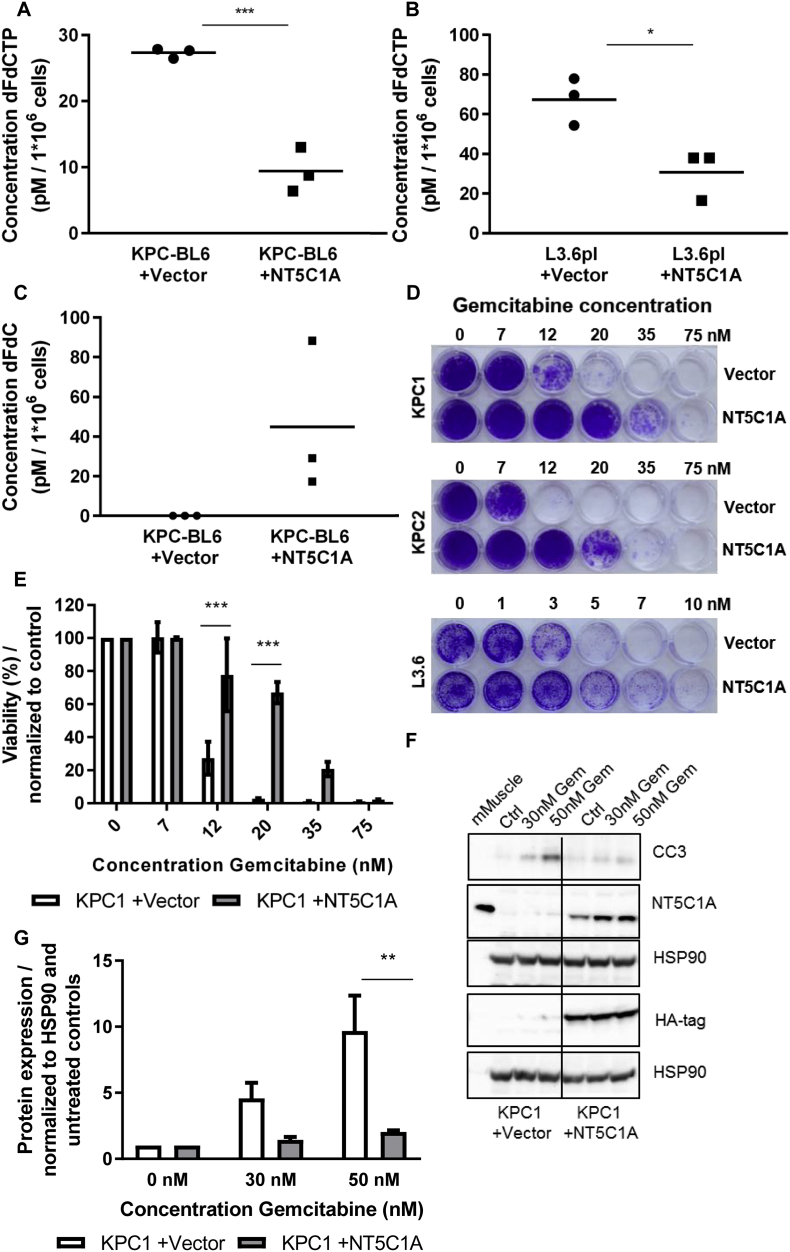
NT5C1A dephosphorylates gemcitabine monophosphate (dFdCMP) to the prodrug dFdC, thus potentially limiting the cytotoxicity of gemcitabine by increasing the pool of dFdC and decreasing the amount of cytotoxic dFdCTP. To test the implication of NT5C1A on gemcitabine metabolism in pancreatic cancer we analyzed gemcitabine metabolites in cell pellets upon gemcitabine treatment using LC-MS/MS. The concentrations of the native form of gemcitabine dFdC as well as the triphosphate, the active metabolite of gemcitabine (dFdCTP) were measured using a previously established protocol [30,31]. Following NT5C1A overexpression in the murine KPC-BL6 cell line, upon 2 h of gemcitabine treatment, the dFdCTP concentration was significantly reduced compared to control cells (27.3 vs. 9.4 pM per 1*106 cells, p = .0008) (Fig. 3A). Equivalent results were seen for the human L3.6pl cell line (67.3 vs. 30.8 pM per 1*106 cells, p = .021) (Fig. 3B). Interestingly, intracellular dFdC levels were only detectable in the NT5C1A-overexpressing KPC-BL6 cells, all values in the vector control cells were below the limit of quantification (Fig. 3C). Thus, our findings suggest a significant contribution of NT5C1A towards the availability of active gemcitabine metabolites in PDAC, and possibly gemcitabine resistance.
Fig. 3.
High levels of NT5C1A increase chemotherapeutic resistance towards gemcitabine in pancreatic cancer cells. A) Pharmacokinetic analysis of gemcitabine metabolites in murine PDAC cells. Murine KPC-BL6 cells (A) and human L3.6pl cells (B) (+NT5C1A) and respective control cells (+vector) were simultaneously treated with 1 μM gemcitabine-hydrochloride for 2 h. The concentration of the active gemcitabine metabolite dFdCTP (KPC-BL6: 27.3 vs. 9.4 pM per 1*106 cells, p = .0008 and L3.6pl: 67.3 vs. 30.8 pM per 1*106 cells, p = .021) and of native gemcitabine dFdC (C) were determined in homogenates of cell pellets using LC-MS/MS-analysis. Three biological replicates and the mean value are shown. Each dot represents the mean of three (KPC-BL6) or two (L3.6pl) technical replicates, respectively. All dFdC measurements of KPC-BL6 control cells were below the level of quantification, thus the values were set to zero. D) Murine and human pancreatic cancer cell lines were incubated with different concentrations of gemcitabine for six days. Crystal violet staining was more pronounced in cell lines with high NT5C1A expression. Three independent experiments, with each two technical replicates, were performed for all cell lines shown. E) Quantification of crystal violet assays for KPC1 cells using 10% acetic acid to solubilize crystal violet stain and photometric measurements at 595 nm. Results were normalized to untreated control cells. Two-way ANOVA with Sidak's multiple comparisons test was performed (12 nM: p = .0007, 20 nM: p < .0001). Graph shows mean ± SEM. F) Western blot analysis revealed reduced cleaved caspase-3 (CC3) protein levels in NT5C1A-expressing cells following gemcitabine treatment for 24 h. Strong NT5C1A and HA-tag expression was demonstrated in the +NT5C1A-cell line. Three independent experiments were performed. G) Quantification of CC3 protein expression in these three Western blot analyses. Two-way ANOVA with Sidak's multiple comparisons test was performed (30 nM: p = .249, 50 nM: p = .002). Graph shows mean ± SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. NT5C1A overexpression confers chemotherapeutic resistance towards gemcitabine in vitro
We showed that NT5C1A decreases the concentration of dFdCTP in vitro, and thus hypothesized that NT5C1A may be an important candidate in mediating gemcitabine resistance. Using crystal violet cytotoxicity assays for two murine KPC cell lines as well as the human L3.6pl pancreatic cancer cell line, we found that NT5C1A expression reverts chemosensitivity in a concentration-dependent manner in all cell lines (Fig. 3D and E, Fig. S2A and B). In contrast, all cell lines were still sensitive to treatment with paclitaxel, which is another important chemotherapeutic agent for the treatment of pancreatic cancer patients (Fig. S3A and B). To confirm and further clarify the involvement of NT5C1A in gemcitabine resistance, we performed Western blot analysis for cleaved caspase-3 (CC3) protein levels upon gemcitabine treatment. Gemcitabine-induced CC3-levels were reduced by factor 4 following recombinant NT5C1A expression in KPC1 cells (Fig. 3F and G). Similar results were obtained for the KPC-BL6 cell line (factor 2, Fig. S2C and D). These results confirmed the impact of NT5C1A on gemcitabine resistance in vitro.
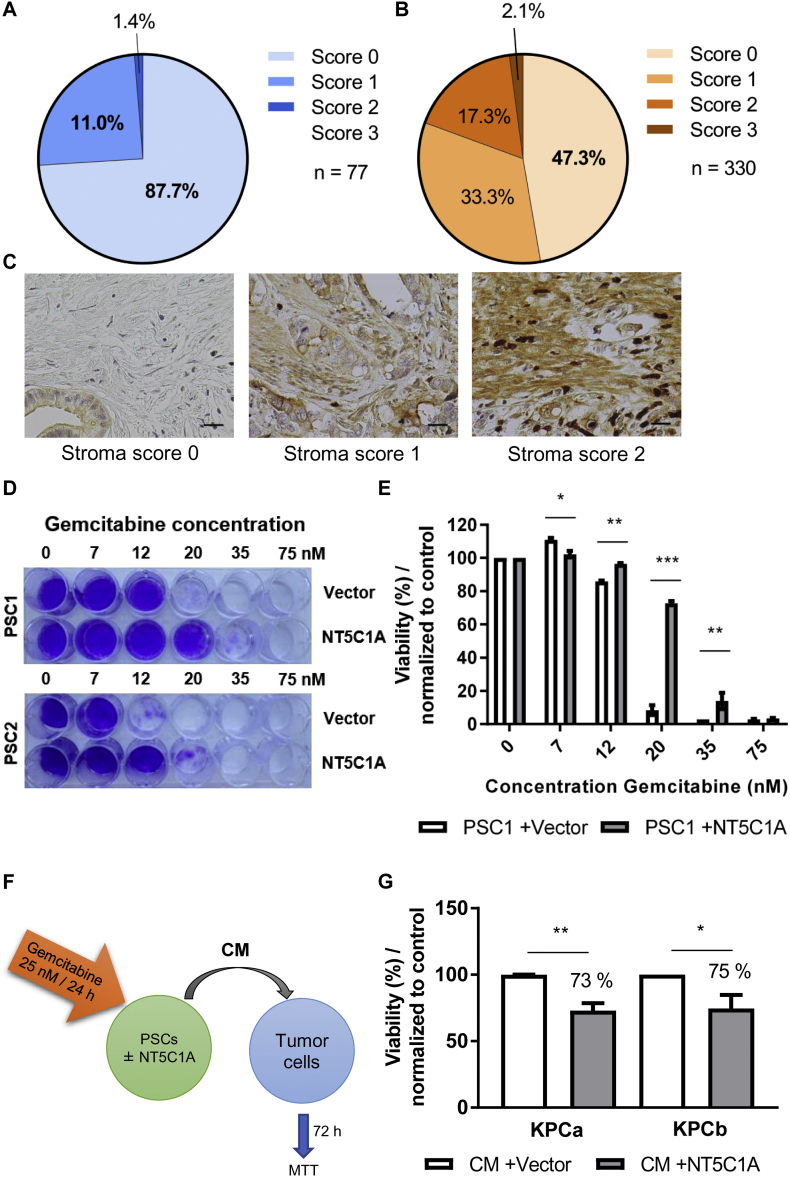
3.5. NT5C1A expression and function in the tumor stroma
With the objective to obtain a more comprehensive picture of the clinical situation regarding NT5C1A, we analyzed both TMAs for stromal NT5C1A expression. The same scoring system was employed with score 0 indicating no stromal expression of NT5C1A and score 3 indicating strong expression of NT5C1A. The proportion of PDAC patients without stromal NT5C1A expression was between 87.7% in TMA-1 (n = 77) and 47.3% in TMA-2 (n = 330). Low expression was detected in 11.0% (TMA-1) and 33.3% (TMA-2) of these patients. Interestingly, high scores [2 and 3] of stromal NT5C1A was only given to the samples of 1.4% (TMA-1) or 19.4% (TMA-2) of all patients (Fig. 4A–C). Consistent with the tumor data, stromal NT5C1A expression was not a prognostic marker. The median post-surgical survival time was 15.2 months for patients that did not express NT5C1A (n = 156) in the tumor stroma and 17.3 months for all other patients (n = 173; p = .3, TMA-2) (Fig. S4F). Due to the lack of more detailed treatment details, it remains to be answered whether stromal NT5C1A might be a predictive marker for gemcitabine response in PDAC patients. As shown by our group previously, NT5C1A is expressed at very low levels in cancer-associated fibroblasts (CAFs) and pancreatic stellate cells (PSCs) [23]. Furthermore, PSCs do not differ significantly regarding intracellular dFdCTP accumulation compared to CAFs [23]. Therefore, two PSC cell lines were stably transfected and + NT5C1A cells and vector control cells were analyzed by Western blot as previously shown [23], and also by immunocytochemistry and qPCR. HA-tag expression and robust expression of NT5C1A was confirmed in the +NT5C1A-cells (Fig. S4A–D). Crystal violet staining showed increased resistance of NT5C1A expressing PSCs to gemcitabine treatment (Fig. 4D and E, Fig. S4E). Considering our previously published findings that +NT5C1A-PSCs accumulate significant lower amounts of intracellular gemcitabine triphosphate [23], we hypothesized that gemcitabine availability would be significantly increased in the supernatant of NT5C1A-PSCs. To test this, MTT assays with conditioned media (CM) of transfected PSCs were performed. PSCs were incubated with 25 nM of gemcitabine-hydrochloride for 24 h and CM was then transferred to two KPC tumor cell lines (Fig. 4F). Cell viability was determined 72 h later and demonstrated significantly decreased tumor cell viability with CM of +NT5C1A-PSCs, compared to vector control cells with only endogenous levels of NT5C1A. In the two KPC cell lines the viability decreased to 73% and 75% respectively (KPCa: p = .003 and KPCb: p = .047) (Fig. 4G).
Fig. 4.
NT5C1A expression and function in PDAC stroma in vitro and in vivo. A) and B) Tissue microarray analysis for NT5C1A expression revealed very low expression in the tumor stroma of resected PDAC patients. A semi-quantitative scoring system indicated no stromal expression with score 0 and strong stromal expression with score 3. TMA-1 from Goettingen (A) with n = 77 patients (none scored with 3) and TMA-2 from Erlangen (B) with n = 330 patient samples. C) Representative immunohistochemistry of NT5C1A expression showing no NT5C1A expression (score 0), low expression (score 1), and robust stromal expression (score 2) of TMA-1. Scale bars 20 μm. D) Murine PSCs were treated with increasing concentrations of gemcitabine for six days and crystal violet assays were performed. The staining was more pronounced in NT5C1A expressing cell lines. Representative images of two independent experiments, with each two technical replicates, are shown. E) Crystal violet stain intensity was quantified and results were normalized to untreated control cells. Graph indicates mean ± SEM, two-way ANOVA with Sidak's multiple comparisons test was performed (7 nM: p < .035, 12 nM: p < .009, 20 nM: p < .0001, 35 nM: p = .007). F) and G) MTT cell viability assay for KPC cell lines treated with conditioned medium (CM) of NT5C1A expressing PSCs and control CM. F) Schematic experimental overview. CM from PSCs (+NT5C1A) and control PSCs (+vector) was obtained by preincubation with 25 nM gemcitabine-hydrochloride for 24 h. Subsequently, tumor cells were treated for 72 h with CM of PSCs and viability was assessed using MTT cell viability assay. G) Tumor cell viability of two different murine KPC cell lines was significantly decreased following treatment with CM of +NT5C1A-expressing PSCs (KPCa: 73%; p = .003 and KPCb: 75%; p = .047). Graphs indicate mean ± SEM of four biological replicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. NT5C1A expression mediates chemoresistance in vivo
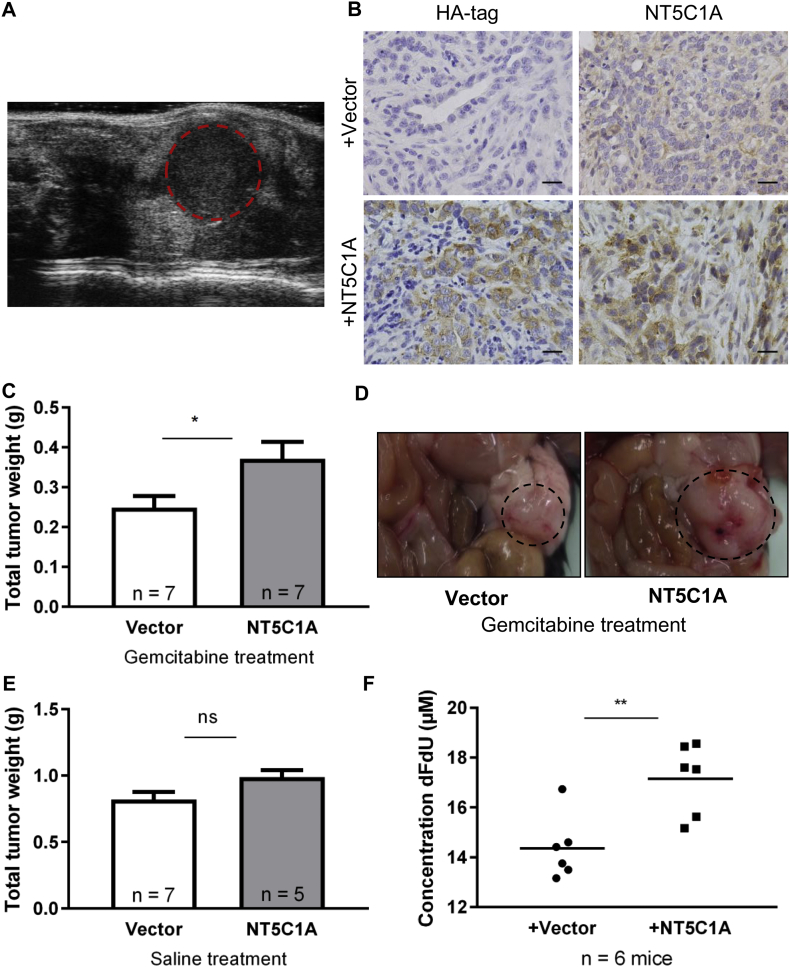
Finally, we aimed to investigate whether NT5C1A mediates chemoresistance in vivo. To this end, we employed a syngeneic, orthotopically transplanted mouse model using C57BL/6-J mice and stably transfected KPC-BL6 cells. Seven days after tumor cell transplantation, mice were treated with gemcitabine or saline for 14 days (Fig. S5A). High-resolution small animal ultrasound screening was performed on day 9 of the treatment to verify tumor growth upon transplantation (Fig. 5A). Intratumoral NT5C1A and HA-tag expression were confirmed by immunohistochemistry and showed robust protein levels in tumors derived from stably transfected cells (Fig. 5B). Tumor growth measured by absolute tumor weights was significantly increased following gemcitabine treatment in NT5C1A-overexpressing tumors compared to tumors derived from vector control cells (0.25 g vs. 0.37 g; p = .03) (Fig. 5C and D). However, the overall number of apoptotic cells within the tumor was not significantly decreased upon NT5C1A overexpression (data not shown). Moreover, serum levels of dFdU, the inactive gemcitabine metabolite, were significantly increased in mice bearing NT5C1A-overexpressing tumors with median concentrations of 14.1 vs. 17.6 μM dFdU (p = .009) (Fig. 5F). These data strongly support our hypothesis that high NT5C1A expression reduces sensitivity to gemcitabine. Accordingly, and in line with the patient data demonstrating NT5C1A not to be a prognostic marker (Fig. 1D and E), we did not observe significant NT5C1A-dependent differences in tumor growth upon saline treatment of mice (0.82 g vs. 0.99 g; p = .07) (Fig. 5E). Additionally, comparing gemcitabine treated tumors to saline treated tumors, we show that NT5C1A expression is not induced by gemcitabine treatment (Fig. S5B).
Fig. 5.
NT5C1A expression mediates chemoresistance in vivo. A) Tumor detection was performed by small animal high-resolution sonography of orthotopically transplanted mice in all groups on day 9 of treatment. Dotted line indicates the tumor. B) Representative immunohistochemistry showing HA-tag (left) and NT5C1A (right) stainings in vector (upper panel) and NT5C1A transfected (lower panel) orthotopic tumors. Scale bars 20 μm. C) Tumor weights upon necropsy are shown with significantly increased tumor weights upon NT5C1A overexpression in the gemcitabine treated cohort (n = 7 each; p = .03). Graph shows mean ± SEM. D) Necropsy pictures of orthotopically transplanted pancreatic tumors upon gemcitabine treatment with vector (left) and stable expression of NT5C1A (right). E) NT5C1A expression in saline treated controls did not have a significant effect on tumor growth (p = .07, n = 7 and 5, respectively). Graph shows mean ± SEM. F) The inactive gemcitabine metabolite dFdU was measured in serum samples of gemcitabine treated mice. Significantly higher values were detected in mice with +NT5C1A tumors (median: 14.1 vs. 17.6 μM, p = .009, Mann-Whitney test). Single values of n = 6 mice per group and the mean values are shown.
4. Discussion
The advent of precision oncology concepts has shown extraordinary treatment responses in several tumor entities such as melanoma, lung and breast cancer. However, there are currently no established biomarkers for PDAC patients in clinical routine that would assist to select the best treatment option or to predict treatment response for individual patients [32]. Notably, several recent preclinical and clinical studies suggested that gemcitabine transport and metabolism proteins are implicated in treatment response and patient outcome and may thus serve as appropriate biomarkers to select patients for gemcitabine treatment. For example, high hENT1 and dCK protein levels have been associated with improved outcome of PDAC patients following gemcitabine treatment [[10], [11], [12],33,34]. In contrast, data from large randomized prospective clinical trials suggest that DCTD and RRM1 are not associated with survival upon gemcitabine treatment [10,34].
Here, we investigated the expression and function of NT5C1A, a previously unrecognized gemcitabine inactivating enzyme that has not been reported to be expressed in any solid carcinoma before. To the best of our knowledge, we are the first group to publish detailed expression data on NT5C1A in pancreatic cancer specimens. Interestingly, NT5C1A was robustly expressed in the epithelial compartment of 64–70% of PDAC patients, whereas 23–26% showed weak NT5C1A expression, and 5–13% were devoid of NT5C1A immunoreactivity. Although the functional implications have not been elucidated in PDAC, NT5C1A expression had no prognostic effect on overall survival of postoperative PDAC patients in our two cohorts. These results are in line with previously published data on hENT and other gemcitabine metabolizing enzymes such as DCTD that also had no prognostic effect in resected PDAC [11,34].
An unexpected finding was that murine and human pancreatic cancer cells showed dramatically reduced expression of NT5C1A when cultured in 2D. As we generated primary murine cell lines from the KPC model, in which epithelial tumor cells showed robust NT5C1A expression in vivo, we speculate that NT5C1A expression is regulated by signaling cues from the tumor microenvironment (TME) that are lacking during cell culture. The TME is abundant in PDAC and consists of large amounts of collagen and hyaluronic acid, activated fibroblasts and immune cells, including tumor associated macrophages (TAMs) [35]. Recent evidence from patient derived organoids (PDOs) have provided compelling insights into tumor biology and tailored therapy approaches [36,37]. This technique could be employed in future studies to understand the differential expression of NT5C1A in 2D and 3D, and possibly to use this knowledge for future patient stratification.
Notably, we provide first evidence that re-expression of NT5C1A, in both murine and human PDAC cells, reduces sensitivity to gemcitabine both in vitro and in vivo. Using LC-MS/MS techniques, we could directly demonstrate the activity of NT5C1A on gemcitabine metabolism and the subsequent reduction of tumor cell apoptosis. As expected, NT5C1A overexpression did not induce chemotherapeutic resistance to paclitaxel, whose mechanism of action is independent of intracellular phosphorylation. We had previously shown that recombinant overexpression of NT5C1A in PSCs reduced the intracellular amounts of active gemcitabine, thus, potentially limiting the described drug scavenging effect of PSCs [23]. Here, we provide a large dataset of patient samples, demonstrating not only overexpression in the majority of tumor cells but also a lack of stromal NT5C1A expression in most cases. In addition, we show for the first time that re-expression of NT5C1A in PSCs leads to an improved sensitivity of gemcitabine in tumor cells suggesting a reciprocal effect of NT5C1A, where high expression in the stroma and low expression in the tumor cells may promote chemosensitivity. As stromal cells are not present in cell culture and tumor cells express NT5C1A at low levels under 2D conditions, our observations may partly explain the conundrum that most human and murine pancreatic cancer cell lines respond very well to gemcitabine in vitro but fail to exert meaningful anti-tumor effects in genetically engineered mice or in human PDAC that both harbor abundant desmoplasia.
It remains to be seen whether NT5C1A has any predictive effect following gemcitabine treatment in PDAC patients, and whether a combination with hENT quantification may yield any predictive benefit over hENT or NT5C1A expression alone. In both TMA cohorts, a relevant fraction of patients did not undergo adjuvant chemotherapy. Therefore, further studies in prospectively collected, randomized and controlled clinical specimens are warranted to determine the role of NT5C1A as a potential novel predictor for gemcitabine-based therapies in PDAC.
Interestingly, a recent study provided evidence that TAMs may mediate chemoresistance in PDAC by upregulation of CDA, an important gemcitabine inactivating enzyme, in tumor cells [13]. Whether this is also true for other gemcitabine metabolizing enzymes such as NT5C1A and DCTD has not been investigated so far. Recent findings suggest multiple cross-talking pathways between tumor cells, macrophages, cancer-associated fibroblasts, and immune cells in stroma-driven chemoresistance in PDAC [[38], [39], [40]]. Consistently, stroma-mediated cancer cell activation correlates with widespread increases in histone acetylation at H3K9 and H3K27 residues, suggestive for profound alterations in promoter and enhancer activation levels, although the transcriptional consequences and therapeutic relevance of these findings remain largely elusive [41]. Future studies will need to address whether the dynamic changes of NT5C1A expression in vitro and in vivo may be orchestrated by epigenetic mechanisms that could also act as potential therapeutic targets.
In conclusion, we have identified NT5C1A to be highly expressed in the epithelial compartment of a relevant subgroup of PDAC patients. Mechanistically, NT5C1A mediates gemcitabine resistance by dephosphorylating gemcitabine monophosphate, thus reducing the amount of cytotoxic gemcitabine metabolites intracellularly. Therefore, our study paves the way for the evaluation of NT5C1A for stratified treatment approaches in PDAC, both for adjuvant as well as palliative therapy regimens.
Acknowledgments
Acknowledgements
We are grateful to Jutta Blumberg and Ulrike Wegner for technical support. We further thank Prof. Zimmermann and Marcel Zoremba (University Medical Center Goettingen) for providing us access to and technical advice on the use of the small-animal ultrasound equipment. Furthermore, we want to thank the veterinarians and animal care takers of the University Medical Center Goettingen.
Funding sources
This work was supported by the Deutsche Krebshilfe (Max Eder group) [110972 and 70113213] to A.N. and PiPAC Consortium [70112505] to S.A.J., J.G., E.H. and V.E; the German Academic Exchange Service (DAAD) to F.H.H.; the Deutsche Forschungsgemeinschaft (DFG) [JO 815/3-2] to S.A.J.; and the Else-Kröner-Fresenius-Stiftung to R.G.G. Cancer Research UK (CRUK) supports the Jodrell Group (DIJ and FMR). LC-MS/MS analyses were performed in the PKB core at the CRUK Cambridge Institute. The CRUK Cambridge Institute (Li Ka Shing Centre) was generously funded by CK Hutchison Holdings Limited, the University of Cambridge, The Atlantic Philanthropies and a range of other donors. None of the funders were involved in study design, data collection, data analysis and interpretation, writing of the manuscript, and in the decision to submit the manuscript for publication.
Author contributions
M.S.P., A.N., and V.E. conceived and designed the experiments. M.S.P., V.K., F.H.H., and S.A.J. participated in the design of and performed cell culture experiments. M.S.P., E.H., S.P., and R.G.G. planned and performed animal experiments. M.S.P. did histology stainings. J.G., M.B., and J.K. provided TMA-1 slides, clinical data, and scored the stainings. C.P., R.G., P.R., and T.K. provided, stained, and scored the TMA-2. F.M.R. and D.I.J. analyzed liquid-chromatography tandem mass-spectrometry samples. M.S.P. performed data analysis. M.S.P. and A.N. assembled the data and wrote the manuscript. All authors reviewed the manuscript.
Declaration of interests
Dr. Richards reports grants from Cancer Research UK, other (funding construction of the building and facilities) from CK Hutchison Holdings Limited, during the conduct of the study; non-financial support from Astra Zeneca, outside the submitted work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.037.
Appendix A. Supplementary data
Supplementary material
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Stathis A., Moore M.J. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Chand S., O'Hayer K., Blanco F.F., Winter J.M., Brody J.R. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int J Biol Sci. 2016;12(3):273–282. doi: 10.7150/ijbs.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Burris H.A.I., Moore J.A., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 9.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V. Pancreatic cancer. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 10.Maréchal R., Bachet J.B., Mackey J.R., Dalban C., Demetter P., Graham K. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012;143(3):664–674. doi: 10.1053/j.gastro.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Greenhalf W., Ghaneh P., Neoptolemos J.P., Palmer D.H., Cox T.F., Lamb R.F. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106(1):djt347. doi: 10.1093/jnci/djt347. [DOI] [PubMed] [Google Scholar]
- 12.Frese K.K., Neesse A., Cook N., Bapiro T.E., Lolkema M.P., Jodrell D.I. nab-Paclitaxel Potentiates Gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weizman N., Krelin Y., Shabtay-Orbach A., Amit M., Binenbaum Y., Wong R.J. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33(29):3812–3819. doi: 10.1038/onc.2013.357. [DOI] [PubMed] [Google Scholar]
- 14.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Supplement 5):v7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y., Xie J. Promising molecular mechanisms responsible for gemcitabine resistance in cancer. Genes Dis. 2015;2(4):299–306. doi: 10.1016/j.gendis.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla S.K., Purohit V., Mehla K., Gunda V., Chaika N.V., Vernucci E. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32(3):71–87. doi: 10.1016/j.ccell.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchi V., Spychala J. Mammalian 5′-nucleotidases. J Biol Chem. 2003;278(47):46195–46198. doi: 10.1074/jbc.R300032200. [DOI] [PubMed] [Google Scholar]
- 18.Kviklyte S., Vertommen D., Yerna X., Andersén H., Xu X., Gailly P. Effects of genetic deletion of soluble 5′-nucleotidases NT5C1A and NT5C2 on AMPK activation and nucleotide levels in contracting mouse skeletal muscles. Am J Physiol Endocrinol Metab. 2017;313(1):E48–E62. doi: 10.1152/ajpendo.00304.2016. [DOI] [PubMed] [Google Scholar]
- 19.Hunsucker S.A., Mitchell B.S., Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107(1):1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Hunsucker S.A., Spychala J., Mitchell B.S. Human cytosolic 5′-nucleotidase I: characterization and role in nucleoside analog resistance. J Biol Chem. 2001;276(13):10498–10504. doi: 10.1074/jbc.M011218200. [DOI] [PubMed] [Google Scholar]
- 21.Saliba J., Zabriskie R., Ghosh R., Powell B.C., Hicks S., Kimmel M. Pharmacogenetic characterization of naturally occurring germline NT5C1A variants to chemotherapeutic nucleoside analogs. Pharmacogenet Genomics. 2016;26(6):271–279. doi: 10.1097/FPC.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumontet C., Bauchu E.C., Fabianowska K., Lepoivre M., Wyczechowska D., Bodin F. Common resistance mechanisms to nucleoside analogues in variants of the human erythroleukemic line K562. Adv Exp Med Biol. 1999;457:571–577. doi: 10.1007/978-1-4615-4811-9_63. [DOI] [PubMed] [Google Scholar]
- 23.Hessmann E., Patzak M.S., Klein L., Chen N., Kari V., Ramu I. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut. 2018;67(3):497–507. doi: 10.1136/gutjnl-2016-311954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Kari V., Mansour W.Y., Raul S.K., Baumgart S.J., Mund A., Grade M. Loss of CHD1 causes DNA repair defects and enhances prostate cancer therapeutic responsiveness. EMBO Rep. 2016;17(11):1609–1623. doi: 10.15252/embr.201642352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feoktistova M., Geserick P., Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016(4):343–346. doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 28.Goetze R.-G., Buchholz S.M., Patil S., Petzold G., Ellenrieder V., Hessmann E. Utilizing high resolution ultrasound to monitor tumor onset and growth in genetically engineered pancreatic cancer models. J Vis Exp JoVE. 2018;134 doi: 10.3791/56979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neesse A., Frese K.K., Bapiro T.E., Nakagawa T., Sternlicht M.D., Seeley T.W. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci. 2013;110(30):12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bapiro T.E., Richards F.M., Jodrell D.I. Understanding the complexity of porous graphitic carbon (PGC) chromatography: modulation of mobile-stationary phase interactions overcomes loss of retention and reduces variability. Anal Chem. 2016;88(12):6190–6194. doi: 10.1021/acs.analchem.6b01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bapiro T.E., Frese K.K., Courtin A., Bramhall J.L., Madhu B., Cook N. Gemcitabine diphosphate choline is a major metabolite linked to the Kennedy pathway in pancreatic cancer models in vivo. Br J Cancer. 2014;111:318–325. doi: 10.1038/bjc.2014.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellenrieder V., König A., Seufferlein T. Current standard and future perspectives in first- and second-line treatment of metastatic pancreatic adenocarcinoma. Digestion. 2016;94(1):44–49. doi: 10.1159/000447739. [DOI] [PubMed] [Google Scholar]
- 33.Bird N.T.E., Elmasry M., Jones R., Psarelli E., Dodd J., Malik H. Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br J Surg. 2017;104(4):328–336. doi: 10.1002/bjs.10482. [DOI] [PubMed] [Google Scholar]
- 34.Elander N.O., Aughton K., Ghaneh P., Neoptolemos J.P., Palmer D.H., Cox T.F. Intratumoural expression of deoxycytidylate deaminase or ribonuceotide reductase subunit M1 expression are not related to survival in patients with resected pancreatic cancer given adjuvant chemotherapy. Br J Cancer. 2018;118(8):1084–1088. doi: 10.1038/s41416-018-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neesse A., Algül H., Tuveson D.A., Gress T.M. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 36.Tiriac H., Belleau P., Engle D.D., Plenker D., Deschênes A., Somerville T. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8(9):1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boj S.F., Il Hwang C., Baker L.A., Engle D.D., Tuveson D.A., Clevers H. Model organoids provide new research opportunities for ductal pancreatic cancer. Mol Cell Oncol. 2016;3(1) doi: 10.1080/23723556.2015.1014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griesmann H., Drexel C., Milosevic N., Sipos B., Rosendahl J., Gress T.M. Pharmacological macrophage inhibition decreases metastasis formation in a genetic model of pancreatic cancer. Gut. 2017;66(7):1278–1285. doi: 10.1136/gutjnl-2015-310049. [DOI] [PubMed] [Google Scholar]
- 39.Oezdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman M.H., Yu R.T., Tseng T.W., Sousa C.M., Liu S., Truitt M.L. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci. 2017;114(5):1129–1134. doi: 10.1073/pnas.1620164114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material