INTRODUCTION
Noninvasive brain stimulation (NIBS) techniques have emerged as alternatives to invasive modalities given the ease of application, safety, tolerability, and reversibility. The 2 most well-studied forms of NIBS are transcranial magnetic stimulation (TMS) and transcranial electrical stimulation. Research protocols began applying brain stimulation techniques to children and adolescents in the early 1990s. Progress has been slow due to practical limitations and safety concerns.1 As of 2017, there is no Food and Drug Administration–approved therapeutic use of NIBS techniques in children. Current evidence suggests potential use of NIBS techniques in children with depression, attention-deficit hyperactivity disorder (ADHD), epilepsy, autism, schizophrenia, dystonia, dyslexia, cerebral palsy, and Tourette syndrome (Table 1).2–4
Table 1.
Neuropsychiatric diseases included in neuromodulation trials in children and adolescents
| Depression | OCD | ADHD | Autism | Tourette Syndrome | Schizophrenia | Addiction | Dyslexia | Migraine | Cerebral Palsy | Dystonia | Epilepsy | Stroke | Headache | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rTMS | X | — | X | X | X | X | — | — | — | X | — | X | X | — |
| TBS | — | — | — | X | X | — | — | — | — | — | — | — | — | — |
| tDCS | — | — | X | X | — | X | — | X | — | X | X | X | — | X |
| ECT | X | — | — | X | — | X | — | — | — | — | — | — | — | — |
| MST | X | — | — | — | — | — | — | — | — | — | — | — | — | — |
| eTNS | — | — | X | — | — | — | — | — | — | — | — | — | — | — |
Abbreviations: ECT, electroconvulsive therapy; eTNS, external trigeminal nerve stimulation; MST, magnetic seizure therapy; OCD, obsessive-compulsive disorder; rTMS, repetitive transcranial magnetic stimulation; TBS, theta burst stimulation; tDCS, transcranial direct current stimulation.
TRANSCRANIAL MAGNETIC STIMULATION
The applications of TMS in children first started in the early 2000s and included both diagnostic and therapeutic approaches. Potential therapeutic applications of TMS in children include epilepsy, ADHD, autism spectrum disorder (ASD), depression, schizophrenia, and Tourette syndrome.2 Single-pulse TMS is also used for presurgical mapping of the motor cortex and language areas.5
Safety and application guidelines for TMS were published in 2009 but focused on adults.1
In children and adolescents, recent systematic reviews suggest that both single-pulse and repetitive TMS have similar adverse effect profiles to adult populations.3,6,7 The most commonly reported side effects are headache (11.5%), scalp discomfort (2.5%), twitching (1.2%), mood changes (1.2%), fatigue (0.9%), and tinnitus (0.6%).3 The most serious side effect is seizure and to date there are 3 reported seizures in adolescents receiving TMS. These events occurred in the context of epileptogenic medication use,8,9 alcohol consumption before the TMS session,9 and application of deep TMS.10 There are 2 reported instances of TMS-induced hypomania8,11 and 2 reported cases of neurocardiogenic syncope, which were associated with preexisting circumstances.12 No changes in cognitive functioning have been reported (Fig. 1).13
Fig. 1.
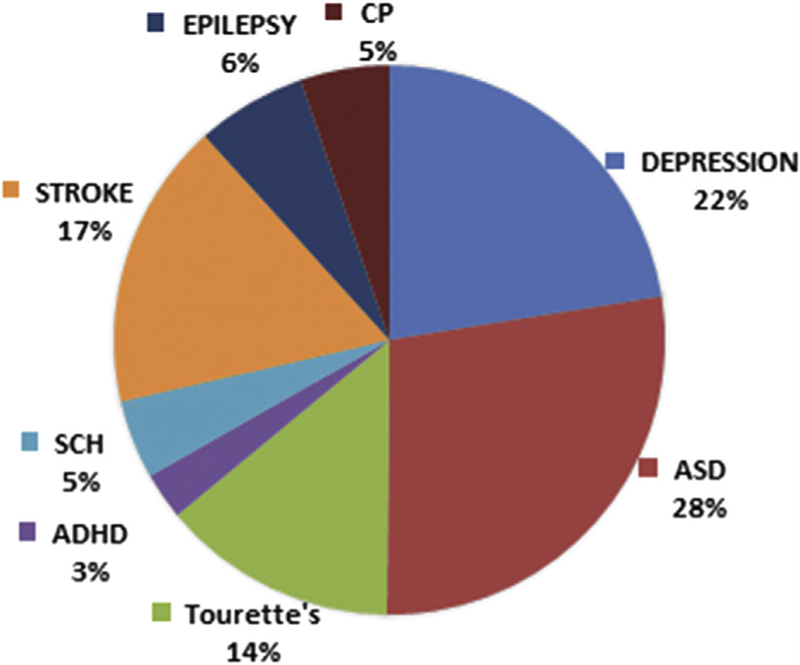
Distribution of subjects in therapeutic TMS studies. SCH, schizophrenia; ASD, autism spectrum disorder; ADHD, attention-deficit hyperactivity disorder; CP, cerebral palsy.
Major Depressive Disorder
Major depressive disorder is one of the most common psychiatric illnesses in children and adolescents. Suboptimal outcomes in the treatment of depression in children and adolescents have sparked interest focused on the study of novel, brain-based approaches such as TMS. Prior therapeutic TMS studies included 73 participants between the ages of 7 to 21 years in open trials, case studies, case series, and small sham-controlled trials. In a systematic review, Donaldson and colleagues14 suggested that TMS may be an effective and well-tolerated treatment for treatment-resistant depression in adolescents. The most common TMS application was high-frequency TMS (10 Hz) over the left dorsolateral prefrontal cortex (L-DLPFC). TMS parameters varied in terms of number of sessions (10–30), session duration (10–37.5 min), and intensity (80%–120% of motor threshold [MT]). Among these studies, 2 open trials by Bloch and colleagues11 (2008) and Wall and colleagues15 (2011) showed statistically significant improvement in depressive symptoms measured by CDRS-R as well as the significant improvement in the Clinical Global Impression Severity of Illness Scales (CGI-S) with high-frequency TMS applied over the DLPFC. The study by Wall and colleagues differed from the study by Bloch and colleagues based on MT intensity (120% vs 80%), total number of pulses per session (3000 vs 400) and number of total TMS sessions (30 vs 14). A follow-up study by Bloch and colleagues11 showed sustained improvement after 3 years.13 Another open-label study by Wall and colleagues16 in 2016 (n 5 10) showed significant improvement (60% of participants) in depressive symptoms measured by CDRS-R, the Quick Inventory for Depressive Symptomatology Adolescent Seventeen-Item Self-Report (QIDS-A17-SR) and CGI-S after treatment and at 6-month follow-up. Initial studies suggest that high-frequency TMS treatments may modulate glutamatergic neurotransmission, and this presents an opportunity for precision medicine approaches to TMS.17,18
Autism Spectrum Disorder
ASD is diagnosed behaviorally by social impairments and the presence of restricted and repetitive patterns of behavior and interests. Early studies focused on ASD used conventional low-frequency (1 Hz) repetitive TMS applied to the prefrontal cortex daily over a period of time and demonstrated positive effects on behavioral and electrophysiological outcomes in children with ASD.19,20 More recently, high-frequency theta-burst stimulation protocols applied to the motor cortex have also been investigated experimentally in this population.21
Tourette Disorder
TD is thought to involve hyperexcitability of the basal ganglia and motor cortex.22,23 Among the few studies conducted in children with TD, low-frequency TMS (1 Hz, 110% MT, 10–20 sessions) applied over supplementary motor area has been shown to improve symptoms up to 6 months and was associated with increase in resting MT in children younger than 16 years.24,25
Attention-Deficit Hyperactivity Disorder
ADHD affects up to 12% of the population.26 Initial treatment strategies include pharmacotherapy; yet, because of the unwanted side effects and risk for potential abuse, alternative treatments have emerged. In a study of 9 subjects (age 15–20 years), high-frequency TMS applied to the right prefrontal cortex (100% MT, 10 sessions) showed no difference between active and sham groups.27
Schizophrenia
Childhood onset schizophrenia is a rare disorder with an incidence less than 0.04%.28 In adults, TMS inhibition of left temporoparietal region reduced auditory hallucinations in double-blind, randomized trials.29,30 In children and young adults (age 18) limited studies showed improvement in positive and negative symptoms of schizophrenia with both high-frequency TMS delivered to the right frontal cortex (10 daily sessions of 20 Hz TMS) and low-frequency TMS applied to the left temporoparietal cortex (10 sessions of 1 Hz TMS).31,32
Neurologic Disorders
Inhibitory TMS (1 Hz for 20 minutes) applied over contralesional primary cortex showed improvement in hand functioning in patients aged 6 to 18 years with pediatric stroke,33 especially when combined with constraint-induced movement therapy (CIMT) in a larger study.34 In epilepsy, there are only a few case reports in children with intractable epilepsy that shows that low-frequency TMS (1 Hz) can lead to temporary reduction of epileptic activity.35
TRANSCRANIAL ELECTRICAL STIMULATION
Transcranial Direct Current Stimulation
In adults, tDCS has shown promise as an intervention for multiple neuropsychiatric disorders.36 Experience in children and adolescents is limited to small randomized controlled trials (RCTs) and pilot studies,4,37,38 but tDCS has potential as a tool to modulate cortical activity and promote neuroplasticity. It is appealing because it may prove more portable, safe, and accessible as compared with other techniques such as TMS (Fig. 2).39
Fig. 2.
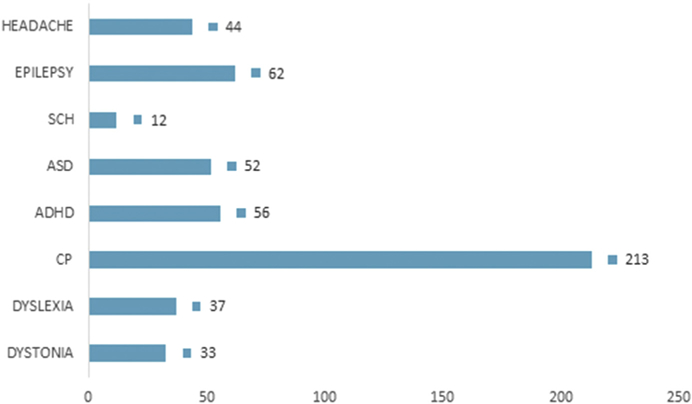
Total number of subjects for each condition in TDCS studies. SCH, schizophrenia; ASD, autism spectrum disorder; ADHD, attention-deficit hyperactivity disorder; CP, cerebral palsy. (Data from Palm U, Segmiller FM, Epple AN, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm 2016;123(10):1219–34; and Muszkat D, Polanczyk GV, Dias TG, et al. Transcranial direct current stimulation in child and adolescent psychiatry. J Child Adolesc Psychopharmacol 2016;26(7):590–7.)
Electroconvulsive therapy (ECT) is safely used in adults with mood disorders with catatonia, psychotic features, and refractory to antidepressant therapy as well as in patients who refuse food and water intake or are acutely suicidal.40 In children and adolescents it is often considered as a last resort likely due to factors such as stigma, lack of clinical experience, concerns about long-term side effects, and legal restrictions.41,42
Major Depressive Disorder
In adults, prior studies demonstrate that depressed patients who underwent tDCS had greater response and remission rates,43 but no prior studies have examined the effects of tDCS on depression in children and adolescents. Prior retrospective reviews suggest that electroconvulsive therapy can be effective in treating depression in children and adolescents with the rate of improvements up to 80% in unipolar depression and up to 90% in treatment-resistant depression.41,42,44 Unfortunately, the dearth of RCTs and safety studies in children limits the use of ECT in younger patients.
Schizophrenia
In a double-blind sham-controlled trial, Mattai and colleagues45 investigated the tolerability of bilateral anodal DLPFC (targeting cognitive difficulties) and bilateral cathodal and superior temporal (targeting auditory hallucinations) (2 mA for 20 min, 10 days) tDCS in 12 children with childhood-onset schizophrenia (age: 10–17 years). They found no difference between groups in terms of adverse effects or clinical measures suggesting that tDCS was well tolerated.
With regard to ECT trials, in a study with 13 participants with schizophrenia spectrum disorder (n 5 13), Baeza and colleagues46 showed that ECT lead to significant improvements in PANSS scores and CGI scores after acute phase of ECT and at 6 months. In a retrospective study of ECT, Puffer and colleagues42 found significant improvement in CGI-I and CGI-S scores in 9 adolescents with psychotic disorder.
Autism Spectrum Disorders
Andrade and colleagues47 targeted language problems in a sample of 14 children with 4 of the subjects with ASD. Across the sample, self-report measures indicate considerable variability in the perceived improvement in symptoms, ranging from “no change” to “very much better.” One pilot study suggested that tDCS may improve syntax acquisition in children and adolescents with ASD.48
Other studies found improvement in the childhood autism rating scale and the Autism Treatment Evaluation Checklist with anodal tDCS applied over F3 (1 mA, 5 days).49 Amatachaya and colleagues50 found significant association between the electroencephalogram alpha activity and improvement in ASD symptoms with anodal tDCS applied over F3 (DLPFC) (2 mA, 20 min).
There are a small number of case reports supporting the safe and efficacious application of electroconvulsive therapy (ECT) to treat catatonia51 and self-injurious behaviors52 in children and adolescents with ASD. Moreover, ECT may also have had positive outcomes for some other characteristics of ASD in these cases, such as eye contact, verbal conversation,53 and engagement in family activities.52
Attention-Deficit Hyperactivity Disorder
The neural basis of ADHD is thought to involve deficient inhibitory mechanisms that could be a potential target for tDCS.54,55 In children, several studies showed improvement in inhibitory control with the stimulation of L-DLPFC.56,57 For example, in a randomized crossover study (n 5 20) anodal tDCS applied over the L-DLPFC improved the accuracy to responses in a Go-No-Go task, whereas cathodal tDCS improved No-Go accuracy suggesting improved inhibitory control.57 Other studies looked at the effects of the slow oscillating tDCS on modulating cortical activity during non–rapid eye movement sleep phase 2. Participants had improved reaction time and memory performance after slow oscillating tDCS.58,59
Epilepsy
Several case series with tDCS revealed reductions in epileptiform discharges in children with continuous spike and Wave during slow-wave sleep and Landau-Kleffner syndrome.60 Initial pilot work in children suggested that tDCS reduced seizure frequency and severity in patients with generalized seizures due to cerebral palsy and other brain lesions,61 Rasmussen encephalitis,62 and focal cortical dysplasia.63 In a randomized sham-controlled trial, Auvichayapat and colleagues64 found a reduction in epileptiform discharges at 24 hours, 48 hours, and 4 weeks posttreatment following a single session of tDCS. In contrast, another study found no reduction in epileptiform activity.65 Overall, tDCS has been well tolerated in patients with epilepsy except that a single case of seizure was reported during a course of tDCS.66
Cerebral Palsy/Dystonia
Dystonia is one of the most common movement disorders in children and does not always respond to classical pharmacologic interventions.67 TDCS studies in dystonia have focused on combination of different therapeutic approaches with tDCS, including CIMT, visual reality, and treadmill. In a randomized clinical study of 20 patients with spastic cerebral palsy, anodal tDCS (1 mA, 20 min, 10 sessions total) applied over C3 combined with virtual reality mobility training improved velocity and cadence, mobility, and gross motor function.68 Other studies showed similar results, including increase of body sway velocity,69 decreased spasticity,70 and improved static balance.71 In contrast to aforementioned findings, Bhanpuri and colleagues72 showed that anodal tDCS placed contralateral to the most affected limb worsened motor performance in patients with dystonia.
Dyslexia
Two studies explored the effects of tDCS in the treatment of dyslexia in children. In a sham-controlled study, Costanzo and colleagues73 showed that anodal tDCS (1 mA, 20 min, 18 sessions) applied over the left parietotemporal region, with the cathode placed over the right homologue region, led to improved reading when combined with reading training. In a subsequent study, the investigators showed that cathodal tDCS applied over the left parietotemporal region increased the number of errors, whereas the anodal tDCS over the same region decreased the number of errors.74
FRONTIERS AND EMERGING TECHNOLOGIES
Trigeminal Nerve Stimulation and Magnetic Seizure Therapy
External stimulation of the trigeminal nerve (eTNS) and magnetic seizure therapy (MST) are emerging neuromodulatory techniques that have been shown to have therapeutic effects in adults. In an 8-week open-label pilot trial including 24 participants (between ages 7 and14 years) with ADHD, eTNS administered at night time led to significant improvement in ADHD-IV Rating Scale and Conners Global Index.75 There is a single case report in which the investigators reported full remission of depressive symptoms in an 18-year-old boy with refractory depression in the context of bipolar II disorder following 18 sessions of 100 Hz MST.76
DEVELOPMENTAL AND SAFETY CONSIDERATIONS
A recent systematic review that examined 48 studies with 513 children younger than 18 years supported the safety and feasibility of TMS and tDCS in children and adolescents.3 Yet, in a recent commentary, Davis identified the potential gaps in translating brain stimulation techniques to children.77 These include the unknown effects of stimulation in developing brains due to differences in anatomy and physiology, unknown side effects, limited translational data, and inherent ethical challenges in work with vulnerable populations.77
One of the most serious possible side effects of NIBS is seizure. MTs are typically higher in young children and reach adult levels by the age of 16 to 18 years.78 As a result, higher stimulus intensities required in younger children might be associated with increased risk for adverse effects.1 Moreover, infants and young children are thought to be especially prone to seizures due to increased glutamate sensitivity, reduced glutamate clearance, and incomplete GABA-mediated inhibition in the developing brain.37 Therefore, further shift toward the excitatory activity induced by TMS could theoretically increase the seizure risk. Moreover, computational modeling studies suggest that typical intensities of tDCS results in higher densities and peak electrical fields in the cortex of children compared with that in adults.79,80 Given the conductivity of the underlying biological tissues plays an important role in determining the maximum intensity and the distribution of the current that reaches to the cortex,79 differences in skull size and composition can result in variability in the amount of the current delivered to the cortex, introducing not only safety concerns but also intersubject variability in dosing, making standardization more difficult. In addition, the relative size of the external auditory canal is smaller in young children resulting in higher resonance frequency,81 which can increase the risk of acoustic injury during the delivery of TMS pulses.1
NEUROETHICS
Early guidelines regarding recruitment of children in TMS trials conclude that, unless there is compelling evidence for treatment of refractory cases, children should not be included in TMS trials due to concerns for interfering with normal neurodevelopment.82
Another important aspect to consider is potential applications of NIBS as a tool for neuroenhancement. It has been demonstrated that brain stimulation can enhance cognitive functions.83 However, it is still unknown if improvement in one domain hinders the functioning of other domains.84 Other concerns include emergence of unexpected effects such as unintentional behavioral responses or the discovery of incidental but clinically nonsignificant findings.85
In addition, tDCS or similar devices can be easily purchased online or constructed at home by simply watching online videos. Advertisement of these techniques without proper regulatory approvals could lead to inappropriate use of these techniques resulting in significant health issues.
SUMMARY
NIBS techniques have emerged as novel tools to promote plasticity and alleviate symptoms in neuropsychiatric disorders. Despite the intrinsic challenges in work with children and adolescents, the growing evidence suggests that brain stimulation will offer powerful and alternative tools to treat early onset neuropsychiatric disorders.
KEY POINTS.
Neuromodulation is a rapidly developing field that will provide opportunities to develop new therapeutic modalities in child and adolescent psychiatry.
Recent research has examined the feasibility and safety of transcranial direct current stimulation and transcranial magnetic stimulation in child and adolescent neuropsychiatric disorders.
Enthusiasm for applying neuromodulatory tools in childhood and adolescent neuropsychiatric disorders must be moderated with systematic study, neurodevelopmental considerations, and rigorous ethical analyses.
Acknowledgments
Financial Disclosures: Dr P.E. Croarkin has received research grant support from Pfizer (WS1926243), National Institute of Mental Health (K23MH10026 and R01MH113700), the Brain and Behavior Research Foundation (20883), and the Mayo Clinic Foundation. He has received equipment support from Neuronetics, Inc and receives supplies and genotyping services from Assurex Health, Inc for investigator-initiated studies. He is the primary investigator for a multicenter study of adolescent TMS funded by Neuronetics, Inc. Drs D.D. Camsari and M. Kirkovski have no financial disclosures.
REFERENCES
- 1.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120(12):2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croarkin PE, Wall CA, Lee J. Applications of transcranial magnetic stimulation (TMS) in child and adolescent psychiatry. Intl Rev of Psyc 2011;23(5):445–53. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan C, Santos L, Peterson MD, et al. Safety of noninvasive brain stimulation in children and adolescents. Brain stimulation 2015;8(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm U, Segmiller FM, Epple AN, et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. J Neural Transm 2016; 123(10):1219–34. [DOI] [PubMed] [Google Scholar]
- 5.Narayana S, Papanicolaou AC, McGregor A, et al. Clinical applications of transcranial magnetic stimulation in pediatric neurology. J Child Neurol 2015;30(9):1111–24. [DOI] [PubMed] [Google Scholar]
- 6.Allen CH, Kluger BM, Buard I. Safety of transcranial magnetic stimulation in children: a systematic review of the literature. Pediatr Neurol 2017;68:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong YH, Wu SW, Pedapati EV, et al. Safety and tolerability of theta burst stimulation vs. single and paired pulse transcranial magnetic stimulation: a comparative study of 165 pediatric subjects. Front Hum Neurosci 2015;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S-H, Wang S-S, Zhang M-M, et al. Repetitive transcranial magnetic stimulation-induced seizure of a patient with adolescent-onset depression: a case report and literature review. J Int Med Res 2011;39(5):2039–44. [DOI] [PubMed] [Google Scholar]
- 9.Chiramberro M, Lindberg N, Isometsä E, et al. Repetitive transcranial magnetic stimulation induced seizures in an adolescent patient with major depression: a case report. Brain Stimul 2013;6(5):830–1. [DOI] [PubMed] [Google Scholar]
- 10.Cullen KR, Jasberg S, Nelson B, et al. Seizure induced by deep transcranial magnetic stimulation in an adolescent with depression. J Child Adolesc Psychopharmacol 2016;26(7):637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloch Y, Grisaru N, Harel EV, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT 2008; 24(2):156–9. [DOI] [PubMed] [Google Scholar]
- 12.Kirton A, deVeber G, Gunraj C, et al. Neurocardiogenic syncope complicating pediatric transcranial magnetic stimulation. Pediatr Neurol 2008;39(3):196–7. [DOI] [PubMed] [Google Scholar]
- 13.Mayer G, Aviram S, Walter G, et al. Long-term follow-up of adolescents with resistant depression treated with repetitive transcranial magnetic stimulation. J Ect 2012;28(2):84–6. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson AE, Gordon MS, Melvin GA, et al. Addressing the needs of adolescents with treatment resistant depressive disorders: a systematic review of rTMS. Brain Stimul 2014;7(1):7–12. [DOI] [PubMed] [Google Scholar]
- 15.Wall CA, Croarkin PE, Sim LA, et al. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: a prospective, open pilot study. J Clin Psychiatry 2011;72(9):1263–9. [DOI] [PubMed] [Google Scholar]
- 16.Wall CA, Croarkin PE, Maroney-Smith MJ, et al. Magnetic resonance imaging-guided, open-label, high-frequency repetitive transcranial magnetic stimulation for adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 2016;26(7):582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XR, Kirton A, Wilkes TC, et al. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT 2014; 30(3):242–7. [DOI] [PubMed] [Google Scholar]
- 18.Caetano SC, Fonseca M, Olvera RL, et al. Proton spectroscopy study of the left dorsolateral prefrontal cortex in pediatric depressed patients. Neurosci Lett 2005; 384(3):321–6. [DOI] [PubMed] [Google Scholar]
- 19.Sokhadze EM, El-Baz AS, Sears LL, et al. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci 2014;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokhadze EM, El-Baz AS, Tasman A, et al. Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: an exploratory study. Appl Psychophysiol Biofeedback 2014;39(3–4):237–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y-Z, Rothwell JC. Theta burst stimulation. In: Marcolin MA, Padberg F, editors. Transcranial brain stimulation for treatment of psychiatric disorders Basel (Switzerland: ): Karger; 2007. p. 187–203. [Google Scholar]
- 22.Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res 2003;55(1):13–22. [DOI] [PubMed] [Google Scholar]
- 23.George MS, Sallee FR, Nahas Z, et al. Transcranial magnetic stimulation (TMS) as a research tool in Tourette syndrome and related disorders. Adv Neurol 2001;85: 225–35. [PubMed] [Google Scholar]
- 24.Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett 2011;492(1):1–4. [DOI] [PubMed] [Google Scholar]
- 25.Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci 2013;20(2):257–62. [DOI] [PubMed] [Google Scholar]
- 26.Faraone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: is it an American condition? World psychiatry 2003;2(2):104. [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver L, Rostain AL, Mace W, et al. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder in adolescents and young adults: a pilot study. J ECT 2012;28(2):98–103. [DOI] [PubMed] [Google Scholar]
- 28.Driver DI, Gogtay N, Rapoport JL. Childhood onset schizophrenia and early onset schizophrenia spectrum disorders. Child Adolesc Psychiatr Clin N Am 2013; 22(4):539–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman RE, Boutros NN, Hu S, et al. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet 2000;355(9209):1073–5. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry 2003;60(1):49–56. [DOI] [PubMed] [Google Scholar]
- 31.Jardri R, Bubrovszky M, Demeulemeester M, et al. Repetitive transcranial magnetic stimulation to treat early-onset auditory hallucinations. J Am Acad Child Adolesc Psychiatry 2012;51(9):947–9. [DOI] [PubMed] [Google Scholar]
- 32.Jardri R, Lucas B, Delevoye-Turrell Y, et al. An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry 2007;12(4):320. [DOI] [PubMed] [Google Scholar]
- 33.Kirton A, Chen R, Friefeld S, et al. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: a randomised trial. Lancet Neurol 2008;7(6):507–13. [DOI] [PubMed] [Google Scholar]
- 34.Kirton A, Andersen J, Herrero M, et al. Brain stimulation and constraint for perinatal stroke hemiparesis The PLASTIC CHAMPS Trial. Neurology 2016;86(18): 1659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotenberg A, Bae EH, Muller PA, et al. In-session seizures during low-frequency repetitive transcranial magnetic stimulation in patients with epilepsy. Epilepsy Behav 2009;16(2):353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szymkowicz SM, McLaren ME, Suryadevara U, et al. Transcranial direct current stimulation use in the treatment of neuropsychiatric disorders: a brief review. Psychiatr Ann 2016;46(11):642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hameed MQ, Dhamne SC, Gersner R, et al. Transcranial magnetic and direct current stimulation in children. Curr Neurol Neurosci Rep 2017;17(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muszkat D, Polanczyk GV, Dias TGC, et al. Transcranial direct current stimulation in child and adolescent psychiatry. J child Adolesc Psychopharmacol 2016; 26(7):590–7. [DOI] [PubMed] [Google Scholar]
- 39.Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation 2012;5(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink M Convulsive therapy: a review of the first 55 years. J Affect Disord 2001; 63(1):1–15. [DOI] [PubMed] [Google Scholar]
- 41.Ghaziuddin N, Kutcher SP, Knapp P. Practice parameter for use of electroconvulsive therapy with adolescents. J Am Acad Child Adolesc Psychiatry 2004;43(12): 1521–39. [DOI] [PubMed] [Google Scholar]
- 42.Puffer CC, Wall CA, Huxsahl JE, et al. A 20 year practice review of electroconvulsive therapy for adolescents. J child Adolesc Psychopharmacol 2016;26(7): 632–6. [DOI] [PubMed] [Google Scholar]
- 43.Brunoni AR, Moffa AH, Fregni F, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry 2016;208(6):522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein D, Weizman A, Bloch Y. Electroconvulsive therapy and transcranial magnetic stimulation: can they be considered valid modalities in the treatment of pediatric mood disorders? Child Adolesc Psychiatr Clin 2006;15(4):1035–56. [DOI] [PubMed] [Google Scholar]
- 45.Mattai A, Miller R, Weisinger B, et al. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain stimulation 2011;4(4):275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baeza I, Flamarique I, Garrido JM, et al. Clinical experience using electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. J child Adolesc Psychopharmacol 2010;20(3):205–9. [DOI] [PubMed] [Google Scholar]
- 47.Andrade AC, Magnavita GM, Allegro JVBN, et al. Feasibility of transcranial direct current stimulation use in children aged 5 to 12 years. J Child Neurol 2014;29(10): 1360–5. [DOI] [PubMed] [Google Scholar]
- 48.Schneider HD, Hopp JP. The use of the bilingual aphasia test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clin Linguist Phon 2011;25(6–7):640–54. [DOI] [PubMed] [Google Scholar]
- 49.Amatachaya A, Auvichayapat N, Patjanasoontorn N, et al. Effect of anodal transcranial direct current stimulation on autism: a randomized double-blind crossover trial. Behav Neurol 2014;2014:173073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amatachaya A, Jensen MP, Patjanasoontorn N, et al. The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: a randomized crossover controlled trial. Behav Neurol 2015;2015: 928631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wachtel LE, Griffin MM, Dhossche DM, et al. Brief report: Electroconvulsive therapy for malignant catatonia in an autistic adolescent. Autism 2010;14(4):349–58. [DOI] [PubMed] [Google Scholar]
- 52.Wachtel LE, Contrucci-Kuhn SA, Griffin M, et al. ECT for self-injury in an autistic boy. Eur Child Adolesc Psychiatry 2009;18(7):458–63. [DOI] [PubMed] [Google Scholar]
- 53.Wachtel LE, Kahng S, Dhossche DM, et al. ECT for catatonia in an autistic girl. Am J Psychiatry 2008;165(3):329–33. [DOI] [PubMed] [Google Scholar]
- 54.Cosmo C, Baptista AF, de Arau´jo AN, et al. A randomized, double-blind, sham-controlled trial of transcranial direct current stimulation in attention-deficit/ hyperactivity disorder. PLoS One 2015;10(8):e0135371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ditye T, Jacobson L, Walsh V, et al. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res 2012;219(3):363–8. [DOI] [PubMed] [Google Scholar]
- 56.Bandeira ID, Guimarães RSQ, Jagersbacher JG, et al. Transcranial direct current stimulation in children and Adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD) a pilot study. J child Neurol 2016;31(7):918–24. [DOI] [PubMed] [Google Scholar]
- 57.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Attent Disord 2015. 1087054715618792. [DOI] [PubMed] [Google Scholar]
- 58.Munz MT, Prehn-Kristensen A, Thielking F, et al. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front Cell Neurosci 2015; 9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prehn-Kristensen A, Munz M, Göder R, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain stimulation 2014;7(6):793–9. [DOI] [PubMed] [Google Scholar]
- 60.Faria P, Fregni F, Sebastião F, et al. Feasibility of focal transcranial DC polarization with simultaneous EEG recording: preliminary assessment in healthy subjects and human epilepsy. Epilepsy Behav 2012;25(3):417–25. [DOI] [PubMed] [Google Scholar]
- 61.Shelyakin AM, Preobrazhenskaya IG, Kassil’ MV, et al. The effects of transcranial micropolarization on the severity of convulsive fits in children. Neurosci Behav Physiol 2001;31(5):555–60. [DOI] [PubMed] [Google Scholar]
- 62.San-Juan D, Calcáneo Jde D, González-Aragón MF, et al. Transcranial direct current stimulation in adolescent and adult Rasmussen’s encephalitis. Epilepsy Behav 2011;20(1):126–31. [DOI] [PubMed] [Google Scholar]
- 63.Yook S-W, Park S-H, Seo J-H, et al. Suppression of seizure by cathodal transcranial direct current stimulation in an epileptic patient: a case report. Ann Rehabil Med 2011;35(4):579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auvichayapat N, Rotenberg A, Gersner R, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimulation 2013;6(4):696–700. [DOI] [PubMed] [Google Scholar]
- 65.Varga ET, Terney D, Atkins MD, et al. Transcranial direct current stimulation in refractory continuous spikes and waves during slow sleep: a controlled study. Epilepsy Res 2011;97(1–2):142–5. [DOI] [PubMed] [Google Scholar]
- 66.Ekici B Transcranial direct current stimulation-induced seizure: analysis of a case. Clin EEG Neurosci 2015;46(2):169. [DOI] [PubMed] [Google Scholar]
- 67.Mink JW. Special concerns in defining, studying, and treating dystonia in children. Mov Disord 2013;28(7):921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collange Grecco LA, de Almeida Carvalho Duarte N, Mendonc a ME, et al. Effects of anodal transcranial direct current stimulation combined with virtual reality for improving gait in children with spastic diparetic cerebral palsy: a pilot, randomized, controlled, double-blind, clinical trial. Clin Rehabil 2015;29(12): 1212–23. [DOI] [PubMed] [Google Scholar]
- 69.Lazzari RD, Politti F, Santos CA, et al. Effect of a single session of transcranial direct-current stimulation combined with virtual reality training on the balance of children with cerebral palsy: a randomized, controlled, double-blind trial. J Phys Ther Sci 2015;27(3):763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aree-uea B, Auvichayapat N, Janyacharoen T, et al. Reduction of spasticity in cerebral palsy by anodal transcranial direct current stimulation. J Med Assoc Thai 2014;97(9):954–62. [PubMed] [Google Scholar]
- 71.Grecco LA, Duarte NA, Zanon N, et al. Effect of a single session of transcranial direct-current stimulation on balance and spatiotemporal gait variables in children with cerebral palsy: A randomized sham-controlled study. Braz J Phys Ther 2014;18(5):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhanpuri NH, Bertucco M, Young SJ, et al. Multiday transcranial direct current stimulation causes clinically insignificant changes in childhood dystonia: a pilot study. J child Neurol 2015;30(12):1604–15. [DOI] [PubMed] [Google Scholar]
- 73.Costanzo F, Varuzza C, Rossi S, et al. Evidence for reading improvement following tDCS treatment in children and adolescents with Dyslexia. Restor Neurol Neurosci 2016;34(2):215–26. [DOI] [PubMed] [Google Scholar]
- 74.Costanzo F, Varuzza C, Rossi S, et al. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. Neuroreport 2016; 27(5):295–300. [DOI] [PubMed] [Google Scholar]
- 75.McGough JJ, Loo SK, Sturm A, et al. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain stimulation 2015;8(2):299–304. [DOI] [PubMed] [Google Scholar]
- 76.Noda Y, Daskalakis ZJ, Downar J, et al. Magnetic seizure therapy in an adolescent with refractory bipolar depression: a case report. Neuropsychiatr Dis Treat 2014;10:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci 2014;8:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eyre J Development and plasticity of the corticospinal system in man. Neural Plast 2003;10(1–2):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kessler SK, Minhas P, Woods AJ, et al. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One 2013;8(9):e76112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minhas P, Bikson M, Woods AJ, et al. Transcranial direct current stimulation in pediatric brain: a computational modeling study. Paper presented at: Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE2012 San Diego, CA, USA, August 28 - September 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kruger B An update on the external ear resonance in infants and young children. Ear and Hearing 1987;8(6):333–6. [DOI] [PubMed] [Google Scholar]
- 82.George MS, Bohning DE, Loberbaum J, et al. Overview of transcranial magnetic stimulation. In: George MS, Belmaker RH, editors. Transcranial magnetic stimulation in clinical psychiatry Washington, DC: American Psychiatric Publication; 2007. p. 1–38. [Google Scholar]
- 83.Snowball A, Tachtsidis I, Popescu T, et al. Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr Biol 2013; 23(11):987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kadosh RC, Levy N, O’Shea J, et al. The neuroethics of non-invasive brain stimulation. Curr Biol 2012;22(4):R108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rajapakse T, Kirton A. Non-invasive brain stimulation in children: applications and future directions. Transl Neurosci 2013;4(2):217–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


