Abstract
Purpose:
Racial disparities in childhood asthma prevalence increased after the 1990s. Obesity, which also varies by race/ethnicity, is an asthma risk factor but its contribution to asthma prevalence disparities is unknown.
Methods:
We analyzed nationally representative National Health Examination and Nutrition Survey data for 2–19 year olds with logistic regression and decomposition analyses to assess contributions of weight status to racial disparities in asthma prevalence, controlling for sex, age and income status.
Results:
From 1988–1994 to 2011–2014, asthma prevalence increased more among non-Hispanic black (NHB) (8.4% to 18.0%) than non-Hispanic white (NHW) youth (7.2% to 10.3%). Logistic regression showed that obesity was an asthma risk factor for all groups but that a three-way “weight status-race/ethnicity-time” interaction was not significant, i.e., weight status did not explain the race/ethnicity association with asthma over time. In decomposition analyses, weight status had a small contribution to NHB/NHW asthma prevalence disparities but most of the disparity remained unexplained by weight status or other asthma risk factors (sex, age and income status).
Conclusions:
NHB youth had a greater asthma prevalence increase from 1988–1994 to 2011–2014 than NHW youth. Most of the racial disparity in asthma prevalence remained unexplained after considering weight status and other characteristics.
Keywords: child, adolescents, asthma, obesity, health status disparities
INTRODUCTION
Asthma and obesity are both common conditions among children, and prevalence of both conditions have increased over the past three decades.(1, 2) Furthermore, obesity has increasingly been recognized as a risk factor for asthma.(3, 4) Racial disparities exist for both asthma and obesity prevalence among children, and it is unclear if rising obesity prevalence has contributed to asthma prevalence disparities.
The disparity in asthma prevalence between non-Hispanic black (NHB) and white (NHW) children grew from little to no disparity in the 1980s and 1990s to 40% higher in 2001, to 100% higher by 2007.(5) Over this period, asthma prevalence increased among NHB children while remaining stable among NHW children.(1) No clear explanation for this recently emerging asthma prevalence disparity has been elucidated, although studies suggest many factors contribute such as poverty, family structure, environmental and housing exposures, gene-environment interactions, low birthweight/premature birth, diet, and psychosocial stress (1, 6–8) as well as diagnostic differences and health care access factors.(1, 7) Yet, no analyses of these factors have completely explained racial disparities in US childhood asthma prevalence.(1, 7) Racial disparities in obesity prevalence also exist: in 2011–2014 obesity prevalence was 19.5% among NHB children versus 14.7% among NHW children.(9) These obesity prevalence disparities emerged earlier than those in asthma prevalence. Obesity prevalence increased four-fold among black compared to two-fold white children from 1971 to 2002,(10) and subsequently from 1999–2010, obesity increased among NHB males but not for other groups.(2)
A recent study found that the asthma prevalence disparity between NHB and NHW children persists, although it stopped increasing by 2013.(1) The potential impact of weight status on asthma prevalence disparities was not evaluated because measured weight and height were not collected in the survey used for that study. Including weight status as a potential factor in racial disparities in asthma is important because prospective studies have observed increased risk of incident asthma among children with obesity.(11–16) Obesity could have contributed racial disparities if NHB children had a higher rate of change in obesity prevalence and thus an increasingly larger proportion was at risk of developing asthma, and/or if the obesity-associated risk of developing asthma was higher among NHB children.
Our objective was to assess if, on a population level, changes in obesity prevalence contributed to the increased racial disparity in childhood asthma prevalence. We conducted a serial cross sectional study of five cycles of the National Health and Nutrition Examination Survey (NHANES), a primary source of nationally representative data on measured height and weight data, from 1988 to 2014 for US children and adolescents aged 2–19 years of age. This method is commonly used to assess secular trends on the population level. While serial cross sectional analysis cannot ascertain causal pathways between weight status and differential prevalence of asthma between race groups, no nationally-representative data exist where the same children were sampled repeatedly over time to allow longitudinal analysis of person-level data. Therefore, we assessed the relationship between weight status and asthma status and disparities using the same methods and same target population (US children) for each survey cycle, but each survey cycle included a different sample of children. We also used decomposition analysis to assess two possible mechanisms by which obesity prevalence could impact racial disparities in asthma prevalence: differential rates of asthma between race groups due to different obesity prevalence, and/or different risk of obesity for asthma between race groups.
METHODS:
The National Health and Nutritional Examination Survey (NHANES), administered by the National Center for Health Statistics (NCHS), uses a complex sampling design to conduct cross-sectional surveys of the US civilian, non-institutionalized population.(17, 18) Parental consent is obtained for participants <18 years of age, and child assent is obtained for 7–17 year olds. The NCHS Ethics Review Board approved the survey protocol.
Data for youth aged 2–19 years were used from NHANES III (1988–1994) and continuous NHANES in 4-year segments (1999–2002, 2003–2006, 2007–2010, and 2011–2014). Current asthma prevalence was defined using the CDC asthma surveillance definition as persons who had asthma at the time of survey participation(19) based on an affirmative response to two questions: “Has a doctor or other health professional ever told you that you have/your child has asthma?” and “Do you/does your child still have asthma?” Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Height and weight were measured using standard protocols.(20, 21) Weight status for all study participants was defined using CDC growth chart sex-specific BMI-for-age percentiles:(22) underweight, BMI <5th percentile; normal weight, 5th-<85th percentile; overweight, 85th -<95th percentile; and obesity, ≥95th percentile. Children <2 years of age were excluded because weight status is defined differently for this group, and asthma diagnosis is more difficult to ascertain in very young children.(23) Conditional examination response rates for 0–19 year olds was 85% in 1998–1994, and ranged between 76% and 87% during 1999–2014.(24)
Covariates
The following covariates were included: sex, age group (2–5 years, 6–11 years, 12–19 years), race/ethnicity (NHW, NHB, Mexican American (MA) and other), and income status, defined as the family income to the federal poverty threshold ratio (FIPR).(25) Income status categories were defined as <=1.85 FIPR, a cut-point used to determine eligibility for some federal programs, including reduced price school lunches, 1.85 - <=3.5 FIPR, and >3.5 FIPR. Highest educational achievement of the head of household was defined as less than high school, high school graduate, some college or greater, and missing. Unlike the covariates above, education was not associated with asthma status in univariate regression (p=0.64) and therefore was not included in the remainder of the analyses.
Exclusions and final sample
Of 42,049 youth aged 2–19 years, 236 were pregnant, 1033 were missing height and/or weight measurements, and 136 were missing asthma status information, leaving 40,644 eligible. Due to the low sample size of underweight participants, this group was also excluded (n=1439). The proportion of records with missing income information was <10% (n=3053, 7.7%), and had asthma prevalence (8.2%, SE 0.8) similar to the two nonpoor groups (those with income 1.85 FIPR and greater) and thus exclusion was less likely to lead to bias.(26) Therefore, these participants were excluded, leaving a final sample of 36,152. To assess the impact of missing income information, sensitivity analyses were performed (see Supplement).
Statistical Analyses
Analyses included assessment of trends in asthma prevalence and obesity, assessment of differences in asthma trends by race/ethnicity and weight status, logistic regression, and decomposition analysis. All analyses used sample weights to account for differential probability of selection and non-response, and accounted for the complex survey design. Statistical significance was determined at P<0.05.
Differences between subgroups were assessed with the chi-squared test. We tested unadjusted asthma and obesity trends (both linear and quadratic) using logistic regression in Stata SE Version 13.0. Differences in trends by race/ethnicity were tested by including an interaction term for race/ethnicity*survey year using an F-test to assess whether the interaction term coefficients were different from zero. The margins statement was used to estimate the change in prevalence per survey period.
Adjusted logistic regression models
Adjusted associations between asthma prevalence and the covariates described above were assessed using logistic regression in SUDAAN.(27) Interaction terms between race/ethnicity and sex, age group and income status were significant, suggesting models should be stratified by race/ethnicity. Variables associated with asthma status (p<0.10) in univariate analyses were included in multivariate models stratified by race/ethnicity. To assess whether the relationship between weight status and asthma status differed by race/ethnicity over time, a three-way interaction (survey year*race/ethnicity *weight status) was entered into the overall unstratified model (in addition to the three two-way interaction terms between these three variables).
Oaxaca-Blinder Decomposition
The contribution of a given risk factor to racial disparities can be decomposed into two main components. First, race/ethnicity groups can have differing prevalence of an asthma risk factor (e.g., an “endowment” component where higher prevalence of obesity (a risk factor for asthma) among NHB children would lead to higher asthma prevalence among NHB children compared to NHW children). Second, a risk factor (obesity) may pose differing levels of risk of asthma between race/ethnicity groups (e.g., “effect” component where the obesity-asthma association may be stronger or in a different direction in NHB versus NHW children). Logistic regression cannot disaggregate the overall contribution of a given risk factor into these two components. We used the Oaxaca Blinder decomposition package in Stata SE version 13.0 by Jann (28) to decompose the adjusted asthma prevalence disparity between NHB and NHW youth into “endowment” and “effect” components. The remaining portion of the difference between the comparison groups is the interaction between the endowment and the effects.(29)
Decomposition analysis can only consider the difference between two groups at a time (e.g., NHB versus NHW, or 1998–1994 cohort versus 2011–2014 cohort). Therefore, we used two separate decomposition analyses to assess the contribution of weight status to: 1) the NHB/NHW prevalence disparity over time, and 2) the asthma prevalence change from 1988–1994 to 2011–2014 in each race/ethnic group. For both models, only the first (1988–1994) and last survey periods (2011–2014) were included given a) that we were primarily interested in assessing the change in NHB/NHW asthma disparity, rather than the averaged disparity over the entire time period and b) the constraint to two comparison groups in decomposition analysis. In the first model, the outcome was the NHB/NHW racial disparity in asthma prevalence measured in percentage points (MA children were not included in this analysis). This disparity was decomposed to assess the contributions of covariates to the racial disparity in the beginning survey period (1988–1994) and end survey period (2011–2014) separately. This model allows comparison of contributions of covariates, including weight status, to the NHB/NHW disparity before and after the disparity grew. In the second model, the outcome was the change in asthma prevalence between 1988–1994 and 2011–2014 within each race/ethnic group. This model allows assessment of how covariates contributed to the growth in asthma prevalence for NHB, NHW and MA children separately.
With decomposition analysis, an issue known as the “identification problem” has been recognized where results depend on the choice of the omitted (reference) category of each variable.(30) A solution is to compute the decomposition using effects that are normalized.(30) We used the “normalize” option in the Stata package by Jann (28) and confirmed that model results did not change with varying reference categories. Also of note is that interpretation of the decomposition with normalized coefficients is only appropriate for the contribution of overall variables--the contribution of each category of a variable cannot be assessed in isolation. For example, results from the decomposition may indicate what percentage of the NHB/NHW disparity is due to differences in weight status, but not what percentage is due specifically to differences in obesity prevalence. Thus, decomposition results are shown only for overall variables and not individual categories. Finally, no omnibus test is available to assess whether the overall variable (e.g., weight status) contributes to the disparity. Instead, if any coefficient for each of the dummy variables representing categories (e.g., normal weight, overweight, obesity) were statistically significantly different from zero, the overall variable was assumed to contribute significantly to the disparity.
RESULTS:
From 1988–1994 to 2011–2014, current asthma prevalence among 2–19 year olds increased from 7.3% (SE 0.5) to 10.9% (SE 0.6) (p<0.001) (Table 1). Similar to patterns in other national data sources,(19) in 1988–1994, asthma prevalence was higher among 12–19 year olds and NHB youth. In 2011–2014, asthma prevalence was higher among 12–19 years olds, NHB youth, those with low family income, and youth with obesity.
Table 1.
Sample Characteristics, Youth 2–19 Years of Age, United States, 1988–1994 and 2011–2014a
| 1988–1994 | 2011–2014 | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Weighted Percent (SE) | Asthma prevalence (SE) | P-valueb | n | Weighted Percent (SE) | Asthma prevalence (SE) | P-valueb | |
| Total | 9437 | 100 | 7.3 (0.5) | 6112 | 100 | 10.9 (0.6) | ||
| Sex | ||||||||
| Male | 4635 | 50.9 (1.0) | 7.6 (0.6) | 0.397 | 3107 | 50.9 (0.9) | 11.4 (0.9) | 0.361 |
| Female | 4802 | 49.1 (1.0) | 7.0 (0.6) | 3005 | 49.1 (0.9) | 10.4 (0.7) | ||
| Age | ||||||||
| 2–5 years | 3820 | 23.2 (0.8) | 5.4 (0.5) | 0.003 | 1511 | 21.8 (0.8) | 6.9 (1.1) | <0.001 |
| 6–11 years | 2908 | 35.0 (0.9) | 7.4 (0.9) | 2308 | 33.2 (0.8) | 11.6 (0.9) | ||
| 12–19 years | 2709 | 41.7 (1.3) | 8.2 (0.8) | 2293 | 45.0 (1.0) | 12.4 (0.9) | ||
| Race/ethnicity | ||||||||
| NH white | 2682 | 66.1 (1.8) | 7.2 (0.8) | 0.094 | 1522 | 54.0 (3.5) | 10.3 (0.9) | <0.001 |
| NH black | 3163 | 15.3 (1.2) | 8.4 (0.8) | 1702 | 14.5 (1.9) | 18.0 (1.2) | ||
| Mex Am | 3145 | 8.4 (0.8) | 5.5 (1.0) | 1240 | 15.0 (2.0) | 8.1 (1.2) | ||
| Other | 447 | 9.7 (1.3) | 7.3 (1.6) | 1648 | 16.5 (1.3) | 9.5 (1.0) | ||
| Income status | ||||||||
| <=1.85 FIPR | 6069 | 45.8 (1.3) | 7.2 (0.6) | 0.983 | 3776 | 49.6 (2.7) | 12.2 (1.2) | 0.021 |
| 1.85-<=3.5 FIPR | 2362 | 33.4 (1.4) | 7.3 (1.1) | 1139 | 22.5 (1.3) | 11.7 (1.2) | ||
| >3.5 FIPR | 1006 | 20.8 (1.4) | 7.4 (1.3) | 1197 | 27.9 (2.3) | 8.0 (1.0) | ||
| Highest household education | ||||||||
| < HS | 3627 | 23.7 (1.1) | 5.6 (0.7) | 0.032 | 1504 | 19.6 (1.4) | 10.0 (1.1) | 0.43 |
| HS graduate | 3105 | 34.9 (1.2) | 8.2 (1.0) | 1360 | 20.6 (1.5) | 12.0 (1.6) | ||
| Some college+ | 2705 | 41.5 (1.5) | 7.4 (1.0) | 3081 | 57.3 (2.2) | 10.8 (0.8) | ||
| Missing | 0 | -- | -- | 167 | 2.6 (0.4) | 13.1 (3.3) | ||
| Weight status | ||||||||
| Normal | 7039 | 76.2 (1.0) | 6.3 (0.5) | 0.030 | 3995 | 66.1(0.9) | 10.3 (0.5) | 0.001 |
| Overweight | 1267 | 13.5 (0.7) | 8.4 (1.2) | 990 | 16.2 (0.6) | 8.2 (1.1) | ||
| Obese | 1131 | 10.3 (0.6) | 13.4 (3.2) | 1127 | 17.6 (0.8) | 15.7 (1.9) | ||
FIPR=family income to poverty ratio; HS=high school; NH=non-Hispanic
Although all survey cycles are included in subsequent analyses, only the first and last cycles are shown to allow assessment of change over the study period. Underweight youth and those missing income status information were excluded.
P-value for chi square comparison of asthma prevalence between categories.
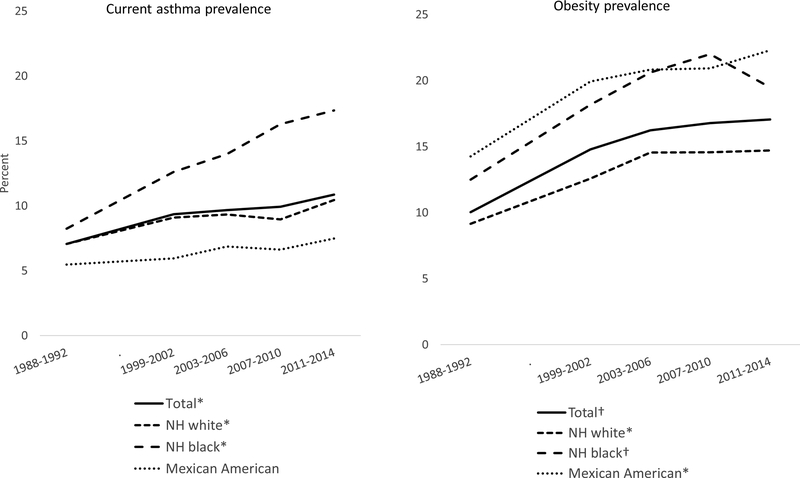
Figure 1 shows asthma and obesity prevalence trends. From 1988–1994 to 2011–2014, asthma prevalence increased among NHW youth from 7.2% (SE 0.8) to 10.3% (SE 0.9), an average 0.6 percentage points per survey period (95% CI 0.1, 1.1). Asthma prevalence increased among NHB youth (8.4% (SE 0.8) to 18.0% (SE 1.2) by an average 2.3 percentage points per period (95% CI 1.6, 3.0). Prevalence did not change significantly among MA youth (p>0.05). The joint F-test showed a significant race/ethnicity difference in asthma prevalence trends (p=0.003). The NHB/NHW asthma prevalence ratio was 1.2 in 1988–1994 and 1.7 in 2011–2014.
Figure 1:
Prevalence of current asthma, obesity among children and adolescents 2–19 years of age, by race/Hispanic origin, 1988–1994 to 2011–2014
* Significant linear trend, p<0.05
† Significant quadratic trend p<0.05
Obesity prevalence followed a quadratic trend for the total population (10.3%, SE 0.6 in 1988–1994, 16.8, SE 0.8 in 2007–2010 and 17.1%, SE 0.7 in 2011–2014). By race/ethnicity, a quadratic trend in obesity prevalence was observed for NHB youth (12.5% (SE 0.8) in 1988–1994, 22.0% (SE 1.2) in 2007–2010, and 19.5% (SE 1.2) in 2011–2014), and an increasing linear trend was seen among NHW (9.6% (SE 0.8) in 1988–1994 and 14.7% (SE 1.2) in 2011–2014) and MA youth (14.3% (SE 1.5) in 1988–1994 and 22.3% (SE 1.2) in 2011–2014). Despite the apparent difference in trend patterns by race/ethnicity, the differences were not statistically significant (joint F-test P=0.85).
Trends in asthma prevalence by sociodemographic characteristics
Asthma prevalence among youth with normal weight increased among all race/ethnicity groups (Figure 2), but asthma prevalence among those with obesity increased only among NHB youth. Among overweight youth, asthma prevalence followed a downward bending quadratic trend among NHW youth, an increasing trend among NHB youth, and no change among MA youth. The association between weight status and asthma status for each race/ethnicity group are shown in Table 2 and Supplemental Figures A–C. Logistic regression models yielded similar associations between asthma status and weight status for all three race/ethnicity groups, with increased odds of asthma (if not always statistically significant) for overweight and obese categories. However, the odds of having asthma increased with age for NHW and MA, but not NHB youth. Females had lower odds of having asthma among NHB and MA, but not NHW youth. Finally, low income status was associated with asthma for NHW and MA, but not NHB youth. The three-way interaction term between weight status, race/ethnicity and survey period was not significant (p=0.525, data not shown). This was true even if the model was limited to NHW and NHB youth (p=0.406). This result suggests that weight status changes did not play a significant role in the increasing NHB/NHW asthma disparity.
Figure 2:
Current asthma prevalence among children and adolescents 2–19 years of age, by race/Hispanic origin and weight status, 1988–2014
Table 2.
Odds Ratios of Having Asthma Among Youth 2–19 Years of Agea, Stratified by Race/Ethnicity, United States, 1988–2014
| NH white | NH black | Mex Am | |
|---|---|---|---|
| Weight status | |||
| Normal | Ref | Ref | ref |
| Overweight | 1.1 (0.9, 1.4) | 1.4 (1.2, 1.7)** | 1.2 (0.9, 1.5) |
| Obese | 1.7 (1.4, 2.2)** | 1.8 (1.6, 2.1)** | 1.4 (1.1, 1.8)* |
| Survey year | |||
| 1988–1994 | Ref | Ref | Fef |
| 1999–2002 | 1.2 (0.9, 1.6) | 1.6 (1.2, 2.1)* | 1.0 (0.7, 1.6) |
| 2003–2006 | 1.3 (1.0, 1.8)* | 1.7 (1.4, 2.2)** | 1.2 (0.8, 1.8) |
| 2007–2010 | 1.2 (0.9, 1.6) | 2.0 (1.6, 2.6)** | 1.2 (0.8, 1.9) |
| 2011–2014 | 1.4 (1.1, 1.9)* | 2.3 (1.8, 3.0)** | 1.4 (0.9, 2.3) |
| Age group | |||
| 2–5 years | Ref | Ref | Ref |
| 6–11 years | 1.4 (1.1, 1.8)* | 0.9 (0.8, 1.1) | 1.6 (1.2, 2.1)** |
| 12–19 years | 1.7 (1.3, 2.1)** | 0.9 (0.8, 1.1) | 1.6 (1.2, 2.0)** |
| Sex | |||
| Male | Ref | Ref | Ref |
| Female | 0.9 (0.8, 1.1) | 0.7 (0.6, 0.7)** | 0.8 (0.7, 1.0)* |
| Income status | |||
| <=1.85 FIPR | 1.3 (1.0, 1.6)* | 1.2 (1.0, 1.5) | 0.6 (0.4, 0.7)** |
| 1.85 - <=3.5 FIPR | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.4) | 0.8 (0.6, 1.1) |
| >3.5 FIPR | Ref | Ref | Ref |
P <0.05,
P <0.001
FIPR=family income to poverty ratio; NH=non-Hispanic; OR=odds ratio
Sample size n=36152. Underweight youth and those missing income status information were excluded.
Decomposition of race/ethnicity asthma prevalence disparities
Table 3 shows the contribution of weight status and other covariates to NHB/NHW asthma prevalence disparities. Average adjusted asthma prevalence in 1988–1994 was 8.4% among NHB youth and 7.2% among NHW youth, with a difference of 1.2 percentage points (p>0.05). These 1.2 percentage points were decomposed into 0 percentage points explained by differences in characteristics (endowments) between NHW and NHB youth, 1.5 percentage points due different association between characteristics and asthma (effects), and −0.3 percentage points due to the interaction between endowments and effects. The only variable with a statistically significant contribution was age group for the effects component. That is, only the differing risk of having asthma by age group between NHW and NHB youth contributed to the small prevalence disparity in 1988–1994. The large intercept term (1.7 percentage points) shows that the majority of the prevalence difference remained unexplained by covariates included in the model.
Table 3.
Adjusted Current Asthma Prevalencea Among Youth 2–19 Years of Age with Decomposition Analysisb of Racial/Ethnic Disparity, United States, 1988–1994 and 2011–2014
| 1988–1994 | 2011–2014 | |
|---|---|---|
| n | 5845 | 3224 |
| NH black | 8.4** | 18.0** |
| NH white | 7.2** | 10.3** |
| Overall difference | 1.2 | 7.7** |
| Endowment | 0.0 | 1.7 |
| Effect | 1.5 | 5.4** |
| Interaction | −0.3 | 0.6 |
| Endowment (difference in prevalence due to varying characteristics between time periods) | ||
| Weight status | −0.2 | 0.0 |
| Age group | 0.0 | 0.0 |
| Sex | 0.0 | 0.0 |
| Income status | 0.2 | 1.7* |
| Effect (difference in prevalence due to impact of characteristic on risk for asthma) | ||
| Weight status | 0.4 | −2.6* |
| Age group | −0.5* | −0.9 |
| Sex | 0.1 | 0.0 |
| Income status | −0.2 | 0.2 |
| Intercept | 1.7 | 8.6** |
P<0.05,
P<0.001 for one or more individual variable categories
FIPR= family income to poverty ratio; NH=non-Hispanic
Asthma prevalence estimates adjusted for covariates shown in the table. Results are rounded to nearest tenth percent, and therefore sums may differ due to rounding.
The prevalence estimates for endowment, effect, and interaction components sum to the overall difference in asthma prevalence between NH black and NH white youth in each period. The endowment contributions for all variables sum to the overall endowment component, and the effect contributions for all variables and the intercept sum to the overall effect component. Estimates of the interaction between explained and unexplained differences were also estimated for each variable (not shown) but were small and not statistically significant.
For 2011–2014, the asthma prevalence difference between NHB (18%) and NHW youth (10.3%) was 7.7 percentage points (p-value <0.001). Of this, 1.7 percentage points were due to endowments, 5.4 percentage points due to effects, and 0.6 percentage points due to the interaction between endowments and effects. Of these, only the effects component was statistically significantly. Among the endowment contributions, income status was statistically significant (1.7% percentage points). Among effect contributions, the intercept term represented the largest contribution (8.6 percentage points) to the NHB/NHW disparity and represents the portion of the disparity that remains unexplained by the covariates in the model. Recall that the overall effects contribution to the racial disparity is the sum of the effects contribution of each covariate as well as the intercept. In this case, the intercept (8.6 percentage points) is larger than the overall disparity of 7.7 percentage points because the contribution of weight status and other covariates was negative (weight status contributed −2.6 percentage points). The result for weight status suggests that if the relationship between weight status and asthma were the same for NHW and NHB children in 2011–2014, the asthma disparity would be even higher. This result is likely due to the decreased prevalence of asthma among overweight NHW children in 2011–2014 (see Figure 2).
Decomposition of trends in asthma prevalence by race/ethnicity
Another way to examine the role of weight status is by decomposing the asthma prevalence difference between 1988–1994 and 2011–2014 separately for each race/ethnicity group (Table 4). Among NHW youth, asthma prevalence grew by 3 percentage points (from 7.2% to 10.3%). Changes in endowments contributed 0.6 percentage points, changes in effects 3.1 percentage points (statistically significant), and the interaction −0.7 percentage points. Weight status was the only variable with a statistically significant effects contribution. That is, the increase in asthma prevalence among NHW youth was not due to changes in weight status among this group over time (endowment), but it was partly attributable to a change in the association between weight status prevalence and asthma over time (effects). NHB youth had a 9.6 percentage point increase in asthma prevalence between 1988–1994 (8.4%) and 2011–2014 (18.0%). Endowments contributed a small but statistically significant portion of this difference (0.7 percentage points), with weight status contributing 0.5 percentage points of the endowment portion. Among effects contributions (8.6 percentage points), income status accounted for 2.3 percentage points. Much of the increase in NHB asthma prevalence remained unexplained (6.2 percentage points were contributed by the intercept term). In sum, weight status had a larger contribution to changes in NHW asthma prevalence through effect contributions, in contrast to the smaller endowment contribution among NHB youth. This finding seems counterintuitive given the patterns observed in Figure 2, but supplemental figures A–C show that asthma prevalence increased among nearly every subgroup of NHB youth (for income status, age group and sex), in contrast to patterns for NHW and MA youth. That is, for NHB youth, neither weight status nor any other included covariate explained trends in asthma prevalence which increased in nearly every NHB subgroup.
Table 4.
Adjusted Current Asthma Prevalence Among Youth 2–19 Years of Age with Decomposition Analysis of Change Between 1988–1994 and 2011–2014, by Race/Ethnicity, United States
| NH White | NH Black | Mexican Am | |
|---|---|---|---|
| n | 4204 | 4865 | 4385 |
| 2011–2014 | 10.3** | 18.0** | 8.1** |
| 1988–1994 | 7.2** | 8.4** | 5.5** |
| Overall difference | 3.0* | 9.6** | 2.6 |
| Endowment | 0.6 | 0.7* | 0.2 |
| Effect | 3.1* | 8.6** | 2.4 |
| interaction | −0.7 | 0.3 | 0.0 |
| Endowment (difference in prevalence due to varying characteristics between time periods) | |||
| Weight status | 0.5 | 0.5* | 0.2 |
| Age group | 0.1 | 0.0 | 0.0 |
| Sex | 0.0 | 0.0 | 0.0 |
| Income status | 0.0 | 0.2 | -0.1 |
| Effect (difference in prevalence due to impact of characteristic on risk for asthma) | |||
| Weight status | 3.1* | 0.0 | 0.8 |
| Age group | 0.2 | 0.1 | 0.3 |
| Sex | 0.0 | 0.0 | 0.0 |
| Income status | 0.3 | 2.3* | -0.8 |
| Intercept | −0.4 | 6.2* | 2.0 |
P<0.05,
P<0.0001
FIPR=federal income to poverty ratio; NH=non-Hispanic
Asthma prevalence estimates adjusted for covariates shown in the table. Results rounded to nearest tenth percent, and therefore sums may differ due to rounding.
The prevalence estimates for endowment, effect, and interaction components sum to the overall change in asthma prevalence for each race/ethnicity group between 1988–1994 to 2011–2014. The endowment contributions for all variables sum to the overall endowment component, and the effect contributions for all variables and the intercept sum to the overall effect component. Estimates of the interaction between explained and unexplained differences are also produced by the model. None were statistically significant and are therefore not shown.
DISCUSSION:
Results from logistic regression and decomposition analyses suggest that changes in weight status did not explain the increasing asthma prevalence disparity between 1988–1994 and 2011–2014. The disparity in US childhood asthma prevalence arose from the greater increase among NHB versus NHW youth,(5) and this analysis also demonstrated that the asthma prevalence growth among NHB youth was not explained by available covariates, including weight status. Thus, it is not surprising that weight status also was not an explanatory variable for the NHB/NHW disparity.
The findings may still seem counterintuitive because among those with obesity, asthma prevalence increased among NHB youth, but not NHW or MA youth. However, asthma prevalence increased among all weight status subgroups of NHB youth versus only among normal weight NHW and MA youth. The supplemental figures show that this pattern of increasing asthma among all subgroups of NHB children was not unique to weight status but occurred for income group, age group and sex. The major difference between NHW and NHB youth was shown by decomposition analysis: NHW youth had a significant “effect” contribution of weight status, while NHB youth had a significant “effect” contribution of income status and had a greater portion of increased prevalence not explained by any covariates.
Despite the findings that increasing obesity prevalence did not contribute to asthma racial disparities, youth with obesity in all race/ethnic groups had higher prevalence compared to those in lower weight status categories. This and other studies show that weight status is a risk factor for asthma.(3, 13, 14, 16, 31–36) Additionally, although some studies found no association between obesity and asthma severity,(34, 37) several studies have shown obesity is associated with more frequent exacerbations, increased health care use, poor asthma control, and decreased response to asthma medications.(14, 33, 38–45)
The increased asthma prevalence disparity between NHB and NHW youth observed in this study is consistent with other nationally representative data,(5) but NHANES data also includes measured weight status,(1) allowing for an assessment of how trends in weight status might related to trends in disparities. However, this serial cross-sectional data and could not directly assess the impact of weight status on incident asthma. Yet, the increased risk for developing childhood asthma due to has been observed in several other studies.(13, 14, 16, 36) In addition, BMI may not be an ideal measure of adiposity: BMI does not measure adiposity equally well in children versus adults and men versus women due to differences in muscle mass, growth, lung development, and pre- and postpubertal sex hormones.(3) Furthermore, BMI does not measure adiposity equally in race/ethnicity groups: at a given BMI, NHB children have lower adiposity compared to NHW children.(46) Yet, BMI z-scores and percentiles have been shown to strongly predict fat mass among children at high levels of BMI,(47, 48) and it remains the standard measure of weight status.(49) A study of an allergy clinic population suggests that measures of central adiposity may be more closely related to asthma and atopy risk.(50) Other studies have found that BMI had a similar or stronger association with asthma status than central adiposity measures, including waist circumference.(32, 33) Other limitations study include possible uncontrolled confounding (e.g., atopic status, environmental exposures, diagnostic practice patterns, and health care utilization) and use of self-reported asthma diagnosis. Although asthma diagnosis was not confirmed with comparison to medical records, respondents were asked about receiving an asthma diagnosis from a health care professional.
In conclusion, while obesity is a risk factor for asthma, differences in weight status between race/ethnicity groups do not explain the increased asthma prevalence disparity between NHB and NHW youth from 1988–1994 to 2011–2014.
Supplementary Material
Acknowledgments
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- BMI
body mass index
- FIPR
federal income to poverty ratio
- MA
Mexican American
- NCHS
National Center for Health Statistics
- NH
non-Hispanic
- NHANES
National Health and Nutrition Examination Survey
- NHB
non-Hispanic black
- NHW
non-Hispanic white
- OR
odds ratio
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References:
- 1.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. American journal of respiratory and critical care medicine. 2006;174(2):112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7(5):325–35. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. The Journal of allergy and clinical immunology. 2014;134(3):547–53 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forno E, Celedon JC. Health disparities in asthma. American journal of respiratory and critical care medicine. 2012;185(10):1033–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gergen PJ, Togias A. Inner city asthma. Immunology and allergy clinics of North America. 2015;35(1):101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph CL, Williams LK, Ownby DR, Saltzgaber J, Johnson CC. Applying epidemiologic concepts of primary, secondary, and tertiary prevention to the elimination of racial disparities in asthma. The Journal of allergy and clinical immunology. 2006;117(2):233–40; quiz 41–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016;315(21):2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity. 2006;14(2):301–8. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland FD, Berhane K, Islam T, McConnell R, Gauderman WJ, Gilliland SS, et al. Obesity and the risk of newly diagnosed asthma in school-age children. American journal of epidemiology. 2003;158(5):406–15. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. American journal of respiratory and critical care medicine. 2001;163(6):1344–9. [DOI] [PubMed] [Google Scholar]
- 13.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatric pulmonology. 2003;36(6):514–21. [DOI] [PubMed] [Google Scholar]
- 14.Black MH, Zhou H, Takayanagi M, Jacobsen SJ, Koebnick C. Increased asthma risk and asthma-related health care complications associated with childhood obesity. American journal of epidemiology. 2013;178(7):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loid P, Goksor E, Alm B, Pettersson R, Mollborg P, Erdes L, et al. A persistently high body mass index increases the risk of atopic asthma at school age. Acta paediatrica. 2015;104(7):707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC pediatrics. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital and health statistics Series 2, Data evaluation and methods research. 2014(162):1–33. [PubMed] [Google Scholar]
- 18.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013(160):1–23. [PubMed] [Google Scholar]
- 19.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital & health statistics Series 3, Analytical and epidemiological studies / [US Dept of Health and Human Services, Public Health Service, National Center for Health Statistics]. 2012(35):1–67. [PubMed] [Google Scholar]
- 20.Zipf G, Chiappa M, Porter KS, et al. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. Hyattsville, MD: Center for Disease Control and Prevention; 2013. [PubMed] [Google Scholar]
- 21.Divison of Health and Nutrition Examination Survey. National Health and Nutrition Examination Survey: Anthropometry Procedures Manual Hyattsville, MD: National Center for Health Statistics; 2013. [updated January 2013. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_Anthropometry.pdf. [Google Scholar]
- 22.National Center for Health Statistics. Growth Charts: WHO Growth Standards Are Recommended for Use in the U.S. for Infants and Children 0 to 2 Years of Age Hyattsville, MD: Centers for Disease Control and Prevention; 2010. [Available from: http://www.cdc.gov/growthcharts/who_charts.htm#.The WHO Growth Charts [Google Scholar]
- 23.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. The New England journal of medicine. 1995;332(3):133–8. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics. NHANES Response Rates and CPS Totals. Hyattsville, MD: Centers for Disease Control and Prevention; [Available from: http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm. [Google Scholar]
- 25.Poverty [Internet]. U.S. Census Bureau. 2011. [cited 5/13/2016]. Available from: http://www.census.gov/hhes/www/poverty/.
- 26.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464–9. [PubMed] [Google Scholar]
- 27.Research Triangle Institute. SUDAAN Language Manual, Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 28.Jann B The Blinder-Oaxaca decomposition for linear regression models. The Stata Journal. 2008;8(4):453–79. [Google Scholar]
- 29.Biewen M Additive Decompositions with Interaction Effects. Bonn, Germany: Institute for the Study of Labor (IZA); 2012. July 2012. Contract No.: 6730. [Google Scholar]
- 30.Yun M A Simple Solution to the Identification Problem in Detailed Wage Decompositions. Economic Inquiry. 2005;43:766–72. [Google Scholar]
- 31.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax. 2001;56(11):845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egan KB, Ettinger AS, DeWan AT, Holford TR, Holmen TL, Bracken MB. General, but not abdominal, overweight increases odds of asthma among Norwegian adolescents: the Young-HUNT study. Acta paediatrica. 2014;103(12):1270–6. [DOI] [PubMed] [Google Scholar]
- 33.Forno E, Acosta-Perez E, Brehm JM, Han YY, Alvarez M, Colon-Semidey A, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. The Journal of allergy and clinical immunology. 2014;133(5):1308–14, 14 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gennuso J, Epstein LH, Paluch RA, Cerny F. The relationship between asthma and obesity in urban minority children and adolescents. Archives of pediatrics & adolescent medicine. 1998;152(12):1197–200. [DOI] [PubMed] [Google Scholar]
- 35.Han YY, Forno E, Celedon JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. American journal of respiratory and critical care medicine. 2014;190(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56(11):835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters JI, McKinney JM, Smith B, Wood P, Forkner E, Galbreath AD. Impact of obesity in asthma: evidence from a large prospective disease management study. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2011;106(1):30–5. [DOI] [PubMed] [Google Scholar]
- 38.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. The Journal of allergy and clinical immunology. 2011;127(3):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belamarich PF, Luder E, Kattan M, Mitchell H, Islam S, Lynn H, et al. Do obese inner-city children with asthma have more symptoms than nonobese children with asthma? Pediatrics. 2000;106(6):1436–41. [DOI] [PubMed] [Google Scholar]
- 40.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. American journal of respiratory and critical care medicine. 2013;187(7):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michelson PH WL, Benjanim DK, Barnato, AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001–2004. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2009;103:381–5. [DOI] [PubMed] [Google Scholar]
- 42.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. The Journal of allergy and clinical immunology. 2010;125(3):584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luder E, Melnik TA, DiMaio M. Association of being overweight with greater asthma symptoms in inner city black and Hispanic children. The Journal of pediatrics. 1998;132(4):699–703. [DOI] [PubMed] [Google Scholar]
- 44.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. The Journal of allergy and clinical immunology. 2011;128(5):964–9. [DOI] [PubMed] [Google Scholar]
- 46.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. The American journal of clinical nutrition. 2010;91(4):1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heo M, Wylie-Rosett J, Pietrobelli A, Kabat GC, Rohan TE, Faith MS. US pediatric population-level associations of DXA-measured percentage of body fat with four BMI metrics with cutoffs. International journal of obesity. 2014;38(1):60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. International journal of obesity. 2005;29(1):1–8. [DOI] [PubMed] [Google Scholar]
- 49.National Center fo Health Statistics. CDC Growth Charts: Centers for Disease Control and Prevention; 2010. [updated 9/9/2010. Available from: http://www.cdc.gov/growthcharts/cdc_charts.htm. [Google Scholar]
- 50.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, et al. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. The Journal of allergy and clinical immunology. 2009;123(6):1321–7 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.