Abstract
The mammalian Fem1b gene encodes a homolog of FEM-1, a protein in the sex-determination pathwayof the nematode Caenorhabditis elegans. Fem1b and FEM-1 proteins each contain a VHL-box motif that mediates their interaction with certain E3 ubiquitin ligase complexes. In C. elegans, FEM-1 negatively regulates the transcription factor TRA-1, and functions as an E3 ubiquitin ligase substrate recognition subunit to target TRA-1 for ubiquitylation. TRA-1 is homologous to the mammalian Gli1 protein, a transcription factor that mediates Hedgehog signaling as well as having Hedgehog-independent functions. Whether the interaction between nematode FEM-1 and TRA-1 proteins is conserved, between corresponding mammalian homologs, has not been reported. Herein, we show that Fem1b interacts with Gli1 within cells, and directly binds Gli1. Fem1b also promotes ubiquitylation of Gli1, suppresses transcriptional activation by Gli1, and attenuates an oncogenic Gli1 autoregulatory loop in cancer cells, all dependent on the VHL-box of Fem1b. These findings have implications for understanding the cellular functions of Fem1b, and the regulation of Gli1 oncoprotein activity.
1. Introduction
Fem1b is a member of the vertebrate Fem1 gene family, consisting of Fem1a, Fem1b, and Fem1c, and is involved in apoptosis regulation, glucose homeostasis, and reproductive physiology [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. Fem1b encodes a homolog of FEM-1, a protein within the sex-determination pathway of the nematode Caenorhabditis elegans[11], [12]. The nematode FEM-1 negatively regulates the transcription factor TRA-1, and functions as an E3 ubiquitin ligase substrate recognition subunit (SRS) to target TRA-1 for ubiquitylation [13]. The nematode FEM-1 and vertebrate Fem1b proteins each contain a C-terminal VHL-box motif that mediates interaction with E3 ubiquitin ligase complexes that contain Cul-2/Elongin-BC (CBC) subunits [13], [14], [15]. Fem1b has been shown to bind and promote ubiquitylation of the protein Ankrd37, a protein of unknown biological function [16].
It has been proposed that the sex-determination pathway in C. elegans is a variant of the Hedgehog signaling pathway of vertebrates [17], based on homology of components upstream and downstream of FEM-1: upstream, the cell-surface receptor TRA-2 has homology to the Hedgehog cell-surface receptor Patched [18]; downstream, the transcription factor TRA-1 has homology to the vertebrate Gli proteins, consisting of Gli1, Gli2, and Gli3, which are transcription factors that mediate Hedgehog signaling [19]. However, FEM-1 homologs in vertebrates have never been reported to operate within the Hedgehog signaling pathway, and TRA-1 homologs such as Gli1 can have Hedgehog-independent functions.
Whether the regulatory interaction between FEM-1 and TRA-1 is conserved, between homologs of these two proteins in vertebrates, remains an open question. A mouse Fem1 gene exhibited weak masculinizing activity when expressed as a transgene in fem-1 mutants of C. elegans[1], [20], consistent with possible evolutionary conservation of a biochemical interaction regulating TRA-1. However, it has never been directly shown that a vertebrate Fem1 protein can bind or modify activity of a Gli protein. In the experiments presented herein, we address this basic question.
2. Materials and methods
2. 1. Cell lines
HEK293T (293T) cells and SW480 (human colon carcinoma) cells were obtained from ATCC and were cultured in Dulbecco’s modified eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Atlantic Biologicals). RMS-13 (human alveolar rhabdomyosarcoma) cells were obtained from ATCC and were cultured in RPMI 1640 medium supplemented with 10% FBS. NIH-3T3 (mouse fibroblast) cells were obtained from ATCC and cultured in DMEM supplemented with 10% calf serum (CS) (Hyclone, Invitrogen). Details of plasmid constructs are given in Supplementary information online.
2.2. Protein purification, in vitro translation of Gli1, and GST pull-down
Recombinant GST and GST-Fem1b (mouse) was generated in Escherichia coli using one shot BL21 (DE3) pLysS (Invitrogen). For in vitro translation of Gli1, pBluescript-Gli1 [21] was used as template for in vitro transcription, followed by in vitro translation with a rabbit reticulocyte lysate (Promega). For GST pull-down of Myc-Gli1 a previously described protocol was employed [22]. For GST pull-down of in vitro translated Gli1, and GST pull-down of endogenous Gli1 in RMS-13 cells, a similar protocol was followed. Further experimental details of these experiments is given in Supplementary information online.
2.3. Dual luciferase assay
NIH 3T3 cells were transfected in 12 well plates with 0.1 μg 8×Gli reporter construct, increasing amounts of HA-Fem1b or HA-Fem1b L597A, 0.2 μg of myc-hGli1 and 0.04 ng renilla luciferase. Empty HA vector was added so that the total DNA per well was 0.325 μg. After 48 h cells were processed following the dual luciferase reporter assay protocol (Promega). Briefly, cells were lysed and loaded in triplicate in 96 well luminescence microplates (Nunc) and luminescence was measured using a Glomax dual injector 96 microplate luminometer (Promega).
2.4. qPCR
Total RNA was isolated using the nucleospin RNAII RNA isolation kit (Clontech) according to the manufacturer’s protocol. Following isolation of total RNA, cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s protocol, and qPCR was performed on an iCycler IQ Real Time PCR (Bio-Rad) using IQ qPCR supermix (Bio-Rad) according to the manufacturer’s protocol.
2.5. Supplementary materials and methods
Materials and methods for plasmid constructs, transfections, co-immunoprecipitation experiments, ubiquitylation experiments, and immunocytochemistry, are given in Supplementary information online, in addition to the supplemental information noted above for protein purification, in vitrotranslation of Gli1, and GST pull-down.
3. Results
3.1. Fem1b interacts with Gli1
To approach the question of whether the interaction between FEM-1 and TRA-1 in nematodes is conserved between corresponding vertebrate homologs, we tested co-immunoprecipitation of epitope-tagged human Fem1b and Gli constructs in 293T cells. Immunoprecipitation of Myc-Gli1 co-immunoprecipitated HA-Fem1b from co-transfected 293T cell lysate, while empty Myc vector or control IgG failed to co-immunoprecipitate HA-Fem1b (Fig. 1A). HA-Fem1b also co-immunoprecipitated with Myc-Gli2 and with Myc-Gli3 (data not shown), but herein we focus on the interaction between Fem1b and Gli1. In GST pull-down experiments, GST-Fem1b, but not GST, pulled-down Myc-Gli1 from transfected 293T cell lysate (Fig. 1B). GST-Fem1b also specifically pulled-down endogenous Gli1 from a lysate of RMS-13 cells (Fig. 1C), in which there is an approximately 30-fold amplification of the Gli1 gene, thereby expressing easily detectable levels of endogenous full length Gli1 (Fig. 1C, input lane) [23].
Fig. 1.
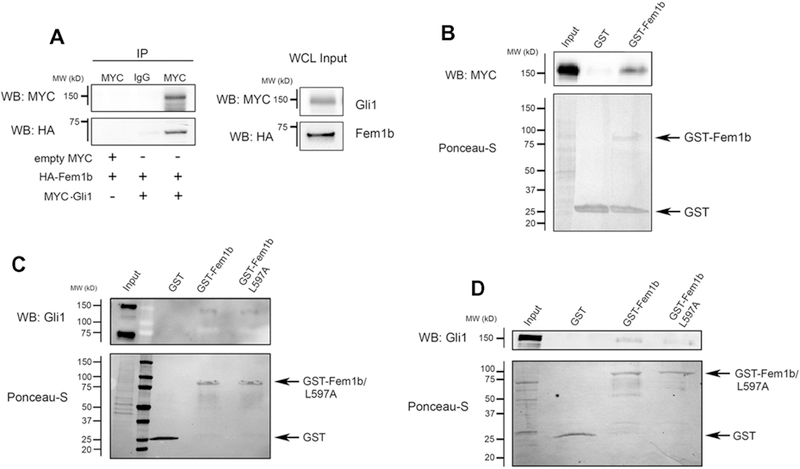
Fem1b interacts with Gli1. (A) Gli1 co-immunoprecipitates with Fem1b. HA-Fem1b and Myc-Gli1 or empty Myc vector were co-transfected into 293T cells and immunoprecipitation was performed after 24 h with a Myc antibody or control IgG followed by Western blot. Input represents 8% of the whole cell lysate (WCL). (B) Recombinant Fem1b binds ectopically expressed Gli1. GST pull-down was performed with GST or GST-Fem1b using 293T lysate expressing Myc-Gli1 20 h post-transfection followed by Western blot. Ponceau-S demonstrates the relative amount of GST fusion bait protein. Input represents 0.25% of total protein. (C) Recombinant Fem1b binds endogenous Gli1. GST pull-down was performed with the indicated constructs using RMS-13 lysate followed by Western blot. Input represents 0.25% of the total protein and (D) Fem1b and Gli1 interact directly. GST pull-down was performed with the indicated constructs using human Gli1 programmed rabbit reticulocyte lysate. Input represents 1.5% of the total rabbit reticulocyte protein.
The nematode FEM-1 and mammalian Fem1b proteins each contain a VHL-box motif, which mediates interaction with CBC-containing E3 ubiquitin ligase complexes [13]. A Fem1b construct carrying a point mutation in the BC-box within the VHL-box motif (L597A) that disrupts interaction with the Elongin-BC complex [15] was also used in pull-down experiments to test if this mutation influenced the interaction of Fem1b with Gli1 (Fig. 1C and D). Accordingly, GST-Fem1b L597A also pulled-down endogenous Gli1 from RMS-13 cell lysate, indicating that the interaction of Fem1b with Gli1 is independent of its interaction with Elongin B and C (Fig. 1C). Also, considering the possibility that the interaction of Fem1b with Gli1 could be bridged by other proteins, rather than direct, we performed GST pull-down with in vitro translated Gli1 (Fig. 1D). GST-Fem1b and GST-Fem1b L597A, but not GST, pulled down in vitro translated Gli1 (Fig. 1D), indicating that Fem1b interacts directly with Gli1, barring some unidentified components in the rabbit reticulocyte lysate that bridge the interaction.
We next sought to identify the potential Gli1 binding site(s) within Fem1b. As shown (Fig. 2A), Fem1b contains: an N-terminal ankyrin repeat domain, known to be a protein–protein interaction motif [24], as well as two ankyrin repeats near the C-terminus [1], [2], [12], [15]; a central region with homology to kinesin light chainsand containing a caspase-3 cleavage site [1], [2]; and the VHL-box motif in the C-terminus [14], [15]. Utilizing HA-tagged truncation mutants of Fem1b in co-transfection experiments, Myc-Gli1 co-immunoprecipitated all of the indicated truncation constructs (Fig. 2A) except amino acids 480–627, which contains the VHL-box (Fig. 2B). These results suggest multiple Fem1b binding contacts involving the N-terminal ankyrin repeat domain and the central region having kinesin light chain homology, but not involving the VHL-box motif or the C-terminal ankyrin repeats.
Fig. 2.
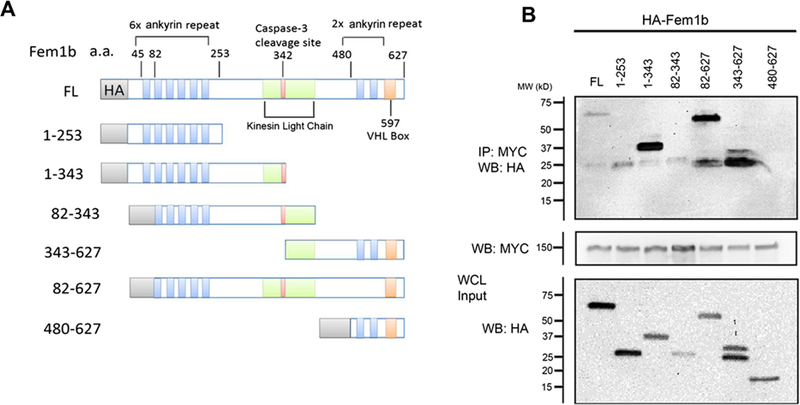
(A) Schematic of the constructs used in co-immunoprecipitation experiments and (B) co- immunoprecipitation assay maps the Gli1 binding interface on Fem1b. Various truncation mutants of HA- Fem1b and Myc-Gli1 were co-transfected into 293T cells and co-immunoprecipitation was performed after 24 h using a Myc antibody followed by Western blot. Input represents 8% of the total protein.
3.2. Fem1b promotes ubiquitylation of Gli1
Given that Fem1b and Fem1b L597A interacted directly with Gli1, we hypothesized that Fem1b promotes Gli1 ubiquitylation, analogous to FEM-1 regulation of TRA-1 in C. elegans. We evaluated ubiquitylation by co-expression of HA-Ubiquitin constructs and Myc-Gli1 with either Flag-Fem1b or Flag-Fem1b L597A in 293T cell cells, followed by immunoprecipitation of Myc-Gli1 from cell lysates, and immunoblot for HA-Ubiquitin (Fig. 3A). Gli1 ubiquitylation was stimulated in the presence of Fem1b (compare Fig. 3A, lane 3 to lane 2), including production of very high molecular weight (>250 kD) poly-ubiquitylated species (Fig. 3A, lane 3). The VHL-box mutant Fem1b L597A eliminated the capacity of Fem1b to promote Gli1 ubiquitylation (compare Fig. 3A, lane 4 to lanes 3 and 2), demonstrating that the VHL-box of Fem1b is required to promote Gli1 ubiquitylation. In the ubiquitin–proteasome pathway, lysine 48-conjugated poly-ubiquitin chains can direct proteins to the proteasome for degradation [25], [26]. HA-Ubiquitin K48R does not support Fem1b-mediated ubiquitylation of Gli1 as does wild-type HA-Ubiquitin (compare Fig. 3A, lanes 3–5), indicating that Fem1b promotes Ubiquitin K48-linked polyubiquitylation, although we can’t rule out that the possibility that Fem1b-mediated ubiquitylation of Gli1 produces mono- or oligo-ubiquitylated intermediates with discrete functional properties.
Fig. 3.
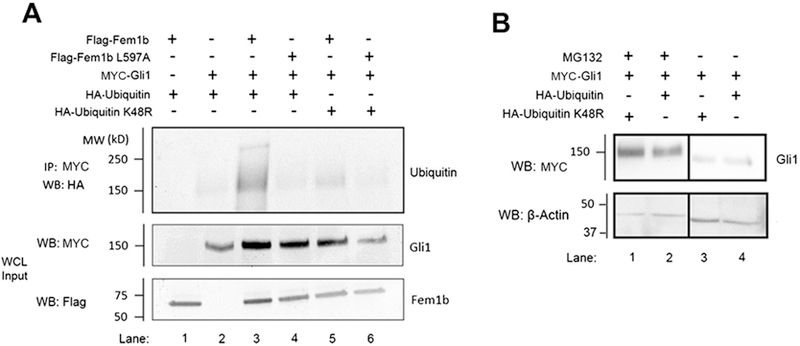
(A) Fem1b promotes the ubiquitylation of Gli1. 293T cells were transfected with the indicated constructs before co-immunoprecipitation with a Myc antibody followed by Western blot. Inputs represent 7% of the WCL. (B) Gli1 protein is stabilized by the proteasome inhibitor MG132. 293T cells were transfected with the indicated constructs for 9 h and then treated or untreated with 10 μM of the proteasome inhibitor MG132 for 3.5 h before Western blotting.
We note that both Flag-Fem1b and Flag-Fem1b L597A appear to stabilize Myc-Gli1 protein levels (Fig. 3A), possibly due to exogenous Fem1b sequestering Gli1 away from endogenous factors, such as endogenous E3 ubiquitin ligases, that promote Gli1 degradation in 293T cells. We confirmed that Gli1 is regulated by the proteasome in 293T cells by treating them with the proteasome inhibitor MG132(Fig. 3B). MG132 stabilized ectopically expressed Gli1 in the presence or absence of ectopically expressed wild-type ubiquitin and the ubiquitin chain-terminating mutant K48R (compare Fig. 3B, lanes 1 and 2 to lanes 3 and 4).
3.3. Fem1b suppresses Gli1 transcriptional activity
Since Fem1b binds and promotes ubiquitylation of Gli1, we wanted to test whether Fem1b affects Gli1 transcriptional activity. Utilizing the Hedgehog-responsive cell line NIH3T3 we performed a Gli1 transcriptional assay with a luciferase reporter construct containing 8 multimerized consensus Gli binding sites [27], [28] co-transfected with a Myc-Gli1 construct and HA-Fem1b or empty HA vector constructs. Fem1b, but not Fem1b L597A, reduced Gli1-dependent transcriptional activity in a dose-dependent manner (Fig. 4A). When we transfected this same cell line individually with either HA-Fem1b or Myc-Gli1 we saw unique predominantly cytoplasmic distributions for both proteins (Fig. 4B, left). However, co-transfection of both HA-Fem1b and Myc-Gli1 resulted in cytoplasmic co-localization of the two proteins (Fig. 4B, right), suggesting that cytoplasmic sequestration could contribute to transcriptional regulation of Gli1 by Fem1b.
Fig. 4.
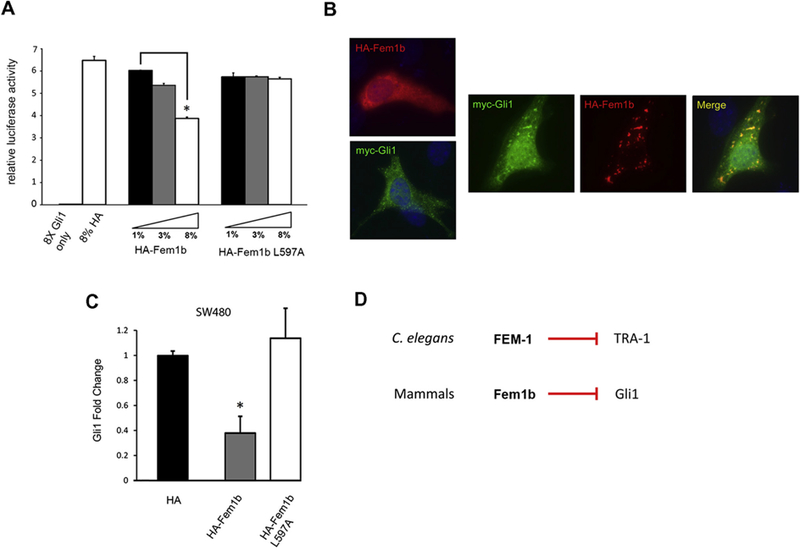
Fem1b suppresses Gli1 transcriptional activity. (A) Luciferase activity of NIH-3T3 cells transfected with a Gli1 luciferase reporter and the indicated constructs was measured (data is a representative experiment with error bars expressing the average relative luciferase activity ± the standard deviation (S.D.) from three repeated measurements); ∗p < 0.01. (B) Ectopically expressed Fem1b and Gli1 co-localize. Left: NIH-3T3 cells were transfected with either HA-Fem1b or Myc-Gli1 for 24 h and fixed and immunostained for HA (red) or Myc (green). Right: NIH-3T3 cells were co-transfected with HA-Fem1b and Myc-Gli1 and fixed and immunostained for HA (red) and Myc (green). Co-localized proteins appear yellow. Nuclei were stained by 4’,6-diamino-2-phenylindole (DAPI) (blue). (C) Transient expression of Fem1b reduces the level of Gli1 message. The qPCR was performed on total RNA isolated from SW-480 cells transfected with the indicated constructs after 48 h. Gli1 message was determined relative to GAPDH message levels. Data are normalized to Gli1 message in empty vector transfected cells (error bars expressed as mean fold induction ± standard deviation (S.D.); ∗p < 0.05) and reflect three independent experiments and (D) in the C. elegans sex determination pathway FEM-1 negatively regulates the zinc finger transcription factor TRA-1 (above), similarly in the mammalian Hedgehog signaling pathway Fem1b negatively regulates the homologous zinc finger transcription factor Gli1 (below).
3.4. Fem1b attenuates a Gli1 autoregulatory loop in cancer cells
To evaluate the effect of Fem1b on an endogenous Gli1 target we chose cancer cells, since Gli1 is not essential during development [29], and is best characterized as an oncogene and part of the “Gli-code” operating in cancer cells [30], [31]. Gli1 activates its own transcription in an autoregulatory positive feedback loop, and transcription of Gli1 message is considered the most reliable read-out of the Gli-code in cancer cells [30], [31]. Therefore, we measured Gli1 mRNA levels by qPCR in response to ectopically expressed Fem1b in the colon cancer cell line SW480, in which Gli1 has been shown to promote cell survival and the DNA damage response [32], [33]. HA-Fem1b expression resulted in an approximately 60% reduction in Gli1 message levels, whereas HA-Fem1b L597A expression resulted in no change in Gli1 message levels (Fig. 4C).
4. Discussion
The FEM-1 protein within the sex-determination pathway of the nematode C. elegans negatively regulates the transcription factor TRA-1 [11], [12], [13]. Herein, we sought to investigate whether a biochemical regulatory interaction may be conserved between the respective mammalian homologs Fem1b and Gli1 (Fig. 4D). Collectively, our results show that Fem1b interacts with Gli1 within cells, and directly binds Gli1. Fem1b also promotes ubiquitylation of Gli1, suppresses transcriptional activation by Gli1, and attenuates an oncogenic Gli1 autoregulatory loop in cancer cells.
Biochemical evidence supports a mechanism in which nematode FEM-1 functions as an SRS to target TRA-1 for ubiquitylation by CBC-containing E3 ubiquitin ligasecomplexes [13]. The C-terminal VHL-box of FEM-1 was shown to interact with nematode CBC homologs, although a requirement of the VHL-box for FEM-1 mediated ubiquitylation of TRA-1 was not established [13]. FEM-1 also contains an N-terminal ankyrin repeat domain, presumed to mediate protein–protein interaction with TRA-1 [13]. The C-terminal VHL-box of mammalian Fem1b is known to mediate interaction with CBC components of E3 ubiquitin ligase complexes [14], [15]. Our findings that the VHL-box is required for Fem1b-mediated ubiquitylation of Gli1, suppression of transcriptional activation by Gli1, and attenuation of an oncogenic Gli1 autoregulatory loop in cancer cells, demonstrates the functional importance of the VHL-box motif within Fem1b. We also find that the N-terminal ankyrin repeat domain and the central kinesin light chain-like region of Fem1b mediate interaction with Gli1. Therefore, homologous to nematode FEM-1 [13], Fem1b has the hallmarks of an SRS that targets Gli1 for ubiquitylation by CBC-containing E3 ubiquitin ligase complexes.
When expressed ectopically in human HEK293T cells, nematode FEM-1 can also promote proteasomal degradation of TRA-1 [13]. It remains to be established whether Fem1b suppression of Gli1 transcriptional activity, and the role of ubiquitylation, occurs through proteasomal degradation of Gli1, or involves other possible mechanisms such as direct inhibition of transcriptional activity of DNA-bound Gli1, cytoplasmic sequestration and inhibition of nuclear translocation, or a combination of mechanisms. As noted above, it is possible that a cytoplasmic sequestration mechanism is involved in Fem1b regulation of Gli1. Such a mechanism has precedent, such as the suppressor-of-fused protein which is known to negatively regulate Gli1-mediated transcription by preventing Gli1 accumulation in the nucleus [34], [35]. We also do not yet know whether a signaling switch may regulate the interaction of Fem1b with Gli1, such as the phosphorylation switches that have been shown to regulate the interaction of some E3 ubiquitin ligase SRS adaptors with their target substrates [36]. Future research will address these important questions, as well as the regulation of Gli2 and Gli3 by Fem1b, and whether the other mammalian Fem1 homologs, Fem1a and Fem1c, are involved in Gli regulation.
Finding that Fem1b regulates Gli1 could have important implications for understanding the functions of both Fem1b and Gli1 in various biological and pathophysiological contexts. This may be especially the case where the cellular and physiological processes in which Fem1b is implicated overlap those in which Gli1 has been implicated. For example, Fem1b is expressed in pancreatic islets, and targeted inactivation of Fem1b leads to defective insulin secretion, but with increased insulin content in the pancreas [7]. Gli1 is known to activate insulin expression in pancreatic islets [37], and therefore future studies can address whether increased Gli1 transcriptional activity mediates the increased insulin expression seen with loss of Fem1b.
In cancer cells, Fem1b (also known as F1Aα) is known to be a pro-apoptotic factor [2], [3], [4], [5]. Therefore, it will be important to establish whether some of the pro-apoptotic activity of Fem1b involves negative regulation of Gli1, which is known to be a pro-survival factor in cancer cells [32], [38]. It is interesting to note that the protein RACK1, which suppresses Fem1b pro-apoptotic activity in cancer cells [5], has been found to promote activation of Gli1 activity in cancer cells [39]. Furthermore, Gli1 is known to display Hedgehog-independent, non-canonical activity in cancer cells [31], [32], [33], [38], and so the negative regulation of Gli1 by Fem1b demonstrated herein could have important implications for therapeutic targeting of Gli1 oncoprotein activity in cancer cells, to be addressed in future studies.
Acknowledgments
We thank the Molecular Core of the Center for Psychiatric Neuroscience, NIGMS – P20-RR- 17701 (UMMC) for use of the luminometer. We thank Joan W. Conaway (Stowers Institute for Medical Research) for the Flag-Fem1b L597A plasmid. We thank Michael Hebert (UMMC) for use of the fluorescence microscope. We also thank Luis Martinez and members of the Maher lab for helpful discussions. This work was supported by the Cancer Institute, University of Mississippi Medical Center Cancer, and the McDermott Center for Human Growth & Development, University of Texas Southwestern Medical Center. We dedicate this article to the memory of our valued colleague M. Cecilia Subauste.
References
- 1.Ventura-Holman T, Seldin MF, Li W, Maher JF. The murine Fem1 gene family: homologs of the Caenorhabditis elegans sex-determination protein FEM-1. Genomics, 54 (1998), pp. 221–230 [DOI] [PubMed] [Google Scholar]
- 2.Chan SL, Tan KO, Zhang L, Yee KS, Ronca F, Chan MY, Yu VC. F1Aalpha, a death receptor-binding protein homologous to the Caenorhabditis eleganssex-determining protein, FEM-1, is a caspase substrate that mediates apoptosis. J. Biol. Chem, 274 (1999), pp. 32461–32468 [DOI] [PubMed] [Google Scholar]
- 3.Chan SL, Yee KS, Tan KM, Yu VC. The Caenorhabditis elegans sex determination protein FEM-1 is a CED-3 substrate that associates with CED-4 and mediates apoptosis in mammalian cells. J. Biol. Chem, 275 (2000), pp. 17925–17928 [DOI] [PubMed] [Google Scholar]
- 4.Subauste MC, Sansom OJ, Porecha N, Raich N, Du L, Maher JF. Fem1b, a proapoptotic protein, mediates proteasome inhibitor–induced apoptosis of human colon cancer cells. Mol. Carcinog, 49 (2010), pp. 105–113 [DOI] [PubMed] [Google Scholar]
- 5.Subauste MC, Ventura Holman T, Du L, Subauste JS, Chan SL, Yu VC, Maher JF. RACK1 downregulates levels of the pro-apoptotic protein Fem1b in apoptosis-resistant colon cancer cells. Cancer Biol. Ther, 8 (2009), pp. 2297–2305 [DOI] [PubMed] [Google Scholar]
- 6.Goodarzi MO, Maher JF, Cui J, Guo X, Taylor KD, Azziz R FEM1A and FEM1B: novel candidate genes for polycystic ovary syndrome. Hum. Reprod, 23 (2008), pp. 2842–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu D, Ventura-Holman T, Li J, McMurray RW, Subauste JS, Maher JF Abnormal glucose homeostasis and pancreatic islet function in mice with inactivation of the Fem1b gene. Mol. Cell. Biol, 25 (2005), pp. 6570–6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Desai N, Hu YP, Price SM, Abate-Shen C, ShenMouse MM Fem1b interacts with the Nkx3.1 homeoprotein and is required for proper male secondary sexual development. Dev. Dyn, 237 (2008), pp. 2963–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventura-Holman T, Lu D, Si X, Izevbigie EB, MaherThe JF Fem1c genes: conserved members of the Fem1 gene family in vertebrates. Gene, 314 (2003), pp. 133–139 [DOI] [PubMed] [Google Scholar]
- 10.Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Par ker SC, Margulies EH, Legro RS, Dunaif A, Strauss JF, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J. Clin. Endocrinol. Metab, 95 (2010), pp. 2306–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doniach T, Hodgkin J A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev. Biol, 106 (1984), pp. 223–235 [DOI] [PubMed] [Google Scholar]
- 12.Spence AM, Coulson A, Hodgkin J. The product of fem-1, a nematode sex-determining gene, contains a motif found in cell cycle control proteins and receptors for cell–cell interactions. Cell, 60 (1990), pp. 981–990 [DOI] [PubMed] [Google Scholar]
- 13.Starostina NG, Lim JM, Schvarzstein M, Wells L, Spence AM, Kipreos ET. A CUL-2 ubiquitin ligase containing three FEM proteins degrades TRA-1 to regulate C. elegans sex determination. Dev. Cell, 13 (2007), pp. 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev, 18 (2004), pp. 3055–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahrour N, Redwine WB, Florens L, Swanson SK, MartinBrown S, Bradford WD, Staehlin g Hampton K, Washburn MP, Conaway RC, Conaway JW. Characterization of cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J. Biol. Chem, 283 (2008), pp. 8005–8013 [DOI] [PubMed] [Google Scholar]
- 16.Shi YQ, Liao SY, Zhuang XJ, Han CS. Mouse Fem1b interacts with and induces ubiquitin-mediated degradation of Ankrd37. Gene, 485 (2011), pp. 153–159 [DOI] [PubMed] [Google Scholar]
- 17.Puoti A, Gallegos M, Zhang B, Wickens MP, Kimble J. Controls of cell fate and pattern by 3’ untranslated regions: the Caenorhabditis eleganssperm/oocyte decision. Cold Spring Harb. Symp. Quant. Biol, 62 (1997), pp. 19–24 [PubMed] [Google Scholar]
- 18.Kuwabara PE, Okkema PG, Kimble J. Tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol. Biol. Cell, 3 (1992), pp. 461–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarkower D, Hodgkin J. Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell, 70 (1992), pp. 237–249 [DOI] [PubMed] [Google Scholar]
- 20.Zarkower D. Establishing sexual dimorphism: conservation amidst diversity? Nat. Rev. Genet, 2 (2001), pp. 175–185 [DOI] [PubMed] [Google Scholar]
- 21.Kinzler KW, Vogelstein B. The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol. Cell. Biol, 10 (1990), pp. 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilder AS, Do PM, Carrero ZI, Cosman AM, Broome HJ, Velma V, Martinez LA, He bert MD. Coilin participates in the suppression of RNA polymerase I in response to cisplatin-induced DNA damage. Mol. Biol. Cell, 22 (2011), pp. 1070–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts WM, Douglass EC, Peiper SC, Houghton PJ, Look AT Amplification of the gli gene in childhood sarcomas. Cancer Res, 49 (1989), pp. 5407–5413 [PubMed] [Google Scholar]
- 24.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci, 13 (2004), pp. 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev, 82 (2002), pp. 373–428 [DOI] [PubMed] [Google Scholar]
- 26.Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelen der S, Ross CA, Dawson VL, Dawson TM. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J. Neurosci, 25 (2005), pp. 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro Development, 124 (1997), pp. 1313–1322 [DOI] [PubMed] [Google Scholar]
- 28.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in smoothened and patched can be reversed by cyclopamine. Nature, 406 (2000), pp. 1005–1009 [DOI] [PubMed] [Google Scholar]
- 29.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyne AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development, 127 (2000), pp. 1593–1605 [DOI] [PubMed] [Google Scholar]
- 30.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol, 17 (2007), pp. 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stecca B, Ruiz i Altaba A. Context-dependent regulation of the GLI code in cancer by Hedgehog and non-Hedgehog signals. J. Mol. Cell Biol, 2 (2010), pp. 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazumdar T, DeVecchio J, Shi T, Jones J, Agyeman A, Houghton JA. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res, 71 (2011), pp. 1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agyeman A, Mazumdar T, Houghton JA. Regulation of DNA damage following termination of Hedgehog (HH) survival signaling at the level of the GLI genes in human colon cancer. Oncotarget, 3 (2012), pp. 854–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Q, Si. Fukami X Meng Y Nishizaki X Zhang H Sasaki A Dlugosz M Nakafuku Cc. Hu i. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr. Biol, 9 (1999), pp. 1119–1122 [DOI] [PubMed] [Google Scholar]
- 35.Kogerman P, Grimm T, Kogerman L, Krause D, Undén AB, Sandstedt B, Toftgård R, Zap hiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat. Cell Biol, 1 (1999), pp. 312–319 [DOI] [PubMed] [Google Scholar]
- 36.Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell, 28 (2007), pp. 730–738 [DOI] [PubMed] [Google Scholar]
- 37.Thomas MK, Lee JH, Rastalsky N, Habener JF. Hedgehog signaling regulation of homeodomain protein islet duodenum homeobox-1 expression in pancreatic beta-cells. Endocrinology, 142 (2001), pp. 1033–1040 [DOI] [PubMed] [Google Scholar]
- 38.Lauth M, Toftgard R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle, 6 (2007), pp. 2458–2463 [DOI] [PubMed] [Google Scholar]
- 39.Shi S, Deng YZ, Zhao JS, Ji XD, Shi J, Feng YX, Li G, Li JJ, Zhu D, Koeffler HP, Zhao Y , Xie D. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J. Biol. Chem, 9 (287) (2012), pp. 7845–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]


