How a protein folds into its native structure has puzzled scientists for the last 40 years, primarily due to the difficulties of directly observing protein folding events. The β-hairpin is a small protein structure motif with the basic physics of protein folding.1 An understanding of its folding mechanism will shed light on the protein folding problem. Herein, we report a successful observation of the folding and unfolding of a β-hairpin structure in explicit water at physiological folding conditions through computer simulation.
The peptide studied here is designed by Blanco et al.,2 which consists of 9 amino acids, Tyr-Gln-Asn-Pro-Asp-Gly-Ser-Gln-Ala. Strong NMR NOE evidence indicates that this peptide folds into a β-hairpin structure in aqueous solution.2 The simulation system contains one molecule of the peptide, a single sodium ion, and 725 TIP3P water3 molecules. The simulation was performed with cubic periodic boundary conditions at constant temperature (274 K) and constant volume (29 × 29 × 29 Å3). The AMBER force field4 was used to describe the system and the Particle-Meshed-Ewald method5 was used for the electrostatic interaction calculation. The self-guided molecular dynamics (SGMD) simulation method6–10 was used with a local sampling time of 0.2 ps and a guiding factor of 0.1.
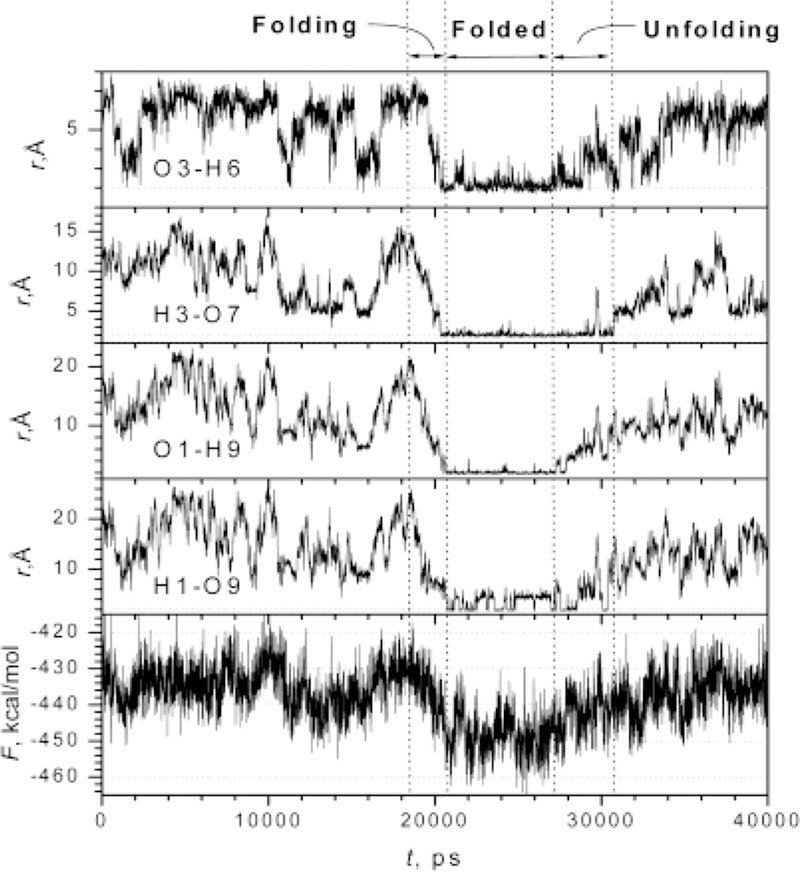
The folding of a β-hairpin is characterized by the formation of hydrogen bonds between its two strands. Figure 1 plots the distances of several hydrogen bonding atom pairs as well as the conformational free energies of the peptide during the simulation. Starting from a fully extended conformation, the peptide explored its conformational space extensively before it began to fold at 18 700 ps. From 18 700 to 20 700 ps, the peptide underwent a cooperative folding process and all the distances declined simultaneously until the peptide reached the folded structure. The peptide remained the folded structure until 27 900 ps when it began to unfold. From 27 900 to 30 750 ps, the unfolding proceeded with the breaking of some interstrand hydrogen bonds. After 30 750 ps, the peptide was completely unfolded. It should be pointed out that the SGMD time scale observed here is enhanced. It is this enhancement that makes β-hairpin folding accessible at this condition.
Figure 1.
The distances (r) of some hydrogen bonding atom pairs and the conformational free energies (F) of the peptide during the self-guide molecular dynamics simulation. Hi and Oj represent the amid hydrogen of residue i and carbonyl oxygen of residue j, respectively. The conformational free energies were calculated using the MM_PBSA module provided with the AMBER6 program,13 which utilizes the Generalized-Born method to estimate the electrostatic contribution to the solvation free energy.14
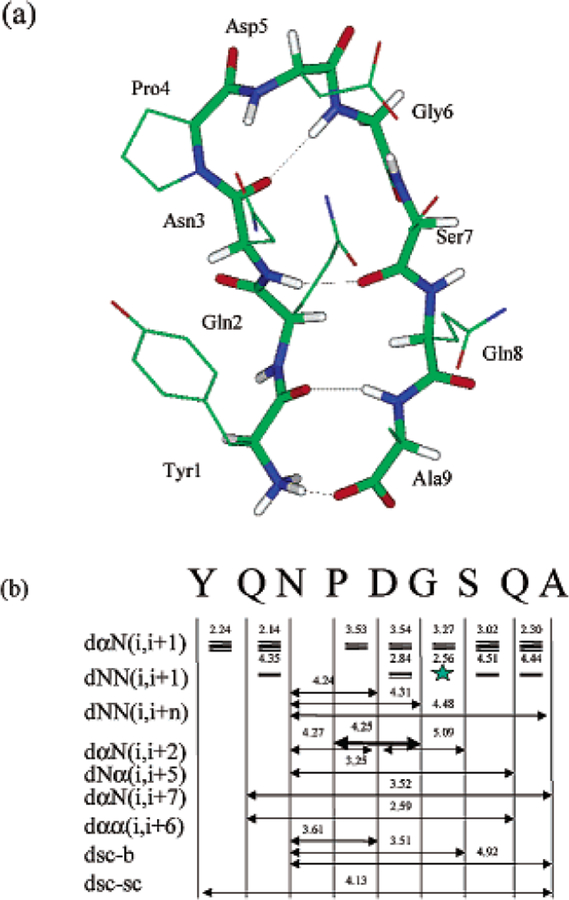
Figure 2a shows a typical folded structure of this peptide. It has a turn involving residues Asn3, Pro4, Asp5, and Gly6 with the carbonyl oxygen of Asn3 hydrogen bonded with the amide hydrogen of Gly6. This turn is a common type turn, which cannot directly link a tight β-hairpin structure.11,12 Therefore, the folded structure is not a tight β-hairpin. The two β-strands, Tyr1-Asn3 and Ser7-Ala9, are hydrogen bonded with each other through hydrogen bonds between Tyr1 and Ala9, and between Asn3 and Ser7. The β-hairpin structure has been detected by NMR experiment2 and the NOEs are shown in Figure 2b. The H-H distances are averaged over the folded conformations from 20 700 to 27 900 ps. As can be seen in Figure 2b, all distances of these atom pairs in the folded conformations are in good agreement with the NMR NOE observations.
Figure 2.
(a) A typical folded structure of the peptide obtained in the simulation (at 21 ,000 ps). For clarity, side chain hydrogens are not shown. The backbone atoms are shown as thick sticks and side chain atoms as thin sticks. Interstrand hydrogen bonds are marked by dashed lines. Atoms are colored red for oxygen, blue for nitrogen, white for hydrogen, and green for the rest. (b) NMR NOEs observed in the peptide aqueous solution2 (arrow bars between residues) and the average hydrogen pair distances (numbers in Å above NOE bars) in the β-hairpin structure obtained in our simulation. R, N, sc, and b represent the hydrogen atoms on R-carbon, amide nitrogen, side chain (β-carbon in our calculation), and backbone (amide nitrogen in our calculation). The thickness of the NOE bars represents the strength of the NOEs reported. Generally, NOEs are strong for hydrogen pair distances within 3 Å, medium between 3 and 4 Å, and week between 4 and 5 Å.
Obviously, the β-hairpin folding process is highly cooperative, as evidenced by the simultaneous decrease in the distances between native hydrogen boding atoms (Figure 1). The turn structure in a β-hairpin has been argued to initiate the β-hairpin folding.1 From Figure 1 it is clear that the turn hydrogen bond, O3-H6, as well as other interstrand hydrogen bonds, did not form until the final stage of the folding. Therefore, the turn structure in the β-hairpin did not play an initiation role in this folding process. After examining the conformations, we find that the side chain interaction, primarily between Gln2 and Gln8, occurred first in the folding process. The interstrand hydrogen bonds only helped to stabilize the folded structure.
These folding and unfolding events are apparent if we examine the conformational free energy profile (Figure 1). The peptide climbed up a conformational free energy hill from 16 000 to 18 700 ps before folding. From 18 700 to 20 700 ps, the cooperative folding process was accompanied by a systematic decrease in conformational free energy. There is a broad free energy barrier prior to the folding process. The peak of the barrier corresponds to the conformations where the hydrogen bonding atom pair distances are almost at their maxima. Detailed examination shows that these conformations are fully hydrated with neither intrapeptide hydrogen bonds nor side chain interaction, which suggests that the fully hydrated conformation could be the transition state during the β-hairpin folding process. Many studies have shown that the unfolded states are partially hydrated with intrapeptide hydrophobic and/or hydrogen bonding interactions.15 By going through the fully hydrated conformations, which are relatively high in conformational free energy, the peptide can get rid of those non-native interactions.
This simulation provides us energetic insights into the β-hairpin folding process. The energies of the unfolded state and the folded state are estimated by averaging over 1000–18000 ps and over 20700–27900 ps, respectively. The conformational free energy decreases from −435.1 ± 0.2 to −447.8 ± 0.2 kcal/mol upon folding, changing about −12.7 ± 0.4 kcal/mol. In this folding procedure, the intrapeptide interaction contributes −66.8 ± 1.4 kcal/ mol, while the GB electrostatic energy contributes 54.4 ± 1.3 kcal/ mol. Solvent accessible surface tension contributes only −1.4 ± 0.1 kcal/mol. It is clear that the driving force for the β-hairpin folding is the intrapeptide interaction. The solvent electrostatic interaction opposes the folding, and the hydrophobic effect favors the folded state but has very limited contribution. To confirm the results, we performed two 20 000 ps regular MD simulations at the same conditions, one started from the fully extended conformation and the other started from the folded conformation obtained in the SGMD simulation. The peptide remained a coil throughout the first simulation and remained in the folded state throughout the second one. Energetic analysis of these two simulations provided similar results as described above.
Even though the cooperative β-hairpin folding remains a rare event in current simulation studies, we observed repeatedly similar folding and unfolding events of this β-hairpin structure in either a continued simulation (occurred from 108 310 to 117 360 ps) or another simulation starting from a random coil structure (occurred from 69 140 to 73 040 ps). These additional observations support the conclusions presented here. To date, β-hairpin folding at native conditions was only observed directly in simulations using implicit solvation models.16–18 With explicit solvent, β-hairpin folding was studied indirectly at high temperature19 or through a temperature exchange technique.20–21 Compared with those studies using implicit solvation models, our simulation shows a stronger cooperativity in β-hairpin folding and a significant free energy barrier prior to the folding. Contrasting studies with explicit water, our simulation shows that β strand hydrogen bonds are the last to form during the folding. Nonetheless, it is clear that the cooperative β-hairpin folding is becoming accessible at native condition with current computing resources.
Acknowledgment.
This study is supported in part by NIH grant GM59188.
Footnotes
Supporting Information Available: The conformations of the peptide at 19 600, 19 750, 20 750, 32 000, and 33 000 ps from the SGMD simulation (PDB link). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Munoz V; Thompson PA; Hofrichter J; Eaton WA Nature 1997, 390, 196–199. [DOI] [PubMed] [Google Scholar]
- (2).Blanco FJ; Jime´nez MA; Herranz J; Rico M; Santoro J; Nieto JL J. Am. Chem. Soc 1993, 115, 5887–5888. [Google Scholar]
- (3).Jorgensen WL; Chandrasekhar J; Madura JD; Impey RW; Klein ML J. Chem. Phys 1983, 79, 926–935. [Google Scholar]
- (4).Cornell WD; Cieplak P; Bayly CI; Gould IR; Merz KM Jr.; Ferguson DM; Spellmeyer DC; Fox T; Caldwell JW; Kollman PA J. Am. Chem. Soc 1995, 117, 5179–5197. [Google Scholar]
- (5).York DM; Wlodawer A; Pedersen LG; Darden TA Proc. Natl. Acad. Sci. U.S.A 1994, 91, 8715–8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wu X-W; Wang SJ Phys. Chem. B 1998, 102, 7238–7250. [Google Scholar]
- (7).Shinoda W; Mikami M Chem. Phys. Lett 2001, 335, 265–272. [Google Scholar]
- (8).Wu X-W; Wang SJ Phys. Chem. B 2000, 104, 8023–8034. [Google Scholar]
- (9).Wu X; Wang SJ Phys. Chem. B 2001, 105, 2227–2235. [Google Scholar]
- (10).Wu X-W; Wang SJ Chem. Phys 1999, 110, 9401–9410. [Google Scholar]
- (11).Sibanda B; Thornton J Nature 1985, 316, 170–174. [DOI] [PubMed] [Google Scholar]
- (12).Richardson JS; Richardson DC Principles and patterns of protein conformation. In Prediction of protein structure and the principles of protein conformation; Fasman GD, Ed.; Plenum Press: New York and London, 1989; pp 1–98. [Google Scholar]
- (13).Perlman DA; Case DA; Caldwell JW; Ross WS; Cheatham TE III; Debolt S; Ferguson D; Seibel GL; Kollman PA Comput. Phys. Commun 1995, 91, 1–41. [Google Scholar]
- (14).Jayaram B; Sprous D; Beveridge DL J. Phys. Chem. B 2000, 102, 9571–9576. [Google Scholar]
- (15).Plaxco KW; Gross M Nat. Struct. Biol 2001, 8, 659–660. [DOI] [PubMed] [Google Scholar]
- (16).Sung SS Biophys. J 1999, 76, 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zagrovic B; Sorin EJ; Pande VJ Mol. Biol 2001, 313, 151–169. [DOI] [PubMed] [Google Scholar]
- (18).Schaefer M; Bartels C; Karplus MJ Mol. Biol 1998, 284, 835–848. [DOI] [PubMed] [Google Scholar]
- (19).Bonvin AM; van Gunsteren WF J. Mol. Biol 2000, 296, 255–268. [DOI] [PubMed] [Google Scholar]
- (20).Zhou R; Berne BJ; Germain R Proc. Natl. Acad. Sci. U.S.A 2001, 98, 14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Garcia AE; Sanbonmatsu KY Proteins 2001, 42, 345–354. [DOI] [PubMed] [Google Scholar]