Abstract
Previous studies investigating prostate cancer (PCa) features in younger men have reported conflicting findings. This study aimed to investigate pathologic outcomes and biochemical recurrence (BCR) status in younger men who underwent radical prostatectomy (RP) for PCa. Records of 2057 patients who underwent RP at Seoul National University Bundang Hospital (Seongnam, Korea) between 2006 and 2015 were reviewed; patients were divided according to age into the younger and older groups (men aged ≤50 and >50 years, respectively). Postoperative BCR status and functional outcomes and clinicopathologic features were compared between both groups. All analyses were repeated after propensity score matching. Younger men were more likely to have low-risk disease (P < 0.001), lower pathologic Gleason score (P < 0.001) and pathologic stages (P < 0.001) than older men. The pathologic Gleason score (P = 0.002) and rates of extracapsular extension (P = 0.004) were lower in younger men after propensity score matching. In multivariate analysis, age at RP was not an independent predictor of BCR-free survival after RP (P = 0.669). Moreover, at 1 year after RP, younger men with preoperative 5-item International Index of Erectile Function score ≥22 (n = 228) showed more favorable results for urinary continence (defined as nonuse of pads daily) (99.4% vs 95%, P = 0.009) and erections sufficient for vaginal intercourse (81.8% vs 55.5%, P = 0.001). Younger men had more favorable clinicopathologic features at RP than their older counterparts. Although age was not an independent predictor of BCR status outcome, younger men had better functional outcomes following RP.
Keywords: age group, prostate cancer, prostatectomy
INTRODUCTION
Prostate cancer (PCa) is predominantly diagnosed in older men, with approximately 80% of cases having been diagnosed in men aged ≥65 years.1 Moreover, autopsy series have reported variable rates of latent PCa in younger men, indicating a prevalence rate as high as 30% in younger men.2 The extensive use of prostate-specific antigen (PSA) testing has contributed to a shift toward younger age at diagnosis and stage migration.3 Recent data have shown that the proportion of younger patients with PCa has increased with time.4 In addition to the increasing prevalence, the longer life expectancy of younger men makes PCa in younger men a clinically significant entity.
Previous studies investigating the features of PCa in younger men have reported conflicting findings. Relatively earlier reports mostly from the pre-PSA era have described that PCa was associated with a more aggressive profile and worse prognosis in younger men than in older counterparts.5,6,7 However, recent studies reported more favorable features and/or outcomes in younger men who underwent radical prostatectomy (RP) in Europe, North America, and Australia than in older men.8,9,10,11,12 Overall, relevant reports have indicated variable results. In addition, there is currently a paucity of published data on PCa in young Asian men.
This study aimed to investigate pathologic outcomes and biochemical recurrence (BCR) status in younger (≤50 years) men diagnosed with PCa and subsequently treated with RP at our institution and to examine functional outcomes, particularly urinary continence and erectile function, following RP in younger men. We performed comparative analyses with older men to analyze various outcomes of interest in younger counterparts.
PATIENTS AND METHODS
Patients
With approval from the Institutional Review Board of Seoul National University Bundang Hospital (Seongnam, Korea) (IRB no. B-1706/405-103), we reviewed the medical records of 2188 patients who underwent RP for PCa between January 2006 and December 2015 at our institution. Indication for prostate biopsy for PCa detection at our institution between 2006 and 2015 was either an elevated serum PSA level (≥3.0 ng ml−1) or abnormal finding on digital rectal examination, as described previously.13 Prostate biopsy was performed based on physician's preference or patients’ concern.
Patients who received neoadjuvant or adjuvant hormonal or radiation therapy and those with missing data were excluded from this study. Finally, a total of 2057 patients were included in our analyses. All RPs were performed by five surgeons using the open (n = 635), laparoscopic (n = 27), and robotic approach (n = 1395). Nerve-sparing procedure was performed based on the clinical decision of each corresponding surgeon. The bladder neck was usually attempted to be preserved in every surgery. However, in the case of severe intravesical prostatic protrusion, large prostate volume, or advanced PCa, bladder neck reconstruction was performed using the tennis racket technique based on the surgeon's decision. Anterior suspension was performed in every surgery. All patients were followed up at 2 weeks and 1, 3, 6, 9, and 12 months postoperatively in an outpatient office and thereafter visited the outpatient office every 6 months for 5 years postoperatively.
Clinicopathologic data
Clinicopathologic data, including patients’ age, preoperative PSA level, body mass index (BMI), prostate volume, Gleason score, tumor stage, surgical margin status, nodal status, postoperative follow-up PSA level, and functional outcomes (urinary continence and erectile function), were collected for each patient.
Pathologic analyses of RP specimens from our patients were performed, as previously reported.14 Specimens were weighed, measured, and fixed in 10% neutral buffered formalin (Samchun Chemicals, Pyeongtaek, Korea). The apex and base were amputated and serially sectioned at 3–5-mm intervals in the vertical parasagittal plane. The remaining specimens were serially sectioned at 3–5-mm intervals perpendicular to the long axis of the gland from the apex to the base. The seminal vesicles were sectioned parallel to their junction with the prostate and submitted entirely for evaluation. All slides were stained with hematoxylin and eosin for histological evaluation.
Biochemical recurrence
In this study, BCR was defined as two consecutive increases in PSA level ≥0.2 ng ml−1 for at least 2 months following RP. Patients were divided into two groups according to age: younger group (men aged ≤50 years) and older group (men aged >50 years). Various clinicopathologic features were compared between the two groups. Moreover, for our analyses, patients were stratified according to their D’Amico risk classification: low-risk (clinical stage T1c–T2a, PSA level ≤10 ng ml−1, and biopsy Gleason score ≥6), intermediate-risk (clinical stage T2b, PSA level of 10.1–20 ng ml−1, or biopsy Gleason score of 7), and high-risk (clinical stage T2c, PSA level >20 ng ml−1, or biopsy Gleason score of 8–10) disease groups.15 Within each disease risk group, outcomes were assessed and compared between the two age groups.
Postoperative urinary continence and erectile function
Patients’ status with respect to urinary continence and erectile function at baseline and postoperative follow-up was assessed by determining the number of pads used per day and the return of erections sufficient for penetrative (vaginal) intercourse regardless of the use of erectile aids, respectively. Patients who did not use any pad daily were considered continent. In general, patients completed the 5-item International Index of Erectile Function (IIEF-5) before RP. With regard to erectile aids, a phosphodiesterase type 5 (PDE-5) inhibitor or intracavernosal injection was prescribed postoperatively, as needed.
Statistical analyses
Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Paired Student's t-test, Chi-squared test, or analysis of variance (ANOVA) were used to compare the patient groups. The BCR-free survival rates of patient cohorts were estimated by employing the Kaplan–Meier method. Log-rank test was used to compare survival curves. Multivariate analysis with Cox proportional hazards regression model was performed to identify independent predictors of BCR-free survival.
In addition, we relied on propensity score matching between the younger and older groups to reduce the selection bias. Both propensity score-matched groups were balanced with respect to BMI, comorbidity, preoperative PSA level, prostate volume, clinical stage, and biopsy Gleason score. All P values were two-sided, with P < 0.05 indicating a significant result.
RESULTS
Patient characteristics before and after matching are summarized in Table 1. Before matching, 169 (8.2%) and 1888 (91.8%) of 2057 patients in total were men aged ≤50 years (younger men) and >50 years (older men) at RP, respectively. On stratification of these 2057 patients into disease risk groups, younger men were noted to more frequently present with low-risk disease than older men (P = 0.001). Moreover, younger men were observed to have lower pathologic Gleason score (P < 0.001) and lower rates of extracapsular extension (P < 0.001) and seminal vesicle invasion (P = 0.016) than their older counterparts. In addition, the nerve-sparing procedure was more frequently performed among younger men during RP. After matching, all differences before matching disappeared.
Table 1.
Clinicopathologic characteristics of patients according to age before and after propensity score matching
| Operation stage | Characteristics | Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=2057) | Patients aged ≤50 years (n=169) | Patients aged >50 years (n=1888) | P | Overall (n=790) | Patients aged ≤50 years (n=158) | Patients aged >50 years (n=632) | P | ||
| Preoperation | Follow-up (month), mean±s.d. | 53.80±29.05 | 57.74±31.74 | 53.45±28.78 | 0.091# | 52.65±28.59 | 59.11±31.79 | 51.03±27.52 | 0.004# |
| BMI (kg m−2) | 34.80±24.30 | 24.34±2.49 | 24.30±2.59 | 0.848# | 24.35±2.56 | 24.27±2.51 | 24.37±2.57 | 0.645# | |
| HTN, n (%) | 878 (42.7) | 30 (17.8) | 848 (44.9) | <0.001* | 157 (19.9) | 30 (19.0) | 127 (20.1) | 0.755* | |
| DM, n (%) | 321 (15.6) | 18 (10.7) | 303 (16.0) | 0.064* | 95 (12.0) | 18 (11.4) | 77 (12.2) | 0.784* | |
| PSA (ng ml−1) | 12.15±14.32 | 9.59±8.61 | 12.38±14.70 | <0.001# | 9.85±9.77 | 9.82±8.84 | 9.86±9.99 | <0.001# | |
| PSA <10, n (%) | 1315 (63.9) | 119 (70.4) | 1196 (63.3) | 0.110* | 554 (70.1) | 109 (69.0) | 445 (70.4) | 0.871* | |
| 10 ≤PSA ≤20, n (%) | 464 (22.6) | 35 (20.7) | 429 (22.7) | 169 (21.4) | 34 (21.5) | 135 (21.4) | |||
| PSA >20, n (%) | 278 (13.5) | 15 (8.9) | 263 (13.9) | 67 (8.5) | 15 (9.5) | 52 (8.2) | |||
| Prostate volume (ml) | 40.37±16.85 | 34.15±9.04 | 40.92±12.27 | <0.001# | 31.27±9.92 | 34.48±9.18 | 31.22±10.10 | 0.773# | |
| Biopsy GS, n (%) | 0.017* | 0.855* | |||||||
| <7 | 957 (46.5) | 94 (55.6) | 863 (45.7) | 421 (53.3) | 87 (55.1) | 334 (52.8) | |||
| =7 | 778 (37.8) | 59 (34.9) | 719 (38.1) | 290 (36.7) | 55 (34.8) | 235 (37.2) | |||
| >7 | 322 (15.7) | 16 (9.5) | 306 (16.2) | 79 (10.0) | 16 (10.1) | 63 (10.0) | |||
| Clinical stage, n (%) | 0.020* | 0.888* | |||||||
| T1c | 1219 (59.3) | 115 (68.0) | 1104 (58.5) | 536 (67.8) | 107 (67.7) | 429 (67.9) | |||
| T2a-b | 605 (29.4) | 44 (26.0) | 561 (29.7) | 203 (25.7) | 42 (26.6) | 161 (25.5) | |||
| T2c | 233 (11.3) | 10 (5.9) | 223 (11.8) | 51 (6.5) | 9 (5.7) | 42 (6.6) | |||
| D’Amico risk stratification, n (%) | 0.001* | 0.756 | |||||||
| Low | 743 (36.1) | 79 (46.7) | 664 (35.2) | 345 (43.7) | 73 (46.2) | 272 (43.0) | |||
| Intermediate | 744 (36.2) | 62 (36.7) | 682 (36.1) | 308 (39.0) | 58 (36.7) | 250 (39.6) | |||
| High | 570 (27.7) | 28 (16.6) | 542 (28.7) | 137 (17.3) | 27 (17.1) | 110 (17.4) | |||
| Perioperation | Nerve sparing (+), n (%) | 1412 (68.6) | 159 (94.1) | 1253 (66.4) | <0.001* | 604 (76.5) | 149 (94.3) | 455 (72.0) | <0.001* |
| Unilateral | 186 (9.0) | 17 (10.1) | 618 (32.7) | 78 (9.9) | 16 (10.1) | 62 (9.8) | |||
| Bilateral | 1226 (59.6) | 142 (84.0) | 635 (33.6) | 526 (66.6) | 133 (84.2) | 393 (62.2) | |||
| Postoperation | Pathologic GS, n (%) | <0.001* | 0.002* | ||||||
| <7 | 245 (11.9) | 36 (21.3) | 209 (11.1) | 107 (13.5) | 34 (21.5) | 73 (11.6) | |||
| =7 | 1565 (76.1) | 117 (69.2) | 1448 (76.7) | 616 (78.0) | 108 (68.4) | 508 (80.4) | |||
| >7 | 247 (12.0) | 16 (9.5) | 231 (12.2) | 67 (8.5) | 16 (10.1) | 51 (8.1) | |||
| ECE, n (%) | 611 (29.7) | 27 (16.0) | 584 (30.9) | <0.001* | 189 (23.9) | 24 (15.2) | 165 (26.1) | 0.004* | |
| SVI, n (%) | 175 (8.5) | 6 (3.6) | 169 (9.0) | 0.016* | 42 (5.3) | 6 (3.8) | 36 (5.7) | 0.341* | |
| PSM, n (%) | 566 (27.5) | 37 (21.9) | 529 (28.0) | 0.088* | 196 (24.8) | 35 (22.2) | 161 (25.5) | 0.387* | |
| Confirmed LNI, n (%) | 38 (1.8) | 3 (1.8) | 35 (1.9) | 0.942* | 11 (1.4) | 3 (1.9) | 8 (1.3) | 0.544* | |
| BCR status (+), n (%) | 436 (21.2) | 35 (20.7) | 401 (21.2) | 0.872* | 127 (16.1) | 33 (20.9) | 94 (14.9) | 0.066* | |
| Follow-up time until BCR (month), mean±s.d. | 20.97±22.45 | 19.91±22.72 | 21.06±22.45 | 0.775# | 20.38±22.30 | 20.24±23.20 | 20.43±22.10 | 0.969* | |
*The P values were calculated using the t-test model, #the P values were calculated using the Chi-square model. BMI: body mass index; HTN: hypertension; DM: diabetes mellitus; PSA: prostate-specific antigen; GS: Gleason score; ECE: extracapsular extension; SVI: seminal vesicle invasion; PSM: positive surgical margin; LNI: lymphatic nodal invasion; BCR: biochemical recurrence; s.d.: standard deviation
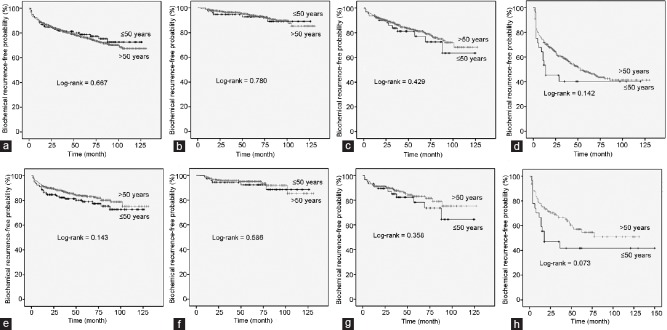
As for the BCR status outcome following RP among patients, younger and older men showed comparable BCR-free survival following RP, as shown in Figure 1a (log-rank P = 0.667). When analyzed according to disease risk, no significant differences in BCR-free survival were observed between younger and older men in the low-, intermediate-, and high-risk groups (P = 0.780, P = 0.429, and P = 0.142, respectively; Figure 1b–1d).
Figure 1.
Biochemical recurrence-free survival curves after prostatectomy according to age (≤50 vs >50 years) before and after propensity score matching. (a) All patients before matching. (b) Patients with D’Amico low-risk disease before matching. (c) Patients with D’Amico intermediate-risk disease before matching. (d) Patients with D’Amico high-risk disease before matching. (e) All patients after matching. (f) Patients with D’Amico low-risk disease after matching. (g) Patients with D’Amico intermediate-risk disease after matching. (h) Patients with D’Amico high-risk disease after matching.
After propensity score matching with 1:4 ratio, 158 younger men (93.5%) could be matched with older men; these younger men had lower pathologic Gleason score (P = 0.002) and lower rates of extracapsular extension (P = 0.004). There were no significant differences in BCR-free survival between younger and older men in the overall, low-, intermediate-, and high-risk groups (P = 0.143, P = 0.586, P = 0.358, and P = 0.073, respectively; Figure 1e–1h).
In multivariate analysis, even when analyzed in each disease risk group, the age of patients at RP was not shown to be an independent predictor of BCR status outcome following RP among all patients (P > 0.05; Table 2). In contrast, other factors such as PSA level, pathologic Gleason score, extracapsular extension, seminal vesicle invasion, lymph node involvement, and positive surgical margin were observed to be independent predictors among all patients (all P < 0.05).
Table 2.
Multivariate Cox proportional hazards regression model of predictive factors for biochemical recurrence-free survival after radical prostatectomy among the overall, low-, intermediate-, and high-risk groups
| Group | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≤50 vs >50 years) | 0.927 | 0.656–1.310 | 0.669 | |||
| BMI | 1.013 | 0.977–1.051 | 0.471 | |||
| PSA | 1.025 | 1.023–1.028 | <0.001 | 1.008 | 1.005–1.012 | <0.001 |
| Prostate volume | 1.003 | 0.998–1.008 | 0.211 | |||
| Clinical stage | 1.515 | 1.435–1.599 | <0.001 | |||
| Pathologic stage | 2.692 | 2.487–2.914 | <0.001 | 1.121 | 0.942–1.332 | 0.197 |
| Pathologic GS | 3.083 | 2.785–3.412 | <0.001 | 1.885 | 1.672–2.126 | <0.001 |
| ECE (+) versus (−) | 7.015 | 5.723–8.599 | <0.001 | 2.410 | 1.753–3.312 | <0.001 |
| SVI (+) versus (−) | 10.793 | 8.721–13.357 | <0.001 | 2.397 | 1.760–3.264 | <0.001 |
| cLNI (+) versus (−) | 11.747 | 8.185–16.860 | <0.001 | 1.800 | 1.221–2.653 | 0.003 |
| PSM (+) versus (−) | 4.759 | 3.929–5.764 | <0.001 | 1.907 | 1.527–2.383 | <0.001 |
| Low-risk group | ||||||
| Age (≤50 vs >50 years) | 1.130 | 0.479–2.669 | 0.780 | |||
| BMI | 1.029 | 0.918–1.153 | 0.621 | |||
| PSA | 1.199 | 1.044–1.377 | 0.010 | 1.081 | 0.939–1.246 | 0.277 |
| Prostate volume | 0.975 | 0.954–0.997 | 0.023 | |||
| Clinical stage | 1.997 | 1.113–3.583 | 0.020 | |||
| Pathologic stage | 1.977 | 1.237–3.159 | 0.004 | 0.664 | 0.416–1.062 | 0.088 |
| Pathologic GS | 2.608 | 1.845–3.685 | <0.001 | 1.741 | 1.156–2.621 | 0.008 |
| ECE (+) versus (−) | 4.573 | 2.510–8.332 | <0.001 | 2.838 | 1.152–6.991 | 0.023 |
| SVI (+) versus (−) | 21.943 | 6.527–73.770 | <0.001 | 8.119 | 2.041–32.305 | 0.003 |
| cLNI (+) versus (−) | 0.050 | – | 0.845 | |||
| PSM (+) versus (−) | 6.911 | 3.854–12.394 | <0.001 | 5.079 | 2.592–9.953 | <0.001 |
| Intermediate-risk group | ||||||
| Age (≤50 vs >50 years) | 1.259 | 0.709–2.237 | 0.431 | |||
| BMI | 1.010 | 0.944–1.081 | 0.774 | |||
| PSA | 1.074 | 1.034–1.116 | <0.001 | 1.042 | 1.000–1.085 | 0.048 |
| Prostate volume | 0.986 | 0.973–0.999 | 0.042 | 0.992 | 0.977–1.008 | 0.334 |
| Clinical stage | 1.528 | 1.119–2.088 | 0.008 | |||
| Pathologic stage | 2.389 | 1.999–2.856 | <0.001 | 1.435 | 1.011–2.038 | 0.043 |
| Pathologic GS | 2.496 | 1.895–3.288 | <0.001 | 2.070 | 1.546–2.771 | <0.001 |
| ECE (+) versus (−) | 3.943 | 2.762–5.629 | <0.001 | 1.462 | 0.832–2.571 | 0.187 |
| SVI (+) versus (−) | 6.137 | 3.750–10.045 | <0.001 | 2.096 | 1.078–4.078 | 0.029 |
| cLNI (+) versus (−) | 6.053 | 1.922–19.069 | 0.002 | 0.740 | 0.203–2.695 | 0.648 |
| PSM (+) versus (−) | 2.838 | 1.997–4.033 | <0.001 | 1.790 | 1.210–2.647 | 0.004 |
| High-risk group | ||||||
| Age (≤50 vs >50 years) | 1.446 | 0.872–2.399 | 0.153 | |||
| BMI | 1.017 | 0.971–1.065 | 0.479 | |||
| PSA | 1.014 | 1.010–1.018 | <0.001 | 1.004 | 1.000–1.009 | 0.049 |
| Prostate volume | 1.004 | 1.000–1.009 | 0.055 | |||
| Clinical stage | 1.124 | 1.053–1.201 | <0.001 | |||
| Pathologic stage | 2.049 | 1.843–2.277 | <0.001 | 1.048 | 0.834–1.315 | 0.689 |
| Pathologic GS | 2.188 | 1.910–2.507 | <0.001 | 1.635 | 1.412–1.892 | <0.001 |
| ECE (+) versus (−) | 4.624 | 3.422–6.248 | <0.001 | 2.105 | 1.365–3.247 | 0.001 |
| SVI (+) versus (−) | 5.006 | 3.876–6.464 | <0.001 | 2.237 | 1.548–3.234 | <0.001 |
| cLNI (+) versus (−) | 5.808 | 3.919–8.607 | <0.001 | 2.034 | 1.346–3.074 | 0.001 |
| PSM (+) versus (−) | 2.958 | 2.290–3.820 | <0.001 | 1.512 | 1.132–2.018 | 0.005 |
HR: hazard ratio; CI: confidence interval; BMI: body mass index; PSA: prostate-specific antigen; GS: Gleason score; ECE: extracapsular extension; SVI: seminal vesicle invasion; cLNI: confirmed lymphatic nodal invasion; PSM: positive surgical margin
The proportion of patients who were reported to be continent at 1 year after RP was significantly higher among younger men than among their older counterparts (99.4% vs 95.0%, P = 0.009). Of 2057 patients in total, 1845 men had an available preoperative IIEF-5 score. Further, of these 1845 men, 228 (including 53 [23.3%] men aged ≤50 years at RP) had a preoperative IIEF-5 score ≥22. There were no significant differences in preoperative IIEF-5 score (23.60 ± 1.17 vs 23.35 ± 1.08, P = 0.149), neurovascular bundle-sparing status (94.3% vs 87.4%, P = 0.158), PDE-5 inhibitor therapy status (77.1% vs 84.7%, P = 0.225), intracavernosal injection therapy status (13.3% vs 7.5%, P = 0.232), and starting point of erectile dysfunction treatment after RP (1.78 ± 2.15 vs 1.92 ± 2.24 months, P = 0.744). The proportion of younger men who reported having erections sufficient for vaginal intercourse at 1 year after RP was significantly higher than that of their older counterparts (81.8% vs 55.5%, P = 0.001).
DISCUSSION
Our study showed that younger men (aged ≤50 years) who underwent RP were more likely to present with low-risk PCa and had tumors characterized by more favorable pathologic features than their older counterparts. After performing propensity score matching to control selection bias, younger men were shown to have lower pathologic Gleason score and pathologic stages than their older counterparts. Moreover, multivariate analysis showed that pathologic Gleason score and extracapsular extension were independent predictors of BCR status outcome following RP. Unlike age, PSA level, pathologic Gleason score, extracapsular extension, seminal vesicle invasion, lymph node involvement, and positive surgical margin were shown to significantly affect postoperative BCR status outcome, which is consistent with the results reported in a previous study.16 With respect to functional outcomes, younger men were observed to have enhanced recovery of urinary continence and erectile function compared with their older counterparts.
Classically, PCa in younger men was generally considered more aggressive, as described by reports published in the 1970s and 1980s.5,6,7 However, a number of more contemporary studies have reported otherwise. Siddiqui et al.8 examined the effect of age at RP on postoperative outcome and reported that survival in younger patients undergoing RP, despite having more favorable clinicopathologic features, was similar to that in their older counterparts. Furthermore, a multi-institutional study from the United States showed that men aged <50 years at diagnosis had relatively more favorable pathologic profile and better BCR status outcome after RP than older patients.9 In contrast, a German study by Becker et al.10 showed that men aged <50 years who underwent RP were more likely to harbor low-risk, organ-confined, and low-grade tumors and that age was not a significant factor for BCR status outcome following RP. Kinnear et al.11 performed a similar study that included Australian men with PCa and reported that men aged ≤50 years had less aggressive clinical characteristics but similar rates of adjusted BCR after primary treatment. Such results from contemporary series can be considered similar to our findings to some extent.
No definite explanation exists for the more favorable clinicopathologic features of PCa in younger men. Moreover, autopsy series have indicated PCa in younger men to be mostly of indolent, low-grade nature.2 Accordingly, it may be simply hypothesized that younger patients are diagnosed at an earlier stage. However, it can also be suggested that doctors may tend to be stricter and more aggressive in the decision-making process for PCa screening, prostate biopsy, and definitive treatment in younger men than in older men.8 A lower PSA level at diagnosis in younger men would be supportive of such suggestions. In addition, tumors presenting at different ages may just have different intrinsic traits, although this remains unknown. Early-onset PCa is linked to a stronger genetic component; however, the molecular basis of such association is not yet fully understood. A recent study suggested that early-onset PCa formation in young men may involve the specific emergence of androgen-driven structural genomic variations, potentially altering the tumor's clinical behavior.17
Despite the observation that younger men had more favorable clinicopathologic profile at RP than older men, no significant difference in postoperative BCR status outcome according to the age of patients was noted in our study. In multivariate analysis incorporating various factors, age was determined to exert no significant effect on BCR status outcome. On stratification of patients into disease risk groups (i.e., low-, intermediate-, and high-risk groups), age was similarly not shown to be an independent predictor of BCR status outcome in each disease risk group. As previously mentioned, some studies have reported similar findings, whereas others have indicated better postoperative BCR status outcome in the younger cohort.8,9,10,11,12 In our study, we could actually note that BCR-free survival was higher in younger men, albeit without statistical significance. Our findings on comparable postoperative BCR status outcome between younger and older men would be supportive evidence for the therapeutic effect of RP in older men rather than a lack of therapeutic effect of RP in younger men. Although older men generally present with worse clinicopathologic features, RP appears to have resulted in equivalent treatment outcome.
BCR status outcome, though widely applied, is not a perfectly accurate predictor of disease progression and/or mortality from PCa. Our results on the outcome following RP may have admittedly been different with longer follow-up duration and assessment of postoperative metastasis and mortality. With longer follow-up duration, factors such as comorbidities may become stronger determinants of survival after RP. Siddiqui et al.8 observed that systemic progression-free survival and cancer-specific survival after RP in younger patients appeared to be worse after adjustment for pathologic features and PSA level. In addition, they found that the risk of systemic progression was higher in younger men among patients with high-risk PCa. In contrast, overall survival was worse in older men, most probably owing to the increasing risk of death from non-PCa causes. Siddiqui et al.8 concluded that the probability of disease progression or cancer death was greater in younger men despite having more favorable clinicopathologic features of PCa, as they are less likely to die of causes other than PCa. In a population-based study, men younger than 45 years in the high-risk group were observed to have significantly worse disease-specific outcomes.18 Although such finding could be reflective of biologically more aggressive PCa occurring in younger men, the relative lack of competing comorbidities may well have played a role in their finding. In our study, the Kaplan–Meier curve showed relatively shorter BCR-free survival in younger men in the high-risk group only, which did not reach statistical significance.
Various series have shown that younger men undergoing RP, in general, exhibited relatively favorable BCR status outcome following RP, as can also be corroborated by our results.9,10,11,12 However, as many would agree, some younger men present with very aggressive disease. Among our patients, a 50-year-old man was shown to have high-grade PCa (pathologic Gleason score of 9) with seminal vesicle invasion and lymphatic nodal invasion at right common iliac lymph node chain. During postoperative follow-up, the patient developed distant metastasis, including bone metastasis, within 4 years.
As our study included only men who underwent RP, younger patients with locally advanced or metastatic disease at presentation who received nonsurgical therapy were not analyzed. Although younger patients deemed not suitable for RP because the extent of their disease may not be numerous, future investigations should also assess younger nonsurgically managed patients to elucidate the true nature of PCa in younger men.
We analyzed BCR status and functional outcomes following RP in our study. As previously reported, we also observed a more favorable recovery of urinary continence and erectile function after RP in younger men than in older men.10 Considering the association of nerve-sparing procedure with functional recovery after RP according to reports,19,20 we assessed the frequency of nerve-sparing procedure being performed during RP. Among our patients, the nerve-sparing procedure was more frequently performed in younger men than in older men (Table 1). Such phenomenon may well be largely due to the more favorable disease features in the younger group. Moreover, to minimize the effect of various potential confounders on erectile function, we only selected men with preoperative IIEF-5 score ≥22 for the evaluation of postoperative erectile function. We observed that the proportion of men who reported having a successful vaginal intercourse within 1 year after surgery was significantly higher in the younger group than in the older group in our study, corroborating other reports that indicated more favorable recovery of erectile function following RP among younger patients.10,21 Overall, a higher rate of nerve-sparing procedure may well have contributed to better functional recovery. Further, we observed that men who underwent robot-assisted RP had enhanced recovery of urinary continence compared with those who underwent open RP, although no significant difference in the recovery of erectile function was observed (data not shown).
Our study may be limited by its retrospective and single-institution design. In addition, younger men who underwent treatment other than RP were not included, and we did not assess progression-free, metastasis-free, or cancer-specific survival owing to the relative lack of relevant events and the length of postoperative follow-up. Moreover, the number of patients aged ≤50 years was rather low, making the present study underpowered. However, to our knowledge, our study is the largest RP series analyzing postoperative outcome in younger Asian men. It should be noted that we also examined functional and oncological outcomes following RP in younger Asian men.
CONCLUSIONS
Our study on a relatively large cohort of contemporary Asian men who underwent RP showed that younger men (aged ≤50 years) had more favorable clinicopathologic features at surgery than their older counterparts. With respect to postoperative oncological outcome, the age of patients was not determined to be an independent predictor of BCR-free survival in multivariate analysis. However, younger men were observed to have better functional outcomes following RP.
AUTHOR CONTRIBUTIONS
BS and MSL performed data collection and statistical analysis. HL provided advice on statistical analysis. SKH conceived the study and its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hussein S, Satturwar S, Van der Kwast T. Young-age prostate cancer. J Clin Pathol. 2015;68:511–5. doi: 10.1136/jclinpath-2015-202993. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Severi G, Giles GG. The epidemiology of prostate cancer. Urol Clin North Am. 2003;30:209–17. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 4.Li J, German R, King J, Joseph D, Thompson T, et al. Recent trends in prostate cancer testing and incidence among men under age of 50. Cancer Epidemiol. 2012;36:122–7. doi: 10.1016/j.canep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Silber I, McGavran MH. Adenocarcinoma of the prostate in men less than 56 years old: a study of 65 cases. J Urol. 1971;105:283–5. doi: 10.1016/s0022-5347(17)61510-6. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DE, Lanieri JP, Jr, Ayala AG. Prostatic adenocarcinoma occurring in men under 50 years of age. J Surg Oncol. 1972;4:207–16. doi: 10.1002/jso.2930040305. [DOI] [PubMed] [Google Scholar]
- 7.Huben R, Natarajan N, Pontes E, Mettlin C, Smart CR, et al. Carcinoma of prostate in men less than fifty years old. Data from American College of Surgeons’ National Survey. Urology. 1982;20:585. doi: 10.1016/0090-4295(82)90304-1. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui SA, Sengupta S, Slezak JM, Bergstralh EJ, Leibovich BC, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol. 2006;175:952–7. doi: 10.1016/S0022-5347(05)00339-3. [DOI] [PubMed] [Google Scholar]
- 9.Parker PM, Rice KR, Sterbis JR, Chen Y, Cullen J, et al. Prostate cancer in men less than the age of 50: a comparison of race and outcomes. Urology. 2011;78:110–5. doi: 10.1016/j.urology.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Becker A, Tennstedt P, Hansen J, Trinh QD, Kluth L, et al. Functional and oncological outcomes of patients aged <50 years treated with radical prostatectomy for localised prostate cancer in a European population. BJU Int. 2014;114:38–45. doi: 10.1111/bju.12407. [DOI] [PubMed] [Google Scholar]
- 11.Kinnear NJ, Kichenadasse G, Plagakis S, O’Callaghan ME, Kopsaftis T, et al. Prostate cancer in men aged less than 50 years at diagnosis. World J Urol. 2016;34:1533–9. doi: 10.1007/s00345-016-1824-4. [DOI] [PubMed] [Google Scholar]
- 12.Prendeville S, Nesbitt ME, Evans AJ, Fleshner NE, van der Kwast TH. Variant histology and clinicopathological features of prostate cancer in men younger than 50 years treated with radical prostatectomy. J Urol. 2017;198:79–85. doi: 10.1016/j.juro.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 13.Park YH, Lee JK, Jung JW, Lee BK, Lee SC, et al. Prostate cancer detection rate in patients with fluctuating prostate-specific antigen levels on the repeat prostate biopsy. Prostate Int. 2014;2:26–30. doi: 10.12954/PI.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong SK, Nam JS, Na W, Oh JJ, Yoon CY, et al. Younger patients have poorer biochemical outcome after radical prostatectomy in high-risk prostate cancer. Asian J Androl. 2011;13:719–23. doi: 10.1038/aja.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Amico AV, Whittington R, Malkowicz SB, Weinstein M, Tomaszewski JE, et al. Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J Urol. 2001;166:2185–8. [PubMed] [Google Scholar]
- 16.Hong JH, Lee HM, Choi HY. [The predictors of biochemical recurrence and metastasis following radical perineal prostatectomy in clinically localized prostate cancer] Korean J Urol. 2005;46:1161–7. [Google Scholar]
- 17.Weischenfeldt J, Simon R, Feuerbach L, Schlangen K, Weichenhan D, et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell. 2013;23:159–70. doi: 10.1016/j.ccr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2009;115:2863. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabbani F, Stapleton AM, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929–34. [PubMed] [Google Scholar]
- 20.Steineck G, Bjartell A, Hugosson J, Axén E, Carlsson S, et al. LAPPRO steering committee. Degree of preservation of the neurovascular bundles during radical prostatectomy and urinary continence 1 year after surgery. Eur Urol. 2015;67:559–68. doi: 10.1016/j.eururo.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Labanaris AP, Zugor V, Witt JH. Robotic-assisted radical prostatectomy in men ≤ 50 years of age.Surgical, oncological and functional outcomes. Anticancer Res. 2012;32:2097–101. [PubMed] [Google Scholar]