Abstract
Dysfunctional sperm maturation is the primary reason for the poor sperm motility and morphology in infertile men. Spermatozoa from infertile men were fractioned on three-layer density gradient (80%, 60%, and 40%). Fraction 1 (F1) refers to the least mature stage having the lowest density, whereas the fraction 4 (F4) includes the most dense and morphologically mature motile spermatozoa. Fraction 2 (F2) and fraction 3 (F3) represent the intermediate stages. Proteins were extracted and separated by 1-dimensional gel. Bands were digested with trypsin and analyzed on a LTQ-Orbitrap Elite hybrid mass spectrometer system. Functional annotations of proteins were obtained using bioinformatics tools and pathway databases. A total of 1585 proteins were detected in the four fractions of spermatozoa. A dysregulated protein turnover and protein folding may lead to accumulation of defective proteins or proteins that otherwise would have been eliminated during the process of maturation, resulting in the impairment of sperm function. Aberrant chaperone expression may be a major contributing factor to the defective sperm function. Androgen receptor was predicted as a transcription regulator in one of the networks and the affected pathways were chaperone-mediated stress response, proteosomal pathway, and sperm function. The downregulation of key pathways and proteins which compromises the fertilizing potential of spermatozoa may provide insight into the mechanisms that lead to male infertility.
Keywords: androgen receptor, chaperone, immature sperm, infertile men, proteasome, spermatozoa
INTRODUCTION
Male infertility is a multifactorial condition and there is no identifiable cause in 50% of the cases. The human testis produces spermatozoa at a rate of 1000 cells per second and these cells are highly differentiated and unique.1 Spermatozoa originate from the complex process of spermatogenesis in three major steps as follows: (1) proliferation and differentiation of spermatogonia; (2) divisions during the spermatocyte stage; and (3) spermiogenesis. Spermiogenesis involves major morphological and molecular changes, including the removal of cytoplasm, formation of the acrosome and flagella, mitochondrial rearrangement, and nuclear remodeling. During mid-spermiogenesis, the nucleus of the round spermatid changes from spherical to a unique elongated and flattened shape. This reshaping protects the male genome during sperm transport and also facilitates the penetration of spermatozoa into ovum. Thus, spermatozoa are terminally differentiated and possess specialized organelles.2 However, they undergo maturation during epididymal transit to acquire the ability to fertilize.3
Spermatozoa have different pathologies from those of somatic cells, which result in different sperm phenotypes in the ejaculated semen. Seven sperm phenotypes have been detected in human semen from electron microscopy, which include spermatozoa with dysplasia of the fibrous sheath, nonspecific flagellar defects, immotile cilia, acrosomal hypoplasia, defective chromatin condensation and compaction, pin head, and even sperm cells without heads.4 These conditions cannot be identified by routine semen analysis or functional tests since the deficiencies demonstrated by these methods are secondary manifestations of an underlying pathology.
The generation of high-quality spermatozoa is governed by a number of selective mechanisms within the testes and epididymis.5 The dramatic changes that occur during spermiogenesis, sperm maturation, and capacitation involve loss and gain of specific proteins.6,7,8 Recently, we have reported that distinct proteomic signatures distinguish high-quality spermatozoa from their low-quality counterparts in fertile donors.9 Improper spermatogenesis produces abnormal spermatozoa that are generally earmarked for elimination by apoptosis and appear in the ejaculate when they escape apoptosis. Furthermore, differential localization of Fas, a membrane receptor of the tumor necrosis factor family that initiates apoptosis, also segregates the spermatozoa into different subsets.10,11 Thus, spermatozoa marked for apoptosis are of lower reproductive potential than their unmarked counterparts. Defects in epididymal maturation lead to increased morphological abnormalities in the spermatozoa and poor sperm motility.2,12,13 In addition, immature spermatozoa exhibit metabolic alterations, presence of excess cytoplasm in the ejaculate, increased production of reactive oxygen species (ROS), lipid peroxidation, and DNA fragmentation.5,14
Sperm preparation methods such as density gradient separation are routinely used to obtain highly motile and morphologically normal sperm for assisted reproductive technology (ART). Spermatozoa separated on a three-layer density gradient (40%, 60%, and 80%) demonstrate cell-to-cell variation in both fertile and infertile men.5 Spermatozoa were separated into four fractions on the basis of their density and maturity. The lowest level of ROS production and DNA damage correlates with morphologically normal, motile spermatozoa obtained in the mature subset from fertile men compared with abnormal spermatozoa from infertile men.14,15,16 Different subsets of spermatozoa obtained from the ejaculate of fertile men after separation on a three-layer density gradient differ in their proteome profile.9 We have demonstrated an increasing trend in proteins involved in key biological processes during sperm maturation such as reproductive cellular process, gamete production, motility, oxidative phosphorylation, and energy metabolism. A decreasing trend was seen in the expression of proteins that were involved in key biological processes such as protein synthesis, protein transport, and response to oxidative stress.9
Division of spermatozoa into phenotypes or subsets is of importance in the evaluation of its true fertility potential, particularly when using testicular spermatozoa for intracytoplasmic sperm injection (ICSI). A recent review by Esteves et al.17 documented that infertile couples may benefit from ICSI with testicular spermatozoa instead of ejaculated spermatozoa if the male partners exhibit high sperm DNA fragmentation (SDF) in the ejaculate. We have also demonstrated that proteins critical for sperm maturation, motility, and fertilization are involved in biological processes that are activated or suppressed in different subsets of spermatozoa from fertile men.9 However, the underlying pathways and the distribution of proteins in immature and mature sperm from infertile men have not been explored utilizing a proteomic approach. The present study is a continuation of our previous report on fertile donors deciphering the proteomic signatures in the spermatozoa of infertile patients to understand the underlying mechanism(s) of defective sperm maturation.
PATIENTS AND METHODS
Patients
Following the approval of the study by the Institutional Review Board (IRB) of Cleveland Clinic (Cleveland, OH, USA), semen samples were collected from 11 infertile men. Men with leukocytospermia (Endtz test positive) and female factor infertility were excluded. All enrolled patients provided written consent to participate in the study.
Sample collection and semen analysis
Semen samples were examined according to 2010 World Health Organization (WHO) criteria.18 All specimens were collected by masturbation after sexual abstinence of 48–72 h and were allowed to liquefy for 20 min at 37°C before further processing. Following liquefaction, manual semen analysis was performed, including evaluation of presence of round cells, presence of white blood cells by the peroxidase test (Endtz test), viability, and morphology as previously described.9
Separation of sperm phenotypes and proteomic analysis
For separating immature and mature spermatozoa, a three-layer density gradient was used as described earlier.9 It consisted of 2 ml of 40%, 60%, and 80% of the upper layer, intermediate layer, and lower layer, respectively. The three layers were reconstituted from the stock (100%) solution of the gradient with the SpermRinse medium (Vitrolife, San Diego, CA, USA). The stock gradient was an antibiotic-free bicarbonate and HEPES-buffered medium containing silane-coated, colloid silica particles. The SpermRinse medium was a bicarbonate and HEPES-buffered medium containing human serum albumin and gentamycin as an antibiotic (Vitrolife, San Diego, CA, USA). The three-layer gradient is a slight modification of the 2-layer density gradient method routinely used for preparing sperm for ART techniques, especially intrauterine insemination.9 Briefly, 1–2 ml of liquefied semen sample was carefully loaded on the 40% gradient and centrifuged at 300 g for 20 min. The resulting interfaces between the seminal plasma and 40% (fraction 1), 40% and 60% (fraction 2), 60% and 80% (fraction 3), and the 80% pellet (fraction 4, mature fraction) were carefully aspirated, resuspended in human tubal fluid media (HTF, Irvine Scientific, Santa Ana, CA, USA), and centrifuged at 300 g for 7 min. The pellets of each fraction were resuspended in 0.5–1 ml HTF, and the total sperm count, motility, and morphology were assessed again.
Sperm proteins from two individual samples and one pooled (from four individuals after normalization for spermatozoa number and protein content) sample were dissolved in RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with proteinase inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein concentration was determined by the Bicinchoninic acid (BCA) kit (Thermo, Rockford, IL, USA). Proteins were extracted and separated by 1-dimensional gel electrophoresis. Bands were digested with trypsin and analyzed on a LTQ-Orbitrap Elite hybrid mass spectrometer (Thermo) system as described.9 Each sample was run in triplicate and the average was taken.
Database searching and protein identification
Tandem mass spectra were extracted by Proteome Discoverer (version 1.4.1.288, Thermo Fisher Scientific, San Jose, CA, USA). Twelve tandem mass spectrometry or MS/MS samples (3 runs per sample) were analyzed by using Mascot (version 2.3.02, Matrix Science, London, UK), Sequest (version 1.4.0.288, Thermo Fisher Scientific), and X! Tandem (TheGPM, thegpm.org; version CYCLONE 2010.12.01.1). Mascot, Sequest, and X! Tandem were set up to search the human database (33 292 entries) assuming the digestion enzyme trypsin. To validate MS/MS-based peptide and protein identifications, Scaffold (version Scaffold 4.0.6.1, Proteome Software Inc., Portland, OR, USA) was used. Peptide identifications were accepted if they could be established at >95.0% probability by the PeptideProphet™ algorithm with Scaffold delta-mass correction.19
Quantitation of the relative abundance of protein in spermatozoa
The relative abundance of sperm proteins was determined by comparing the number of spectra, termed spectral counts (SC), used to identify each protein. The numerical values used in the quantitation correspond to the normalized spectral abundance factor (NSAF, SC/[ΣSC] × protein length). NSAF approach was applied before relative protein quantification.20 Differentially expressed proteins (DEPs) were obtained by applying different constraints for significance tests or fold change cutoffs from the average SC of the protein from multiple runs.9
The categorization of overall abundance along with the filtering criteria used for differential expression analysis is summarized below:
Very low abundance: spectral count range 1.7–7; P ≤ 0.001; and NSAF ratio ≥2.5 for overexpressed and ≤0.4 for underexpressed proteins
Low abundance: spectral count range 8–19; P ≤ 0.01; and NSAF ratio ≥2.5 for overexpressed and ≤0.4 for underexpressed proteins
Medium abundance: spectral count range 20–79; P ≤ 0.05; and NSAF ratio ≥2.0 for overexpressed and ≤0.5 for underexpressed proteins
High abundance: spectral counts >80; P ≤ 0.05; and NSAF ratio ≥1.5 for overexpressed and ≤0.67 for underexpressed proteins.
Bioinformatic analyses
Functional bioinformatics analyses were done with publicly available tools such as Gene Ontology (GO) annotations from GO Term Finder (http://search.cpan.org/dist/GO-TermFinder/),21 GO Term Mapper (http://go.princeton.edu/cgi-bin/GOTermMapper), UNIPROT (The UniProt Consortium; http://www.uniprot.org/), Database for Annotation, Visualization and Integrated Discovery (DAVID; http://david.niaid.nih.gov), and proprietary software packages such as Ingenuity Pathway Analysis (IPA from Ingenuity® Systems; https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/) and MetaCore™ (GeneGo Inc., Encinitas, CA, USA) to identify the cellular distribution of proteins and differentially affected processes and pathways.
Statistical analyses
The results were expressed as mean ± standard deviation (s.d.). To compare the differences between different fractions of the ejaculate, we used Jonckheere–Terpstra test (or Jonckheere's trend test). It is similar to the Kruskal–Wallis test where the null hypothesis is that several independent samples are from the same population. However, there is no priori ordering of the populations from which the samples are drawn. When there is a priori ordering, the Jonckheere–Terpstra test has more power than the Kruskal–Wallis test. In this test, there is no issue of normality and does not require log transformation of the data. The statistical analysis was performed using the MedCalc (version 17.9.7, MedCalc Software bvba, Ostend, Belgium). For IPA and MetaCore™, the right-tailed Fisher's exact testwas used. Differences were considered statistically significant for P < 0.05.
RESULTS
Semen analysis
Total sperm count (TSC), presence of round cells, motility, and total motile sperm count (TMS) before and after density gradient centrifugation are shown in Table 1 and Figure 1. The TSC (×106, mean ± s.d.) recovered in F3 and F4 was similar (32.51 ± 25.78 and 34.32 ± 32.53, respectively) and significantly different (P = 0.004665) from the TSC in the prewash sample (79.98 ± 84.58) (Figure 1a). There was a significant decrease (P < 0.000001) in the number of round cells (median, 25th and 75th percentiles) from the prewash sample (1.90 [1.07, 3.75]) to that of F3 (0 [0, 0]) and F4 (0 [0, 0]) (Figure 1b). Spermatozoa recovered from F4 displayed the highest average motility (63.0 [53.5, 83.1]) compared with F1, F2, and F3 followed by F3 (36.6 [32.3, 43.2]) compared with F1, F2, and F4 (P = 0.000001) (Figure 1c). A higher recovery of TMS (×106 spermatozoa) was observed in F4 (23.46 ± 23.39) than F3 (12.68 ± 11.90) (Figure 1d).
Table 1.
Semen parameters before and after separation on a 3-layer density gradient
| Parameter | PW | F1 | F2 | F3 | F4 | P |
|---|---|---|---|---|---|---|
| Motility (%) | 45.40±18.84 | 7.71±6.82 | 21.21±7.35 | 35.78±13.48 | 64.46±18.12 | 0.000001a |
| 47.0 (29, 61) | 6.6 (3.5, 7.8) | 21.4 (17.1, 24.4) | 36.6 (32.3, 43.2) | 63.0 (53.5, 83.1) | ||
| F1, F2, F4 | F2, F3, F4, (PW) | F1, F3, F4, (PW) | F1, F2, F4 | F1, F2, F3 | <0.00001b | |
| Total sperm count (×106) | 79.98±84.58 | 12.96±18.35 | 20.14±17.70 | 34.32±32.53 | 32.51±25.78 | 0.004665a |
| 61.80 (30.20, 81.60) | 8.85 (1.78, 15.40) | 17.00 (7.75, 22.80) | 26.50 (13.30, 35.20) | 27.45 (11.60, 52.20) | ||
| F1, F2 | F3, F4, (PW) | (PW) | F1 | F1 | 0.00007b | |
| Total motile sperm (×106) | 102.87±92.06 | 0.79±1.17 | 3.98±3.75 | 12.68±11.90 | 23.46±23.39 | 0.000002a |
| 65.93 (33.65, 154.87) | 0.199 (0.16, 1.00) | 2.79 (1.59, 4.20) | 10.79 (4.00, 18.18) | 10.90 (7.54, 35.96) | ||
| F1, F2, F3, F4 | F2, F3, F4, (PW) | F1, F3, F4, (PW) | F1, F2, (PW) | F1, F2, (PW) | <0.00001b | |
| Round cells (×106 ml−1) | 1.90 (1.07, 3.75) | 1.35 (0.60, 3.20) | 0.30 (0.10, 0.70) | 0 (0, 0) | 0 (0, 0) | <0.000001a |
| F2, F3, F4 | F2, F3, F4 | F1, F3, F4 | F1, F2, (PW) | F1, F2, (PW) | 0.28496b |
Values are mean±s.d. and median (25th and 75th percentiles). aComparison between PW and different fractions was done by Kruskal–Wallis test; bpost hoc analysis for differences was done by Jonckheere–Terpstra trend test. P<0.05 was considered statistically significant. F1: least mature stage having the lowest density; F2, F3: intermediate stages; F4: includes the most dense and morphologically mature motile spermatozoa. s.d.: standard deviation; PW: prewash
Figure 1.
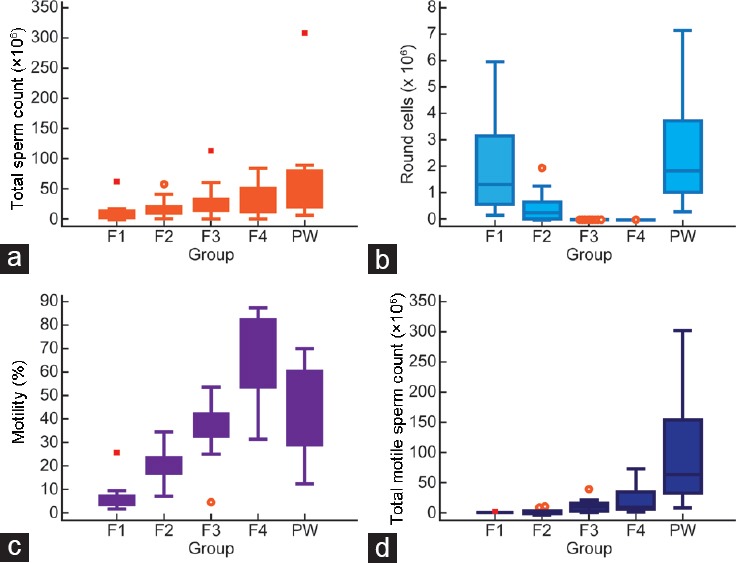
Semen parameters in four fractions compared to PW sample. (a) Total sperm count (×106); (b) round cell count (×106); (c) motility (%); and (d) total motile count (×106) in F1–F4. PW: prewash; F1: least mature stage having the lowest density; F2, F3: intermediate stages; F4: includes the most dense and morphologically mature motile spermatozoa.
Overall protein abundance
The relative abundance of the identified proteins in the sperm samples was quantified. For higher accuracy of proteomic results, the protein abundance should be similar between the analyzed samples. The range of total SC was 31188–51131 which showed that the abundance of proteins in these samples was similar.
Distribution of proteins in different fractions
All fractions, i.e. F1, F2, and F3 were compared with mature fraction F4. A total of 1585 proteins were identified in the four fractions together. Among those, 1202, 1140, 1129, and 890 proteins were detected in F1, F2, F3, and F4, respectively. By comparing the F1 and F4 proteomes, 136 proteins were overexpressed in F1, 177 were underexpressed in F1, 158 were unique to F1, and 51 were unique to F4. When F2 was compared with F4, 113 proteins were overexpressed in F2, 111 were underexpressed in F2, 114 were unique to F2, and 24 were unique to F4. Comparison of F3 versus F4 showed 89 proteins overexpressed in F3, 53 underexpressed in F3, 38 unique to F3, and 8 unique to F4. All the four fractions were compared to identify the differentially expressed proteins (DEPs). Of the 656 DEPs, 75 proteins showed an increasing trend from fraction F1 to F4 (Supplementary Table 1), while 279 showed a decreasing trend (Supplementary Table 2).
Supplementary Table 1.
Proteins showing an increasing trend during sperm maturation
| Protein | Uniprot number |
MW kDa |
F1 Average SC |
F2 Average SC |
F3 Average SC |
F4 Average SC |
|---|---|---|---|---|---|---|
| Actin-like protein 9 | Q8TC94 | 46 | 0.0 | 5.0 | 8.3 | 50.3 |
| AMY-1-associating protein expressed in testis 1 | Q7Z4T9 | 90 | 0.0 | 0.0 | 3.0 | 4.3 |
| Calcium-binding tyrosine phosphorylation-regulated protein isoform C | O75952 | 41 | 0.0 | 104.7 | 138.0 | 175.3 |
| Calicin | Q13939 | 67 | 0.0 | 0.0 | 33.7 | 50.7 |
| Cartilage acidic protein 1 isoform B precursor | Q9NQ79 | 70 | 0.0 | 0.0 | 5.0 | 10.7 |
| Coiled-coil domain-containing protein 39 | Q9UFE4 | 110 | 0.0 | 3.7 | 4.0 | 5.7 |
| Disintegrin and metalloproteinase domain-containing protein 29 preproprotein | Q9UKF5 | 93 | 0.0 | 1.3 | 7.0 | 17.3 |
| Dynein heavy chain 1, axonemal | Q9P2D7 | 488 | 0.0 | 18.3 | 18.3 | 18.7 |
| Dynein heavy chain 12, axonemal isoform 1 | Q6ZR08 | 357 | 0.0 | 8.7 | 12.0 | 15.7 |
| Fibronectin type III domain-containing protein 8 | Q8TC99 | 36 | 0.0 | 3.0 | 15.0 | 26.7 |
| Fibrous sheath-interacting protein 2 | J3QTJ6 | 790 | 0.0 | 0.0 | 2.3 | 13.0 |
| GLIPR1-like protein 2 | Q4G1C9 | 29 | 0.0 | 15.3 | 16.7 | 20.0 |
| Isochorismatase domain-containing protein 2, mitochondrial isoform 1 | P50213 | 22 | 0.0 | 56.3 | 60.7 | 60.7 |
| Isocitrate dehydrogenase [NAD] subunit gamma, mitochondrial isoform A precursor | O75874 | 43 | 0.0 | 5.7 | 2.0 | 5.3 |
| Leucine-rich repeat and IQ domain-containing protein 4 | Q53EV4 | 64 | 0.0 | 3.0 | 6.7 | 13.3 |
| Leucine-rich repeat-containing protein 23 isoform A | A6NMS7 | 40 | 0.0 | 11.0 | 16.3 | 18.0 |
| leucine-rich repeat-containing protein 48 isoform A | Q08722 | 61 | 0.0 | 15.7 | 30.7 | 52.7 |
| Mitochondrial thiamine pyrophosphate carrier | Q10713 | 36 | 0.0 | 2.3 | 8.7 | 9.0 |
| Poly(ADP-ribose) glycohydrolase ARH3 | Q15365 | 39 | 0.0 | 2.7 | 2.3 | 9.0 |
| Potassium channel subfamily U member 1 | A8MYU2 | 130 | 0.0 | 0.0 | 4.0 | 5.7 |
| Protein FAM205A | Q6ZU69 | 148 | 0.0 | 0.0 | 11.7 | 39.3 |
| Septin-7 isoform 1 | Q16181 | 51 | 0.0 | 0.0 | 5.7 | 6.0 |
| Sodium/potassium-transporting ATPase subunit beta-1 | P05026 | 35 | 0.0 | 0.0 | 2.7 | 2.7 |
| Speriolin isoform 1 | Q9HBV2 | 62 | 0.0 | 6.0 | 6.3 | 6.3 |
| SUN domain-containing protein 5 | Q8TC36 | 43 | 0.0 | 0.0 | 3.7 | 4.0 |
| Tripartite motif-containing protein 42 | Q8IWZ5 | 83 | 0.0 | 0.0 | 1.3 | 10.7 |
| Uncharacterized protein LOC730159 precursor | 21 | 0.0 | 0.0 | 5.7 | 6.7 | |
| WD repeat-containing protein 52 isoform 1 | Q9GZS3 | 214 | 0.0 | 15.7 | 17.0 | 24.0 |
| Bovine seminal plasma protein homolog 1 precursor | Q075Z2 | 16 | 0.7 | 4.0 | 7.3 | 9.7 |
| Probable Xaa-Pro aminopeptidase 3 isoform 1 | Q9NQH7 | 57 | 0.7 | 6.3 | 16.0 | 16.7 |
| ADP/ATP translocase 1 | P12235 | 33 | 1.0 | 2.0 | 4.7 | 6.7 |
| 3-hydroxyisobutyryl-CoA hydrolase, mitochondrial isoform 1 precursor | Q6NVY1 | 43 | 2.0 | 7.3 | 10.0 | 13.0 |
| Actin-like protein 7A | Q9Y615 | 49 | 2.0 | 8.0 | 15.3 | 42.0 |
| Isobutyryl-CoA dehydrogenase, mitochondrial | Q9UKU7 | 45 | 2.0 | 7.0 | 12.7 | 15.7 |
| Alpha-aminoadipic semialdehyde synthase, mitochondrial | Q9UDR5 | 102 | 2.3 | 1.0 | 3.7 | 15.7 |
| Izumo sperm-egg fusion protein 1 precursor | Q8IYV9 | 39 | 2.7 | 19.7 | 23.0 | 50.0 |
| Mitochondrial-processing peptidase subunit alpha precursor | Q10713 | 58 | 2.7 | 4.7 | 5.7 | 16.7 |
| Radial spoke head protein 3 homolog | Q86UC2 | 64 | 2.7 | 20.0 | 24.7 | 31.3 |
| Adenylate kinase domain-containing protein 1 isoform 1 | Q5TCS8 | 221 | 3.3 | 39.0 | 58.3 | 58.7 |
| Methylmalonyl-CoA mutase, mitochondrial precursor | P22033 | 83 | 3.3 | 10.3 | 11.7 | 16.0 |
| Sorting and assembly machinery component 50 homolog | Q9Y512 | 52 | 3.3 | 5.7 | 10.7 | 18.3 |
| Choline dehydrogenase, mitochondrial | Q8NE62 | 65 | 3.7 | 13.3 | 15.0 | 24.7 |
| Cytochrome b5 domain-containing protein 1 | Q6P9G0 | 27 | 4.0 | 9.7 | 10.7 | 11.7 |
| Dynein intermediate chain 2, axonemal isoform 1 | Q9GZS0 | 69 | 4.0 | 17.3 | 20.0 | 21.7 |
| Peptidyl-prolyl cis-trans isomerase-like 6 isoform 1 | Q8IXY8 | 35 | 4.0 | 13.7 | 18.3 | 22.7 |
| Putative transferase CAF17, mitochondrial precursor | Q5T440 | 38 | 5.3 | 11.7 | 14.7 | 17.7 |
| Dipeptidase 1 precursor | P16444 | 46 | 6.0 | 9.3 | 11.3 | 17.0 |
| Glutathione S-transferase omega-2 isoform 1 | Q9H4Y5 | 28 | 7.3 | 15.3 | 38.7 | 43.7 |
| Integrin alpha-M isoform 1 precursor | P11215 | 127 | 7.3 | 19.0 | 0.0 | 0.0 |
| Oxidoreductase HTATIP2 isoform A precursor | Q9BUP3 | 30 | 7.3 | 9.3 | 10.3 | 5.0 |
| Adenylate kinase 7 | Q96M32 | 83 | 7.7 | 17.3 | 24.0 | 38.3 |
| Actin-related protein M1 | Q9BYD9 | 41 | 9.0 | 19.7 | 44.7 | 70.0 |
| Sodium/potassium-transporting ATPase subunit alpha-1 isoform A | P05023 | 113 | 9.0 | 12.3 | 16.3 | 19.0 |
| SPRY domain-containing protein 7 isoform 1 | Q5W111 | 22 | 9.3 | 10.3 | 13.0 | 20.7 |
| EF-hand domain-containing protein KIAA0494 | O75071 | 55 | 9.7 | 28.0 | 34.7 | 41.7 |
| Uncharacterized protein KIAA1683 isoform A | Q9H0B3 | 147 | 12.0 | 49.3 | 62.0 | 81.0 |
| Serine/threonine-protein phosphatase with EF-hands 1 isoform 1 | O14829 | 76 | 13.0 | 31.7 | 32.7 | 61.0 |
| Casein kinase II subunit beta | P67870 | 25 | 16.7 | 20.0 | 26.0 | 34.3 |
| Deoxyguanosine kinase, mitochondrial isoform A precursor | Q16854 | 32 | 17.0 | 20.0 | 32.0 | 40.3 |
| Fibrinogen-like protein 1 precursor | Q08830 | 36 | 17.0 | 19.7 | 30.0 | 39.7 |
| Dynein light chain 2, cytoplasmic | Q96FJ2 | 10 | 17.7 | 31.7 | 33.0 | 54.7 |
| Disintegrin and metalloproteinase domain-containing protein 30 preproprotein | Q9UKF2 | 89 | 23.0 | 25.0 | 31.3 | 42.0 |
| Radial spoke head 1 homolog | Q8WYR4 | 35 | 27.0 | 36.3 | 49.0 | 49.0 |
| Izumo sperm-egg fusion protein 2 precursor | Q6UXV1 | 25 | 29.7 | 29.7 | 36.7 | 46.3 |
| Coiled-coil domain-containing protein 147 | Q5T655 | 103 | 32.7 | 53.3 | 60.0 | 76.7 |
| Actin-related protein T2 | Q8TDY3 | 42 | 33.0 | 59.0 | 64.7 | 87.0 |
| Protein FAM71B | Q8TC56 | 65 | 41.3 | 60.0 | 81.0 | 103.3 |
| Sodium/potassium-transporting ATPase subunit alpha-4 isoform 1 | Q13733 | 114 | 46.0 | 93.0 | 105.3 | 131.3 |
| Beta-galactosidase-1-like protein precursor | Q6UWU2 | 74 | 48.3 | 63.7 | 71.3 | 105.3 |
| Carnitine O--acetyltransferase precursor | P43155 | 71 | 48.3 | 51.3 | 66.7 | 112.3 |
| 4-trimethylaminobutyraldehyde dehydrogenase | P49189 | 56 | 61.0 | 74.7 | 113.0 | 115.0 |
| Glycerol kinase 2 | Q14410 | 61 | 118.3 | 148.3 | 151.0 | 171.7 |
| Stress-70 protein, mitochondrial precursor | P38646 | 74 | 121.7 | 130.3 | 140.7 | 206.7 |
| Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | O14556 | 45 | 128.7 | 139.0 | 207.7 | 254.7 |
F1: fraction 1; F2: fraction 2; F3: fraction 3; F4: fraction 4; SC: spectral count; MW: molecular weight
Supplementary Table 2.
Proteins showing a decreasing trend during sperm maturation
| Protein | Uniprot number |
MW kDa |
F1 Average SC |
F2 Average SC |
F3 Average SC |
F4 Average SC |
|---|---|---|---|---|---|---|
| Lactotransferrin isoform 1 precursor | P02788 | 78 | 2260.0 | 1810.3 | 1407.3 | 299.3 |
| Endoplasmin precursor | P14625 | 92 | 1002.0 | 609.0 | 436.7 | 246.0 |
| Tubulin beta-4B chain | P68371 | 50 | 927.3 | 707.3 | 457.3 | 406.3 |
| 78 glucose-regulated protein precursor | P11021 | 72 | 875.7 | 528.0 | 506.7 | 368.7 |
| Heat shock protein HSP 90-alpha isoform 1 | Q86SX1 | 98 | 777.7 | 680.7 | 531.0 | 385.7 |
| Semenogelin-2 precursor | P07900 | 65 | 712.0 | 397.7 | 170.0 | 70.0 |
| Heat shock-related 70 protein 2 | P54652 | 70 | 707.7 | 596.0 | 326.7 | 276.0 |
| Protein disulfide-isomerase A3 precursor | P30101 | 57 | 622.3 | 389.3 | 367.7 | 212.0 |
| Tubulin alpha-3C/D chain | Q13748 | 50 | 598.0 | 474.3 | 284.7 | 264.7 |
| Hypoxia up-regulated protein 1 precursor | Q9Y4L1 | 111 | 509.3 | 331.7 | 173.7 | 80.3 |
| 60 HSP, mitochondrial | P10809 | 61 | 482.3 | 283.3 | 66.3 | 57.0 |
| Aminopeptidase N precursor | P15144 | 110 | 363.7 | 258.3 | 95.0 | 54.0 |
| Importin-5 | B3KWG6 | 126 | 348.3 | 172.7 | 61.0 | 48.7 |
| Semenogelin-1 preproprotein | O00410 | 52 | 336.7 | 212.3 | 166.0 | 87.7 |
| Calreticulin precursor | P04279 | 48 | 291.7 | 195.0 | 192.0 | 110.3 |
| RuvB-like 2 | P27797 | 51 | 287.0 | 208.3 | 110.3 | 71.7 |
| Actin, cytoplasmic 1 | Q9Y230 | 42 | 285.3 | 164.0 | 84.0 | 61.3 |
| Uncharacterized protein C1orf56 precursor | P60709 | 37 | 263.7 | 103.3 | 30.7 | 29.7 |
| Heat shock 70 protein 1-like | Q9BUN1 | 70 | 258.3 | 236.3 | 183.3 | 130.0 |
| Prostatic acid phosphatase isoform TM-PAP precursor | P34931 | 48 | 249.7 | 151.7 | 69.7 | 33.3 |
| Elongation factor 1-gamma | P15309 | 50 | 247.3 | 167.0 | 128.0 | 80.7 |
| Phosphoglycerate kinase 2 | P26641 | 45 | 240.7 | 199.0 | 176.7 | 145.0 |
| Calmegin precursor | P07205 | 70 | 229.7 | 94.7 | 14.0 | 0.0 |
| Calnexin precursor | O14967 | 68 | 227.3 | 130.7 | 64.7 | 39.3 |
| Protein disulfide-isomerase precursor | P27824 | 57 | 222.7 | 131.0 | 79.7 | 35.3 |
| Importin subunit beta-1 | P07237 | 97 | 217.0 | 78.3 | 47.7 | 28.7 |
| T-complex protein 1 subunit beta isoform 1 | Q14974 | 57 | 200.3 | 139.7 | 46.0 | 27.0 |
| T-complex protein 1 subunit gamma isoform A | P78371 | 61 | 199.0 | 106.7 | 27.0 | 24.7 |
| T-complex protein 1 subunit eta isoform A | P49368 | 59 | 193.0 | 144.0 | 58.3 | 35.0 |
| Vesicular integral-membrane protein VIP36 precursor | Q99832 | 40 | 192.7 | 118.3 | 82.0 | 59.7 |
| T-complex protein 1 subunit theta | Q12907 | 60 | 189.3 | 126.7 | 48.0 | 33.0 |
| Valyl-tRNA synthetase | P50990 | 140 | 176.3 | 95.7 | 91.0 | 20.0 |
| Cullin-associated NEDD8 dissociated-protein 1 | P26640 | 136 | 168.0 | 138.0 | 82.3 | 53.7 |
| T-complex protein 1 subunit delta | Q86VP6 | 58 | 162.0 | 98.7 | 44.3 | 30.3 |
| Heat shock 70 protein 1A/1B | P50991 | 70 | 147.7 | 93.7 | 39.7 | 11.7 |
| Heme oxygenase 2 | P08107 | 36 | 145.3 | 30.3 | 0.0 | 0.0 |
| Neprilysin | P30519 | 86 | 141.7 | 107.0 | 37.0 | 23.0 |
| Histone H2B type 1-A | P08473 | 14 | 137.3 | 73.7 | 35.3 | 0.0 |
| Cytoplasmic dynein 1 heavy chain 1 | Q96A08 | 532 | 136.7 | 135.3 | 2.3 | 0.0 |
| 60S acidic ribosomal protein P0 | Q14204 | 34 | 136.3 | 61.0 | 33.0 | 30.3 |
| Heat shock cognate 71 protein isoform 1 | P05388 | 71 | 122.7 | 72.7 | 47.7 | 41.0 |
| Transmembrane emp24 domain-containing protein 10 precursor | P11142 | 25 | 122.7 | 72.0 | 71.0 | 54.0 |
| Protein disulfide-isomerase A6 precursor | P49755 | 48 | 119.0 | 84.0 | 43.7 | 23.7 |
| Peroxiredoxin-4 precursor | Q15084 | 31 | 118.0 | 85.7 | 83.3 | 56.0 |
| Calcium-binding tyrosine phosphorylation-regulated protein isoform A | Q13162 | 53 | 117.0 | 26.7 | 22.0 | 21.7 |
| Fatty-acid amide hydrolase 1 | O75952 | 63 | 114.0 | 83.3 | 77.7 | 73.3 |
| Peroxiredoxin-6 | O00519 | 25 | 109.7 | 77.3 | 14.7 | 2.0 |
| Stomatin-like protein 2 | P30041 | 39 | 105.3 | 72.7 | 28.0 | 25.7 |
| T-complex protein 1 subunit epsilon | Q9UJZ1 | 60 | 104.0 | 58.7 | 31.3 | 15.3 |
| T-complex protein 1 subunit zeta isoform A | P48643 | 58 | 103.7 | 74.3 | 23.0 | 11.7 |
| cAMP-dependent protein kinase type II-alpha regulatory subunit | P40227 | 46 | 100.0 | 82.7 | 59.0 | 42.3 |
| ATP synthase subunit b, mitochondrial precursor | P13861 | 29 | 99.7 | 86.0 | 35.3 | 37.3 |
| Ras-related protein Rab-14 | P24539 | 24 | 95.0 | 46.3 | 30.0 | 12.7 |
| Dehydrogenase/reductase SDR family member 7 precursor | P61106 | 38 | 93.3 | 47.7 | 10.0 | 0.0 |
| Prostate-specific antigen isoform 1 preproprotein | Q9Y394 | 29 | 91.7 | 23.0 | 3.0 | 0.0 |
| Voltage-dependent anion-selective channel protein 2 isoform 2 | P07288 | 32 | 91.0 | 19.7 | 11.3 | 8.3 |
| Arachidonate 15-lipoxygenase B isoform D | P45880 | 76 | 90.7 | 89.3 | 27.3 | 7.7 |
| Heat shock 70 protein 4L | O15296 | 95 | 88.7 | 43.3 | 28.3 | 17.0 |
| Bifunctional aminoacyl-tRNA synthetase | O95757 | 171 | 87.3 | 65.0 | 45.3 | 19.7 |
| Histone H1t | P07814 | 22 | 86.0 | 37.3 | 8.7 | 3.7 |
| Peroxiredoxin-1 | P22492 | 22 | 85.0 | 42.7 | 34.3 | 17.0 |
| T-complex protein 1 subunit alpha isoform A | Q06830 | 60 | 83.7 | 77.3 | 37.7 | 15.7 |
| Transmembrane emp24 domain-containing protein 9 precursor | P17987 | 27 | 82.0 | 51.7 | 23.0 | 19.0 |
| Endoplasmic reticulum resident protein 44 precursor | Q9BVK6 | 47 | 81.3 | 50.7 | 41.7 | 26.7 |
| Importin subunit alpha-2 | Q9BS26 | 58 | 80.3 | 56.7 | 45.0 | 35.0 |
| Nuclear pore complex protein Nup93 isoform 1 | P52292 | 93 | 78.7 | 65.7 | 31.7 | 22.0 |
| General vesicular transport factor p115 | Q8N1F7 | 108 | 75.0 | 14.3 | 4.7 | 1.0 |
| Polyadenylate-binding protein 1 | P11940 | 71 | 72.0 | 5.3 | 0.0 | 0.0 |
| Creatine kinase B-type | P12277 | 43 | 71.0 | 51.0 | 6.0 | 0.0 |
| Elongation factor 1-delta isoform 1 | P29692 | 71 | 69.7 | 26.3 | 6.3 | 5.7 |
| Mesencephalic astrocyte-derived neurotrophic factor precursor | P55145 | 21 | 69.7 | 34.0 | 26.0 | 8.3 |
| Reticulocalbin-2 precursor | Q14257 | 37 | 69.7 | 26.0 | 0.0 | 0.0 |
| Peroxisomal membrane protein 11B isoform 1 | O96011 | 28 | 67.7 | 23.0 | 10.7 | 4.3 |
| Calmodulin | P62158 | 17 | 66.7 | 52.7 | 41.3 | 38.3 |
| Heat shock protein beta-1 | P04792 | 23 | 66.0 | 41.7 | 4.3 | 1.0 |
| 14-3-3 protein theta | P27348 | 28 | 64.3 | 55.7 | 18.0 | 5.3 |
| 26S proteasome non-ATPase regulatory subunit 13 isoform 1 | Q9UNM6 | 43 | 64.3 | 48.3 | 19.7 | 5.0 |
| Peptidyl-prolyl cis-trans isomerase B precursor | P23284 | 24 | 63.0 | 28.3 | 20.3 | 9.0 |
| Annexin A4 | P09525 | 36 | 62.0 | 33.3 | 25.0 | 15.3 |
| Protein ERGIC-53 precursor | P49257 | 58 | 61.7 | 29.0 | 7.0 | 1.3 |
| 60S ribosomal protein L12 | P30050 | 18 | 60.3 | 23.3 | 13.0 | 11.3 |
| Exportin-2 | P55060 | 110 | 60.0 | 39.7 | 16.0 | 11.3 |
| cAMP-dependent protein kinase type I-alpha regulatory subunit | P10644 | 43 | 58.3 | 37.7 | 20.7 | 8.0 |
| Ras-related protein Rab-11B | Q15907 | 24 | 58.3 | 26.0 | 9.7 | 0.0 |
| Testis-expressed protein 101 isoform 1 | Q9BY14 | 29 | 58.3 | 43.0 | 28.3 | 20.7 |
| Ecto-ADP-ribosyltransferase 3 isoform A precursor | Q13508 | 44 | 58.0 | 48.7 | 46.0 | 12.3 |
| Ras-related protein Rab-6A isoform A | P20340 | 24 | 56.3 | 18.0 | 1.3 | 0.0 |
| T-complex protein 1 subunit zeta-2 isoform 1 | Q92526 | 58 | 56.3 | 32.0 | 22.7 | 11.3 |
| Voltage-dependent anion-selective channel protein 3 isoform 1 | Q9Y277 | 31 | 56.0 | 10.0 | 5.7 | 0.0 |
| CD177 antigen precursor | Q8N6Q3 | 46 | 55.7 | 35.3 | 5.3 | 0.0 |
| Uncharacterized protein KIAA2013 precursor | Q8IYS2 | 69 | 54.3 | 34.0 | 33.3 | 23.7 |
| Glutamate carboxypeptidase 2 isoform 1 | Q04609 | 84 | 53.0 | 39.0 | 1.3 | 1.0 |
| Sperm surface protein Sp17 | Q15506 | 17 | 53.0 | 35.3 | 19.7 | 14.0 |
| Endoplasmic reticulum resident protein 29 isoform 1 precursor | P30040 | 29 | 52.3 | 33.0 | 19.3 | 5.7 |
| NADH-cytochrome b5 reductase 3 isoform 2 | P00387 | 32 | 51.7 | 34.0 | 31.3 | 20.7 |
| Protein sel-1 homolog 1 isoform 1 precursor | Q9UBV2 | 89 | 51.3 | 23.3 | 9.3 | 0.0 |
| 26S proteasome non-ATPase regulatory subunit 11 | O00231 | 47 | 49.7 | 36.0 | 8.0 | 7.0 |
| Annexin A2 isoform 2 | P07355 | 39 | 48.3 | 24.3 | 13.3 | 2.0 |
| DnaJ homolog subfamily B member 11 precursor | Q9UBS4 | 41 | 48.0 | 29.3 | 27.7 | 7.7 |
| 26S proteasome non-ATPase regulatory subunit 7 | P51665 | 37 | 47.3 | 36.0 | 13.0 | 2.0 |
| Phosphoglycerate mutase 2 | P15259 | 29 | 47.3 | 29.3 | 24.7 | 20.0 |
| Histone H2A type 1-A | Q96QV6 | 14 | 47.0 | 28.0 | 19.0 | 11.3 |
| Receptor expression-enhancing protein 6 | Q96HR9 | 21 | 45.3 | 31.0 | 20.7 | 12.0 |
| Alpha-actinin-4 | O43707 | 105 | 44.7 | 29.3 | 21.7 | 14.7 |
| Melanoma inhibitory activity protein 3 precursor | Q5JRA6 | 214 | 44.3 | 10.7 | 7.0 | 1.3 |
| Axonemal dynein light intermediate polypeptide 1 | O14645 | 32 | 43.3 | 35.0 | 29.7 | 0.0 |
| Chitinase domain-containing protein 1 isoform A | Q9BWS9 | 45 | 43.3 | 25.3 | 8.0 | 6.0 |
| Ras-related protein Rab-1A isoform 1 | P62820 | 23 | 42.7 | 12.0 | 5.3 | 3.3 |
| Cytochrome c oxidase subunit 5B, mitochondrial precursor | P10606 | 14 | 42.3 | 22.7 | 20.0 | 15.7 |
| 60S ribosomal protein L4 | P36578 | 48 | 41.3 | 4.0 | 0.0 | 0.0 |
| Peroxiredoxin-2 isoform A | P32119 | 22 | 40.3 | 20.0 | 7.0 | 3.7 |
| Annexin A1 | P04083 | 39 | 39.3 | 27.3 | 19.7 | 14.0 |
| Ribonuclease inhibitor | P13489 | 50 | 39.3 | 12.7 | 12.3 | 3.3 |
| DnaJ homolog subfamily A member 2 | O60884 | 46 | 38.3 | 29.7 | 17.7 | 13.0 |
| 60S acidic ribosomal protein P2 | P05387 | 12 | 38.0 | 12.0 | 9.7 | 8.7 |
| Prostate and testis expressed protein 1 precursor | Q8WXA2 | 14 | 37.7 | 29.7 | 24.7 | 22.7 |
| 60S ribosomal protein L6 | Q02878 | 33 | 37.0 | 18.0 | 4.0 | 1.0 |
| Lon protease homolog, mitochondrial | P36776 | 106 | 36.7 | 24.3 | 11.3 | 17.0 |
| Stromal cell-derived factor 2-like protein 1 precursor | Q9HCN8 | 24 | 36.3 | 27.0 | 23.7 | 13.0 |
| Proteasome subunit beta type-1 | P20618 | 26 | 36.0 | 31.0 | 24.7 | 12.3 |
| Myosin light polypeptide 6 isoform 1 | P60660 | 17 | 35.0 | 21.3 | 0.0 | 0.0 |
| Histone H2A-Bbd type 2/3 | P0C5Z0 | 13 | 34.7 | 20.3 | 0.0 | 0.0 |
| Voltage-dependent anion-selective channel protein 1 | P21796 | 31 | 34.3 | 21.0 | 7.7 | 0.0 |
| Serine/threonine-protein phosphatase 2A 65 regulatory subunit A alpha isoform | P30153 | 65 | 34.0 | 28.0 | 5.7 | 4.7 |
| Alpha-centractin | P61163 | 43 | 33.7 | 26.3 | 10.0 | 0.0 |
| 40S ribosomal protein S9 | P46781 | 23 | 33.3 | 12.3 | 4.0 | 0.0 |
| Signal peptidase complex subunit 3 | P61009 | 20 | 33.0 | 21.3 | 18.3 | 14.0 |
| Importin subunit alpha-3 | O00505 | 58 | 32.7 | 22.0 | 18.3 | 10.7 |
| Calpain small subunit 1 | P04632 | 28 | 32.3 | 20.3 | 1.0 | 0.0 |
| 26S proteasome non-ATPase regulatory subunit 8 | P48556 | 40 | 31.7 | 24.7 | 5.7 | 4.3 |
| Nucleobindin-2 precursor | P80303 | 50 | 31.3 | 21.0 | 18.7 | 0.0 |
| Synaptogyrin-2 | O43760 | 25 | 31.3 | 17.0 | 9.0 | 9.0 |
| Cytoskeleton-associated protein 4 | Q07065 | 66 | 30.7 | 17.7 | 0.0 | 0.0 |
| Myosin regulatory light chain 12A | P19105 | 20 | 30.7 | 21.3 | 2.0 | 0.0 |
| Ras-related protein Rab-7a | P51149 | 23 | 30.7 | 13.7 | 11.7 | 6.7 |
| Syntaxin-12 | Q86Y82 | 32 | 30.7 | 10.7 | 0.0 | 0.0 |
| DnaJ homolog subfamily B member 1 | P25685 | 38 | 30.0 | 12.3 | 10.3 | 10.0 |
| Leucine zipper transcription factor-like protein 1 | Q9NQ48 | 35 | 30.0 | 14.0 | 2.3 | 0.0 |
| Peroxisomal membrane protein 11C | Q96HA9 | 27 | 30.0 | 19.3 | 2.7 | 0.0 |
| Nicastrin precursor | Q92542 | 78 | 29.7 | 23.0 | 18.3 | 11.0 |
| Cytosolic nonspecific dipeptidase isoform 1 | Q96KP4 | 53 | 29.3 | 19.3 | 5.0 | 0.0 |
| 40S ribosomal protein S8 | P62241 | 24 | 29.0 | 4.3 | 2.0 | 0.0 |
| Protein FAM162A | Q96A26 | 17 | 28.7 | 18.0 | 16.0 | 12.3 |
| Ras-related protein Rab-18 | Q9NP72 | 23 | 28.0 | 7.0 | 0.7 | 0.0 |
| Erlin-1 | O75477 | 39 | 27.7 | 21.7 | 11.7 | 8.7 |
| 26S protease regulatory subunit 10B | P62333 | 46 | 27.3 | 20.3 | 8.3 | 5.7 |
| Eukaryotic translation initiation factor 3 subunit A | Q14152 | 167 | 27.0 | 15.0 | 2.0 | 0.0 |
| Peptidyl-prolyl cis-trans isomerase A | P62937 | 18 | 27.0 | 4.7 | 0.0 | 0.0 |
| Nucleophosmin isoform 1 | P06748 | 33 | 26.7 | 10.0 | 0.0 | 0.0 |
| Hsc70-interacting protein | P50502 | 41 | 26.3 | 24.7 | 16.3 | 7.3 |
| Heat shock protein 75 , mitochondrial precursor | Q12931 | 80 | 26.0 | 17.3 | 0.0 | 0.0 |
| Platelet-activating factor acetylhydrolase precursor | Q13093 | 50 | 25.0 | 16.7 | 0.0 | 0.0 |
| Galectin-3-binding protein precursor | Q08380 | 65 | 24.7 | 12.7 | 11.7 | 5.0 |
| Hemoglobin subunit beta | D9YZU5 | 16 | 24.7 | 13.0 | 8.3 | 7.3 |
| 40S ribosomal protein SA | P08865 | 33 | 24.0 | 2.7 | 0.0 | 0.0 |
| Poly(rC)-binding protein 1 | Q15365 | 37 | 24.0 | 3.7 | 0.0 | 0.0 |
| Serine/threonine-protein phosphatase 2A catalytic subunit beta isoform | P62714 | 36 | 24.0 | 15.0 | 7.7 | 0.0 |
| Probable inactive serine protease 37 isoform 1 precursor | A4D1T9 | 26 | 23.7 | 13.3 | 1.7 | 0.0 |
| 40S ribosomal protein S16 | P62249 | 16 | 23.0 | 5.7 | 1.3 | 0.0 |
| 40S ribosomal protein S25 | P62851 | 14 | 23.0 | 3.0 | 1.7 | 0.0 |
| Cytoplasmic dynein 1 light intermediate chain 1 | Q9Y6G9 | 57 | 23.0 | 17.0 | 0.0 | 0.0 |
| Normal mucosa of esophagus-specific gene 1 protein | Q9C002 | 10 | 23.0 | 10.7 | 7.3 | 3.0 |
| Sorbitol dehydrogenase | Q00796 | 38 | 23.0 | 17.0 | 12.7 | 0.0 |
| 26S protease regulatory subunit 6A | P17980 | 49 | 22.7 | 20.7 | 13.0 | 3.0 |
| Alkyl dihydroxyacetone phosphate synthase, peroxisomal precursor | O00116 | 73 | 22.7 | 5.0 | 0.0 | 0.0 |
| Mitochondrial import receptor subunit TOM22 homolog | Q9NS69 | 16 | 22.7 | 10.7 | 0.0 | 0.0 |
| Anterior gradient protein 2 homolog precursor | O95994 | 20 | 22.3 | 6.7 | 0.7 | 0.0 |
| Gamma-glutamyltranspeptidase 1 precursor | P19440 | 61 | 22.3 | 4.0 | 0.0 | 0.0 |
| 40S ribosomal protein S13 | P62277 | 17 | 22.0 | 4.3 | 0.0 | 0.0 |
| 60S ribosomal protein L14 | P50914 | 23 | 21.3 | 5.0 | 0.0 | 0.0 |
| Dynactin subunit 2 | Q13561 | 45 | 21.3 | 14.3 | 8.0 | 0.7 |
| Prostate stem cell antigen preproprotein | D3DWI6 | 12 | 21.3 | 13.0 | 0.0 | 0.0 |
| 40S ribosomal protein S15a | P62244 | 15 | 21.0 | 10.3 | 6.7 | 6.0 |
| 60S acidic ribosomal protein P1 isoform 1 | P84098 | 12 | 21.0 | 11.7 | 8.7 | 0.0 |
| 60S ribosomal protein L19 | P84098 | 23 | 21.0 | 5.0 | 0.0 | 0.0 |
| Arginyl-tRNA synthetase, cytoplasmic | P54136 | 75 | 21.0 | 9.7 | 4.3 | 0.7 |
| Glutathione S-transferase P | P09211 | 23 | 21.0 | 11.3 | 6.3 | 0.0 |
| GTP-binding nuclear protein Ran | P62826 | 24 | 21.0 | 6.3 | 4.3 | 0.0 |
| 60S ribosomal protein L13 isoform 1 | P26373 | 24 | 20.7 | 2.3 | 0.0 | 0.0 |
| Protein S100-A9 | P06702 | 13 | 20.3 | 11.3 | 0.0 | 0.0 |
| Talin-1 | Q9Y490 | 270 | 20.3 | 19.3 | 0.0 | 0.0 |
| Endoplasmic reticulum-Golgi intermediate compartment protein 1 | Q969X5 | 33 | 20.0 | 6.7 | 0.0 | 0.0 |
| F-actin-capping protein subunit alpha-1 | P52907 | 33 | 20.0 | 11.3 | 5.7 | 0.0 |
| 60S ribosomal protein L15 isoform 1 | P61313 | 24 | 19.7 | 3.7 | 0.0 | 0.0 |
| Nucleoporin NUP53 | Q8NFH5 | 35 | 19.3 | 6.0 | 0.0 | 0.0 |
| 26S protease regulatory subunit 6B isoform 1 | P43686 | 47 | 19.0 | 13.7 | 10.3 | 3.7 |
| Lipoprotein lipase precursor | P06858 | 53 | 19.0 | 18.3 | 0.0 | 0.0 |
| Perilipin-3 isoform 1 | O60664 | 47 | 19.0 | 4.3 | 0.0 | 0.0 |
| Gastricsin isoform 1 preproprotein | P20142 | 42 | 18.7 | 22.3 | 11.0 | 7.7 |
| ERO1-like protein beta precursor | Q86YB8 | 54 | 18.3 | 14.7 | 0.0 | 0.0 |
| Transmembrane emp24 domain-containing protein 1 precursor | Q13445 | 25 | 18.3 | 11.7 | 5.3 | 3.0 |
| Glutamate dehydrogenase 1, mitochondrial precursor | P00367 | 61 | 17.7 | 6.0 | 0.7 | 0.0 |
| Importin subunit alpha-4 | O00629 | 58 | 17.7 | 17.3 | 9.0 | 5.3 |
| NADH dehydrogenase (ubiquinone) 1 alpha subcomplex subunit 10, mitochondrial precursor | O95299 | 41 | 17.3 | 9.0 | 1.3 | 1.3 |
| Peptidyl-prolyl cis-trans isomerase FKBP2 precursor | P26885 | 16 | 17.3 | 10.0 | 0.0 | 0.0 |
| Protein S100-A8 | P05109 | 11 | 17.3 | 9.7 | 0.0 | 0.0 |
| Stress-induced-phosphoprotein 1 | P31948 | 63 | 17.3 | 11.0 | 0.0 | 0.0 |
| Transmembrane emp24 domain-containing protein 5 isoform 1 precursor | Q9Y3A6 | 26 | 16.7 | 10.3 | 0.0 | 0.0 |
| 60S ribosomal protein L27a | P46776 | 17 | 16.3 | 7.7 | 0.0 | 0.0 |
| Isocitrate dehydrogenase [NADP] cytoplasmic | O75874 | 47 | 16.3 | 9.0 | 2.7 | 0.0 |
| Lysosome membrane protein 2 isoform 1 precursor | Q14108 | 54 | 16.0 | 6.7 | 0.0 | 0.0 |
| Mitochondria-eating protein | Q8TC71 | 61 | 16.0 | 10.7 | 7.3 | 6.0 |
| NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8, mitochondrial precursor | O95169 | 22 | 16.0 | 7.0 | 0.0 | 0.0 |
| 40S ribosomal protein S14 | P62263 | 16 | 15.7 | 2.3 | 0.0 | 0.0 |
| 60S ribosomal protein L11 isoform 1 | P62913 | 20 | 15.7 | 7.3 | 0.0 | 0.0 |
| Protein canopy homolog 2 isoform 1 precursor | Q9Y2B0 | 21 | 15.3 | 7.3 | 4.0 | 0.0 |
| Translocon-zassociated protein subunit alpha precursor | P43307 | 32 | 15.3 | 12.0 | 8.0 | 7.7 |
| 60S ribosomal protein L22 proprotein | P35268 | 15 | 15.0 | 8.3 | 4.0 | 1.3 |
| Cysteinyl-tRNA synthetase, cytoplasmic isoform C | P49589 | 95 | 15.0 | 2.7 | 0.0 | 0.0 |
| Abhydrolase domain-containing protein 16A isoform A | O95870 | 63 | 14.7 | 11.3 | 0.0 | 0.0 |
| COP9 signalosome complex subunit 8 isoform 2 | Q99627 | 18 | 14.7 | 5.0 | 0.0 | 0.0 |
| Golgi apparatus protein 1 isoform 2 precursor | Q92896 | 136 | 14.7 | 14.0 | 7.7 | 2.3 |
| S-phase kinase-associated protein 1 isoform B | P63208 | 19 | 14.7 | 7.3 | 0.0 | 0.0 |
| Transcription elongation factor B polypeptide 1 isoform A | Q15369 | 12 | 14.7 | 5.7 | 3.7 | 1.3 |
| 26S proteasome non-ATPase regulatory subunit 5 | Q16401 | 56 | 14.0 | 5.7 | 0.0 | 0.0 |
| 14-3-3 protein sigma | P31947 | 28 | 13.7 | 10.7 | 0.0 | 0.0 |
| Coatomer subunit zeta-1 | P61923 | 20 | 13.7 | 4.3 | 0.0 | 0.0 |
| Eukaryotic translation initiation factor 3 subunit E | P60228 | 52 | 13.7 | 3.3 | 1.3 | 0.0 |
| Neutrophil gelatinase-associated lipocalin precursor | P80188 | 23 | 13.7 | 7.0 | 0.0 | 0.0 |
| Aspartyl-`tRNA synthetase, cytoplasmic | P14868 | 57 | 13.3 | 12.0 | 5.3 | 1.0 |
| Chloride intracellular channel protein 1 | O00299 | 27 | 13.3 | 5.7 | 3.7 | 0.0 |
| Programmed cell death protein 6 | O75340 | 22 | 13.3 | 11.3 | 8.7 | 3.3 |
| Signal recognition particle receptor subunit beta | Q9Y5M8 | 30 | 13.3 | 5.0 | 0.0 | 0.0 |
| 14-3-3 protein beta/alpha | P31946 | 28 | 13.0 | 6.3 | 0.0 | 0.0 |
| Cathepsin D preproprotein | P07339 | 45 | 13.0 | 6.0 | 0.0 | 0.0 |
| Phosphoglycerate kinase 1 | P00558 | 45 | 13.0 | 12.0 | 8.7 | 0.0 |
| Protein-tyrosine phosphatase mitochondrial 1 isoform 1 | Q8WUK0 | 23 | 12.7 | 7.7 | 0.0 | 0.0 |
| Ras-related protein Ral-A precursor | P11233 | 24 | 12.7 | 8.3 | 3.7 | 0.0 |
| Low molecular weight phosphotyrosine protein phosphatase isoform C | P24666 | 18 | 12.3 | 3.3 | 0.0 | 0.0 |
| NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 4 isoform 1 | O95168 | 15 | 12.3 | 9.0 | 0.0 | 0.0 |
| ATP synthase-coupling factor 6, mitochondrial isoform A precursor | P18859 | 13 | 12.0 | 7.0 | 7.0 | 3.3 |
| CDGSH iron-sulfur domain-containing protein 2 | Q8N5K1 | 15 | 12.0 | 6.0 | 0.0 | 0.0 |
| HD domain-containing protein 2 | Q7Z4H3 | 23 | 12.0 | 6.7 | 0.0 | 0.0 |
| Ubiquitin carboxyl-terminal hydrolase isozyme L3 | P15374 | 26 | 12.0 | 3.3 | 2.3 | 0.0 |
| 15 selenoprotein isoform 1 precursor | O60613 | 18 | 11.7 | 7.7 | 0.7 | 0.0 |
| 40S ribosomal protein S6 | P62753 | 29 | 11.7 | 4.3 | 0.0 | 0.0 |
| Coiled-coil-helix-coiled-coil-helix domain-containing protein 3, mitochondrial precursor | Q9NX63 | 26 | 11.7 | 6.0 | 5.3 | 4.3 |
| Protein FAM166A | Q6J272 | 36 | 11.7 | 6.0 | 0.0 | 0.0 |
| Serine/threonine-protein phosphatase PGAM5, mitochondrial isoform 1 | Q96HS1 | 32 | 11.7 | 8.0 | 0.0 | 0.0 |
| DnaJ homolog subfamily A member 1 | P31689 | 45 | 11.3 | 4.3 | 0.0 | 0.0 |
| Eukaryotic translation elongation factor 1 epsilon-1 isoform 1 | O43324 | 20 | 11.3 | 8.0 | 2.7 | 2.7 |
| Protein S100-A11 | P31949 | 12 | 11.3 | 8.7 | 2.3 | 0.0 |
| Histone H1.4 | P10412 | 22 | 10.7 | 5.0 | 0.0 | 0.0 |
| Peroxisomal biogenesis factor 16 isoform 1 | Q9Y5Y5 | 39 | 10.7 | 1.7 | 0.0 | 0.0 |
| Selenoprotein T precursor | P62341 | 22 | 10.7 | 4.7 | 0.0 | 1.0 |
| ATP synthase subunit e, mitochondrial | P56385 | 8 | 10.3 | 8.3 | 3.0 | 0.0 |
| Torsin-1A-interacting protein 1 | Q5JTV8 | 66 | 10.0 | 5.7 | 0.0 | 0.0 |
| DnaJ homolog subfamily C member 3 precursor | Q13217 | 58 | 9.7 | 8.0 | 0.7 | 0.0 |
| Myoferlin isoform B | Q9NZM1 | 233 | 9.7 | 2.7 | 0.0 | 0.0 |
| NADH dehydrogenase [ubiquinone] iron sulfur-protein 5 | O43920 | 13 | 9.7 | 9.0 | 1.0 | 2.0 |
| Up-regulated during skeletal muscle growth protein 5 | Q96IX5 | 6 | 9.7 | 6.0 | 6.0 | 4.7 |
| Neutrophil defensin 1 preproprotein | P59665 | 10 | 9.3 | 6.7 | 3.0 | 0.0 |
| Protein FAM3C precursor | Q92520 | 25 | 9.3 | 3.3 | 0.0 | 0.0 |
| Cytochrome b-c1 complex subunit 9 isoform A | Q9UDW1 | 7 | 9.0 | 4.7 | 0.0 | 0.0 |
| Eukaryotic translation initiation factor 3 subunit F | O00303 | 38 | 8.7 | 5.7 | 0.0 | 0.0 |
| Sorcin isoform B | P30626 | 20 | 8.7 | 4.7 | 0.0 | 0.0 |
| Thioredoxin isoform 1 | P10599 | 12 | 8.7 | 4.7 | 0.0 | 0.0 |
| Apolipoprotein A-I preproprotein | P02647 | 31 | 8.3 | 5.3 | 3.7 | 0.0 |
| Calcium and integrin-binding protein 1 | Q99828 | 22 | 8.3 | 2.7 | 0.0 | 0.0 |
| Sperm-associated antigen 4 protein | Q9NPE6 | 48 | 8.3 | 7.3 | 5.7 | 3.3 |
| D-3-phosphoglycerate dehydrogenase | O43175 | 57 | 8.0 | 3.7 | 0.0 | 0.0 |
| Metalloproteinase inhibitor 1 precursor | P01033 | 23 | 8.0 | 3.0 | 0.0 | 0.0 |
| Eukaryotic translation initiation factor 3 subunit M | Q7L2H7 | 43 | 7.7 | 6.3 | 0.0 | 0.0 |
| Glutaredoxin-related protein 5, mitochondrial precursor | Q86SX6 | 17 | 7.7 | 3.0 | 5.0 | 2.0 |
| Guanine nucleotide-binding protein G (I)/G (S)/G (T) subunit beta-1 | P62873 | 37 | 7.7 | 3.3 | 0.0 | 0.0 |
| UPF0733 protein C2orf88 | Q9BSF0 | 11 | 7.7 | 3.0 | 0.0 | 0.0 |
| Phosphoglycolate phosphatase | A6NDG6 | 34 | 7.3 | 2.7 | 0.0 | 0.0 |
| Prostate-and testis-expressed protein 4 | P0C8F1 | 11 | 7.3 | 4.7 | 0.0 | 0.0 |
| Synaptogyrin-4 | O95473 | 26 | 7.3 | 6.7 | 5.7 | 0.0 |
| Macrophage migration inhibitory factor | P14174 | 12 | 7.0 | 4.0 | 1.3 | 0.0 |
| LYR motif-containing protein 4 isoform 1 | Q9HD34 | 11 | 6.7 | 3.0 | 0.0 | 0.0 |
| Uncharacterized protein C13orf16 | Q8N6K0 | 17 | 6.7 | 3.3 | 1.7 | 1.3 |
| 26S proteasome non-ATPase regulatory subunit 4 | P55036 | 41 | 6.3 | 4.7 | 0.0 | 0.0 |
| thioredoxin domain-containing protein 2 isoform 2 | Q86VQ3 | 60 | 6.3 | 4.0 | 0.0 | 0.0 |
| Serine/threonine-protein phosphatase 4 regulatory subunit 1 isoform A | Q8TF05 | 107 | 6.0 | 4.3 | 0.0 | 0.0 |
| WD repeat-containing protein 61 | Q9GZS3 | 34 | 5.7 | 5.0 | 0.0 | 0.0 |
| DDB1-and CUL4-associated factor 7 | P61962 | 39 | 5.3 | 3.0 | 0.0 | 0.0 |
| Translin-associated protein X | Q99598 | 33 | 5.0 | 3.3 | 0.7 | 1.0 |
| Protein SEC13 homolog isoform 2 | P55735 | 34 | 3.7 | 2.3 | 1.3 | 0.0 |
F1: fraction 1; F2: fraction 2; F3: fraction 3; F4: fraction 4; SC: spectral count; HSP: heat shock protein; MW: molecular weight
Identification of functional proteins
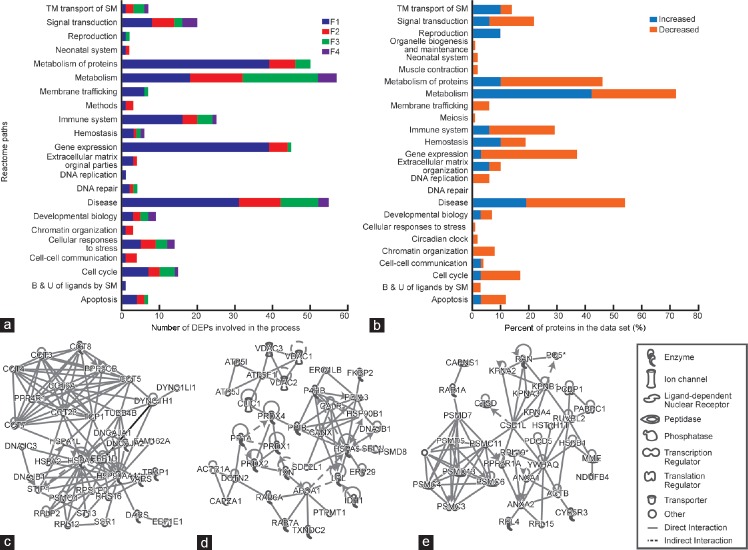
The Reactome pathway analysis showed that the majority of DEPs were involved in metabolism, particularly protein metabolism, disease processes, gene expression, and signal transduction (Figure 2a). The percentage of proteins involved in various pathways that were either underexpressed or showed a decreasing trend or were overexpressed and showed an increasing trend from F1 to F4 is shown in Figure 2b.
Figure 2.
DEPs in fraction 1 (F1) through fraction 4 (F4). (a) Reactome pathway showing the number of DEPs involved in various pathways in the four fractions. F1 being the most immature and F4 the most mature. (b) Percentage of proteins in the data set that were increasing or decreasing for the different pathways. (c) IPA network showing molecular chaperons that were involved in developmental disorder, posttranslational modifications, and protein folding. (d) IPA network showing proteins involved in molecular transport, protein trafficking, and cell cycle and (e) IPA network showing molecular chaperons involved in cell death and survival, posttranslational modifications, and protein folding. IPA: ingenuity pathway analysis; DEPs: differentially expressed proteins; F1: least mature stage having the lowest density; F2, F3: intermediate stages; F4: includes the most dense and morphologically mature motile spermatozoa. Full names of abbreviated proteins are presented in Supplementary Information (39.6KB, pdf) .
The functional annotations from use of the DAVID tool and their enrichment analysis with the number of key proteins involved are presented in Supplementary Table 3. F1 was characterized by proteins involved in translation, elongation, protein transport, oxidoreductase activity, reproductive processes, spermatid development/differentiation, and regulation of apoptotic pathways. The proteins identified in F2 are involved in cell differentiation, protein–protein interactions, protein transport and localization, oxidoreductase activity, spermatogenesis, gonad development, and proteolytic pathways. The key processes of F3 proteins include generation of precursor metabolites and integration of energy metabolism, oxidative phosphorylation, protein catabolic process, and protein ubiquitination. Finally, the proteins identified in F4 were largely involved in reproductive functions (Table 2). The proteins involved in intracellular transport, oxidation–reduction, cellular amino acid catabolic process, and alternative splicing showed an increasing trend from F1 to F4 (Supplementary Table 2). On the other hand, the proteins involved in spermatogenesis, protein metabolism, cell cycle, integration of energy metabolism, regulation of apoptosis, cell redox homeostasis, translational elongation, and response to protein folding showed a decreasing trend from F1 to F4 (Supplementary Table 3).
Supplementary Table 3.
Functional annotations using Database for Annotation, Visualization and Integrated Discovery for proteins expressed from immature through spermatozoa maturation process (F1 through F4)
| Sample dataset | Key pathways | Key processes | Enriched functional categories | Cellular location | Functional categories that are associated with majority of proteins | Top TFBS | Key functions | Summary highlights of key processes/functions/pathways affected |
|---|---|---|---|---|---|---|---|---|
| F1 | Ribosome (21), protein export (3), SNARE interactions in vesicular transport (4), glutathione metabolism (4), influenza infection (27), UTR-mediated translational regulation (21), Met of proteins (27), GX (29), diabetes pathway (12), membrane trafficking (4) | Translational elongation (22), translation (27), intracellular transport (25), Golgi vesicle transport (10), cellular macromolecular complex assembly (15), protein localization (24), oxidation reduction (18), regulation of translation (8), cell death (19), regulation of apoptosis (17) | Translational elongation (22), ribosome (21), protein biosynthesis (21), RNA binding (28), structural molecule activity (26), ER (26), IC transport (925), Golgi vesicle transport (10), ER-Golgi transport (6), vesicle-mediated transport (15), macromolecular complex assembly (18), protein complex biogenesis (11), SRP (3), protein localization in organelle (8), NT binding (44), methylation (11), RasGTPase (6), lipoprotein (11), generation of precursor metabolites and energy (6) cell redox homeostasis (7), cell death (19), regulation of apoptosis (17), sexual reproduction (9), spermatogenesis (7), multicellular organism reproduction (8), spermatid development/differentiation (3) microtubule cytoskeleton (17), cytoskeletal part (21), mitochondrion (26) | Ribosomal subunit (20), ribonucleo protein complex (34), cytosol (53), nonmembrane bounded organelle (63), ER (33), organelle envelope (22), microtubule cytoskeleton (17), mitochondrion (26), Golgi apparatus (19), intracellular organelle lumen (33), nucleolus (15) | Acetylation (102), translational elongation (22), ribonucleo-protein (28), ribosome (21), protein biosynthesis (22); cytosol (53), translation (27), ER (26) | Pa×4 (143), AML1 (140), YY1 (129) | Structural constituent of ribosome (21), RNA binding (28), GTP binding (17), GTPase activity (12), nucleotide binding (44), HSP binding (5), purine ribonucleotide binding (35) | Translation elongation, protein transport, oxidoreductase activity, reproductive process, spermatid development/differentiation, regulation of apoptosis |
| F2 | Telomere maintenance (3), systemic lupus erythematosus (4), pyruvate metabolism (3), val-leu-ilu degradation (3) | Epidermis development (8), ectoderm development (8), oxidation–reduction (10), epithelial cell differentiation (5), protein transport (9), protein localization (9), intermediate filament organization (2), sexual reproduction (6) | Intermediate filament (8), keratin (8), protein transport (9), protein localization (9), membrane-bounded vesicle (8), sexual reproduction (6), reproductive process in a multicellular organism (6), EF-Hand2 domain (4), nucleosome assembly (3), chromatin (3), gonad development (3), sex differentiation (3), actin filament binding (3), ER membrane (3), proteolysis involved in cellular protein catabolic process (3), regulation of apoptosis (3) | Keratin filament (7), IF (8), mitochondrion (16), cytoskeletal part (13), membrane bounded vesicle (8), intracellular nonmembrane bounded organelle (21), ER (10) | Acetylation (23), intracellular nonmembrane bounded organelle (21), disease mutation (18), mitochondrion (16), cytoskeleton (15), coiled coil (15), structural molecular activity (12), transit peptide (10), oxidoreductase (10), ER (10), sexual reproduction (6) | SP1 (24), ROAZ (38) | Structural constituent of cytoskeleton (6), Structural molecular activity (12), coenzyme binding (5), actin filament binding (3), cofactor binding (5) | Epidermis and ectoderm development, cell differentiation, protein–protein interactions, protein transport and localization, oxidoreductase activity, gamete generation, gonad development, proteolysis |
| F3 | Huntington’s disease (6), pyrimidine metabolism (4), oxidative phosphorylation (4), ubiquitin-mediated proteolysis (4), metabolism of protein import into nucleus (2), metabolism of vitamins and cofactors (3), integration of energy metabolism (5), metabolism of nucleotides (3) | Nucleoside metabolic process (4), generation of precursor metabolites and energy (7), oxidation–reduction (9), vitamin metabolic process (3) | Mitochondrial inner membrane (9), oxidoreductase activity (3), oxidative phosphorylation (3), protein ubiquitination (3), protein modification by small protein conjugation (3), phospholipid metabolic process (3), spermatogenesis (4), male gamete generation (4), nucleotide biosynthetic process (3), lysosome (3), lytic vacuole (3), ubl conjugation pathway (5), proteolysis involved in cellular protein catabolic process (5), GTP binding (3), ER membrane (3), mitotic cell cycle (3) | Organelle inner membrane (10), organelle envelope (13), mitochondrion (12), respiratory chain (3), nuclear pore (3) | Alternative splicing (44), acetylation (22), cytoplasm (22), transport (15), secreted (14), organelle envelope (13), mitochondrion (12), oxidoreductase (9), generation of precursor metabolites and energy (7) | Cytochrome-c oxidase activity (3), transmembrane transporter activity (4), nucleotidyl transferase activity (4) | Generation of precursor metabolites and integration of energy metabolism, oxidative phosphorylation, protein catabolic process, protein ubiquitination | |
| F4 | Hedgehog signaling pathway (2), metabolism of amino acids (3), nuclear factor of activated T-cells, cytoplasmic, calcineurin dependent 2 (2) | Reproductive process in a multicellular organism (5), spermatogenesis (4), male gamete generation (4), sexual reproduction (4), phosphorus metabolic process (5), regulation of protein complex disassembly (2) heterocyclic biosynthetic process (2) | Multicellular organism reproduction (5), spermatogenesis (4), male gamete generation (4), sexual reproduction (4), transit peptide (5), mitochondrion (5), phosphate metabolic process (5), protein kinase activity (3), secreted (5), signal (8), cell surface (4), membrane (14), cation binding (8), cytoskeleton (3) | Cell surface (4) | Acetylation (11), hydrolase (8), multicellular organism reproduction (5), mitochondrion (5), phosphorus metabolic process (5), transit peptide (5), spermatogenesis (4), lipid catabolic process (3), hedgehog signaling pathway (2), regulation of protein complex | HAND1E47 (22), ATF (16), CDPCR3 (24); Domains-serine/threonine protein kinases active site signatures (3) | Oxidoreductase activity, acting on sulfur group of donors (2) | Multicellular organism reproduction |
| Increasing trend | Metabolism of amino acids (4), Huntington disease (3), valine-leucine-isoleucine degradation (4), propionate metabolism (3), aldosterone-regulated sodium reabsorption (3), beta-alanine metabolism (2) | Carboxylic acid catabolic process (5), oxidation–reduction (8), sexual reproduction (6), spermatogenesis (4), male gamete generation (4), integrin-mediated signaling pathway (3), generation of precursor metabolites and energy (5), amine catabolic process (4), sperm motility (2), cell motility (4) | Dynein (4), microtubule motor activity (4), purine nucleotide metabolic process (4), cilium axoneme (3), sodium/potassium transport (3), metallopeptidase activity (5), potassium ion binding (4), ion transport (4), cell motility (4), male gamete generation (4), spermatogenesis (4), multicellular organism reproduction (4), protein complex assembly (3) | Mitochondrion (17), dynein complex (4), cytoskeleton (12), cell projection (7), cilium axoneme (3), microtubule based flagellum (3) | Alternative splicing (34), cytoplasm (19), mitochondrion (17), acetylation (15), mitochondrion (15), nucleotide binding (15), coiled coil (13), cytoskeleton (12), transit peptide (10), sexual reproduction (6), generation of precursor metabolites and energy (5) | YY1 (51), CREBP1CJUN (13), CDPCR1 (30), AML1 (54), HSF1 (24) | Sodium: potassium exchanging ATPase activity (3), microtubule motor activity (4), nucleotide binding 915), purine nucleoside binding (11), metallo endopeptidase activity (3), purine nucleotide binding (12), coenzyme binding (5), potassium ion binding (4), magnesium ion binding (6) | Intracellular transport, oxidation reduction, cellular amino acid catabolic process, alternative splicing |
| Decreasing trend | Metabolism of proteins (38), UTR-mediated translational regulation (25), signaling by Wnt (13), apoptosis (13), integration of energy metabolism (16), cell cycle (19), metabolism of amino acids (12), diabetes pathways (22), regulation of activated PAK-2p34 by proteasome-mediated degradation (10), ubiquitin proteasome pathway (9), antigen processing and presentation (9), proteasome (9), ribosome (20), oxidative phosphorylation 99), role of Ran in mitotic spindle regulation (3), Prion pathway (3), mechanism of gene regulation by peroxisome proliferators via PPARa (4) | Protein folding (34), translational elongation (25), cell redox homeostasis (12), proteasomal protein catabolic process (13), protein transport (35), protein localization (36), regulation of ligase activity (11), proteolysis (28), regulation of apoptosis (27), homeostatic process (25), protein complex biogenesis (21), cell cycle (21), sexual reproduction (15) | Protein folding (34), translational elongation (25), protein biosynthesis (27), HSP70 (6), proteasome (10), stress response (12), response to unfolded protein (16), ER (38), mitochondrion (36), negative regulation of protein ubiquitination (10), regulation of ligase activity (11), oxidoreductase (17), intracellular protein transport (20), secreted (28), vesicle-mediated transport (15), cellular protein localization (20), EF-hand type domain (13), peroxidase activity (5), regulation of cell death (27), spermatogenesis (12), male gamete generation (12), sexual reproduction (15), spermatid development (3), spermatid differentiation (3), sperm cell development (3) | Cytosol (83), nonmembrane bounded organelle (65), mitochondrion (36), melanosome (19), ribosomal subunit (19), ER (46), ribonucleo protein complex (30), vesicle (33) | Phosphoprotein (151), acetylation (147), cytoplasm (97), cytosol (83), nonmembrane bounded organelle (65), signal (60), nucleotide binding (53), ER (46), unfolded protein binding (31), protein biosynthesis (29), proteolysis (28), regulation of apoptosis (27), translational elongation (25), homeostatic process (25), protein transport (24), mitochondrion (23), protein complex assembly (21), cell cycle (21), oxidoreductase (17) | NFY (123) | Unfolded protein binding (31), structural constituent of ribosome (20), structural molecule activity (26), nucleotide binding (60), calcium ion binding (24), peptidase activity (15), ATPase activity (12), protein transporter activity (9), GTPase activity (11), peptide binding (8), antioxidant activity (6) | Spermatogenesis, protein metabolism, cell cycle, integration of energy metabolism, regulation of apoptosis, cell redox homeostasis, translational elongation, response to protein folding |
The number of proteins is in the parenthesis. ER: endoplasmic reticulum; HSP: heat shock protein; TFBS: transcription factor binding sites
DEPs involved in various networks
The DEPs were further subjected to network analysis by using IPA. A total of 161 pathways were linked to 279 proteins showing a decreasing trend from F1 to F4. Among these proteins, 58 were involved in cell death and survival, posttranslational modification, and protein folding (Figure 2c). In addition, there were 58 proteins, mostly molecular chaperones, which were involved in developmental disorder, posttranslational modification, and protein folding (Figure 2d). A key network was identified with 58 focal proteins involved in molecular transport, protein trafficking, and cell cycle (Figure 2e), in which 18 proteins were involved in cell signaling, cancer, and cellular development processes. The proteins showing an increasing trend from immature to mature fraction (F1 to F4) were linked to 66 networks. The top networks were lipid metabolism, small molecule biochemistry, and molecular transport that included 25 proteins. Of these, 22 proteins participated in cell-to-cell signaling and interaction, cellular function and maintenance, and inflammatory response and 12 proteins were involved in cell death and survival, cellular compromise, and cancer (Table 2).
Table 2.
Key proteins showing an increasing trend of expression from fraction 1 (F1: most immature) through faction 4 (F4: most mature) involved in structural assembly of spermatozoa, spermatogenesis, reproduction, sperm motility, energy metabolism, and oxidation–reduction processes
| Processes | Proteins | Protein Name | Normalized Spectral Count | NASF ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Reproduction and spermatogenesis | ADAM29 | Disintegrin and metalloproteinase domain-containing protein 29 | 0 | 1.3 | 7.0 | 17.3 | 0 | 0.08 | 0.40 |
| ADAM30 | Disintegrin and metalloproteinase domain-containing protein 30 | 23.0 | 25.0 | 31.3 | 42.0 | 0.55 | 0.59 | 0.75 | |
| AK7 | Adenylate kinase 7 | 7.7 | 17.3 | 24.0 | 38.3 | 0.20 | 0.45 | 0.63 | |
| ATP1A4 | Sodium/potassium-transporting ATPase subunit alpha-4 | 46.0 | 93.0 | 105.3 | 131.3 | 0.35 | 0.71 | 0.80 | |
| BSPH 1 | Binder of sperm protein homolog 1 | 0.7 | 4.0 | 7.3 | 9.7 | 0.069 | 0.417 | 0.76 | |
| CABYR | Calcium-binding tyrosine phosphorylation-regulated protein | 0 | 104.7 | 138.0 | 175.3 | 0 | 0.60 | 0.79 | |
| CCIN | Calicin | 0 | 0 | 33.7 | 50.7 | 0 | 0 | 0.66 | |
| GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 128.7 | 139.0 | 207.7 | 254.7 | 0.50 | 0.55 | 0.81 | |
| IDH1 | Isocitrate dehydrogenase [NADP] cytoplasmic | 0 | 5.7 | 2.0 | 5.3 | 0 | 1.06 | 0.37 | |
| IZUMO1 | Izumo sperm-egg fusion protein 1 | 2.7 | 19.7 | 23.0 | 50.0 | 0.05 | 0.39 | 0.46 | |
| KCNU1 | Potassium channel subfamily U member 1 | 0 | 0 | 4.0 | 5.7 | 0 | 0 | 0.71 | |
| RSPH 1 | Radial spoke head 1 homolog | 27.0 | 36.3 | 49.0 | 49.0 | 0.55 | 0.74 | 1.00 | |
| SPACA1 | Sperm acrosome membrane-associated protein 1 | 0 | 6.0 | 6.3 | 6.3 | 0 | 0.95 | 0 | |
| SUN5 | SUN domain-containing protein 5 | 0 | 0 | 3.7 | 4.0 | 0 | 0 | 0.92 | |
| Sperm motility | ATP1A4 | Sodium/potassium-transporting ATPase subunit alpha-4 | 46.0 | 93.0 | 105.3 | 131.3 | 0.35 | 0.71 | 0.80 |
| CCDC39 | Coiled-coil domain-containing protein 39 | 0 | 3.7 | 4.0 | 5.7 | 0 | 0.65 | 0.71 | |
| DNAH1 | Dynein heavy chain 1, axonemal | 0 | 18.3 | 18.3 | 18.7 | 0 | 0.98 | 0.98 | |
| GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 128.7 | 139.0 | 207.7 | 254.7 | 0.50 | 0.55 | 0.81 | |
| Cilium morphogenesis, axoneme assembly and cilium movement | AK7 | Adenylate kinase 7 | 7.7 | 17.3 | 24.0 | 38.3 | 0.2 | 0.45 | 0.63 |
| CABYR | Calcium-binding tyrosine phosphorylation-regulated protein | 0 | 104.7 | 138.0 | 175.3 | 0 | 0.60 | 0.79 | |
| CCDC39 | Coiled-coil domain-containing protein 39 | 0 | 3.7 | 4.0 | 5.7 | 0 | 0.65 | 0.71 | |
| DNAH1 | Dynein heavy chain 1, axonemal | 0 | 18.3 | 18.3 | 18.7 | 0 | 0.98 | 0.98 | |
| DNAI2 | Dynein intermediate chain 2, axonemal | 0 | 8.7 | 12.0 | 15.7 | 0 | 0.55 | 0.77 | |
| RSPH 1 | Radial spoke head 1 homolog | 27.0 | 36.3 | 49.0 | 49.0 | 0.55 | 0.74 | 1 | |
| SEPT7 | Septin-7 | 0 | 0 | 5.7 | 6.0 | 0 | 0 | 0.94 | |
| Cell recognition | ADAM30 | Disintegrin and metalloproteinase domain-containing protein 30 | 23.0 | 25.0 | 31.3 | 42.0 | 0.55 | 0.59 | 0.75 |
| CRTAC1 | Radial spoke head 1 homolog | 0 | 0 | 5.0 | 10.7 | 0 | 0 | 0.47 | |
| DYNLL2 | Dynein light chain 2, cytoplasmic | 17.7 | 31.7 | 33.0 | 54.7 | 0.32 | 0.58 | 0.60 | |
| IZUMO1 | Izumo sperm-egg fusion protein 1 | 2.7 | 19.7 | 23.0 | 50.0 | 0.05 | 0.39 | 0.46 | |
| KCNU1 | Potassium channel subfamily U member 1 | 0 | 0 | 4.0 | 5.7 | 0 | 0 | 0.71 | |
| Carboxylic acid metabolic process | AASS | Alpha-aminoadipic semialdehyde synthase, mitochondrial | 2.3 | 1.0 | 3.7 | 15.7 | 0.15 | 0.06 | 0.23 |
| ACAD8 | Isobutyryl-CoA dehydrogenase, mitochondrial | 2.0 | 7.0 | 12.7 | 15.7 | 0.13 | 0.45 | 0.81 | |
| ALDH9A1 | 4-trimethylaminobutyraldehyde dehydrogenase | 61.0 | 74.7 | 113.0 | 115.0 | 0.53 | 0.65 | 0.98 | |
| CRAT | Carnitine O-acetyltransferase | 48.3 | 51.3 | 66.7 | 112.3 | 0.43 | 0.46 | 0.59 | |
| DPEP1 | Dipeptidase 1 | 6.0 | 9.3 | 11.3 | 17.0 | 0.35 | 0.55 | 0.67 | |
| GAPDHS | Glyceraldehyde 3-phosphate dehydrogenase, testis-specific | 128.7 | 139.0 | 207.7 | 254.7 | 0.50 | 0.55 | 0.81 | |
| GSTO2 | Glutathione S-transferase omega-2 | 7.3 | 15.3 | 38.7 | 43.7 | 0.17 | 0.35 | 0.88 | |
| HIBCH | 3-hydroxyisobutyryl CoA hydrolase, mitochondrial | 2.0 | 7.3 | 10.0 | 13.0 | 0.15 | 0.56 | 0.77 | |
| IDH1 | Isocitrate dehydrogenase [NADP] cytoplasmic | 0 | 5.7 | 2.0 | 5.3 | 0 | 1.06 | 0.37 | |
| MUT | Methylmalonyl-CoA mutase, mitochondrial | 3.3 | 10.3 | 11.7 | 16.0 | 0.21 | 0.64 | 0.73 | |
| Oxidation-reduction process | AASS | Alpha-aminoadipic semialdehyde synthase, mitochondrial | 2.3 | 1.0 | 3.7 | 15.7 | 0.15 | 0.06 | 0.23 |
| ACAD8 | Isobutyryl-CoA dehydrogenase, mitochondrial | 2.0 | 7.0 | 12.7 | 15.7 | 0.13 | 0.45 | 0.81 | |
| ALDH9A1 | 4-trimethylaminobutyraldehyde dehydrogenase | 61.0 | 74.7 | 113.0 | 115.0 | 0.53 | 0.65 | 0.98 | |
| CHDH | Choline dehydrogenase, mitochondrial | 3.7 | 13.3 | 15.0 | 24.7 | 0.15 | 0.54 | 0.61 | |
| CRAT | Carnitine O-acetyltransferase | 48.3 | 51.3 | 66.7 | 112.3 | 0.43 | 0.46 | 0.59 | |
| GAPDHS | Glyceraldehyde-3-phosphate dehydrogenase, testis-specific | 128.7 | 139.0 | 207.7 | 254.7 | 0.50 | 0.55 | 0.81 | |
| GSTO2 | Glutathione S-transferase omega-2 | 7.3 | 15.3 | 38.7 | 43.7 | 0.17 | 0.35 | 0.88 | |
| HTATIP2 | Oxidoreductase HTATIP2 | 7.3 | 9.3 | 10.3 | 5.0 | 1.47 | 1.87 | 2.07 | |
| IDH1 | Isocitrate dehydrogenase [NADP] cytoplasmic | 0 | 5.7 | 2.0 | 5.3 | 0 | 1.06 | 0.37 | |
| MUT | Methylmalonyl-CoA mutase, mitochondrial | 3.3 | 10.3 | 11.7 | 16.0 | 0.21 | 0.64 | 0.73 | |
| SLC25A4 | ADP/ATP translocase 1 | 1.0 | 2.0 | 4.7 | 6.7 | 0.15 | 0.30 | 0.70 | |
NASF: normalized spectral abundance factor.
Proteins involved in top pathways and upstream regulators
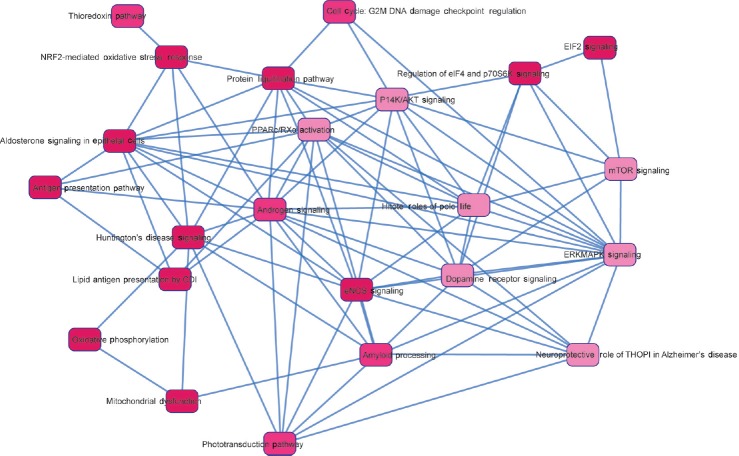
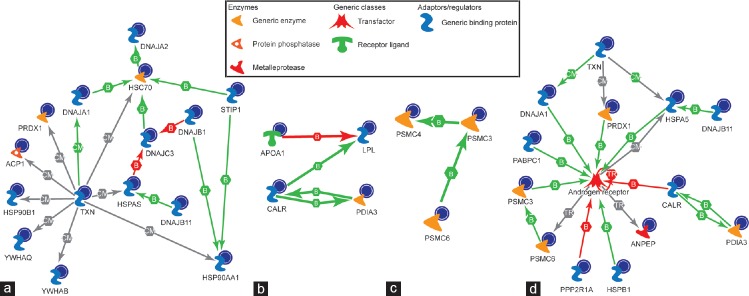
On the basis of the common genes, 15 top pathways showed an overlap with each other and their translated protein product was shown to be gradually underexpressed from immature to mature fractions, while 8 connecting signaling pathways were predicted (Figure 3). The proteins identified in our data set were analyzed using MetaCore™ to predict the upstream regulator(s) and the pathways involved (Figure 4a–4c). The androgen receptor was predicted as one of the transcription regulators and the pathways affected were chaperone-mediated stress response, proteosomal pathway, and sperm function (Figure 4d).
Figure 3.
Fifteen overlapping pathways and eight connecting signaling pathways were predicted based on the identified common genes. Their translated protein products were underexpressed starting from F1 to F4. F1: least mature stage having the lowest density; F2, F3: intermediate stages; F4: includes the most dense and morphologically mature motile spermatozoa. Full names of abbreviated proteins are presented in Supplementary Information (39.6KB, pdf) .
Figure 4.
The biochemical process networks of the differentially expressed proteins generated by MetaCore™ software using a transcription regulation algorithm. The biochemical process regulation networks were ranked by a value and interpreted in terms of GO. Gene network illustrates proteins and interactions of the differentially expressed proteins. MetaCore™ pathway analysis predicting androgen receptor as the major regulator and pathways involved were; (a) chaperone-mediated stress response; (b) sperm function; (c) proteosomal pathway; and (d) predicted impaired androgen receptor signaling in spermatozoa of infertile patients. GO: gene ontology; F1: least mature stage having the lowest density; F2, F3: intermediate stages; F4: includes the most dense and morphologically mature motile spermatozoa. Full names of abbreviated proteins are presented in Supplementary Information (39.6KB, pdf) .
DISCUSSION
We have previously reported the proteome profile changes in these four subsets of immature and mature spermatozoa from fertile men separated by three layer density gradient. Our results demonstrated that proteins critical for sperm maturation are altered in stage specific manner in fertile men. Of these, 4 proteins, namely, heat shock protein HSP701A, clusterin, tektin 2, and tektin 3 were validated by western blot to corroborate the proteomic findings.9 In this study, the goal was to compare the same four subsets of immature and mature spermatozoa in infertile men to understand why spermatozoa obtained in the mature fraction (F4), which separate the good quality spermatozoa in terms of motility, TMC, and even TSC, are still dysfunctional in achieving pregnancy. Therefore, in this study, a high-throughput shotgun proteomic approach followed by pathway analysis was used to understand the impaired molecular mechanism involved in sperm dysfunction.
Despite the absence of effective transcription and translation, spermatozoa undergo important functional transformation during epididymal transit. These modifications are essential to produce functionally efficient spermatozoa and depend on loss, modification, or remodeling of existing sperm proteins in response to the signals conveyed by the male reproductive tract. These signals are carefully regulated by an array of gene products in a stage-specific manner in which molecular chaperones play a key role.22,23 Molecular chaperones are structurally diverse proteins that are expressed nearly in all cells in response to cellular stress.24 In the present study, we observed that two of the top three networks identified by IPA were associated with underexpression of molecular chaperones. These networks were related to posttranslational modification, protein folding, and molecular transport. Furthermore, these networks were involved in protein trafficking related to cellular processes such as cell cycle, cell survival, cell death, and developmental disorder.
Proposed pathway 1
In the first network, eight proteins of the chaperonin-containing T-complex/TCP1-ring complex (CCT2, CCT3, CCT4, CCT5, CCT6A, CCT7, CCT8, and TCP1), four HSP 70 family chaperones (HSPA2, HSPA1L, HSP90AA1, and HSP8), and three members of the co-chaperonin DNAJ (DNAJA1, DNAJB1, and DNAJC3) were identified as key proteins (Figure 2c). Vesicular trafficking is conducted by gene products of Bardet–Biedl syndrome (BBS) and McKusick–Kaufman syndrome (MKKS).25,26 Disruptions of BBS or MKKS genes result in male infertility owing to the failure of flagellum formation in spermatozoa.26,27,28,29 The chaperonin-containing T-complex/TCP1-ring complex as part of the BBS/CCT complex may play a role in the assembly of BBSome, a complex involved in ciliogenesis regulating transport vesicles to the cilia. These proteins also have a role in the folding of actin and tubulin in vitro, and TUBB4B protein was identified in this network. Our liquid chromatography (LC)-MS/MS data also showed a decrease in tubulin (TUBB4B) as well as in both dynein heavy and light chains (DYNC1H1 and DYNC1LI1), further corroborating the concept of flagellar disassembly. Owing to the defects in flagellar disassembly, we did not find any change in Tektin (structural components of outer doublet microtubules) proteins as reported by us in spermatozoa of fertile men.9
The ribosomal subunits that include two translation elongation factors (EEF1E1 and EEF1D) and two tRNA ligases (DARS and VARS) identified in the network were also underexpressed. These are responsible for linking aspartate and valine to their cognate tRNAs, thus inhibiting the incorporation of these amino acids into the protein. In fact, Talevi et al.30 have reported that treatment of spermatozoa with aspartate in vitro enhances sperm motility in oligozoospermic samples along with inhibition of DNA damage and membrane lipid peroxidation.
The HSP family proteins identified in the network were inducible by stress (e.g. HSPA1), constitutively expressed, or both (e.g. HSPH1, HSPA8, and HSP90AA1). Expression of some HSPs is developmentally regulated or restricted to specific cells.31 HSPA2 is expressed on the sperm surface, and it is essential for sperm membrane remodeling during sperm–oocyte interaction. It may be used as a biochemical marker of human sperm function and male fertility.32 Levels of human HSPA2 expression have been correlated with sperm maturity and male fertility.32,33 Therefore, a decline in HSPA2 expression may be responsible for improper maturation, which was not so in our previous study in fertile men.9
We found HSPA1A to be downregulated from F1 to F4 in this study in infertile men as well as in our previous study in fertile donors. HSPA1A plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, re-folding of misfolded proteins, and controlling the targeting of proteins for subsequent degradation. It maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation.34
The other HSPs and DNAJ family proteins are reported to affect spermatogenesis or sperm function.12 Proteins responsible for mitochondria-mediated cell death (FAM162) and the negative regulator of mitochondrial respiration (TRAP1) were also downregulated (Figure 2c). Therefore, this network indicates that during the process of sperm production and maturation in infertile men, there is an impairment of the stress response protein that is demonstrated by the underexpression of chaperones in the network. This may lead to defects in the formation of the flagella and abortive apoptosis. Therefore, though the spermatozoa obtained in the most mature fraction (F4) appear normal, they are incapable of proper functioning after ejaculation.
The above findings were corroborated by the second predicted network (Figure 2d) showing deregulated posttranslational modification and protein folding, leading to developmental disorder.
Proposed pathway 2
The most important proteins were thioredoxins and peroxiredoxins which are reported to be abundantly expressed in developing spermatocytes.35 Thioredoxin domain-containing protein 2 (TXNDC2) is a spermatid-specific thioredoxin-1 (also known as Sptrx-1) which is found exclusively in the tail of elongating spermatids and spermatozoa.36 TXNDC2 transiently associates with the longitudinal columns of the fibrous sheath during sperm tail assembly but does not remain as a permanent component of the fibrous sheath in the mature sperm cell.37 Although the expression of this protein showed a gradual decline from F1 to F4, still its presence in the most mature fraction implies defects in sperm flagella, which corroborates the findings in pathway 1 and our previous study regarding the expression of tektins.9 Similarly, peroxiredoxin 4 (PRDX4) (Figure 2d) is present as a membrane-bound form only in testes, and the unprocessed form may be involved in acrosome formation during spermiogenesis.38 Peroxiredoxins are involved in hydrogen peroxide-mediated signaling in spermatozoa39 and prevent oxidative stress during capacitation.40 PRDX4 knockout mice showed elevated spermatogenic cell death via oxidative stress.41 We observed a decrease in the expression of voltage-dependent mitochondrial outer membrane ion channels (VDAC1, VDAC2, and VDAC3) along with mitochondrial ATP synthase complex subunits (ATP5I, ATP5F1, and ATP5J). These findings suggest mitochondrial dysfunction, which may lead to oxidative stress and cell death as mentioned above. In addition, underexpression of isocitrate dehydrogenase (IHD1), lipoprotein lipase (LPL), and apolipoprotein A1 (APOA1) may be responsible for impaired lipid metabolism. This may result in altered lipid profile of the sperm membrane that in turn may affect fertilization.42 Furthermore, underexpression of dynactin (DCTN2), contractin (actin-related protein 1 homolog A [ACTR1A]), and F-actin capping protein (capping protein Z line alpha [CAPZA]) observed in our study may lead to defects in microtubule packaging and centrosome formation. Since the spermatozoon donates its centriole during fertilization and is essential for cleavage, these spermatozoa from infertile patients may not be able to initiate cleavage in embryos after fertilization. One of the major groups from the network that we identified were the endoplasmic reticulum-specific protein suppressor enhancer lin-12 1 like (SEL1L) and endoplasmic reticulum protein 29 (ERP29) along with the Ras-related proteins RBA6A and RAB7A. The presence of these proteins suggests impaired membrane traffic from the Golgi apparatus toward the endoplasmic reticulum. In addition, proteins involved in posttranslational modification and folding of proteins such as chaperones (HSP90B1, HSPA5, calnexin [CANX], calreticulin [CALR]), protein disulfide isomerase (prolyl 4-hydroxylase, beta polypeptide [P4HB], and protein disulfide isomerase-associated 3 [PDIA3]), peptidyl prolyl-cis-trans isomerase (FK506-binding protein [FKBP]), and serine-threonine phosphatase (peptidylpropyl isomerase B [PPIB]) were also identified in this network. Thus, an endoplasmic reticulum stress may be responsible for improper protein formation leading to developmental disorders in the proteins obtained in F4 fraction in infertile men.
Proposed pathway 3
The third network demonstrated a deregulated molecular transport, protein trafficking, and cell cycle. In the present study, we identified nuclear transport receptor IOP5 or importin-5 and alpha karyopherins such as KPNA2, KPNA3, KPNA4, and KNPNB. These karyopherins are expressed in specific stages during spermatogenesis in the spermatogonium (KNNA1, KPNA2, and KPNA3), spermatocyte (KPNA2, KPNA3, and KPNA4), round spermatid (KPNA3 and KPNA4), and elongating spermatids (KPNA2 and IPO5).43 These importins were all underexpressed in ejaculated sperm in our study. This finding suggests that together with RNA GTPase they may be responsible for impaired import of testis specific H1 histone (HIST1H1T). HIST1H1T is required for less compaction of nuclear DNA during meiosis and is subsequently replaced by protamines. Thus, these importins may play a role in nuclear abnormalities that may eventually render the spermatozoa incapable of fertilization or successful completion of postfertilization steps.
The other two important proteins involved in exosome-mediated cargo delivery were ANXA1 and ANXA2.44 These were underexpressed in this network. It is hypothesized that sperm maturation in the epididymis and regulation of sperm function are mediated by regulatory RNAs that are delivered by exosomes.45,46 In fact, Yang et al.46 reported the presence of annexins in the seminal exosomes. Thus, an improper delivery of regulatory factors to the spermatozoa via exosomes might result in sperm dysfunction.
Proteasomes present in mammalian sperm play a pivotal role during fertilization. The enzymatic activity of the proteasome is modulated by protein kinase A and is involved in the progesterone-induced acrosome reaction. Furthermore, after capacitation, the acrosomal proteasomes facilitate the degradation of zona pellucida glycoproteins leading up to fertilization.47 In this network, we identified eight subunits of 26S proteasome (PSMC3, PSMC4, PSMC6, PSMD5, PSMD7, PSMD8, PSMD11, and PSMD13). One of the nodal proteins, matrix-metalloproteinase (MME) or Neprilysin, was also identified in the network. Pinto et al.48 reported that tachykinins present in human spermatozoa participate in the regulation of sperm motility, and their activity is regulated by Neprilysin. Another nodal protein involved in this network was 14-3-3 protein theta (YWHAQ) adapter protein which has been implicated in the regulation of a large spectrum of both general and specialized signaling pathways,49 thus implying a signaling failure in F4 fraction in our study. We identified 15 overlapping networks on the basis of mutual genes that were affected during the process of maturation in these infertile patients. These networks may be responsible for poor sperm quality.
MetaCore™ pathway analysis
While IPA reports protein–protein interactions, MetaCore™ is an integrated software suite for functional analysis of experimental data of human protein–protein interactions, protein–DNA interactions, transcription factors, and signaling and metabolic pathways including disease and toxicity. The top pathway affected during maturation was identified to be mediated through TXN, PRDX1, signaling adaptor molecules (YWHAQ and YWHAB), and the molecular chaperons (HSP90AA1, HSP90B1, HSC70, HSPA5, DNAJA1, DNAJ2, DNAJB1, DNAJB11, DNAJC3, and STIP1). Taken together, all the networks and pathways described above suggest that the spermatozoa at different stages of maturation suffer from endoplasmic reticulum stress (ER stress) due to accumulation of unfolded or misfolded proteins as a result of underexpressed chaperone and proteasome activities, which will lead to induction of oxidative stress and abortive apoptosis. This is corroborated by the fact that the Clusterin is not differentially expressed in the spermatozoa of infertile men like that reported for fertile men.9 Clusterin isoforms are responsible for differential regulation of apoptosis where the nuclear form promotes the process and the mitochondrial form opposes the process (Uniprot). In fertile men, its proper expression prevented apoptosis of mature spermatozoa where the mitochondria are intact and eliminated the spermatozoa with nuclear defects. Therefore, it is suggested that the process was impaired in infertile men leading to abortive apoptosis. Taken together, the cells that may appear morphologically mature may carry structural anomalies in the cytoskeleton, microtubules, and flagellum, thereby affecting the function of the spermatozoa. Androgen receptor is a transcription regulator. The presence of the AR in human spermatozoa has been demonstrated by Western blot and immunofluorescence assay.50 AR is also localized in the head region.51 In addition to stimulating cell growth, androgens or the AR plays an important role in apoptosis involving both intrinsic and extrinsic pathways. Short exposure of ejaculated spermatozoa to androgens produces an increase in AR phosphorylation, especially on the 110 kDa band which is the less expressed isoform in sperm cells.51
Taken together, all the networks and pathways described in the present study show that the spermatozoa at different stages of maturation suffer from ER stress due to accumulation of unfolded or misfolded proteins as a result of underexpressed chaperone and proteasome activities. This may lead to induction of oxidative stress and abortive apoptosis.52 Thus, although the spermatozoa in F4 fraction may appear morphologically mature and exhibit good motility and morphology following separation on density gradient as is the case during intrauterine insemination or in vitro fertilization, these sperm can potentially carry structural anomalies in the cytoskeleton, microtubule, and flagellum, thereby affecting the functional capabilities of the spermatozoa. A limitation of our study was that we could not perform the western blot validation of the MS-spectrometry data as the quantity of protein from the fractions, particularly F3 and F4, was insufficient.
In conclusion, the results of the present study show that a defective signaling cascade is responsible for the defective sperm function observed in infertile patients, particularly a decline in mitochondrial function and oxidative phosphorylation in the most mature fraction (F4), implying a state of energy deprivation. Furthermore, a dysregulated protein turnover and protein folding may lead to accumulation of defective proteins or proteins that otherwise would have been eliminated during the process of maturation and thus result in the impairment of sperm function. The present study provides mounting evidence that aberrant chaperone expression may be a major contributing factor to the defective sperm function seen in many cases of male infertility. Our study also identified the involvement of AR as the core player in the downregulation of the signaling leading to production of defective spermatozoa incapable of fertilization. Future studies conducted in larger study population are necessary to examine the distribution of proteins in infertile men with different clinical diagnosis such as varicocele and validate the important proteins to further help in understanding the underlying pathology of male infertility.
AUTHOR CONTRIBUTIONS
AA and RS planned the experiments. ZC selected the samples according to the clinical histories. ZC and LS analyzed the collected data. LS was involved in the manuscript preparation. RS, AA, and LS critically reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to the Andrology Center technologists for scheduling the study subjects; Cleveland Clinic Arts Department for creating the four figures; Belinda Willard, Director, Proteomic Core Lab, Lerner Research Institute for providing assistance with proteomic analysis and Banu Gopalan for the bioinformatics analysis of the results. The Orbitrap Elite mass spectrometer used in this study was purchased with funds from an NIH shared instrument grant 1S10RR031537-01 to Belinda Willard. Financial support was provided by the American Center for Reproductive Medicine, Cleveland Clinic. Dr. Zhihong Cui's visit was supported by a fellowship from the Chinese Government and the Institute of Toxicology, College of Preventive Medicine, the Third Military Medical University, Chongqing, China.
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Jones RE, Kristen L. The male reproductive system. In: Jones R, Kristen L, editors. Human Reproductive Biology. Cambridge: Academic Press, Elsevier; 2013. [Google Scholar]
- 2.Sharma R, Agarwal A. Spermatogenesis: An overview. In: Armand Zini A, editor. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. New York: Springer; 2011. pp. 19–44. [Google Scholar]
- 3.Kishigami S, Wakayama S, Nguyen VT, Wakayama T. Similar time restriction for intracytoplasmic sperm injection and round spermatid injection into activated oocytes for efficient offspring production. Biol Reprod. 2004;70:1863–9. doi: 10.1095/biolreprod.103.025171. [DOI] [PubMed] [Google Scholar]
- 4.Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 5.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod. 2001;16:1922–30. doi: 10.1093/humrep/16.9.1922. [DOI] [PubMed] [Google Scholar]
- 6.Gaucher J, Reynoird N, Montellier E, Boussouar F, Rousseaux S, et al. From meiosis to postmeiotic events: the secrets of histone disappearance. FEBS J. 2010;277:599–604. doi: 10.1111/j.1742-4658.2009.07504.x. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Qu F, Xia L, Guo Q, Ying X, et al. Identification and characterization of ERp29 in rat spermatozoa during epididymal transit. Reproduction. 2007;133:575–84. doi: 10.1530/REP-06-0301. [DOI] [PubMed] [Google Scholar]
- 8.Govin J, Gaucher J, Ferro M, Debernardi A, Garin J, et al. Proteomic strategy for the identification of critical actors in reorganization of the post-meiotic male genome. Mol Hum Reprod. 2012;18:1–13. doi: 10.1093/molehr/gar063. [DOI] [PubMed] [Google Scholar]
- 9.Cui Z, Sharma R, Agarwal A. Proteomic analysis of mature and immature ejaculated spermatozoa from fertile men. Asian J Androl. 2016;18:735–46. doi: 10.4103/1008-682X.164924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res. 1999;251:350–5. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, et al. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–7. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 12.Dun MD, Aitken RJ, Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum Reprod Update. 2012;18:420–35. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Tholen E, Jennen D, Ponsuksili S, Schellander K, et al. Evidence for effects of testis and epididymis expressed genes on sperm quality and boar fertility traits. Reprod Domest Anim. 2006;41:538–43. doi: 10.1111/j.1439-0531.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Ollero M, Gil-Guzman E, Lopez MC, Sharma RK, Agarwal A, et al. Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum Reprod. 2001;16:1912–21. doi: 10.1093/humrep/16.9.1912. [DOI] [PubMed] [Google Scholar]
- 15.Brahem S, Mehdi M, Elghezal H, Saad A. Semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity. Andrologia. 2011;43:196–202. doi: 10.1111/j.1439-0272.2010.01050.x. [DOI] [PubMed] [Google Scholar]
- 16.Castillo J, Simon L, de Mateo S, Lewis S, Oliva R. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J Androl. 2011;32:324–32. doi: 10.2164/jandrol.110.011015. [DOI] [PubMed] [Google Scholar]
- 17.Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108:456–67.e1. doi: 10.1016/j.fertnstert.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 5th ed. Geneva: WHO Press; 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 19.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 20.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–24. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 21.Boyle EI, Weng S, Gollub J, Jin H, Botstein D, et al. GO: termfinder – open source software for accessing gene ontology information and finding significantly enriched gene ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–5. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–78. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- 23.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germs cells and sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech. 2010;73:409–94. doi: 10.1002/jemt.20786. [DOI] [PubMed] [Google Scholar]
- 24.Ellis RJ. Discovery of molecular chaperones. Cell Stress Chaperones. 1996;1:155–60. doi: 10.1379/1466-1268(1996)001<0155:domc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 26.Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, et al. The chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–32. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–9. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, et al. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77:1021–33. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–7. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talevi R, Barbato V, Fiorentino I, Braun S, Longobardi S, et al. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Rep Biol Endocrinol. 2013;11:81. doi: 10.1186/1477-7827-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupik W, Jasik K, Bembenek J, Widlak W. The expression patterns of heat shock genes and proteins and their role during vertebrate's development. Comp Biochem Physiol A Mol Integr Physiol. 2011;159:349–66. doi: 10.1016/j.cbpa.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Naaby-Hansen S, Diekman A, Shetty J, Flickinger CJ, Westbrook A, et al. Identification of calcium-binding proteins associated with the human sperm plasma membrane. Reprod Biol Endocrinol. 2010;8:6. doi: 10.1186/1477-7827-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamelak D, Lingwood C. Expression and sulfogalactolipid binding specificity of the recombinant testis-specific cognate heat shock protein 70. Glycoconj J. 1997;14:715–22. doi: 10.1023/a:1018569417218. [DOI] [PubMed] [Google Scholar]
- 34.Seo JH, Park JH, Lee EJ, Vo TT, Choi H, et al. ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation. Nat Commun. 2016;7:12882. doi: 10.1038/ncomms12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins – molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y, Oko R, Miranda-Vizuete A. Developmental expression of spermatid-specific thioredoxin-1 protein: transient association to the longitudinal columns of the fibrous sheath during sperm tail formation. Biol Reprod. 2002;67:1546–54. doi: 10.1095/biolreprod.102.004838. [DOI] [PubMed] [Google Scholar]
- 37.Ranney MK, Ahmed IS, Potts KR, Craven RJ. Multiple pathways regulating the anti-apoptotic protein clusterin in breast cancer. Biochim Biophys Acta. 2007;1772:1103–11. doi: 10.1016/j.bbadis.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuki S, Sasagawa I, Iuchi Y, Fujii J. Impaired expression of peroxiredoxin 4 in damaged testes by artificial cryptorchidism. Redox Report. 2002;7:276–8. doi: 10.1179/135100002125000785. [DOI] [PubMed] [Google Scholar]
- 39.O’Flaherty C, de Souza AR. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod. 2011;84:238–47. doi: 10.1095/biolreprod.110.085712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee D, Moawad AR, Morielli T, Fernandez MC, O’Flaherty C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol Hum Reprod. 2017;23:106–15. doi: 10.1093/molehr/gaw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iuchi Y, Okada F, Tsunoda S, Kibe N, Shirasawa N, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–58. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 42.Koh HJ, Lee SM, Son BG, Lee SH, Ryoo ZY, et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J Biol Chem. 2004;279:39968–74. doi: 10.1074/jbc.M402260200. [DOI] [PubMed] [Google Scholar]
- 43.Major AT, Whiley PA, Loveland KL. Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim Biophys Acta. 2011;1813:1668–88. doi: 10.1016/j.bbamcr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215–27. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290–304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Guo WB, Zhang WS, Bian J, Yang JK, et al. Comprehensive proteomics analysis of exosomes derived from human seminal plasma. Andrology. 2017;5:1007–15. doi: 10.1111/andr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerns K, Morales P, Sutovsky P. Regulation of sperm capacitation by the 26S proteasome: an emerging new paradigm in spermatology. Biol Reprod. 2016;94:117. doi: 10.1095/biolreprod.115.136622. [DOI] [PubMed] [Google Scholar]
- 48.Pinto FM, Ravina CG, Subiran N, Cejudo-Roman A, Fernandez-Sanchez M, et al. Autocrine regulation of human sperm motility by tachykinins. Reprod Biol Endocrinol. 2010;8:104. doi: 10.1186/1477-7827-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: localization of ERbeta and AR in mitochondria of the midpiece. Hum Reprod. 2005;20:3481–7. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- 51.Aquila S, Middea E, Catalano S, Marsico S, Lanzino M, et al. Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod. 2007;10:2594–605. doi: 10.1093/humrep/dem243. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.