Abstract
Structural alterations in fibroelastic components of the penile corpus cavernousum (CC) may impair its compliance, resulting in venous leakage and erectile dysfunction (ED). Our study evaluated the effectiveness of noninvasive two-dimensional shear-wave elastography (2-D SWE) in quantifying penile CC lesions in rabbits with hyperlipidemia-induced ED. A total of 12 New Zealand white rabbits were randomly divided into two groups. Six were fed a high-cholesterol diet containing 2% cholesterol and 8.5% lard for 10 weeks and the other six were fed normal diet as controls. We measured the shear-wave elastic quantitative (SWQ) value of penile CC by 2-D SWE. Erectile function was investigated by intracavernous injection of papaverine, and immunohistochemical (IHC) staining and the western blot analysis to determine the penile CC lesions. After 10 weeks, the SWQ values obtained from penile CC were remarkably higher in the high-cholesterol-fed compared with the control group, and the ΔICP (ICP plateau minus ICP baseline)/MAP (ICP: intracavernous pressure, MAP: mean arterial pressure) was markedly decreased. The IHC staining and western blot revealed extracellular matrix (ECM) accumulation in penile cavernous tissues, and the smooth muscle cell (SMC) phenotypic transition was affected, as indicated by reduced alpha-smooth muscle actin and calponin-1 expression and increased phospho-myosin light chain20 (p-MLC20)/MLC20 and osteopontin expression. Hyperlipidemia resulted in ECM accumulation accompanied with SMC phenotypic transition in penile CC and impaired the erectile function eventually. These might, in turn, lead to variations in the SWQ values. It suggests that 2-D SWE may be a novel, noninvasive and effective approach that distinguishes penile CC lesions secondary to hyperlipidemia from normal.
Keywords: corpus cavernosum, erectile dysfunction, extracellular matrix accumulation, phenotypic transition, shear-wave elastography, smooth muscle cells
INTRODUCTION
Erectile dysfunction (ED) is defined as the persistent inability to attain or maintain a penile erection sufficient for satisfactory sexual performance. This is a worldwide health problem that is associated with broad aspects of a man's life beyond erectile function, including emotional and psychological well-being, as well as their partner's relationship.1 According to a recent meta-analysis, surveys found an overall ED prevalence of 16% in Western countries and 27% to 68% in Asia.2 Compliance of the corpus cavernosum (CC) is essential throughout the entire process of penile erection. In some pathological circumstances, such as aging, diabetes mellitus, hyperlipidemia, and cavernosal nerve damage, penile CC structural changes may impair smooth muscle cell (SMC) relaxation, resulting in corporal veno-occlusive dysfunction (CVOD) and ultimately an insufficient erection.3 The most common pathological change is known as penile CC fibrosis, which includes loss of SMC content and collagen deposition.3 Recent research also demonstrated that the SMC phenotypic transition might also affect cellular relaxation and be accompanied by extracellular matrix (ECM) accumulation.4,5 Therefore, understanding penile CC structures is very important for the diagnosis and further management of ED.
Current specialized testing, such as penile duplex ultrasonography, cavernosometry, and cavernosography, can only predict the existence of structural alterations in penile CC by identifying CVOD rather than directly understanding the texture of penile CC.6 Biopsy analysis was once used to evaluate the smooth muscle composition of corporal tissue; however, it is controversial and clinically unacceptable because of its invasive and painful nature, and may exacerbate patient's psychological problems.6 In previous studies, we tested two-dimensional shear-wave elastography (2-D SWE, ShearWave™ Elastography) for the detection of penile CC tissue stiffness, and found that the obtained values were correlated with age and serum testosterone levels in male volunteers aged from 19 to 81 years old.7 These findings were supported by a recent study by Inci et al.,8 and 2-D SWE showed future potential for evaluating penile CC lesions. Two-dimensional SWE is a new quantitative ultrasound technology that makes the target tissue generate transverse shear waves by ultrasonic pulses. The collection of shear-wave velocity information allows precise estimation of tissue stiffness and quantitative analysis of tissue structural characteristics.9 Using a rat model, we discovered that 2-D SWE could differentiate between the penile CC of aged compared with sexually immature rats.10,11,12 However, whether 2-D SWE also detects pathological changes of CC during the diagnosis of ED remains unknown.
In this study, we used rabbits with hyperlipidemia-induced ED as our experimental model. Hyperlipidemia is one of the risk factors for ED13 and cholesterol feeding is an established model for examining aspects of organic ED.14,15,16,17 Our study aimed to illustrate the relationship between 2-D SWE obtained values and penile CC structural alterations, which may be secondary to hyperlipidemia and lead to organic ED. Our future goal is to assess whether 2-D SWE would be an effective, convenient, repeatable, and noninvasive technique to evaluate penile CC lesions in the clinical management of ED.
MATERIALS AND METHODS
Animal model
A total of 12 male New Zealand white rabbits (aged 14–16 weeks old) were used in this study. After one week of feeding a normal diet, rabbits were divided randomly into two groups. Hyperlipidemia group rabbits (n = 6) were treated by feeding a high-cholesterol diet containing 2% cholesterol and 8.5% lard. Control group rabbits (n = 6) were fed a standard chow for 10 weeks. All protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Shanghai University of Traditional Chinese Medicine, Shanghai, China (IRB No.: SZY2-1706018).
Serum lipid profile analysis
At the start of the study and after feeding for 10 weeks, blood samples were drawn from the rear leg vein of all rabbits the morning after 12 h of fasting. All blood samples were placed at room temperature until clotted, then centrifuged at 2000 g for 15 min to separate the serum. Fasting plasma glucose (FPG) and lipid profile analyses, including triglyceride (Tg), total cholesterol (Tc), and low-density lipoprotein cholesterol (LDL), were conducted by immunoturbidometric assay on a Beckman Coulter AU5800 system (Beckman Coulter, Inc., Brea, CA, USA) according to the manufacturer's instructions.
2-D SWE scanning
At the end of 10 weeks, all rabbits were injected with a dose of 0.8 ml kg−1 1% pentobarbital sodium (Shanghai Westang Biotech Co. Ltd., Shanghai, China) via the ear vein. After sedation, rabbits were placed in the supine position with all limbs fixed, and at 20°C–24°C room temperature. A single dose of 0.5–1 ml 1% pentobarbital was used to maintain sedation. All 2-D SWE imaging was performed by the same doctor in the ultrasound department, who was blind to the animal grouping, with over 20 years of academic and clinical experience in ultrasound imaging. With the probe SuperLinear SL 15-4, the ultrafast ultrasound device Aixplorer (SuperSonic Imagine Co. Ltd., Aix-en-Provence, France) was used for 2-D SWE imaging. A transverse section of the penis at the borderline of skin and hair was chosen for scanning. The 2-D SWE was started when the penile CC and urethral sponge structures were recognized by 2-D ultrasound. The selected 2-D SWE imaging box was larger than the transverse section of the penis and the penetration mode selected for 2-D SWE imaging was “Pen”. The recording system stored video data of the 2-D SWE imaging process.
The sonographer played back and reviewed the whole recording of the 2-D SWE images to select suitable images. The criteria for a selected 2-D SWE image was that each area of penile CC was filled with color and had no mosaic-like colored points. The shear-wave elastic quantitative (SWQ) value (in kPa) of penile CC was measured using the selected 2-D SWE images. The region of measurement (circle) was delineated within the tunica albuginea of the penile CC, which was included as completely as possible. Each rabbit was analyzed three times, using one SWQ value from the left CC (SWQ-L) and one from the right CC (SWQ-R) each time.
Erectile function evaluation
On the next day of 2-D SWE scanning, we examined intracavernous pressure (ICP) according to the protocols described by Lin YM and Lin JS.18 A dose of 1.5 ml kg−1 1% pentobarbital sodium was injected via the ear vein for anesthetization. Rabbits were placed in the supine position at 20°C–24°C room temperature, and a single bulk of 1–2 ml 1% pentobarbital was used to maintain anesthesia. We incised the anterior midline skin of the neck, and the exposed right carotid artery was cannulated with a Gould 23 ID pressure transducer (Bridge AMP, ADInstruments Pty. Ltd., Sydney, Australia) for continuous monitoring of mean arterial pressure (MAP). Then, we denuded the penile skin and fascia, and exposed the penile CC back to the root at the symphysis pubis. A 25G needle (Changzhou Yuekang Medical Equipment Co. Ltd., Changzhou, China) was inserted into the penile CC for recording pressure by a separate monitoring set, and connected to a three-way stopcock, thus permitting the intracavernous injection (ICI) of drugs. All connecting tubes were filled with heparinized saline (200 IU ml−1; SPH No.1 Biochemical and Pharmaceutical Co. Ltd., Shanghai, China) to prevent clotting.
At the start of recording measurements, stable baseline ICP values were recorded and analyzed by LabChart 7 software (Bridging amplifier mode, ADInstruments Pty. Ltd.). We then injected 1 mg kg−1 papaverine hydrochloride (PAP; Shenyang No.1 Pharmaceutical Co. Ltd., Shenyang, China) into the penile CC with a sealing dose of 4.5 mg. We observed the penile response for at least 10 min, and the plateau ICP value and MAP were recorded. The ΔICP (ICP plateau minus ICP baseline)/MAP were calculated as the evaluation of the erectile function.
Histological examination
When ICP evaluation was finished, the penile CC was washed with saline for 30 min via the same 25G needle. The animal was then sacrificed and the penis detached at the root. After quickly removing the skin, mucosa, and corpus spongiosum, a penile transverse section of 1–2 mm thickness was cut off near the borderline between the skin and hair. We selected this region for histological examination because it corresponded to the location used for 2-D SWE scanning. Penile specimens were then fixed in 10% formalin, and embedded in paraffin (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China), for hematoxylin and eosin (H and E) and immunohistochemical (IHC) staining (IHC kit purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Remaining penile tissues were cut open, and penile CC was carefully dissected from the surrounding tunica albuginea. Specimens were cut into small pieces and quickly immersed in liquid nitrogen for storage at −80°C before further protein analysis.
For IHC, sections (6 μm) were incubated overnight at 4°C with primary antibodies (Abcam plc., Cambridge, MA, USA), and the dilutions of these antibodies were as follows: anti-transforming growth factor-beta (TGF-β, Catalog No. ab179695), 1:500; anti-collagen I (Catalog No. ab138492), 1:1500; anti-collagen III (Catalog No. ab7778), 1:1000; anti-alpha-smooth muscle actin (α-SMA, Catalog No. ab32575), 1:200; anti-calponin-1 (Catalog No. ab46794), 1:200; anti-osteopontin (OPN, Catalog No. ab166709), 1:500. After the slides were washed, a biotinylated secondary antibody in the IHC kit was added and incubated. Finally, antigen detection was visualized using diaminobenzidine (DAB) in the IHC kit. The slides were photographed using a Nikon Microphot-FXA microscope (Nikon Co. Ltd., Kogaku, Tokyo, Japan).
Western blot analysis
Frozen samples were washed three times in PBS (Beyotime Biotechnology Co. Ltd., Shanghai, China), cut into small pieces, then suspended in 1 mmol l−1 phenylmethanesulfonyl fluoride (PMSF; Amresco Inc., Huston, TX, USA) and radioimmunoprecipitation (RIPA) buffer (Beyotime Biotechnology Co. Ltd.) on ice. Thirty minutes later, the lysed homogenate was centrifuged at 10 000 g for 5 min and the supernatant was collected for protein measurement using a BCA kit (Beyotime Biotechnology Co. Ltd.). A total of 30 μg protein from each sample was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Inc., Hercules, CA, USA) for protein separation. After transfer to nitrocellulose membranes (Millipore Corp., Bedford, MA, USA), the membranes were blocked in PBS and 5% nonfat dry milk for 1 h at room temperature, followed by incubation with primary antibody at 4°C overnight. After washing three times with the blocking buffer, specific proteins were revealed by peroxidase-conjugated secondary IgG (Amersham Pharmacia Biotech, Inc., Buckinghamshire, UK) at a final dilution of 1:5000 for 1 h at room temperature. The primary antibodies of anti-phospho-myosin light chain20 (p-MLC20, Catalog No.3675) and anti-MLC20 (Catalog No.3672) were purchased from Cell Signaling Technology (CST, Inc., Danvers, MA, USA), and the dilutions were both 1:1000. Other primary antibodies were the same as that of IHC staining, with the adjusted dilutions as follows: anti-TGF-β, 1:1000; anti-collagen I, 1:1000; anti-collagen III, 1:5000; anti-α-SMA, 1:1000; anti-calponin-1, 1:15 000; anti-OPN, 1:1000; anti-β-actin (Catalog No. ab8227; Abcam plc.), 1:1000.
Statistical analyses
All data were examined for normal distribution and expressed as the mean ± standard deviation (s.d.). Statistical analysis was performed by SPSS 24.0 software (IBM Corp., Armonk, NY, USA). Distribution of variables was evaluated using the Kolmogorov–Smirnov test. The paired t-test was used to compare SWQ values between the left side and the right side penile CC of each rabbit. The t-test was performed on the remaining data to compare differences between the hyperlipidemia group and control group rabbits. A value of P < 0.05 was considered statistically significant.
RESULTS
Serum lipid profiles
The baseline values of FPG, lipid profile, and weight were equivalent in the two groups. However, after 10 weeks, the levels of Tc, Tg, and LDL in the high-cholesterol diet fed rabbits were markedly increased compared with levels from the normal diet rabbits (Table 1).
Table 1.
Weight, fasting plasma glucose, serum total cholesterol, triglyceride, and low-density-lipoprotein cholesterol in hyperlipidemia and control group rabbits (mean±standard deviation)
| Group | Weight (g) | FPG (mmol l−1) | Tc (mmol l−1) | Tg (mmol l−1) | LDL (mmol l−1) |
|---|---|---|---|---|---|
| CO (n=6) | |||||
| Baseline | 1853.33±54.19 | 5.94±0.84 | 1.55±0.67 | 1.22±0.23 | 0.89±0.55 |
| 10th week | 3052.17±115.18* | 5.39±0.92 | 1.12±0.27 | 1.13±0.22 | 0.60±0.24 |
| HL (n=6) | |||||
| Baseline | 1862.50±37.25 | 4.49±1.68 | 1.17±0.25 | 1.28±0.61 | 0.62±0.15 |
| 10th week | 2974.83±144.53* | 4.70±1.46 | 33.51±6.17*# | 4.26±2.87*# | 13.51±4.97*# |
*P<0.01, baseline versus 10th week of diet for the same group. #P<0.01, HL versus CO group at 10th week of diet. FPG: fasting plasma glucose; Tc: total cholesterol; Tg: triglyceride; LDL: low-density-lipoprotein; HL: hyperlipidemia; CO: control
2-D SWE scanning
Clear 2-D SWE images of penile transverse sections were obtained using the 2-D SWE scanning technique. The imaging box covered with color displayed the penile CC structures with a clear boundary, complete capsule, and homogeneous hypoechoic interior. When the SWQ values were calculated, a paired t- test showed no difference between the left and right side of the penile CC (SWQ-L−SWQ-R: −1.33 ± 0.87 kPa, P = 0.154, n = 12). The mean SWQ values [(SWQ-L+SWQ-R)/2] from hyperlipidemia group were significantly higher than those of the control group (24.53 ± 2.64 kPa vs 9.97 ± 0.54 kPa, P < 0.01) (Figure 1).
Figure 1.
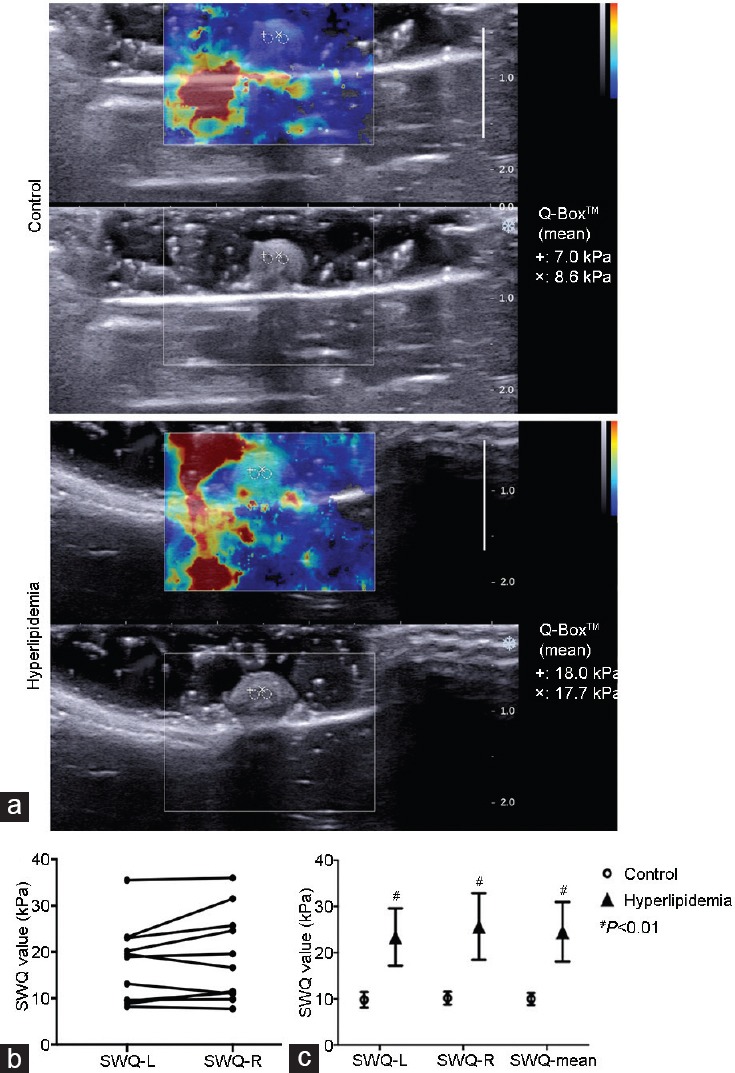
2-D SWE images of the penile CC. (a) The 2-D image displayed the transverse section with a clear boundary, complete capsule and homogeneous hypo-echoic interior. (b) SWQ values were calculated. Paired t-test showed no difference between the left (SWQ-L) and the right side (SWQ-R) of penile CC (n = 12). (c) SWQ values of hyperlipidemia group were significantly higher than those of control group (n = 6 in each group). #P < 0.01, hyperlipidemia group versus control group. Q-Box™: measurement results of SWQ; 2-D SWE: two-dimensional shear wave elastography; CC: corpus cavernousum; SWQ: shear wave elastic quantitative.
Erectile function evaluation
Figure 2 illustrates the differences in erectile function between the two groups. After PAP injection, the ICP in the control group was increased from the baseline and maintained at a high plateau. On the contrary, the ICP of hyperlipidemia group was increased at first but decreased quickly and dramatically later. The mean ΔICP/MAP of hyperlipidemia group was remarkably lower than the control group (0.17 ± 0.02 vs 0.40 ± 0.04, P < 0.01) (Figure 2).
Figure 2.
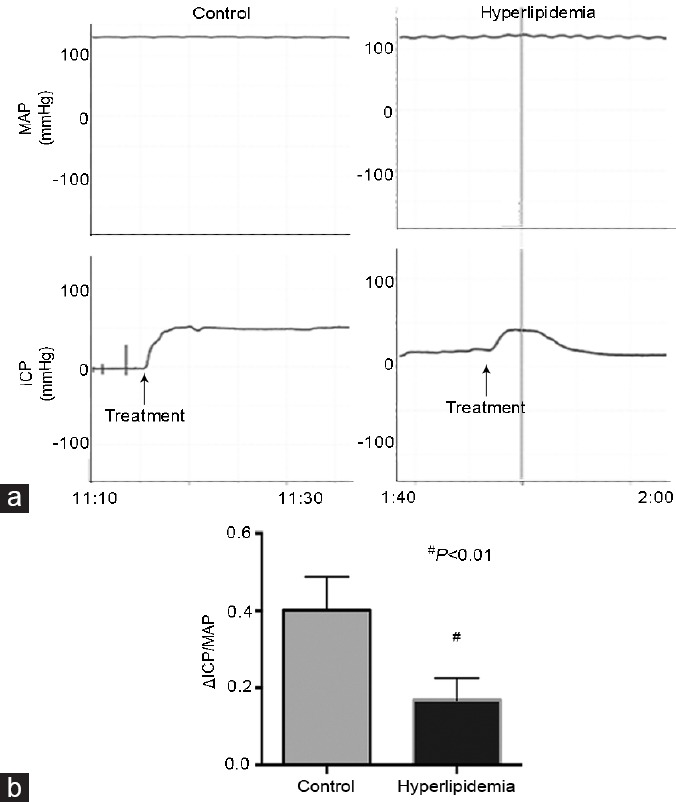
Evaluation of erectile function induced by ICI. (a) Representative of ICP and MAP tracings were obtained for 10 min at plateau in control (n = 6) and hyperlipidemia (n = 6) group rabbits. (b) The ΔICP/MAP was calculated for each group. #P < 0.01, hyperlipidemia group versus control group. ICI: intracavernous injection; ICP: intracavernous pressure; MAP: mean arterial pressure.
Histological examination
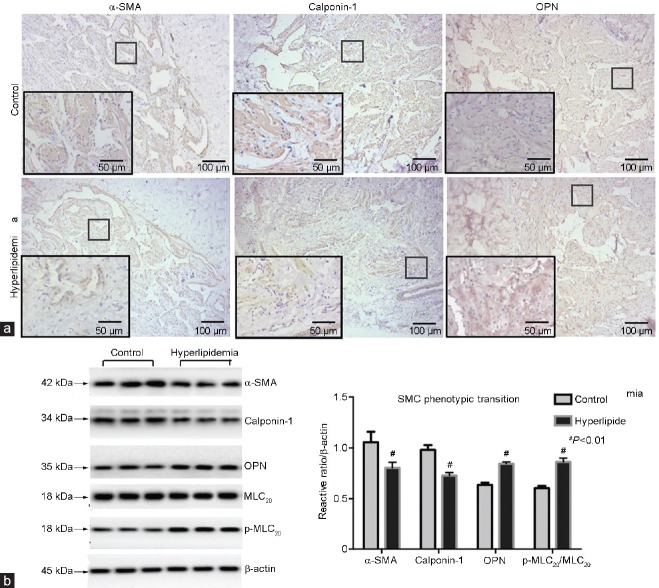
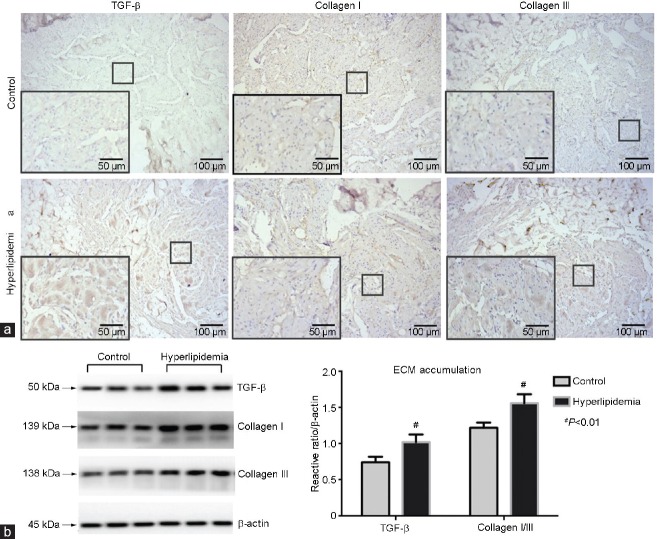
The H and E staining showed the structures involved in transverse sections of penile CC, which was cut at the borderline between the skin and hair. Compared with the control group, the sinusoid lacunae were larger, and the arrangements of SMCs and fibrocytes were looser in the hyperlipidemia group. Numerous empty cellular structures (probably adipocytes) were observed under the tunica albuginea in the hyperlipidemia group rabbits (Figure 3). Photomicrographs of corpus cavernosum smooth muscle (CCSM) cells stained by IHC showed SMC phenotypic transition and ECM accumulation. Cavernous tissues from hyperlipidemic rabbits had reduced staining for α-SMA and calponin-1 compared with controls. On the contrary, the cavernous tissues showed stronger staining for collagen I, collagen III, OPN, and TGF-β, in endothelial cells and/or SMCs of the vessels and sinusoid lacunar spaces found in the hyperlipidemia group compared with the control group (Figure 4 and 5).
Figure 3.
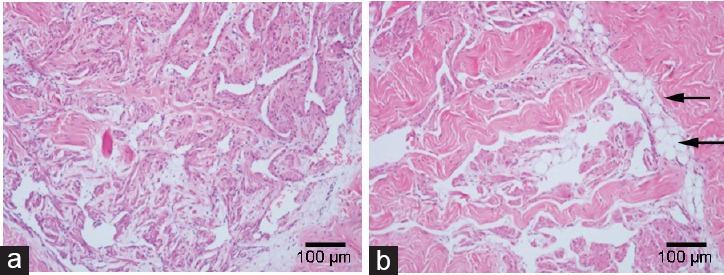
The H and E staining of penile corpus cavernousum. Transverse sections of penile tissues at the borderline between skin and hair was fixed in 10% formalin buffer solution, embedded in paraffin, sectioned (6 μm) and stained with H and E. Compared with (a) control rabbits, the sinusoid lacunae of (b) hyperlipidemic rabbits were larger, and the arrangements of smooth muscle cells and fibrocytes were looser. Numerous empty cellular structures, probably adipocytes (arrow) were seen under the tunica albuginea in hyperlipidemia samples. Magnification ×100; scale bars = 100 μm.
Figure 4.
(a) Immunohistochemical staining of penile cavernous tissues for α-SMA, calponin-1 and OPN in control and hyperlipidemia groups (magnification ×100, scale bars = 100 μm; ×200, scale bars = 50 μm). (b) Western blot analysis showed phenotypic transition in SMCs. Lanes 1–3: control; lanes 4–6: hyperlipidemia. Reduced expression of α-SMA and calponin-1 was demonstrated in hyperlipidemia samples. On the contrary, OPN and p-MLC20 were overexpressed in hyperlipidemia compared with control samples (n = 6 per group). #P < 0.01, hyperlipidemia group versus control group. α-SMA: smooth muscle α-actin; OPN: osteopontin; SMC: smooth muscle cell; p-MLC20: phospho-myosin light chain20.
Figure 5.
(a) Immunohistochemical staining of penile cavernous tissues for collagen I, collagen III and TGF-β, from control and hyperlipidemia group rabbits (magnification ×100, scale bars = 100 μm; ×200, scale bars = 50 μm). (b) Western blot analysis showed ECM in the CCSM cells. Lanes 1–3: control; lanes 4–6: hyperlipidemia. Collagen I/III and TGF-β were over expressed in hyperlipidemia compared with control (n = 6 per group). #P < 0.01, hyperlipidemia group versus control group. TGF-β: transforming growth factor-beta; CCSM: corpus cavernosum smooth muscle; ECM: extracellular matrix.
Western blot assessment
Western blot examined the SMC phenotypic transition and ECM accumulation. Similar to the IHC results, the phenotypic transition of CCSM cells was found in the hyperlipidemia group. Reduced α-SMA and calponin-1 and overexpression of OPN and p-MLC20/MLC20 were observed in the hyperlipidemia group compared with the control group (α-SMA/β-actin: 0.80 ± 0.05 vs 1.06 ± 0.10, P < 0.01; calponin-1/β-actin: 0.73 ± 0.03 vs 0.98 ± 0.04, P < 0.01; p-MLC20/MLC20: 0.86 ± 0.04 vs 0.61 ± 0.02, P < 0.01; OPN/β-actin: 0.84 ± 0.02 vs 0.64 ± 0.02, P < 0.01; Figure 4). Furthermore, ECM accumulation was shown by overexpression of TGF-β and collagen I/III ratio in the hyperlipidemia group compared with the control group (TGF-β/β-actin: 1.02 ± 0.11 vs 0.74 ± 0.08, P < 0.01; collagen I/III: 1.56 ± 0.12 vs 1.22 ± 0.74, P < 0.01; Figure 5).
DISCUSSION
The present study revealed that the SWQ values of penile CC tissue were very different between hyperlipidemic and normal diet fed rabbits. After 10 weeks of cholesterol feeding, the SWQ values obtained by 2-D SWE scanning were significantly higher compared with those of the control group. Shear-wave imaging is currently the most approved state-of-the-art ultrasound scanner by the USA FDA for diagnostic imaging of the musculoskeletal system.19 Shear waves are generated using acoustic radiation force from a linear ultrasound array, which by itself provides local stress and generates local displacement in the tissue. On the ultrasound screen, quantitative shear modulus maps are represented in a color-coded elastogram that display the shear-wave velocities in ms−1 or tissue elasticity in kPa. Shear waves propagate faster in stiffer and contracted tissues, allowing quantification of mechanical and elastic tissue properties. This technique can recognize various musculoskeletal soft-tissue injuries and diseases.19
Our results suggested that cholesterol feeding had induced the penile CC lesions, including SMC phenotypic transition and/or structural alterations. These changes in SMCs elasticity, and/or the SMC/collagen content may lead to different shear wave responses, which could be easily recognized by the ultrasound scanners. Currently, the 2-D SWE technique is widely used in diagnosing liver fibrosis, focal breast lesions, thyroid nodules, focal renal masses, lymph nodes, and grading renal fibrosis with good sensitivity and specificity, as well as transrectal ultrasound (TRUS)-guided prostate biopsies.9,19 In the field of andrology, Riversi et al.20 and Richards et al.21 studied its application in the evaluation of Peyronie's disease, and concluded that lesions in the tunica albuginea could be recognized by higher stiffness compared with the surrounding tissues by real-time elastosonography, no matter if the plaque existed or not. In our previous research of the rat penis, we found that 2-D SWE values could differentiate the penile CC in aged compared with sexually immature rats.10,11,12 The under-developed penis of younger rats showed a higher density of collagen III by IHC and higher SWQ values compared with the developed penis of aged rats.10,11 We also found that the SWQ values were negatively correlated with the content of SMCs.12 Thus, we concluded that the 2-D SWE technique was useful in recognizing differences in tissue stiffness because of the altered penile structures. Our clinical study also found a negative correlation between the SWQ values and age in 40 men ranging from 19 to 81 years of age.7 However, Inci et al.7 had drawn a different conclusion, finding a positive correlation between SWQ values and age. This difference may be due to small sample size, and most probably, the erectile function may not be well characterized in patients of the earlier study (sexually healthy men and those with ED were both included in the study). Therefore, we designed our study to investigate if the 2-D SWE technique could distinguish ED penile CC lesions from normal tissue, and our results are promising.
Hyperlipidemia is an important risk factor of ED.13 Epidemiological studies showed a 42.4% comorbidity in ED patients.22,23 Circulating levels of high-density lipoprotein (HDL) and LDL, and Tc/HDL ratios were found to be good predictors of ED.24 Hyperlipidemia animal models could also induce ED;15,25,26,27 however, the mechanism remains unclear. It is well accepted that endothelial dysfunction and nitric oxide (NO) suppression plays an important role in the impairment of CCSM relaxation.13,16 However, the pathological changes of penile CC remain controversial. Li et al.15 revealed that the effect of hyperlipidemia on corporal fibrosis includes SMC apoptosis followed by collagen replacement. However, Qiu et al.27 demonstrated hyperplasia of CCSM cells with disordered arrangements that restrict relaxation. In our study, we used cholesterol-fed rabbits as the experimental model. After 10 weeks of the high-cholesterol diet, hyperlipidemia models were successfully established, and erectile function had markedly degenerated. Analyses by IHC and western blot revealed the phenotypic transition of CCSM cells. Reduced expression of α-SMA and calponin-1 proteins and overexpression of OPN and p-MLC20 proteins indicated a shift from a contractile to synthetic phenotype. Although no significant difference in collagen and SMC content was observed by H and E staining, ECM accumulation was determined by the increased expression of collagen I/III and TGF-β. We propose this finding indicates the early stage of penile CC lesions, and it may lead to fibrosis with the development of ECM accumulation and an irreversible penile CC condition.
The limitation of our research is that the rabbit's penis is too small to collect data from multiple positions for 2-D SWE scanning. To solve this problem, we selected the same penile transverse section using the skin and hair borderline to minimize sampling error among different rabbits. The 2-D imaging was able to display a clear CC structure so that measurements were able to avoid the tunica albuginea and fat or other tissues. This technique is more noninvasive and convenient compared with the biopsy method. Furthermore, this technique repeatedly selected the equivalent sample in the interested area and calculated the mean value to make the results more representative. In theory, 2-D SWE scanning is dependent on tissue anisotropy. However, our previous studies found no differences between transverse and longitudinal scanning.7,10,11,12 Therefore, we used unified transverse sections, so all rabbits were examined using a single criterion. Other limitations were the small sample size, and no protein agonists were used to investigate the effect that ameliorates the penile CC lesions. Further studies should address these problems and clarify the relationship between SWQ values and penile CC lesions.
CONCLUSION
Hyperlipidemia may result in ECM accumulation accompanied with SMC phenotypic transition in penile CC, which may impair tissue compliance and eventually lead to ED. Such pathological changes may give rise to variations in a certain aspect of SWQ values. Therefore, 2-D SWE may distinguish penile CC lesions that are secondary to hyperlipidemia, compared with normal tissue, by quantifying SWQ values. In conclusion, 2-D SWE scanning is a novel, convenient, noninvasive and effective approach that we expect will be applied as a complementary approach to current specialized testing and may allow detection of penile CC lesions to guide ED management in the near future.
AUTHOR CONTRIBUTIONS
JLH and JFX conceived of the study and designed the protocol. JLH carried out the histological examination and protein analysis, performed data and statistical analysis, and drafted the manuscript. HXC and HRC established the animal model establishment and investigated the ICP. YW, XWS and ZL participated in the study design and coordination and helped draft the manuscript. JFX conducted the 2-D SWE scanning and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 81401195) and Nature Science Foundation of Shanghai (Grant No. 17ZR1422400).
REFERENCES
- 1.McCabe MP, Althof SE. A systematic review of the psychosocial outcomes associated with erectile dysfunction: does the impact of erectile dysfunction extend beyond a man's inability to have sex? J Sex Med. 2014;11:347–63. doi: 10.1111/jsm.12374. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Fan J, Huang G, Zhu X, Tian Y, et al. Meta-analysis of prevalence of erectile dysfunction in Mainland China: evidence based on epidemiological surveys. Sex Med. 2017;5:e19–30. doi: 10.1016/j.esxm.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–35. doi: 10.2164/jandrol.109.008730. [DOI] [PubMed] [Google Scholar]
- 4.Wei AY, He SH, Zhao JF, Liu Y, Hu YW, et al. Characterization of corpus cavernosum smooth muscle cell phenotype in diabetic rats with erectile dysfunction. Int J Impot Res. 2012;24:196–201. doi: 10.1038/ijir.2012.16. [DOI] [PubMed] [Google Scholar]
- 5.Lv B, Zhao J, Yang F, Huang X, Chen G, et al. Phenotypic transition of corpus cavernosum smooth muscle cells subjected to hypoxia. Cell Tissue Res. 2014;357:823–33. doi: 10.1007/s00441-014-1902-0. [DOI] [PubMed] [Google Scholar]
- 6.Wein AJ. Campbell-Walsh Urology. 11th ed. Philadelphia: Elsevier Publishers; 2015. pp. 650–4. [Google Scholar]
- 7.Zhang JJ, Qiao XH, Gao F, Li F, Bai M, et al. A new method of measuring the stiffness of corpus cavernosum penis with ShearWave™ elastography. Br J Radiol. 2015;88:20140671–7. doi: 10.1259/bjr.20140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inci E, Turkay R, Nalbant MO, Yenice MG, Tugcu V. The value of shear wave elastography in the quantification of corpus cavernosum penis rigidity and its alteration with age. Eur J Radiol. 2017;89:106–10. doi: 10.1016/j.ejrad.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: review of techniques and clinical applications. Theranostics. 2017;7:1303–29. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JJ, Qiao XH, Gao F, Bai M, Li F, et al. Smooth muscle cells of penis in the rat: noninvasive quantification with shear wave elastography. Biomed Res Int. 2015;2015:595742. doi: 10.1155/2015/595742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao XH, Zhang JJ, Gao F, Li F, Liu Y, et al. An experimental study: evaluating the tissue structure of penis with 2D-ShearWave™ elastography. Int J Impot Res. 2017;29:12–6. doi: 10.1038/ijir.2016.37. [DOI] [PubMed] [Google Scholar]
- 12.Qiao XH, Zhang JJ, Gao F, Li F, Bai M, et al. An experimental study: quantitatively evaluating the change of the content of collagen fibres in penis with two-dimensional ShearWave ™Elastography. Andrologia. 2017;49:e12653–5. doi: 10.1111/and.12653. [DOI] [PubMed] [Google Scholar]
- 13.Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5:1066–78. doi: 10.1111/j.1743-6109.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 14.Henry GD, Byrne R, Hunyh TT, Abraham V, Annex BH, et al. Intracavernosal injections of vascular endothelial growth factor protects endothelial dependent corpora cavernosal smooth muscle relaxation in the hypercholesterolemic rabbit: a preliminary study. Int J Impot Res. 2000;12:334–9. doi: 10.1038/sj.ijir.3900621. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Cui K, Wang T, Wang S, Li X, et al. Hyperlipidemia impairs erectile function in rats by causing cavernosal fibrosis. Andrologia. 2017;49:e12693. doi: 10.1111/and.12693. [DOI] [PubMed] [Google Scholar]
- 16.Xie D, Kontos CD, Donatucci CF, Annex BH. Cholesterol feeding reduces vascular endothelial growth factor signaling in rabbit corporal tissues. J Sex Med. 2005;2:634–40. doi: 10.1111/j.1743-6109.2005.00111.x. [DOI] [PubMed] [Google Scholar]
- 17.Musicki B, Liu T, Lagoda GA, Strong TD, Senzen SF, et al. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med. 2010;7:3023–32. doi: 10.1111/j.1743-6109.2010.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YM, Lin JS. The rabbit as an intracavernous injection study model. Urol Res. 1996;24:27–32. doi: 10.1007/BF00296730. [DOI] [PubMed] [Google Scholar]
- 19.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiol Graphics. 2017;37:855–70. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riversi V, Tallis V, Trovatelli S, Belba A, Volterrani L, et al. Realtime-elastosonography of the penis in patients with Peyronie's disease. Arch Ital Urol Androl. 2012;84:174–7. [PubMed] [Google Scholar]
- 21.Richards G, Goldenberg E, Pek H, Gilbert BR. Penile sonoelastography for the localization of a non-palpable, non-sonographically visualized lesion in a patient with penile curvature from Peyronie's disease. J Sex Med. 2014;11:516–20. doi: 10.1111/jsm.12396. [DOI] [PubMed] [Google Scholar]
- 22.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Wei M, Macera CA, Davis DR, Hornung CA, Nankin HR, et al. Total cholesterol and high density lipoprotein cholesterol as important predictors of erectile dysfunction. Am J Epidemiol. 1994;140:930–7. doi: 10.1093/oxfordjournals.aje.a117181. [DOI] [PubMed] [Google Scholar]
- 25.Xie D, Odronic SI, Wu F, Pippen AM, Donatucci CF, et al. A mouse model of hypercholesterolemia-induced erectile dysfunction. J Sex Med. 2007;4:898–907. doi: 10.1111/j.1743-6109.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 26.Behr-Roussel D, Darblade B, Oudot A, Compgnies S, Bernabé J, et al. Erectile dysfunction in hypercholesterolemic atherosclerotic apolipoprotein e knockout mice. J Sexl Med. 2011;3:596–603. doi: 10.1111/j.1743-6109.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 27.Qiu X, Fandel TM, Lin G, Huang YC, Dai YT, et al. Cavernous smooth muscle hyperplasia in a rat model of hyperlipidaemia-associated erectile dysfunction. BJU Int. 2011;108:1866–72. doi: 10.1111/j.1464-410X.2011.10162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]