Abstract
The purpose of this study was to determine the diagnostic accuracy of serum inhibin B (INHB) as a predictor of the retrieval outcome of testicular haploid gametes (spermatids and testicular spermatozoa) in nonobstructive azoospermic men. Serum hormone levels, testicular volume, and histological evaluation were performed in 403 Chinese nonobstructive azoospermic men. Testicular haploid gamete was successfully retrieved in 213 of 403 patients (52.85%). The haploid gamete group always had higher INHB levels than the non-haploid gamete group. According to the receiver operating characteristic (ROC) curve analysis, INHB was a good predictor of testicular haploid gamete retrieval outcome in all patients (sensitivity: 77.93% and specificity: 91.58%) and patients with normal follicle-stimulating hormone (FSH; sensitivity: 88.52% and specificity: 70.83%). The area under the ROC curve (AUC) of INHB was similar to that of FSH in all patients or patients with normal FSH. In patients with elevated FSH, INHB was superior to FSH in predicting the presence of haploid gamete (AUC: 0.73 vs 0.55, P < 0.05), with a sensitivity of 60.00% and a specificity of 80.28%. It concluded that serum INHB as an effective marker for spermatogenesis was a significant predictor of testicular haploid gamete retrieval outcomes in nonobstructive azoospermic men. Especially, INHB is superior to FSH in predicting the presence of haploid gamete in the patients with elevated FSH.
Keywords: azoospermia, biomarker, follicle-stimulating hormone, inhibin B, testicular sperm extraction
INTRODUCTION
The incidence of male infertility is increasing by year, of which azoospermia patients are accounted for about 10%–20%.1 There are two types of azoospermia: obstructive azoospermia (OA) and nonobstructive azoospermia (NOA). Around 60% of these cases are due to NOA.2 Since the introduction of intracytoplasmic sperm injection (ICSI) into clinical practice, testicular spermatozoa have made fatherhood a possibility for patients with NOA.3,4,5 The sperm retrieval rate of testicular sperm extraction (TESE) or microdissection testicular sperm extraction (micro-TESE) in NOA patients is only about 50%.6 However, there are no precise clinical biomarkers that can predict the haploid gamete retrieval rate. Finding such an indicator is particularly important, which to some extent can reduce the risk of testicular dysfunction after testicular surgery. In the current clinical practice, serum follicle-stimulating hormone (FSH), testosterone (T), and testicular volume have been commonly used as indicators of sperm retrieval rate in preoperative evaluation, but the clinical value of these parameters is limited.7,8,9,10 FSH levels are easily influenced by gonadotropin-releasing hormone (GnRH), estradiol, and T. Testosterone and testicular volume cannot reflect the presence of focal spermatogenesis.7,11 Even if the testicular volume is <5 ml, spermatozoa can also be successfully retrieved from the testis, and the measurement of testicular volume is easily affected by subjective factors.
Serum inhibin B (INHB) is a heterodimeric glycoprotein consisting of an α-subunit and a βB-subunit, and it is possibly a joint product of Sertoli cells and germ cells.12 The level of INHB is negatively correlated with gonadotropin and FSH in feedback regulation.12,13,14 Compared with FSH, INHB should be a more direct and more sensitive predictor of spermatogenesis.15,16,17 However, it is still controversial whether serum INHB can predict sperm retrieval outcomes of TESE or micro-TESE in NOA and whether INHB is superior to FSH in predicting the presence of spermatozoa.
In patients who cannot obtain spermatozoa for ICSI, round spermatid injection (ROSI) or elongated spermatid injection (ELSI) after cell cultured is another choice for getting blood descendants.18,19,20,21,22,23 However, the testicular haploid gamete (including spermatids and spermatozoa) retrieval rate is rarely reported. In addition, little information is available on predictive factors of successful haploid gamete retrieval in nonobstructive azoospermic men. In this study, we collected the clinical data of 403 patients with NOA in Reproductive Hospital Affiliated to Shandong University (Jinan, China), analyzed the predictive value of serum INHB on testicular haploid gamete retrieval outcome, and compared it with other parameters such as serum FSH, luteinizing hormone (LH), T, and testicular volume.
PATIENTS AND METHODS
Patients
Data from a total of 403 Chinese nonobstructive azoospermic men who underwent TESE (n = 312) or micro-TESE (n = 91) were collected from October 2015 to April 2017 in Reproductive Hospital Affiliated to Shandong University. The World Health Organization (WHO) manual for standardized investigation, diagnosis, and management of the infertile male was followed in this study.24 The azoospermic men were confirmed by at least three semen analyses. All patients underwent detailed medical history acquisition, physical examination, chromosome karyotyping, and azoospermia factor (AZF) microdeletion screening. Testicular volume was measured by ultrasound. Patients with obstructive factors who have sperm retrieved through epididymal puncture were excluded from this study. Patients with chromosomal abnormalities were also not included in this study. This study was approved by the Institutional Review Board (IRB) of Shandong University (Jinan, China). Written informed consent was obtained from the patients at the time of presentation for TESE or micro-TESE.
The patients were categorized into different groups depending on their FSH levels or the histological evaluation of testicular tissue. (1) Patients were categorized into two groups depending on the histological evaluation of testicular tissues (Table 1): haploid gamete group (n = 213) and non-haploid gamete group (n = 190); (2) patients were categorized into four groups depending on their FSH levels and the histological evaluation of testicular tissues (Table 2): Group I (normal FSH, haploid gamete), Group II (normal FSH, non-haploid gamete), Group III (elevated FSH, haploid gamete), and Group IV (elevated FSH, non-haploid gamete); (3) patients were categorized into two groups depending on the FSH levels (Supplementary Table 1): elevated FSH group (n = 172) and normal FSH group (n = 231); (4) patients were categorized into two groups depending on testicular histological results (Supplementary Table 2): sperm group (n = 196) and azoospermia group (n = 207); and (5) patients were categorized into two groups depending on the type of surgical procedure (Supplementary Table 3): TESE group (n = 312) and micro-TESE group (n = 91).
Table 1.
Age, reproductive hormones, and testicular volume differences between groups, which were grouped according to testicular histological results, with Mann–Whitney U test
| Variable | Haploid gamete group (n=213) | Non-haploid gamete group (n=190) | P |
|---|---|---|---|
| Age (year) | 29.91 (26.00, 32.00) | 28.66 (26.00, 31.00) | 0.052 |
| INHB (pg ml−1) | 112.53 (79.01, 146.07) | 28.96 (11.00, 30.62) | <0.001 |
| FSH (IU l−1) | 7.06 (3.56, 7.49) | 18.02 (12.40, 21.67) | <0.001 |
| LH (IU l−1) | 7.43 (3.29, 6.79) | 13.56 (6.03, 11.30) | <0.001 |
| T (ng dl−1) | 500.67 (353.70, 638.00) | 364.88 (260.00, 446.25) | <0.001 |
| Testicular volume (ml) | 13.70 (12.00, 15.00) | 10.83 (8.49, 12.30) | <0.001 |
Data were shown as median (quartile range). INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Table 2.
Age, reproductive hormones, and testicular volume differences between groups, which were grouped according to follicle-stimulating hormone level and testicular histopathological results
| Variable | Group I (n=183) | Group II (n=48) | aP | Group III (n=30) | Group IV (n=142) | bP |
|---|---|---|---|---|---|---|
| Age (year) | 30.02 (26.00, 32.00) | 27.85 (26.00, 29.00) | 0.031 | 29.20 (27.00, 31.00) | 28.93 (26.00, 31.00) | 0.610 |
| INHB (pg ml−1) | 125.90 (95.79, 154.33) | 61.05 (13.30, 97.64) | <0.001 | 30.97 (14.13, 44.88) | 18.11 (10.50, 17.67) | <0.001 |
| FSH (IU l−1) | 5.00 (3.38, 6.25) | 8.34 (5.16, 11.35) | <0.001 | 19.60 (14.87, 22.68) | 21.29 (15.92, 23.63) | 0.365 |
| LH (IU l−1) | 7.19 (3.25, 6.24) | 5.73 (3.19, 7.16) | 0.001 | 8.89 (6.97, 9.95) | 16.20 (7.62, 11.90) | 0.058 |
| T (ng dl−1) | 514.68 (380.30, 646.00) | 402.26 (228.80, 491.35) | <0.001 | 415.17 (275.00, 518.93) | 352.25 (251.45, 437.50) | 0.157 |
| Testicular volume (ml) | 14.17 (12.00, 15.10) | 12.51 (9.10, 13.87) | <0.001 | 10.78 (8.50, 12.38) | 10.27 (8.10, 12.00) | 0.255 |
Data were shown as median (quartile range). Group I: normal FSH, haploid gamete; Group II: normal FSH, non-haploid gamete; Group III: elevated FSH, haploid gamete; and Group IV: elevated FSH, non-haploid gamete. aGroup I versus Group II, bGroup III versus Group IV, with Mann–Whitney U test. INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Supplementary Table 1.
Age, reproductive hormones, and testicular volume differences between groups, which were grouped according to follicle-stimulating hormone level, with Mann–Whitney U-test
| Normal FSH group (n=231) | Elevated FSH group (n=172) | P | |
|---|---|---|---|
| Age (year) | 28.00 (26.00, 31.00) | 28.00 (26.00, 31.00) | 0.552 |
| INHB (pg ml−1) | 111.12 (79.84, 145.03) | 13.60 (11.00, 21.85) | <0.001 |
| LH (IU l−1) | 4.82 (3.26, 6.32) | 9.25 (7.25, 11.50) | <0.001 |
| T (ng dl−1) | 466.00 (351.00, 634.70) | 331.85 (255.75, 444.80) | <0.001 |
| Testicular volume (ml) | 13.20 (12.00, 15.00) | 10.00 (8.23, 12.00) | <0.001 |
Data were shown as median (upper quartile, lower quartile). INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Supplementary Table 2.
Age, reproductive hormones, and testicular volume differences between groups, which were grouped according to testicular histological results, with Mann–Whitney U-test
| Sperm group (n=196) | Azoospermia group (n=207) | P | |
|---|---|---|---|
| Age (year) | 28.00 (26.00, 32.00) | 28.00 (26.00, 31.00) | 0.199 |
| INHB (pg ml−1) | 111.86 (84.83, 152.66) | 14.30 (11.00, 37.34) | <0.001 |
| FSH (IU l−1) | 5.06 (3.53, 6.85) | 16.34 (11.91, 21.57) | <0.001 |
| LH (IU l−1) | 4.83 (3.29, 6.59) | 8.52 (6.00, 11.30) | <0.001 |
| T (ng dl−1) | 483.20 (354.50, 639.00) | 349.00 (262.00, 453.60) | <0.001 |
| Testicular volume (ml) | 13.20 (12.00, 15.00) | 10.30 (8.60, 12.60) | <0.001 |
Data were shown as median (upper quartile, lower quartile). INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Supplementary Table 3.
Age, reproductive hormones, and testicular volume differences between groups, which were grouped according to the type of surgical procedure, with Mann–Whitney U-test
| TESE (n=312) | Micro-TESE (n=91) | P | |
|---|---|---|---|
| Age (year) | 28.00 (26.00, 31.00) | 29.00 (27.00, 33.00) | 0.043 |
| INHB (pg ml−1) | 55.54 (12.32, 120.53) | 38.84 (14.68, 109.89) | 0.938 |
| FSH (IU l−1) | 9.42 (4.76, 17.77) | 9.41 (4.74, 17.06) | 0.998 |
| LH (IU l−1) | 6.31 (4.16, 8.91) | 6.57 (4.35, 9.37) | 0.782 |
| T (ng dl−1) | 426.00 (306.00, 563.00) | 362.00 (275.00, 509.28) | 0.011 |
| Testicular volume (ml) | 12.00 (10.00, 13.96) | 13.70 (10.99, 15.90) | <0.001 |
Data were shown as median (upper quartile, lower quartile). INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone; TESE: testicular sperm extraction
Physical and laboratory examination
For semen collection and analysis, semen samples were collected by masturbation after 2–7 days of sexual abstinence, and manual semen analysis was performed within 30 min according to the WHO guidelines.24 Each patient had his semen analyzed at least three times.
To determine the reproductive hormones, venous blood samples were obtained from the antecubital veins between 8:00 a.m. and 10:00 a.m. Serum samples were separated and assayed for INHB, FSH, LH, and T. The serum INHB concentration was measured by the DS2 automatic immunoassay analyzer (DYNEX Technologies Inc., Chantilly, VA, USA), using the INHB enzyme-linked immunosorbent assay (ELISA) kit (Guangzhou Kang Run Biotech Co., Ltd., Guangdong, China). The detection range of the assay was 10–1500 pg ml−1, and the intra- and inter-assay coefficient of variation was ≤10% and ≤15%, respectively. The minimum detectable concentration for INHB was 10 pg ml−1. The serum FSH, LH, and T concentrations were measured by the Cobas e601 automatic electrochemical luminescence immunoassay system (F. Hoffmann-La Roche Ltd., Basel, Switzerland) according to the manufacturer's instructions. The normal ranges established in our laboratory were as follows: INHB: 54–235 pg ml−1, FSH: 1.5–12.4 IU l−1, LH: 1.7–8.6 IU l−1, and T: 249–836 ng dl−1.
During testicular assessment, ultrasound measurement was repeated three times for each testis and the average volume was taken. Testicular tissue was sent to the pathology laboratory for the histological evaluation after TESE or micro-TESE.
Statistical analyses
Data were analyzed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version 15.2 (MEDCALC Inc., Ostend, Belgium), and expressed as median and quartile range. Differences of parameters between groups were compared using the Mann–Whitney U test. The receiver operating characteristic (ROC) curve was used to analyze the predictive value of each parameter for the testicular haploid gamete retrieval outcomes. The area under the ROC curve (AUC) indicated predictive power (low predictive power: 0.50 < AUC ≤ 0.70, medium predictive power: 0.70< AUC ≤0.90, and high predictive power: 0.9< AUC ≤1.0). P < 0.05 indicated statistical significance.
RESULTS
The haploid gamete retrieval rates were 52.85%, 79.22%, and 17.44% in all patients, the normal FSH group, and the elevated FSH group, respectively. All parameters except age had significantly different values in the haploid gamete group and the non-haploid gamete group in all patients (Table 1) or patients with normal FSH (Table 2) (all P ≤ 0.001). In the patients with elevated FSH, only serum INHB was significantly higher in the haploid gamete group than that in the non-haploid gamete group (Table 2; P < 0.001).
To evaluate the predictive values of serum INHB, FSH, LH, T, and testicular volume for the retrieval outcome of testicular haploid gamete, the ROC curve was established on the basis of the histological evaluation of testicular tissue taken to be “gold standard.” The cutoff value corresponding to the maximum Youden index was selected as the best diagnostic threshold. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. In all patients or patients with normal FSH, the AUC of serum INHB was similar to that of FSH (P > 0.05). However, INHB and FSH performed better than T, LH, and testicular volume in predicting the presence of haploid gamete (all P < 0.05). In all patients, the best discriminating cutoff value of INHB for the retrieval outcomes of testicular haploid gamete was 68.53 pg ml−1 (AUC: 0.90, sensitivity: 77.93%, specificity: 91.58%, PPV: 91.21%, and NPV: 78.73%) and that of FSH was 9.00 IU l−1 (AUC: 0.87, PPV: 90.05%, and NPV: 80.66%(Table 3 and Figure 1). In patients with elevated FSH, the predictive value of INHB for the retrieval outcome of testicular haploid gamete was better than that of FSH and other parameters (LH, T, and testicular volume) (P < 0.05), and the best discriminating cutoff value of INHB was 20.38 pg ml−1 (AUC: 0.73, sensitivity: 60.00%, and specificity: 80.28%; Table 4). Owing to the low haploid gamete retrieval rate (17.44%) in these patients, PPV was only 39.13% and NPV was 90.48%. In patients with normal FSH, the best discriminating cutoff value of INHB was 75.64 pg ml−1 (AUC: 0.82, sensitivity: 88.52%, specificity: 70.83%, PPV: 92.04%, and NPV: 61.81%) and that of FSH was 7.51 IU l−1 (AUC: 0.79, PPV: 91.01%, and NPV: 60.37%) (Table 5).
Table 3.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of testicular haploid gamete in all patients
| Variable | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| INHB | 0.90 (0.86–0.92)a | >68.53 | 77.93 | 91.58 | 91.21 | 78.73 |
| FSH | 0.87 (0.83–0.90)b | ≤9.00 | 80.75 | 90.00 | 90.05 | 80.66 |
| LH | 0.79 (0.75–0.83)c | ≤7.84 | 86.85 | 60.00 | 70.88 | 80.28 |
| T | 0.71 (0.66–0.75) | >391.30 | 68.08 | 64.74 | 68.40 | 64.41 |
| Testicular volume | 0.76 (0.72–0.80) | >11.9 | 83.10 | 65.79 | 73.14 | 77.64 |
aP<0.05: INHB versus LH, INHB versus T, INHB versus testicular volume; bP<0.05: FSH versus LH, FSH versus T, FSH versus testicular volume; cP<0.05: LH versus T. AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Figure 1.
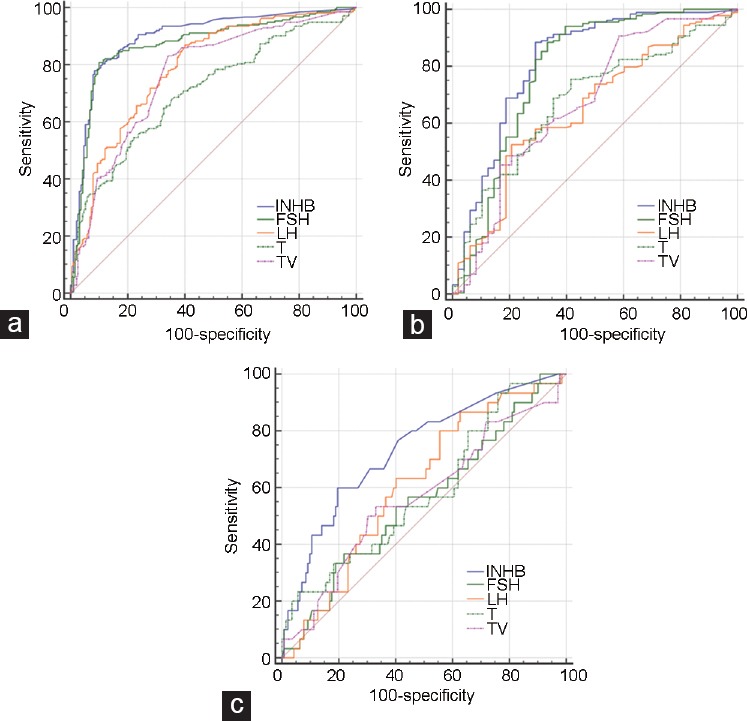
ROC curves of reproductive hormones and testicular volume for the retrieval outcomes of testicular haploid gamete. The ROC curves were used to describe the diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of testicular haploid gamete. In (a) all patients or (b) patients with normal FSH, the area under the ROC curve of INHB was similar to that of FSH (P > 0.05). However, both of them performed better than T, LH, and TV (all P < 0.05). (c) In patients with elevated FSH, the predictive value of INHB for the retrieval outcome of testicular haploid gamete was better than that of FSH and other parameters (LH, T, and TV) (P < 0.05). ROC: receiver operating characteristic; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone; TV: testicular volume.
Table 4.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of testicular haploid gamete in patients with elevated follicle-stimulating hormone
| Variable | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| INHB | 0.73 (0.66–0.80)a | >20.38 | 60.00 | 80.28 | 39.13 | 90.48 |
| FSH | 0.55 (0.48–0.63) | ≤15.45 | 36.67 | 78.17 | 26.19 | 85.39 |
| LH | 0.61 (0.53–0.68) | ≤10.02 | 80.00 | 44.37 | 23.30 | 91.30 |
| T | 0.58 (0.50–0.66) | >563.00 | 23.33 | 94.37 | 46.68 | 85.35 |
| Testicular volume | 0.57 (0.49–0.64) | >10.97 | 53.33 | 66.90 | 25.39 | 87.16 |
aP<0.05: INHB versus FSH, INHB versus T, INHB versus testicular volume. AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Table 5.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of testicular haploid gamete in patients with normal follicle-stimulating hormone
| Variable | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| INHB | 0.82 (0.76–0.87)a | >75.64 | 88.52 | 70.83 | 92.04 | 61.81 |
| FSH | 0.79 (0.73–0.84)b | ≤7.51 | 88.52 | 66.67 | 91.01 | 60.37 |
| LH | 0.65 (0.59–0.71) | ≤4.39 | 52.46 | 79.17 | 90.57 | 30.40 |
| T | 0.69 (0.61–0.74) | >379.00 | 75.41 | 58.33 | 87.34 | 38.36 |
| Testicular volume | 0.67 (0.61–0.73) | >11.35 | 90.71 | 41.67 | 85.57 | 54.06 |
aP<0.05: INHB versus LH, INHB versus T, INHB versus testicular volume; bP<0.05: FSH versus LH, FSH versus testicular volume. AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
In addition, we observed the predictive value of parameters in different types of surgical procedure (TESE and micro-TESE; (Supplementary Table 4 and 5). We also performed the predictive significance of INHB and other parameters on testicular sperm retrieval outcomes (Supplementary Table 6)]. The predictive value of INHB was predominately stable in different situations.
Supplementary Table 4.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of haploid gamete in patients who underwent testicular sperm extraction
| AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| INHB (pg ml−1) | 0.91 (0.87–0.94)a | >75.67 | 80.86 | 93.29 | 92.34 | 82.98 |
| FSH (IU l−1) | 0.89 (0.85–0.93)b | ≤9.19 | 84.57 | 91.28 | 90.65 | 85.54 |
| LH (IU l−1) | 0.80 (0.75–0.84) | ≤7.84 | 90.12 | 61.74 | 70.20 | 86.20 |
| T (ng dl−1) | 0.75 (0.70–0.80) | >458.00 | 61.11 | 79.19 | 74.60 | 67.06 |
| Testicular volume (ml) | 0.77 (0.72–0.82) | >11.90 | 82.10 | 69.80 | 73.11 | 79.59 |
aP<0.05: INHB versus LH, INHB versus T, INHB versus testicular volume; bP<0.05: FSH versus LH, FSH versus T, FSH versus testicular volume. TESE: testicular sperm extraction; AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone
Supplementary Table 5.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of haploid gamete in patients who underwent microdissection testicular sperm extraction
| AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| INHB (pg ml−1) | 0.84 (0.75–0.91)a | >43.40 | 76.47 | 87.80 | 86.24 | 78.86 |
| FSH (IU l−1) | 0.80 (0.70–0.88)b | ≤6.75 | 72.55 | 92.68 | 90.84 | 77.15 |
| LH (IU l−1) | 0.76 (0.66–0.85)c | ≤4.97 | 54.90 | 90.24 | 84.91 | 66.68 |
| T (ng dl−1) | 0.58 (0.47–0.68)d | >399.50 | 49.02 | 70.73 | 62.61 | 58.11 |
| Testicular volume (ml) | 0.74 (0.64–0.83) | >11.00 | 92.16 | 48.78 | 64.28 | 86.15 |
aP<0.05: INHB versus T, INHB versus testicular volume; bP<0.05: FSH versus T; cP<0.05: LH versus T; dP<0.05: T versus testicular volume. micro-TESE: microdissection testicular sperm extraction; AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone; CI: confidence interval
Supplementary Table 6.
Diagnostic accuracy of reproductive hormones and testicular volume for the retrieval outcomes of testicular sperm in all patients
| AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| INHB (pg ml−1) | 0.89 (0.86–0.92)a | >54.20 | 85.20 | 85.02 | 85.05 | 85.17 |
| FSH (IU l−1) | 0.88 (0.84–0.91)b | ≤8.80 | 83.67 | 87.44 | 86.95 | 84.26 |
| LH (IU l−1) | 0.80 (0.76–0.84)c | ≤7.84 | 89.29 | 58.45 | 68.24 | 84.51 |
| T (ng dl−1) | 0.71 (0.66–0.75) | >391.30 | 69.39 | 63.29 | 65.40 | 67.40 |
| Testicular volume (ml) | 0.75 (0.70–0.79) | >11.90 | 83.67 | 62.32 | 68.95 | 79.24 |
aP<0.05: INHB versus LH, INHB versus T, INHB versus testicular volume; bP<0.05: FSH versus LH, FSH versus T, FSH versus testicular volume; cP<0.05: LH versus T. AUC: area under the curve; PPV: positive predictive value; NPV: negative predictive value; INHB: inhibin B; FSH: follicle-stimulating hormone; LH: luteinizing hormone; T: testosterone; CI: confidence interval
DISCUSSION
In this study, our data showed significant differences in the mean levels of INHB and FSH between the haploid gamete group and the non-haploid gamete group in all patients, which was similar to those found in most previous studies.25,26,27,28,29 However, the majority of these studies were to investigate diagnostic accuracy on TESE outcomes. In our study, the successful surgical outcomes included obtaining not only testicular spermatozoa but also testicular spermatids. This is one of the largest differences with other studies.1,2,11,13,15,16,25,26,27,28,29 For this reason, the haploid gamete (including spermatid and testicular spermatozoa) retrieval rate (52.85%) was slightly higher than those in these studies (about 50%). In addition, LH, T, and testicular volume also had statistical difference between groups. It is noteworthy that only serum INHB was significantly higher in the haploid gamete group than that in the non-haploid gamete group (P < 0.001) in patients with elevated FSH. In these patients, the FSH level and other parameters had no statistical difference between groups. This suggests that these parameters (FSH, LH, T, and testicular volume) may not predict the presence of haploid gametes in this special group of patients. The proportion of patients with elevated FSH was 42.68% (172/403) of the total patients, but the haploid gamete retrieval rate was only 17.44%. Therefore, for them, a noninvasive marker, predicting successful haploid gamete retrieval before surgery, would be more urgent.
To further analyze the diagnostic accuracy of each parameter as a predictor of the testicular haploid gamete retrieval outcomes, we regarded the histological results of testicular tissue as the “gold standard” to establish the ROC curve. In total patients or patients with normal FSH, serum INHB all had a good sensitivity and specificity for predicting the testicular haploid gamete retrieval outcome in nonobstructive azoospermic men. Different from Huang's study29 (sensitivity: 83.5%, specificity: 79.1%, and AUC: 0.862), the present study showed a lower sensitivity (77.93%), with a higher specificity (91.58%) and a higher AUC (0.90) in total patients. Ballesca et al.25 demonstrated that the AUC of INHB was 0.98, with the sensitivity of 90% and the specificity of 100%. However, this study included only 17 NOA patients who underwent TESE, which limits the significance of these data. The AUC of serum INHB was similar to that of FSH in total patients or patients with normal FSH, which is in accordance with some previous reports.29,30,31 Serum INHB was not a more sensitive predictor of successful haploid gamete retrieval outcome than FSH in total patients or patients with normal FSH. Although LH, T, and testicular volume had acceptable AUC (0.65–0.79), INHB and FSH were obviously superior to them in predicting the presence of haploid gamete (P < 0.05).
However, in patients with elevated FSH, the AUC of INHB was larger than that of FSH (P < 0.05), and the best discriminating cutoff value of INHB was 20.38 pg ml−1 (AUC: 0.73, sensitivity: 60.00%, and specificity: 80.28%), with a NPV of 90.48% and a PPV of 39.13%. The reason of the low PPV may be that the haploid gamete retrieval rate in patients with elevated FSH was only 17.44%, and most of these patients have a strong intention of having surgery. In these patients, the AUC of FSH, LH, T, and testicular volume was <0.70, which indicated low predictive power. The AUC of INHB was 0.73, which indicated medium predictive power (AUC: 0.70–0.90). It is undeniable that the diagnostic accuracy of serum INHB was also limited in patients with elevated FSH, but INHB, which was better than FSH, was the best biomarker which we can choose. Under this diagnostic threshold (20.38 pg ml−1) of serum INHB, with a high PPV (90.48%), we can effectively exclude patients who are not recommended to undergo surgery and reduce the potential surgical risks.
When we observed the predictive values of parameters in different types of surgical procedure and performed the predictive significance of these parameters on testicular sperm retrieval outcomes, the predictive value of INHB was predominately stable in different situations. In patients who underwent micro-TESE, the diagnostic accuracy of INHB for the retrieval outcome of haploid gamete declined slightly. However, the technique of micro-TESE was only recently employed. When the doctor selected patients for this surgery, it may result in selection bias. Moreover, the proportion of patients who underwent micro-TESE was small.
In 1995, ROSI and ELSI after germ cell culture were reported by Fishel et al.18 and Tesarik et al.,19 respectively. This was another choice for acquiring blood descendants, but owing to the low success rate of ELSI and ROSI in particular, most assisted reproductive medical centers have discontinued the clinical application of these techniques in the mid-1990s. With advances in assisted reproductive and culture in vitro techniques, ROSI and ELSI still have huge potential in clinical applications. In 2002, Sousa et al.22 showed that the fertilization rate by ELSI was 48.4%, and the pregnancy rate of women reached 28.9%. In 2015, Tanaka et al.23 reported the health status of 14 infants born at their center through ROSI between September 2011 and March 2014, and none of these infants showed any physical or psychological problems.
Now, little information is available on predictive factors of successful haploid gamete retrieval in nonobstructive azoospermic men, so we conducted this research. The downside was that the proportion of patients who underwent micro-TESE was small. This is because this technique has been applied at our center just for a short time. Micro-TESE is now one of the more popular sperm retrieval techniques for men with NOA. As the number of patients who undergo micro-TESE increases, we may have new findings in the future.
From the previous studies, researchers need to pay attention to the following points when comparing different studies or conducting new research. First, age affects hormonal levels. INHB shows an inverse U-shaped dependence on age, whereas FSH shows a U-shaped dependence on age (optimum 20–40 years).32 In addition, INHB showed a highly significantly negative correlation with age in patients older than 30 years.32 Second, the time of blood sampling may influence hormonal concentrations. INHB concentrations were higher in samples collected in the morning than those collected in the afternoon.33,34 Jorgensen et al.34 reported that, from 8:00 a.m. to 12:00 a.m., INHB fell to 92%, and in the following 4 h, the hormone level decreased further to 79%. Jorgensen et al.34 also demonstrated that FSH level was not affected by the time of blood sampling, and season was not significantly associated with INHB or FSH level. However, Meeker et al.33 considered that INHB levels were lower in men who had blood samples collected in winter than in men with samples collected in the spring, summer, or fall. Last, researchers should pay attention to whether or not patients with karyotype abnormalities or AZF microdeletions, and patients who received hormonotherapy within 3 months of the prestudy assessments were excluded. Y chromosome microdeletions are the second most common genetic reason for male infertility, and this microdeletion often occurs in the AZF region.35,36 AZF a/b microdeletion often portends the failure of sperm retrieval.37,38,39 Patients with AZF a/b microdeletion are not recommended to undergo surgery. Hormone therapy will affect the basic levels of hormones, influencing the credibility of the study. The above factors will affect the comparability of different studies.
CONCLUSIONS
Serum INHB as an effective marker for spermatogenesis is a significant predictor of testicular haploid gamete retrieval outcome in nonobstructive azoospermic men. Especially, INHB was superior to FSH in predicting the presence of haploid gamete in the patients with elevated FSH.
AUTHOR CONTRIBUTIONS
ZG Zhu and XJX participated in the design of the study, analyzed data, and wrote the manuscript. ZG Zhao participated in its design and coordination and helped to draft the manuscript. XJX modified the paper. QYP and TC participated in data collection and analyzed data. QYP, TJZ, CX, and XJX performed testicular assessment and surgery. JMZ performed the statistical analysis and helped to draft the manuscript. HBZ participated in data collection. WL performed pathological analysis. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We are very grateful to our patients and all participants in the data collection.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Feng K, Zhang CL, Li HS, Guo HB. [Predictive value of reproductive hormone levels and testicular volume for sperm existence in non-obstructive azoospermia patients] J Third Mil Med Univ. 2015;37:69–73. [Article in Chinese] [Google Scholar]
- 2.Cetinkaya M, Onem K, Zorba OU, Ozkara H, Alici B. Evaluation of microdissection testicular sperm extraction results in patients with non-obstructive azoospermia: independent predictive factors and best cutoff values for sperm retrieval. Urol J. 2015;12:2436–43. [PubMed] [Google Scholar]
- 3.Tournaye H, Liu J, Nagy PZ, Camus M, Goossens A, et al. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum Reprod. 1996;11:127–32. doi: 10.1093/oxfordjournals.humrep.a019004. [DOI] [PubMed] [Google Scholar]
- 4.Cha KY, Oum KB, Kim HJ. Approaches for obtaining sperm in patients with male factor infertility. Fertil Steril. 1997;67:985–95. doi: 10.1016/s0015-0282(97)81428-8. [DOI] [PubMed] [Google Scholar]
- 5.Boitrelle F, Robin G, Marcelli F, Albert M, Leroy-Martin B, et al. A predictive score for testicular sperm extraction quality and surgical ICSI outcome in non-obstructive azoospermia: a retrospective study. Hum Reprod. 2011;26:3215–21. doi: 10.1093/humrep/der314. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T. Surgical recovery of sperm in non-obstructive azoospermia. Asian J Androl. 2012;14:109–15. doi: 10.1038/aja.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramasamy R, Padilla WO, Osterberg EC, Srivastava A, Reifsnyder JE, et al. A comparison of models for predicting sperm retrieval before microdissection testicular sperm extraction in men with nonobstructive azoospermia. J Urol. 2013;189:638–42. doi: 10.1016/j.juro.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Aydin T, Sofikerim M, Yucel B, Karadag M, Tokat F. Effects of testicular histopathology on sperm retrieval rates and ICSI results in non-obstructive azoospermia. J Obstet Gynaecol. 2015;35:829–31. doi: 10.3109/01443615.2015.1009879. [DOI] [PubMed] [Google Scholar]
- 9.Khelaia A, Saker Z, Tsintsadze O, Managadze L. Nonobstructive azoospermia, follicle-stimulating hormone as a marker of successful sperm retrieval. Georgian Med News. 2015;249:34–7. [PubMed] [Google Scholar]
- 10.Yang Q, Huang YP, Wang HX, Hu K, Wang YX, et al. Follicle-stimulating hormone as a predictor for sperm retrieval rate in patients with nonobstructive azoospermia: a systematic review and meta-analysis. Asian J Androl. 2015;17:281–4. doi: 10.4103/1008-682X.139259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierik FH, Vreeburg JT, Stijnen T, De Jong FH, Weber RF. Serum inhibin B as a marker of spermatogenesis. J Clin Endocrinol Metab. 1998;83:3110–4. doi: 10.1210/jcem.83.9.5121. [DOI] [PubMed] [Google Scholar]
- 12.Marchetti C, Hamdane M, Mitchell V, Mayo K, Devisme L, et al. Immunolocalization of inhibin and activin alpha and betaB subunits and expression of corresponding messenger RNAs in the human adult testis. Biol Reprod. 2003;68:230–5. doi: 10.1095/biolreprod.102.004424. [DOI] [PubMed] [Google Scholar]
- 13.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–5. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 14.Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, et al. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–5. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- 15.Kumanov P, Nandipati K, Tomova A, Agarwal A. Inhibin B is a better marker of spermatogenesis than other hormones in the evaluation of male factor infertility. Fertil Steril. 2006;86:332–8. doi: 10.1016/j.fertnstert.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Frydelund-Larsen L, Krausz C, Leffers H, Andersson AM, Carlsen E, et al. Inhibin B: a marker for the functional state of the seminiferous epithelium in patients with azoospermia factor C microdeletions. J Clin Endocrinol Metab. 2002;87:5618–24. doi: 10.1210/jc.2002-020737. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell V, Robin G, Boitrelle F, Massart P, Marchetti C, et al. Correlation between testicular sperm extraction outcomes and clinical, endocrine and testicular histology parameters in 120 azoospermic men with normal serum FSH levels. Int J Androl. 2011;34:299–305. doi: 10.1111/j.1365-2605.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 18.Fishel S, Green S, Bishop M, Thornton S, Hunter A, et al. Pregnancy after intracytoplasmic injection of spermatid. Lancet. 1995;345:1641–2. doi: 10.1016/s0140-6736(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 19.Tesarik J, Mendoza C, Testart J. Viable embryos from injection of round spermatids into oocytes. N Engl J Med. 1995;333:525. doi: 10.1056/NEJM199508243330819. [DOI] [PubMed] [Google Scholar]
- 20.Cremades N, Sousa M, Bernabeu R, Barros A. Developmental potential of elongating and elongated spermatids obtained after in vitro maturation of isolated round spermatids. Hum Reprod. 2001;16:1938–44. doi: 10.1093/humrep/16.9.1938. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa H, Terada Y, Ugajin T, Yaegashi N, Sato K. A novel culture system for mouse spermatid maturation which produces elongating spermatids capable of inducing calcium oscillation during fertilization and embryonic development. J Assist Reprod Genet. 2010;27:565–70. doi: 10.1007/s10815-010-9442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa M, Cremades N, Silva J, Oliveira C, Ferraz L, et al. Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod. 2002;17:1800–10. doi: 10.1093/humrep/17.7.1800. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka A, Nagayoshi M, Takemoto Y, Tanaka I, Kusunoki H, et al. Fourteen babies born after round spermatid injection into human oocytes. Proc Natl Acad Sci U S A. 2015;112:14629–34. doi: 10.1073/pnas.1517466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rome PJ, Comhaire FH, Hargreave TB, Mahmoud AM. New York: Cambridge University Press; 2000. WHO manual for standardized investigation, diagnosis and management of the infertile male; pp. 43–4. [Google Scholar]
- 25.Ballesca JL, Balasch J, Calafell JM, Alvarez R, Fabregues F, et al. Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2000;15:1734–8. doi: 10.1093/humrep/15.8.1734. [DOI] [PubMed] [Google Scholar]
- 26.Brugo-Olmedo S, De Vincentiis S, Calamera JC, Urrutia F, Nodar F, et al. Serum inhibin B may be a reliable marker of the presence of testicular spermatozoa in patients with nonobstructive azoospermia. Fertil Steril. 2001;76:1124–9. doi: 10.1016/s0015-0282(01)02866-7. [DOI] [PubMed] [Google Scholar]
- 27.Bohring C, Schroeder-Printzen I, Weidner W, Krause W. Serum levels of inhibin B and follicle-stimulating hormone may predict successful sperm retrieval in men with azoospermia who are undergoing testicular sperm extraction. Fertil Steril. 2002;78:1195–8. doi: 10.1016/s0015-0282(02)04259-0. [DOI] [PubMed] [Google Scholar]
- 28.Goulis DG, Polychronou P, Mikos T, Grimbizis G, Gerou S, et al. Serum inhibin-B and follicle stimulating hormone as predictors of the presence of sperm in testicular fine needle aspirate in men with azoospermia. Hormones (Athens) 2008;7:140–7. doi: 10.1007/BF03401505. [DOI] [PubMed] [Google Scholar]
- 29.Huang X, Bai Q, Yan LY, Zhang QF, Geng L, et al. Combination of serum inhibin B and follicle-stimulating hormone levels can not improve the diagnostic accuracy on testicular sperm extraction outcomes in Chinese non-obstructive azoospermic men. Chin Med J (Engl) 2012;125:2885–9. [PubMed] [Google Scholar]
- 30.Halder A, Fauzdar A, Kumar A. Serum inhibin B and follicle-stimulating hormone levels as markers in the evaluation of azoospermic men: a comparison. Andrologia. 2005;37:173–9. doi: 10.1111/j.1439-0272.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 31.Goulis DG, Tsametis C, Iliadou PK, Polychronou P, Kantartzi PD, et al. Serum inhibin B and anti-Müllerian hormone are not superior to follicle-stimulating hormone as predictors of the presence of sperm in testicular fine-needle aspiration in men with azoospermia. Fertil Steril. 2009;91:1279–84. doi: 10.1016/j.fertnstert.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Grunewald S, Glander HJ, Paasch U, Kratzsch J. Age-dependent inhibin B concentration in relation to FSH and semen sample qualities: a study in 2448 men. Reproduction. 2013;145:237–44. doi: 10.1530/REP-12-0415. [DOI] [PubMed] [Google Scholar]
- 33.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen N, Liu F, Andersson AM, Vierula M, Irvine DS, et al. Serum inhibin-b in fertile men is strongly correlated with low but not high sperm counts: a coordinated study of 1,797 European and US men. Fertil Steril. 2010;94:2128–34. doi: 10.1016/j.fertnstert.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–43. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- 36.Carrell DT, De Jonge C, Lamb DJ. The genetics of male infertility: a field of study whose time is now. Arch Androl. 2006;52:269–74. doi: 10.1080/01485010500503603. [DOI] [PubMed] [Google Scholar]
- 37.Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18:1660–5. doi: 10.1093/humrep/deg348. [DOI] [PubMed] [Google Scholar]
- 38.Stahl PJ, Masson P, Mielnik A, Marean MB, Schlegel PN, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril. 2010;94:1753–6. doi: 10.1016/j.fertnstert.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Chu QJ, Hua R, Luo C, Chen QJ, Wu B, et al. Relationship of genetic causes and inhibin B in non obstructive azoospermia spermatogenic failure. BMC Med Genet. 2017;18:98. doi: 10.1186/s12881-017-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


