Abstract
This study investigated the clinical activity of abiraterone plus prednisone in docetaxel-naïve and docetaxel-resistant Chinese patients with metastatic castration-resistant prostate cancer (mCRPC). A total of 146 patients with docetaxel-naïve group (103 cases) and docetaxel-resistant group (43 cases) were enrolled from the Shanghai Cancer Center (Shanghai, China) in this retrospective cohort study. The efficacy endpoints were prostate-specific antigen response rate, prostate-specific antigen progression-free survival, clinical/radiographic progression-free survival, and overall survival in response to abiraterone plus prednisone. Significantly higher prostate-specific antigen response rate was found in docetaxel-naïve group (54.4%, 56/103) compared to docetaxel-resistant group (34.9%, 15/43) (P = 0.047). In addition, significantly higher median prostate-specific antigen progression-free survival (14.0 vs 7.7 months, P = 0.005), clinical or radiographic progression-free survival (17.0 vs 12.5 months, P = 0.003), and overall survival (27.0 vs 18.0 months, P = 0.016) were found in docetaxel-naïve group compared to docetaxel-resistant group, respectively. The univariate and multivariate analyses indicated that lower albumin and visceral metastases were independent significant predictors for shorter overall survival. To sum up, our data suggested that abiraterone plus prednisone was efficient in both docetaxel-naïve and docetaxel-resistant Chinese patients. Moreover, higher PSA response rate and longer overall survival were observed in the docetaxel-naïve group, which suggested that abiraterone was more effective for docetaxel- naïve patients than for docetaxel failures.
Keywords: abiraterone acetate, castration-resistant prostate cancer, clinical activity, docetaxel-naïve, docetaxel-pretreated
INTRODUCTION
Prostate cancer is the most common malignancy among men and second leading cause of cancer-related death in Western countries.1 In the last decade, the incidence of prostate cancer has gradually increased in China and other Asian countries due to increased life expectancy, as well as the Westernized diet.2 Moreover, the proportion of patients with metastatic prostate cancer is substantially higher in Asian countries compared to Western countries.3 Androgen deprivation therapy is the backbone of metastatic prostate cancer management; nevertheless, almost all patients ultimately develop a more aggressive disease known as metastatic castration-resistant prostate cancer (mCRPC), which is accompanied by worsening of symptoms and poor outcomes.4
In recent years, several novel therapeutic agents, including abiraterone, enzalutamide, cabazitaxel, radium-223, and sipuleucel-T have shown to improve overall survival (OS) in mCRPC patients.5 Abiraterone, a CYP17 inhibitor, has shown to increase radiographic progression-free survival and OS, as well as delay the opiate use for both docetaxel-naïve and docetaxel-resistant mCRPC patients.6 However, most of the patients included in these trials were from Western countries, while only few studies investigated the efficacy of abiraterone plus prednisone in Asian mCRPC patients.7
In the present study, we retrospectively analyzed clinical data of 146 Chinese mCRPC patients who received abiraterone plus prednisone with or without prior exposure to docetaxel chemotherapy, and evaluate the efficacy of abiraterone and identify potential variables associated with prostate-specific antigen (PSA) response and long term OS in the Chinese population.
METHODS
Study population and data collection
This retrospective study included 146 Chinese mCRPC patients who were treated with abiraterone plus prednisone (Johnson & Johnson, NewBrunswick, NJ, USA) at Fudan University Shanghai Cancer Center (Shanghai, China) between July 2013 and February 2017. Before the initiation of abiraterone, all patients underwent a complete evaluation including: physical examination, routine clinical laboratory tests, measurement of serum PSA and testosterone levels, thoracic, abdominal, and pelvic computed tomography (CT) scans, and single- photon emission computed tomography (ECT). During treatment, all patients received ongoing androgen deprivation therapy or had undergone prior bilateral orchiectomy. Serum PSA and testosterone levels were evaluated every month. Imaging studies were requested by the treating physician, based on increasing PSA values and/or new clinical signs or symptoms.
The following clinical and pathological characteristics were analyzed: patient's age at the time of abiraterone initiation, the Eastern Cooperative Oncology Group (ECOG) performance status, serum levels of PSA, hemoglobin, alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and albumin (ALB), Gleason score and clinical stage at initial diagnosis, site and extent of metastatic disease, and based on types of previous hormonal therapeutic regimens.
Clinical endpoints were defined according to the Prostate Cancer Working Group 2 (PCWG2) criteria.8 Confirmed PSA response was defined as a reduction in the PSA level from baseline by ≥50%, and maintained for ≥4 weeks. Maximal PSA response was defined as the maximal percentage decrease in PSA level from baseline. PSA progression-free survival was defined as the time interval from abiraterone initiation to first documented PSA progression (defined as a 25% increase in PSA from baseline or nadir, and by ≥2 ng ml−1). Clinical or radiographic progression-free survival was defined as the interval from abiraterone initiation to symptomatic progression (worsening disease- related symptoms or new cancer-related complications), radiographic progression (≥20% increase in the sum of the diameters of soft-tissue target lesions on CT scanning or ≥2 new bone lesions on bone scanning), or death, whichever occurred first. Overall survival (OS) was defined as the interval from abiraterone initiation to death from any cause.
This study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (No. 050432-4-1212B). Before the initiation of this study, informed consent was obtained from each patient. All the methods were carried out in accordance with the approved guidelines.
Statistical analyses
The descriptive statistics were reported as medians with ranges for continuous variables and as populations and frequencies for categorical variables. The t-test (normal distribution data) and Mann-Whitney U test (nonnormal distribution data) were used to compare continuous variables. The Fisher's exact (small size sample) or Pearson's Chi-squared test were used to compare categorical variables. Survival was calculated using the Kaplan-Meier method with the Log- rank test to assess differences between groups. Multivariate analysis of prognostic factors was performed using the Cox proportional-hazard model. Due to the limited number of patients, only variables that appeared to have a significant impact on survival by univariate analysis were included in the multivariate analysis. All statistical tests were done using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA); P < 0.05 was considered statistically significant.
RESULTS
The baseline clinicopathological characteristics of the patients are summarized in Table 1. Among 146 mCRPC patients, 103 (70.5%) patients received abiraterone plus prednisone without prior exposure to docetaxel (docetaxel-naïve cohort) and 43 (29.5%) previously received chemotherapy with docetaxel (docetaxel-resistant cohort). The site of metastasis included as follows: bone (92.5%), lymph node (18.5%), liver (4.1%), lung (3.4%), brain (1.4%), and other soft tissue (1.4%). In addition, 121 patients developed multi-organ metastasis and none of the patients has previously received enzalutamide.
Table 1.
Baseline clinicopathologic characteristics of 146 metastatic castration resistant prostate cancer patients
| Characteristics | Values |
|---|---|
| Age (year), median (range) | 73.1 (53.5–89.2) |
| PSA (ng ml−1), median (range) | 74.5 (2.9–340.9) |
| Hemoglobin (g l−1), median (range) | 124.0 (40.0–161.0) |
| ALP (U l−1), median (range) | 88.6 (36.0–719.0) |
| LDH (U l−1), median (range) | 197.0 (47.0–752.0) |
| ALB (U l−1), median (range) | 41.0 (22.0–76.0) |
| Duration of previous hormonal therapy (month), median (range) | 16.0 (1.0–183.0) |
| ECOG performance status, n (%) | |
| 0–1 | 131 (89.7) |
| ≥2 | 15 (10.3) |
| Stage at initial diagnosis, n (%) | |
| M0 | 13 (8.9) |
| M1 | 133 (91.1) |
| Gleason score of primary lesion, n (%) | |
| 5–7 | 39 (26.7) |
| 8–10 | 107 (73.3) |
| Site of metastatic diseasea, n (%) | |
| Bone | 135 (92.5) |
| Lymph node | 27 (18.5) |
| Liver | 6 (4.1) |
| Lung | 5 (3.4) |
| Brain | 2 (1.4) |
| Other soft tissue | 2 (1.4) |
| Extent of disease, n (%) | |
| 0–5 metastatic sites | 91 (62.3) |
| >5 metastatic sites | 55 (37.7) |
| Previous therapies, n (%) | |
| Flutamide | 103 (70.5) |
| Bicalutamide | 139 (95.2) |
| Estrogen | 17 (11.6) |
| Docetaxel chemotherapy | 43 (29.5) |
a121 patients developed multi-organ metastasis. PSA: prostate-specific antigen; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; ALB: albumin; ECOG: Eastern Cooperative Oncology Group
Patient characteristics, stratified by prior docetaxel use, are shown in Table 2. The two cohorts were well balanced with respect to age, PSA level, ECOG status, Gleason score, tumor node metastasis (TNM) stage at diagnosis, duration of previous hormonal therapy, the extent of disease, and visceral metastatic rate. The albumin level was higher in the docetaxel-naïve cohort (P = 0.039), and no statistical difference was found in hemoglobin, ALP, and LDH level between the two cohorts.
Table 2.
Clinicopathologic characteristics compared by previous docetaxel chemotherapy
| Characteristics | Docetaxel-naïve cohort (n=103) | Docetaxel-resistant cohort (n=43) | P |
|---|---|---|---|
| Age (year), mean (s.d.) | 72.5 (7.6) | 73.6 (7.4) | 0.423 |
| PSA (ng ml−1), median (IQR) | 73.9 (66.2–77.7) | 72.9 (68.3–78.7) | 0.668 |
| Hemoglobin (g l−1), mean (s.d.) | 120.2 (20.9) | 114.9 (22.7) | 0.178 |
| ALP (U l−1), mean (s.d.) | 142.2 (132.0) | 124.9 (98.7) | 0.444 |
| LDH (U l−1), mean (s.d.) | 214.2 (91.1) | 258.3 (133.4) | 0.056 |
| ALB (g l−1), mean (s.d.) | 40.3 (11.6) | 35.3 (11.9) | 0.039* |
| Duration of previous hormonal therapy (month), median (IQR) | 14.0 (6.0–36.8) | 17.0 (10.5–51.5) | 0.129 |
| ECOG performance status, n (%) | |||
| 0–1 | 91 (88.3) | 40 (93.0) | 0.554 |
| ≥2 | 12 (11.7) | 3 (7.0) | |
| Stage at initial diagnosis, n (%) | |||
| M0 | 10 (9.7) | 3 (7.0) | 0.756 |
| M1 | 93 (90.3) | 40 (93.0) | |
| Gleason score of primary lesion, n (%) | |||
| 5–7 | 28 (27.2) | 11 (25.6) | 0.842 |
| 8–10 | 75 (72.8) | 32 (74.4) | |
| Extent of disease, n (%) | |||
| 0–5 metastatic sites | 66 (64.1) | 25 (58.1) | 0.500 |
| >5 metastatic sites | 37 (35.9) | 18 (41.9) | |
| Visceral metastases, n (%) | |||
| Yes | 12 (11.7) | 3 (7.0) | 0.554 |
| No | 91 (88.3) | 40 (93.0) | |
PSA: prostate-specific antigen; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; ALB: albumin; ECOG: Eastern Cooperative Oncology Group; s.d.: standard deviation; IQR: interquartile range. *Statistically significant
Confirmed PSA response was observed in 48.6% (71/146) patients. Waterfall plots depicting PSA changes after initiation of abiraterone grouped according to previous docetaxel chemotherapy are presented in Supplementary Figure 1 (620.3KB, tif) . Significantly higher PSA response rates were found in docetaxel-naïve patients (54.4%, 56/103) compared to docetaxel-resistant patients (34.9%, 15/43) (P = 0.047, Table 3). Variable associated with confirmed PSA response are shown in Table 3. Higher levels of ALP (P = 0.031) and LDH (P = 0.014), higher Gleason score of primary lesion (P = 0.001) and patients who previously received docetaxel chemotherapy (P = 0.047) were associated with a lower response to abiraterone (Table 3).
Table 3.
Analysis of variables associated with confirmed prostate-specific antigen response
| Characteristics | Patient (n) | Confirmed PSA response, n (%) | P |
|---|---|---|---|
| All patients | 146 | 71 (48.6) | |
| Age (year) | |||
| <70 | 45 | 17 (37.8) | 0.106 |
| ≥70 | 101 | 54 (53.5) | |
| Hemoglobin (g l−1) | |||
| <120 | 67 | 29 (43.3) | 0.249 |
| ≥120 | 79 | 42 (53.2) | |
| ALP (U l−1) | |||
| <160 | 83 | 47 (56.6) | 0.031 |
| ≥160 | 63 | 24 (38.1) | |
| LDH (U l−1) | |||
| <250 | 75 | 44 (58.7) | 0.014 |
| ≥250 | 71 | 27 (38.0) | |
| ALB (g l−1) | |||
| <35 | 29 | 16 (55.2) | 0.534 |
| ≥35 | 117 | 55 (47.0) | |
| ECOG performance status | |||
| 0–1 | 131 | 67 (51.1) | 0.102 |
| ≥2 | 15 | 4 (26.7) | |
| Gleason score of primary lesion | |||
| 5–7 | 39 | 28 (71.8) | 0.001 |
| 8–10 | 107 | 43 (40.2) | |
| Duration of previous hormonal therapy (month) | |||
| <16.0 | 73 | 32 (43.8) | 0.320 |
| ≥16.0 | 73 | 39 (53.4) | |
| Extent of disease | |||
| 0–5 metastatic sites | 91 | 58 (63.7) | <0.001 |
| >5 metastatic sites | 55 | 13 (23.6) | |
| Visceral metastases | |||
| Yes | 15 | 4 (26.7) | 0.102 |
| No | 131 | 67 (51.1) | |
| Previous docetaxel chemotherapy | |||
| Yes | 43 | 15 (34.9) | 0.047 |
| No | 103 | 56 (54.4) |
ALP: alkaline phosphatase; LDH: lactate dehydrogenase; ALB: albumin; ECOG: Eastern Cooperative Oncology Group; PSA: prostate-specific antigen
Waterfall plots of maximal PSA responses during abiraterone treatment according to previous docetaxel chemotherapy. (a) The 113 docetaxel-naïve patients. (b) The 43 docetaxel-pretreated patients. The dotted line shows the threshold for defining a confirmed PSA response (≥50% reduction in PSA level from baseline and maintained for ≥4 weeks). + Bars are truncated due to >100% PSA increase. PSA: prostate-specific antigen.
After a median (range) follow-up of 17 (6-50) months, 81 (55.5%) patients developed PSA progression, 68 (46.6%) patients developed clinical or radiographic progression and 52 (35.6%) patients died by the end of the study. Kaplan–Meier analyses for PSA progression-free survival, clinical or radiographic progression-free survival and OS are shown in Figure 1, respectively. The median PSA progression-free survival among docetaxel- resistant patients, was significantly shorter compared to docetaxel-naïve patients(7.7 vs 14.0 months, P = 0.005, Figure 1a). Furthermore, themedian clinical or radiographic progression-free survival among docetaxel- resistant patients was significantly shorter compared to docetaxel-naïve patients (12.5 vs 17.0 months, P = 0.003, Figure 1b). Moreover, themedian OS among docetaxel-resistant patients was also significantly shorter than thatof docetaxel-naïve patients (18.0 vs 27.0 months, P = 0.016, Figure 1c).
Figure 1.
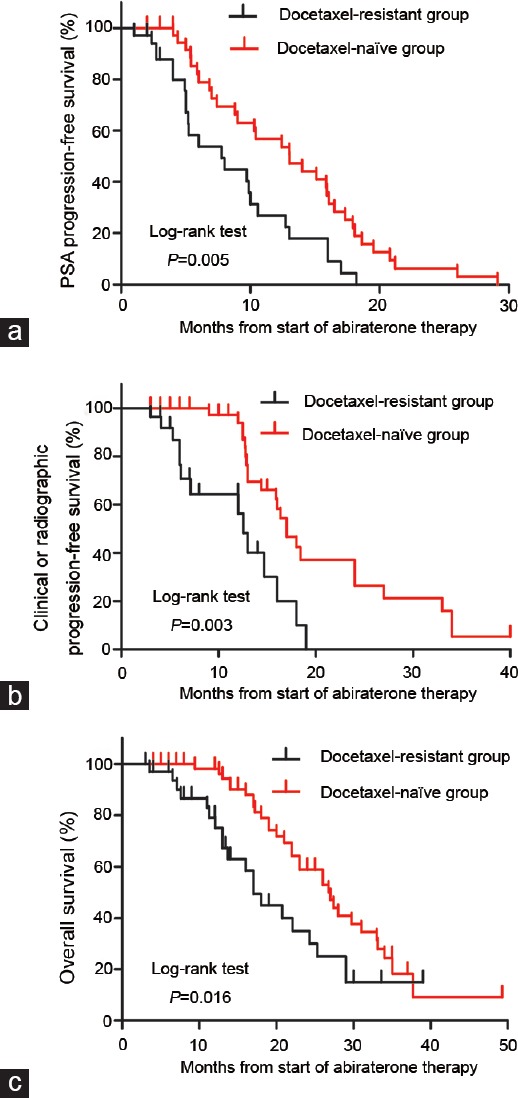
Kaplan–Meier analysis of (a) PSA progression-free survival, (b) clinical or radiographic progression-free survival and (c) overall survival. PSA: prostate-specific antigen.
Table 4 lists the variables associated with OS in our patients. In univariate analyses, higher LDH, lower ALB, with visceral metastases and docetaxel-resistant were associated with shorter OS. In further multivariate analyses for OS, only lower ALB and visceral metastases were independent significant predictors for shorter OS.
Table 4.
Univariate and multivariate analyses of variables associated with overall survival
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (year), ≥70 versus <70 | 1.29 (0.70–2.40) | 0.413 | / | / |
| Hemoglobin (g l−1), <120 versus ≥120 | 1.70 (0.90–3.24) | 0.104 | / | / |
| ALP (U l−1), ≥160 versus <160 | 1.75 (0.91–3.35) | 0.095 | / | / |
| LDH (U l−1), ≥250 versus <250 | 1.33 (1.03–1.72) | 0.026 | 1.52 (0.79–2.08) | 0.321 |
| ALB (g−1), <35 versus ≥35 | 2.64 (1.29–5.42) | 0.008 | 2.43 (1.87–5.32) | 0.016 |
| ECOG performance status, ≥2 versus <2 | 1.84 (0.85–3.95) | 0.091 | / | / |
| Gleason score of primary lesion, ≥8 versus <8 | 1.09 (0.58–2.02) | 0.797 | / | / |
| Duration of previous hormonal therapy (month), <16.0 versus ≥16.0 | 1.05 (0.6–1.86) | 0.864 | / | / |
| Extent of metastatic sites, >5 versus 0–5 | 1.11 (0.64–1.94) | 0.714 | / | / |
| Visceral metastases, yes versus no | 2.38 (1.10–5.15) | 0.028 | 3.10 (1.32–4.88) | 0.042 |
| Previous docetaxel chemotherapy, yes versus no | 2.01 (1.14–3.54) | 0.016 | 1.46 (0.79–3.91) | 0.097 |
ALP: alkaline phosphatase; LDH: lactate dehydrogenase; ALB: albumin; ECOG: Eastern Cooperative Oncology Group; CI: confidence interval; HR: hazard ratios
DISCUSSION
In the last few years, some new treatment options for mCRPC have shown a survival benefit in clinic trials besides docetaxel: abiraterone, enzalutamide, cabazitaxel, radium-223, and sipuleucel-T. However, the prognosis for Chinese mCRPC patients treated with abiraterone plus prednisone remains unclear, since so far there have only been few studies on the topic including a large number of patients.
In the present study, we reported clinicopathological and survival data for 146 Chinese patients with mCRPC who were treated with abiraterone plus prednisone with or without prior exposure to docetaxel. Our data indicated that prognostic indicators were better in docetaxel-naïve group than in docetaxel-resistant group and lower ALB and visceral metastases are independent prognostic factors. The possible explanation is: (1) docetaxel-resistant patients have one less life-prolonging therapy available to them compared to docetaxel- naïve patients; (2) docetaxel is mostly used for symptomatic CRPC, whereas docetaxel-naïve patients treated with abiraterone might be asymptomatic. (3) the treatment of abiraterone has a slighter adverse reactions, more convenient treatment (oral) and more controlled therapeutic doses.
This study reported a large cohort of Chinese mCRPC patients who received abiraterone plus prednisone without prior exposure to docetaxel (docetaxel-naïve).9 The efficacy of abiraterone group in our 103 docetaxel-naïve mCRPC patients was slightly inferior compared to those reported in COU-AA-302 trial.10 In the COU-AA-302 trial, the confirmed PSA response rate was 62% and after long-term follow-up the median OS extended to 34.7 months in abiraterone group.11 Yet, in the current study, the PSA response rate was 54.4% and the OS was 27.0 months in docetaxel-naïve patients. This difference can be explained by the following reason: more patients with poor performance were included in this study. Twelve of 103 (11.7%) patients had an ECOG performance status ≥2 (Table 2). Furthermore, no more new drug treatments available for follow-up treatment options for Chinese patients because of the country's new drug approval policy.
Although abiraterone has shown its life-prolonging efficacy in the treatment of mCRPC, the best sequential strategy for managing these patients remains unclear. Ryan et al. suggests that the top three successors treatment options in the COU-AA-302 trial after the failure of abiraterone were docetaxel (57%), cabazitaxel (18%), and enzalutamide (16%).12 Further studies found that abiraterone- then-enzalutamide strategy seems to be more advantageous in PSA- progression-free survival.13,14
In recent years, there have been many new changes in the treatment options for mCRPC. Currently, systemic treatment options for mCRPC include hormonal therapy (abiraterone, enzalutamide), chemotherapy (cabazitaxel, docetaxel), immunotherapy, and radionuclide therapy as well as bone-modifying agents and palliative or supportive measures.15 Further, new drugs such as poly(adenosine Diphosphate-ribose) polymerase (PARP) inhibitors and PD-1 inhibitors, are under clinical investigation. Individualized management and information on how to select and sequence existing therapies began to appear.16,17,18,19 Early use of active agents even under castrate-sensitive conditions may change the clinical management of the disease according to LATITUDE and STAMPEDE trials.20,21
There are some limitations in this study. First, due to the inherent limitations of a single center retrospective cohort study, the previous treatments of our patients varied significantly in both docetaxel-resistant cohort and docetaxel-naïve cohort. These variations led to imbalance in baseline characteristics between the two cohorts, which could have influenced the final statistical results of the study. Yet, for the reason that docetaxel has been approved for mCRPC patients much earlier than abiraterone, in real clinical practice, mCRPC patients are always divided into docetaxel-resistant and docetaxel-naïve cohort regardless of other previous therapeutic regimens. Therefore, our data reflected the real everyday practice, and the obtained results are useful for clinical application. Second, the sample size was relatively small, especially in the docetaxel-resistant subgroup. Third, the median follow-up time was only 17 months, which is comparatively short.
China or Asia need to have guidelines for the use of drugs for ethnic groups and populations. Considering that there is no authoritative guideline for the diagnosis and treatment of Chinese populations now, this study provides a reference for China to formulate relevant guidelines and further studies.
CONCLUSIONS
Previous randomized trials have shownthatabiraterone plus prednisone is life-prolonging in both docetaxel-naïve and docetaxel-resistant patients. The present study, which included Chinese patients, is consistent with this practice. Higher PSA response rate and longer median OS were observed in the docetaxel-naïve subgroup, which suggested that abiraterone is more effective for docetaxel-naïve patients than for docetaxel failures for Chinese patients.
AUTHOR CONTRIBUTION
GWL and GXL collected, analyzed clinical data and wrote the manuscript. BD and DWY designed the study, supervised the project, and revised the manuscript. YYK and YJS assisted with detailed statistical analysis and mapping. YW helped with patients follow-up and interpreted the clinical data. All authors read and approved the final draft.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation (No. 81702535, 81572531), Natural Science Foundation of Shanghai Municipality (No. 16ZR1406500), and Outstanding Young Talent Training Plan of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013102).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: evolving trend over the last decade. Asian J Androl. 2015;17:48–57. doi: 10.4103/1008-682X.132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:1755–6. doi: 10.1056/NEJMc1410239. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Nilsson S, Heinrich D, Helle SI, O'sullivan JM, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 11.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 13.Maughan BL, Luber B, Nadal R, Antonarakis ES. Comparing sequencing of abiraterone and enzalutamide in men with metastatic castration-resistant prostate cancer: a retrospective study. Prostate. 2017;77:33–40. doi: 10.1002/pros.23246. [DOI] [PubMed] [Google Scholar]
- 14.Miyake H, Hara T, Terakawa T, Ozono S, Fujisawa M. Comparative assessment of clinical outcomes between abiraterone acetate and enzalutamide in patients with docetaxel-naïve metastatic castration-resistant prostate cancer: experience in real-world clinical practice in Japan. Clin Genitourin Cancer. 2017;15:313–9. doi: 10.1016/j.clgc.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2018 doi: 10.1016/j.eururo.2018.03.028. pii: S0302-2838(18)30246-X. doi: 10.1016/j.eururo.2018.03.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Poorthuis MH, Vernooij RW, van Moorselaar RJ, de Reijke TM. Second-line therapy in patients with metastatic castration-resistant prostate cancer with progression after or under docetaxel: a systematic review of nine randomized controlled trials. Semin Oncol. 2017;44:358–71. doi: 10.1053/j.seminoncol.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378:645–57. doi: 10.1056/NEJMra1701695. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenburg DW, Morgans AK. Emerging therapies in metastatic prostate cancer. Curr Oncol Rep. 2018;20:46. doi: 10.1007/s11912-018-0692-z. [DOI] [PubMed] [Google Scholar]
- 19.Bremmer F, Jarry H, Unterkircher V, Kaulfuss S, Burfeind P, et al. Testosterone metabolites inhibit proliferation of castration-and therapy-resistant prostate cancer. Oncotarget. 2018;9:16951–61. doi: 10.18632/oncotarget.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguezantolin A, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–61. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 21.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Waterfall plots of maximal PSA responses during abiraterone treatment according to previous docetaxel chemotherapy. (a) The 113 docetaxel-naïve patients. (b) The 43 docetaxel-pretreated patients. The dotted line shows the threshold for defining a confirmed PSA response (≥50% reduction in PSA level from baseline and maintained for ≥4 weeks). + Bars are truncated due to >100% PSA increase. PSA: prostate-specific antigen.


