Abstract
High-fat diets affect male reproduction and sexual function. Therefore, we evaluated the effects of prolonged resveratrol administration on the metabolic, sperm, and testicular parameters of rats fed a cafeteria diet. Male Wistar rats were divided at weaning into control (C, n = 20) and cafeteria (CAF, n = 16) groups. At 3 months, half of them were given daily supplementations of resveratrol (C-R, n = 10; CAF-R, n = 8) at a dosage of 30 mg kg−1 body mass for 2 months. Animals were killed at 5 months of age, and blood, spermatozoa, and testes were collected for further analysis. Data were analyzed by one-way ANOVA, and P < 0.05 was considered statistically significant. The CAF diet promoted hyperglycemia (P < 0.0001), and treatment with resveratrol reversed this condition (P < 0.0001). The CAF diet reduced sperm viability and motility, while resveratrol improved these parameters (P < 0.05). Regarding testicular morphology, the height of the seminiferous epithelium was reduced in the CAF group compared with that of the C group (P = 0.0007). Spermatogenic cell proliferation was also reduced in the CAF group compared with that of the C group. However, the CAF-R showed an increase in cell proliferation rate compared with that of the untreated CAF group (P = 0.0024). Although it did not modify body mass, the consumption of a CAF diet promoted hyperglycemia, adverse testicular morphology remodeling, and abnormal sperm, which were attenuated by treatment with resveratrol, thus suggesting a protective effect of this antioxidant on spermatogenesis.
Keywords: cafeteria diet, morphology, resveratrol, spermatozoa, testicle
INTRODUCTION
In recent years, the consumption of a hyperenergetic, highly palatable cafeteria-style diet (CAF diet) has been increasing in Western countries. This dietary pattern is associated with sedentarism and may be related to an increase in weight gain1 and the appearance of some diseases, such as hyperinsulinemia, hyperglycemia, and glucose intolerance.2 The obesogenic and inflammatory effects of the CAF diet are likely produced by several mechanisms.3 The mechanism most discussed in the literature is that high-fat/refined carbohydrate intake triggers an increase in the expression of some pro-inflammatory cytokines, such as tumor necrosis factor-α4 and prostaglandin E2,5 in addition to increasing the production of reactive oxygen species (ROS).6
The association between CAF diet, oxidative stress, and the deterioration of male reproductive function is quite common.7,8,9 Oxidative stress from adiposity10 or CAF diet alone1 exerts negative effects on sperm function.9 It has been reported that ROS and nitrogen species negatively interfere with testicular immunity, spermatogenesis, and sperm motility.8,11,12 Therefore, it is necessary to create a balance between the production and metabolism of free radicals to improve the function of testicular cells. To achieve this balance, antioxidants can neutralize free radicals and attenuate damage to the testicles.9
Resveratrol is a polyphenol that is present in many food sources, has a potent inhibitory activity against ROS, and increases the bioavailability of nitric oxide.13 Although many studies have shown that satisfactory results are achieved with the use of this antioxidant,14,15 no quantitative evaluation of the testicular parenchyma or sperm parameters has been performed in the proposed experimental model. It is important to study the sperm parameters in this same model. Therefore, this work aimed to evaluate the testicular morphology and the metabolic and sperm parameters of rats fed a CAF diet and treated with resveratrol.
MATERIALS AND METHODS
Experimental protocol
Thirty-six male Wistar rats (21 days old) were housed in polypropylene boxes in climatized colony rooms (21°C ± 2°C; 60% humidity) on a 12/12 h light/dark cycle with an air exhaustion cycle (15 per hour). All procedures were approved by the local Ethics Committee for the Care and Use of Animals of the Institute of Biology Roberto Alcantara Gomes – UERJ, Rio de Janeiro, Brazil (CEUA, IBRAG/049/16). The animals were randomly divided into two experimental groups: the control diet (C, n = 20; commercial diet [Nuvilab®, Curitiba, Paraná, Brazil]; 6 g lipid per 100 g diet, 1800 kJ) and the CAF diet (CAF, n = 16; rat chow that was manipulated in the laboratory by mixing commercial diet [Nuvilab®] 60 g per 100 g diet, condensed milk [Nestle®, São Paulo, São Paulo, Brazil] 25 g per 100 g diet, and hydrogenated vegetable fat [Primor®, São Paulo, São Paulo, Brazil] 15 g per 100 g diet; totally 30 g lipid per 100 g diet, 2300 kJ). It is important to mention that this hydrogenated vegetable fat is rich in saturated fatty acids (3.5 g/15 g) and trans-fatty acids (3.0 g/15 g). Rats from each group received their respective diet until they were 3 months old. At 3 months of age, the groups were re-divided, and treatment with resveratrol (99% purity, Terraternal®, Santa Clara, CA, USA) was initiated, resulting in two additional experimental groups: the control diet supplemented with resveratrol (C-R; n = 10) and the CAF diet supplemented with resveratrol (CAF-R; n = 8). Resveratrol was administered daily through orogastric gavage at a dose of 30 mg kg−1 body mass (BM) for 2 months.16 The nonsupplemented groups received water by orogastric gavage. All diets were given ad libitum. Food intake was recorded daily, and BM was monitored weekly until the end of the experiment (5 months old).
Blood pressure and oral glucose tolerance test
At 3 months of age, systolic blood pressure (SBP) was measured weekly at night. The animals were heated in an acrylic chamber at a temperature of 37°C for 10 min to dilatate the caudal artery. Next, the rats were packed in acrylic pads, and SBP was measured from the caudal artery using a tail plethysmograph version 2.11 (Insight®, Ribeirão Preto, São Paulo, Brazil). Three measurements were performed, and the final SBP value was obtained from the mean of the three measurements.
The oral glucose tolerance test (OGTT) was performed before (3 months old) and after resveratrol administration (5 months old). For this, the animals were fasted for 12 h and a hypertonic glycosated solution was administered by orogastric gavage (2 g kg−1 BM of glucose 50%). This percentage refers to the concentration of glucose in the solution. The blood was collected directly from the caudal vein before glucose administration (time 0) and 15, 30, 60, and 120 min after glucose overload, and the dose was verified by means of an appropriate glucometer (Accu-Chek, Roche, São Paulo, Brazil). To assess glucose tolerance, the area under the curve was analyzed.
Death of animals and obtaining the samples
At 5 months of age, the rats were killed by intraperitoneal administration of thiopental at a dosage of 100 mg kg−1 BM. Immediately after death, blood was collected by cardiac puncture for subsequent biochemical analysis, and the tail of the epididymis was dissected and cleaved three times to remove the spermatozoa. Then, it was immersed in 5 ml of phosphate-buffered saline with 0.5% bovine serum albumin (0.5% PBS-BSA; A9647, albumin from bovine serum, Sigma, Frederick, MD, USA) at 37°C. Subsequently, the solution containing the tail of the epididymis was carefully shaken for dissemination of the spermatozoa from the organ to the liquid medium. The resulting solution, called the sperm suspension, was then used for all analyses of spermatozoa (concentration and motility in a Neubauer chamber and sperm viability).16,17,18 All materials used in the analyses were maintained at 37°C for the conservation of spermatozoa. We used 100 μl of the sperm solution, previously diluted (between 1 and 5 times) according to its turbidity, to facilitate the counting of the spermatozoa. This dilution was recorded and used in the final calculation to determine the concentration of spermatozoa. Then, 10 μl was collected from this solution and deposited on a Neubauer chamber, covered by a cover slip. This sample was visualized in phase contrast and recorded by a Basler camera (Vision Technology TM®, Ahrensburg, Germany) coupled to an H550S microscope (Nikon, Tokyo, Japan) under magnification of ×100. From the videos, the sperm concentration was determined, counting the number of spermatozoa on five quadrants of the Neubauer chamber (recorded separately in two videos), making a total volume of 2 × 10−5 ml. Sperm viability was determined by the hypo-osmotic test, and 200 spermatozoa were evaluated per animal.19
Next, the testes were dissected and weighed, and their volumes were determined by the Scherle method.20 Then, the organs were fixed and included in paraffin.
Biochemistry and hormone levels
The concentrations of triacylglycerol (TAG), very-low-density lipoprotein (VLDL-c), high-density lipoprotein (HDL-c), and total cholesterol (TC) were determined by spectrophotometry with a commercially available kit (cat. 11506, BioSystems®, Barcelona, Spain). Serum insulin and testosterone levels were analyzed by the ELISA method with commercially available kits from Millipore® (cat. EZRMI-13K, St. Charles, MO, USA) and Cloud-Clone Corp® (cat. CEA458Ge, Wuhan, China), respectively. The samples were analyzed in duplicate and had a coefficient of variation of 1.4%.
Histomorphometry and immunohistochemistry
After the tissues were processed, 5-μm-thick sections were cut. Morphometric analyses were performed on slides stained with hematoxylin and eosin and photographed with an Olympus BX51 light microscope with a coupled DP70 digital camera (Olympus, Tokyo, Japan). The diameter of the seminiferous tubules and the height of the seminiferous epithelium were measured with ImageJ software (image processing and analysis in Java, https://imagej.nih.gov/ij/index.html). The diameters of 125 seminiferous tubules per animal (×10 objective) were measured in each testicle with a perpendicular line that passed through the center of the tubule, excluding the less circular tubules. The height of the seminiferous epithelium was measured by constructing three equidistant lines from each transverse section of the seminiferous tubules. A total of 125 tubules per animal were evaluated with a ×20 objective.
The volume density (Vv) of the tubular (tunica propria, seminiferous epithelium, and tubular lumen) and intertubular (blood vessels and other interstitial space structures) compartments were measured by a point-counting method. A grid of 100 points was superimposed on the photomicrographs, and all the testicular structures that touched the points were quantified, resulting in the density being determined as a percentage of the field analyzed.16 We evaluated 25 fields per animal with a ×40 objective. The absolute volume (Av) was calculated from each structure mentioned above by dividing the testicular volume by the Vv of the structure; the result was expressed in ml.21 To determine the cell proliferation rate, all cell nuclei of the spermatogenic lineages were immunolabeled with an antiproliferating cell nuclear antigen (PCNA) antibody (PC10, Ref: 180110, Invitrogen, Camarillo, CA, USA). The measurement was performed with a cell counter, and the quantification area was delimited to the free hand selection tool (ImageJ software). For these analyses, 25 fields were evaluated for each testicle from photomicrographs obtained with a ×40 objective. PCNA is essential for cellular DNA synthesis. Therefore, it marks the nuclei of proliferating cells and it is not associated with any specific seminiferous stage. Through the immunohistochemical technique employed, all the proliferating spermatogenic lineage nuclei in the seminiferous tubules were immunolabeled and revealed by 3,3’-diaminobenzidine (DAB), which gave a brown color.
Statistical analyses
Data were reported as the mean ± standard deviation (s.d.) and were analyzed by Student's t-test and one-way ANOVA, followed by Bonferroni post hoc. Data that did not follow the normal distribution curve were analyzed by the Kruskal–Wallis test, followed by Dunn's post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Food intake and body mass
There were no differences in either BM or energy intake among the groups. Corroborating these results, the animals showed no differences in weight gain throughout the experiment.
The CAF diet promoted an increase in retroperitoneal fat (mean ± s.d.: 9.42 ± 3.27 g) compared with that of the C group (5.03 ± 1.26 g, P = 0.0042). There were no differences in subcutaneous and epididymal fat deposits among the groups (Table 1).
Table 1.
Data of experimental groups at 5 months of age
| Fat deposits and testicle | C | CAF | C-R | CAF-R | P |
|---|---|---|---|---|---|
| Retroperitoneal fat (g) | 5.02±1.26 | 9.41±3.27a | 4.41±1.70 | 8.39±4.54 | 0.0042 |
| Subcutaneous fat (g) | 2.85±0.86 | 4.36±1.38 | 4.14±1.40 | 5.12±2.44a | 0.0322 |
| Epididymal fat (g) | 5.43±1.15 | 7.41±1.77 | 5.91±1.74 | 6.35±3.05 | 0.2156 |
| Right testicle weight (g) | 1.86±0.16 | 1.88±0.11 | 2.03±0.21 | 1.95±0.15 | 0.1430 |
| Right testicle volume (ml) | 1.79±0.12 | 1.85±0.12 | 1.95±0.16 | 1.91±0.13 | 0.0706 |
| Left testicle weight (g) | 1.84±0.17 | 1.89±0.12 | 2.03±0.17 | 1.98±0.15 | 0.0707 |
| Left testicle volume (ml) | 1.80±1.80 | 1.84±1.83 | 1.91±1.91 | 1.95±1.95 | 0.2940 |
Data are presented as mean±s.d. Differences were tested by ANOVA and Bonferroni post hoc test. P<0.05. aThe indicated group versus C group. C: control diet; CAF: cafeteria diet; C-R: control diet treated with resveratrol; CAF-R: cafeteria diet treated with resveratrol; ANOVA: analysis of variance; s.d.: standard deviation
Systolic blood pressure, carbohydrate metabolism, and blood biochemistry
Table 2 shows the results from blood biochemistry and carbohydrate metabolism. SBP did not differ among the experimental groups. For OGTT, the CAF diet promoted glucose intolerance at 3 months of age, before the initiation of resveratrol treatment. The CAF group showed an increase of 17.5% in the area under the glucose curve compared with that of the C group (P < 0.0001). In contrast, treatment with resveratrol was able to improve glycemic control compared with that of groups that did not receive resveratrol. The C-R group showed a reduction of 13.7% in the area under the glucose curve compared with that of the counterpart, while the CAF-R group had a 16.3% reduction in this parameter compared with that of their respective control (the CAF group) (P < 0.0001). Corroborating these results, the CAF group showed a 54.9% increase in serum glucose values compared with that of the C group, while the CAF-R group reduced this parameter by 43.8% compared with that of the CAF group (P = 0.0023). In addition, treatment with resveratrol decreased the insulinemia of the animals receiving the CAF diet. The CAF-R group showed a 64.4% reduction of this parameter compared with that of the untreated group (P = 0.0001). We did not observe any differences among the other groups studied.
Table 2.
Blood biochemistry
| Biochemical parameters | C | CAF | C-R | CAF-R | P |
|---|---|---|---|---|---|
| OGTT before-resveratrol (u.a.) | 746.00±44.80 | 876.40±65.90a | NA | NA | <0.0001 |
| OGTT after-resveratrol (u.a.) | 745.00±42.84 | 869.50±67.21a | 643.10±33.12a | 728.00±58.09b | <0.0001 |
| Cholesterol total (mg dl−1) | 59.89±5.64 | 60.75±8.01 | 64.22±8.01 | 57.00±12.65 | 0.1810 |
| Triacylglycerol (mg dl−1) | 85.60±29.51 | 103.80±38.69 | 30.44±12.89a | 66.38±41.76 | 0.0003 |
| Glucose (mmol l−1) | 10.47±4.32 | 16.22±5.62a | 8.79±2.56 | 9.12±2.45b | 0.0023 |
| HDL (mg dl−1) | 38.90±7.01 | 40.44±7.00 | 35.44±6.44 | 33.80±6.26 | 0.1382 |
| VLDL (mg dl−1) | 17.10±6.97 | 20.78±8.02 | 6.11±2.80a | 17.60±11.82c | 0.0025 |
| Insulin (ng ml−1) | 3.92±1.58 | 6.37±0.75 | 2.22±0.79 | 2.27±1.95b | 0.0001 |
| Testosterone (ng ml−1) | 10.09±0.67 | 10.30±1.02 | 10.96±1.12 | 10.65±1.46 | 0.5206 |
Data are presented as mean±s.d. The differences were tested by ANOVA and Bonferroni post hoc test. P<0.05. aThe indicated group versus C group; bThe indicated group versus CAF group; and cThe indicated group versus C-R group. C: control diet; CAF: cafeteria diet; C-R: control diet treated with resveratrol; CAF-R: cafeteria diet treated with resveratrol. OGTT: oral glucose tolerance test; HDL: high-density lipoprotein; VLDL: very-low-density lipoprotein; ANOVA: analysis of variance; s.d.: standard deviation; NA: not applicable
Regarding the serum levels of TC, HDL-c, and testosterone, there were no differences among the groups. However, the TAG and VLDL-c values decreased by 64.4% and 64.3% in the C-R group compared with those of the C group (P = 0.0003 and P = 0.0025, respectively). The CAF-R group presented an increase (188.0%) in the serum values of this lipoprotein (VLDL-c) compared with the C-R group (P = 0.0025), as illustrated in Table 2.
Sperm analysis and morphometry of the testicles
The group fed with the cafeteria diet showed a reduction in the sperm viability (53.0%) and motility (80.6%) compared with the C group (P = 0.0052 and P < 0.0001, respectively). However, treatment with resveratrol was able to improve these parameters. The CAF-R group showed an increase of sperm viability (117.7%) and motility (340.3%) compared with the levels in the CAF group (P = 0.0052 and P < 0.0001, respectively). The concentration of spermatozoa was the same among the groups studied (Figure 1).
Figure 1.

Spermatozoa analysis. The image shows (a) the concentration, (b) the motility, and (c) the viability analysis of the spermatozoa in animals at 5 months of age. Data were presented as mean ± standard deviation. The differences were tested by analysis of variance and Bonferroni post hoc test. P<0.05 was considered statistically significant. aThe indicated group versus C group and bthe indicated group versus CAF group. C: control diet; CAF: cafeteria diet; C-R: control diet treated with resveratrol; CAF-R: cafeteria diet treated with resveratrol.
There were no differences in testes (weight and volume) among the animals from different experimental groups (Table 1). Similarly, the diameter of the seminiferous tubules was equal among the groups. Regarding the height of the epithelium of the seminiferous tubules, the group that received the cafeteria diet (without resveratrol treatment) showed a 33.8% reduction compared with that of the C group (P=0.0007; Figure 2). In addition, the Vv of the seminiferous epithelium was lower in the CAF (11.5%), C-R (10.2%), and CAF-R (6.2%) groups than the C group (P < 0.0001).
Figure 2.
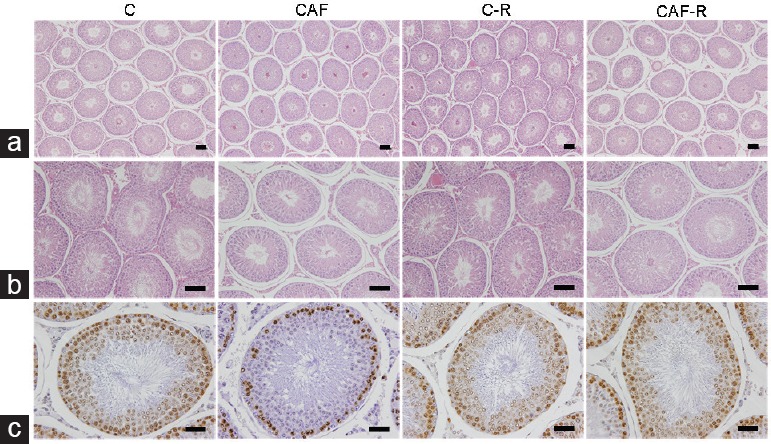
Photomicrographs showing (a) the seminiferous tubule diameter (×100, scale bars=100 μm), (b) the seminiferous epithelium height (×200, scale bars=100 μm), and (c) the proliferation of a spermatogenic cell line (×400, scale bars=50 μm) in animals at 5 months of age. The seminiferous tubule diameter was similar among the groups. However, CAF diet reduced the height of the seminiferous epithelium and the proliferation of a spermatogenic cell line in relation to C, which may affect sperm production. The treatment of resveratrol attenuated this damage in proliferative cells, as observed in the anti-PCNA antibody-immunolabeled image. PCNA: proliferating cell nuclear antigen. C: control diet; CAF: cafeteria diet; C-R: control diet treated with resveratrol; CAF-R: cafeteria diet treated with resveratrol.
In contrast, treatment with resveratrol increased the Vv of the tubular lumen by 20.1% (C-R) and 11.3% (CAF-R) compared with that of the control group (P < 0.0001). However, after comparing the Vv of the tubular compartment as a whole, a reduction only in the CAF group was observed in relation to the C group (4.5%), and treating with resveratrol raised this parameter to 5.1%, in relation to their counterpart (P < 0.0001). There was no difference in the Vv of the tunica propria, the intertubular compartment (interstitial space), the blood vessels, or the stroma among the groups. The Av of the tubular lumen was higher in the C-R group (21.3%) than that in the C group (P = 0.0074). The other parameters were not different. For spermatogenic cell proliferation, the cafeteria diet reduced the number of cells (34.1%) compared with that of the C group, and resveratrol treatment in the same group increased the rate of cell proliferation by 87.9% (Figure 2). Table 3 details all morphometric and stereological data.
Table 3.
Spermatozoa and testicle parameters
| Spermatozoa and testicle parameters | C | CAF | C-R | CAF-R | P |
|---|---|---|---|---|---|
| Spermatozoa concentration (×104 ml−1) | 10.00±0.39 | 10.00±0.32 | 10.00±0.33 | 11.00±0.19 | 0.7832 |
| Spermatozoa motility (%) | 47.82±19.17 | 9.28±8.63a | 49.25±10.06 | 40.86±6.96b | <0.0001 |
| Spermatozoa viability (%) | 7.10±3.24 | 3.33±2.33a | 7.38±2.74 | 7.25±1.53b | 0.0052 |
| Diameter of the seminiferous tubules (μm) | 281.10±6.90 | 280.30±10.95 | 285.80±16.32 | 269.40±13.63 | 0.0720 |
| Seminiferous epithelium | |||||
| Height (μm) | 56.33±3.44 | 37.27±8.39a | 58.23±2.82 | 53.25±8.14 | 0.0007 |
| Vv (%) | 45.24±2.20 | 40.03±1.86a | 40.63±1.14a | 42.43±1.66a | <0.0001 |
| Av (ml) | 0.83±0.06 | 0.73±0.05 | 0.78±0.09 | 0.81±0.06 | 0.0609 |
| Tubular lumen | |||||
| Vv (%) | 33.81±2.37 | 33.70±2.73 | 40.62±1.68a | 37.50±2.22a, b | <0.0001 |
| Av (ml) | 0.61±0.09 | 0.62±0.06 | 0.74±0.11a | 0.72±0.072 | 0.0074 |
| Tunica propria | |||||
| Vv (%) | 5.99±1.34 | 6.51±1.25 | 5.03±0.78 | 5.92±0.88 | 0.0867 |
| Av (ml) | 0.10±0.02 | 0.12±0.03 | 0.09±0.02 | 0.11±0.01 | 0.1095 |
| Tubular compartment | |||||
| Vv (%) | 85.04±1.72 | 81.22±2.43a | 87.22±1.23 | 85.38±2.40b | <0.0001 |
| Av (ml) | 1.54±0.17 | 1.49±0.10 | 1.65±0.26 | 1.65±0.12 | 0.1979 |
| Blood vessels | |||||
| Vv (%) | 0.72±0.29 | 0.63±0.09 | 0.70±0.38 | 0.98±0.39 | 0.1395 |
| Av (ml) | 0.01±0.01 | 0.01±0.00 | 0.01±0.00 | 0.01±0.01 | 0.0643 |
| Stroma | |||||
| Vv (%) | 7.92±0.89 | 7.92±0.76 | 7.29±0.69 | 7.92±0.99 | 0.2372 |
| Av (ml) | 0.14±0.02 | 0.15±0.01 | 0.13±0.02 | 0.15±0.01 | 0.4936 |
| Intertubular compartment | |||||
| Vv (%) | 8.65±0.99 | 8.54±0.47 | 7.75±0.54 | 8.56±0.78 | 0.0755 |
| Av (ml) | 0.15±0.02 | 0.16±0.01 | 0.14±0.02 | 0.17±0.02 | 0.2188 |
| Cell proliferation (cells per mm²) | 2102.00±352.70 | 1386.00±177.30a | 1750.00±278.50 | 2605.00±1436.00b | 0.0024 |
Data are presented as mean±s.d. Differences were tested by ANOVA and Bonferroni post hoc test. P<0.05 was considered statistically significant. aThe indicated group versus C group and bthe indicated group versus CAF group. C: control diet; CAF: cafeteria diet; C-R: control diet treated with resveratrol; CAF-R: cafeteria diet treated with resveratrol; ANOVA: analysis of variance; s.d.: standard deviation; Vv: volume density; Av: absolute volume
DISCUSSION
Our work showed that the CAF diet was able to promote glucose intolerance and important morphological changes in the reproductive system, independent of an increase in BM. However, resveratrol improved carbohydrate metabolism, sperm parameters, such as motility and viability, and testicular morphology and may be a therapeutic option for promoting normal fertility.
The consumption of diets rich in lipids and simple carbohydrates (CAF diet) has increased in the Western world.22 Although not always associated with obesity/overweight,23,24 metabolic complications such as hypertension, dyslipidemia, and hyperglycemia may result from the intake of these foods and aggravate health status.25 For these complications, the use of antioxidants, in particular resveratrol, has been identified as an important and useful tool against the metabolic damage incurred by this type of diet.26,27,28,29 Animal studies related that the doses used and the treatment time are crucial to showing the beneficial effects of this antioxidant.28,29 In our case, and corroborating other studies,23,30 the CAF diet did not alter blood pressure, TC, HDL-c, TAG, or VLDL-c concentration of the animals, and treatment with resveratrol reduced only the serum levels of TAG and VLDL-c in animals receiving normal diets. Differences were not found in the CAF-R group undoubtedly, and the time of administration and the composition of the experimental diets contributed to these results.
In contrast, the CAF diet promoted glucose intolerance at 3 and 5 months of age. However, at 5 months of age, treatment with resveratrol reduced serum glucose and insulin levels. The relationship between the CAF diet and hyperglycemia is well established. Over time, the prediabetic state overloads the pancreas and induces insulin resistance, further compromising carbohydrate metabolism.23,25 However, resveratrol has been used to minimize this metabolic damage.31,32 The use of this antioxidant in diabetic animals potentiates the action of insulin33 and the uptake of peripheral glucose,32,34 in addition to suppressing hepatic glucose production,35 which justifies our findings.
Regarding reproductive parameters, the adverse effects associated with obesity and a high fat (HF) diet are extensively documented in the literature.36,37 Studies involving a cafeteria diet, although in a smaller quantity, showed a relationship between increased oxidative stress and inflammation,1,10,38 and their role in negatively influencing testicular health has already been well described. Brunetti et al.5 demonstrated that the administration of the CAF diet in young patients promoted an increase in prostaglandin E2 levels of the testicular parenchyma, which may induce the disordered proliferation of testicular germ cells (cancer).
Although we did not measure inflammatory indexes, our study is the first to show that, regardless of an increase in BM, CAF diet consumption reduced the viability and motility of spermatozoa and decreased both the height of the seminiferous epithelium and the proliferation of spermatogenic lineage cells. In addition, the morphology of the testis was altered, as shown by a reduction in the Vv of the seminiferous epithelium and of the whole tubular compartment. The deposits of retroperitoneal fat were higher in the CAF group, indicating a redistribution of fat mass. In humans, increased fat accumulation can lead to oxidative stress.39,40 Recent advances in the field of reproductive medicine showed that ROS contributes to sperm dysfunction and infertility.41,42
Although we did not evaluate oxidative stress at the tissue or systemic level, it has been documented that ROS affects sperm count, motility, and morphology and causes sperm DNA fragmentation.41,43,44,45 It has already been well established in the literature that the elevation of serum glucose levels induces increased oxidative stress in the male reproductive system, which causes apoptosis and degeneration of the germ cells, increased lipid peroxidation of the sperm membrane, and a reduction in spermatogenesis, contributing to male infertility.46,47,48,49,50,51,52 Sexton and Jawron, in a review, indicated that diabetic individuals show a reduction in spermatogenesis, sperm count, and sperm motility.53 From these findings, we believe that the hyperglycemia observed in our animals that received the CAF diet may have triggered the increasing of ROS, and thus caused the sperm alterations. However, treatment with resveratrol can increase the synthesis and the activity of some antioxidant enzymes, such as superoxide dismutase, catalase, and glutathione peroxidase,14,54,55,56 thus inhibiting oxidative stress in testicular cells.50 In addition, other studies indicated that resveratrol improves insulin sensitivity and increases the translocation of the type 4 glucose transporter (GLUT4) in the skeletal muscle, which improves the uptake of glucose molecules50,57 and corroborates our findings. In addition, we suggest that this antioxidant activated activating cell proliferation in spermatogenic lineage cells, which positively influences and improves the aforementioned sperm parameters.
Sperm viability was determined from the hypo-osmotic test, which evaluates the integrity and function of the plasma membrane of this cell. Normally, spermatozoa that present tail folding are considered viable as a consequence of the entry of fluid into the intracellular environment.16,19 The exposure to the HF diet promotes oxidative damage in the testis, resulting in lipid peroxidation of the sperm membrane.51,52 Therefore, once again, the CAF diet interfered negatively this sperm parameter, which was attenuated with resveratrol treatment.
Spermatogenesis can also be studied by testicular morphology.58 Although we did not find differences in testicular weight or volume, seminiferous tubule diameter, Vv of the tunica propria, intertubular compartment (interstitial space), blood vessels, stroma, or in serum testosterone concentrations, we did find that the CAF diet reduced the height of the seminiferous epithelium and was not affected by treatment with resveratrol. As in a HF diet, the CAF diet that we made in the laboratory is partially composed of cholesterol, a constitutive molecule of cellular membranes in animals. The increased intake of this type of the fat may have impaired the physical and chemical properties of the plasma membrane and thus compromised spermatogenesis. It is likely that the lack of a positive effect from resveratrol is linked to the time of the experiment. Perhaps, if we had increased the study time, this morphological alteration of the testicle might have been attenuated.
As mentioned previously, the Vv of the seminiferous epithelium was reduced in all experimental groups except for the control group. In addition, treatment with resveratrol promoted an increase in the Vv of the tubular lumen in both the control and CAF groups. Interpreting these data separately is difficult, given the scarcity of works in the literature. However, when we evaluated the Vv of the tubular compartment as a whole, we observed that the CAF diet caused a reduction in this parameter compared with that of the group fed a control diet; once again, treatment with resveratrol reversed this condition. The tubular compartment is fundamental for the occurrence of spermatogenesis. Germ and Sertoli cells are found in this compartment, which are responsible for regulating spermatogenesis and performing various functions in sperm production.59,60 It is known that hyperenergetic diets can cause sperm damage.51 However, we did not know how the CAF diet affects testicular morphology or causes this damage. Recently, evidence for a relation between reproduction and the CAF diet was presented by Bazzano et al.,30 who showed that the consumption of a CAF diet from weaning to adulthood reduced reproductive capacity and altered ovarian function. Likewise, fetal programming studies have shown that female offspring from animals fed a CAF diet have a deficient reproductive system, with or without increased abdominal fat, and have associated metabolic changes.61 Thus, we believe that injury to the tubular compartment in our work is one of the main morphological alterations related to the impairment of spermatogenesis and reproductive function.
CONCLUSION
The consumption of a cafeteria-style diet, although it did not modify BM, promoted hyperglycemia and compromised spermatogenesis as demonstrated by alterations in testicular morphology and sperm parameters. Treatment with resveratrol was able to mitigate these areas of damage, encouraging the scientific community to use it as an adjunctive therapy in testicular cell dysfunction.
AUTHOR CONTRIBUTIONS
FAO carried out the conception and wrote the manuscript. WSC participated in the statistical analysis and interpretation. FJBS participated in its coordination. BMG conceived the study and revised the manuscript. All authors read and approved the final manuscript and agreed with the order of presentation of the authors.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by Brazilian agencies FAPERJ (E-26/010.002569/2014). We would like to thank Priscila Fernandes dos Santos for her technical assistance.
REFERENCES
- 1.Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Terra X, et al. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br J Nutr. 2017;117:218–29. doi: 10.1017/S0007114516004608. [DOI] [PubMed] [Google Scholar]
- 2.Sampey PB, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–17. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savastano MD, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135:1953–9. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 4.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, et al. Propensity to highfat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetti L, Leone S, Chiavaroli A, Orlando G, Recinella L, et al. Cafeteria diet increases prostaglandin E2 levels in rat prostate, kidney and testis. Int J Immunopathol Pharmacol. 2010;23:1073–8. doi: 10.1177/039463201002300411. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res. 2000;33:819–30. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh CA, Karimi F, Jorsaraei SG. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran Red Crescent Med J. 2013;15:780–5. doi: 10.5812/ircmj.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arafa M, Agarwal A, Al Said S, Majzoub A, Sharma R, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2017;50 doi: 10.1111/and.12881. Doi: 10.1111/and.12881 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. 2017;11:IE01–5. doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carillon J, Romain C, Bardy G, Fouret G, Feillet-Coudray C, et al. Cafeteria diet induces obesity and insulin resistance associated with oxidative stress but not with inflammation: improvement by dietary supplementation with a melon superoxide dismutase. Free Radic Biol Med. 2013;65:254–61. doi: 10.1016/j.freeradbiomed.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Doshi SB, Khullar K, Sharma RK, Agarwal A. Role of reactive nitrogen species in male infertility. Reprod Biol Endocrinol. 2012;10:109. doi: 10.1186/1477-7827-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko EY, Sabanegh ES, Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102:1518–27. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Vitaglione P, Ottanelli B, Milani S, Morisco F, Caporaso N, et al. Dietary trans-resveratrol bioavailability and effect on CCl4-induced liver lipid peroxidation. J Gastroenterol Hepatol. 2009;24:618–22. doi: 10.1111/j.1440-1746.2008.05598.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang HJ, Wang Q, Lv ZM, Wang CL, Li CP, et al. Resveratrol appears to protect against oxidative stress and steroidogenesis collapse in mice fed high-calorie and high cholesterol diet. Andrologia. 2015;47:59–65. doi: 10.1111/and.12231. [DOI] [PubMed] [Google Scholar]
- 15.Ramprasath VR, Jones PJ. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660–8. doi: 10.1038/ejcn.2010.77. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro CT, Milhomem R, de Souza DB, Costa WS, Sampaio FJ, et al. Effect of antioxidants on outcome of testicular torsion in rats of different ages. J Urol. 2014;191:1578–84. doi: 10.1016/j.juro.2013.09.066. [DOI] [PubMed] [Google Scholar]
- 17.Seed J, Chapin RE, Clegg ED, Dostal LA, Foote RH, et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: a consensus report. ILSI Risk Science Institute Expert Working Group on Sperm Evaluation. Reprod Toxicol. 1996;10:237–44. doi: 10.1016/0890-6238(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 18.Motrich RD, Rivero VE. Effect of tamoxifen treatment on the semen quality and fertility of the male rat. Fertil Steril. 2007;88:452–61. doi: 10.1016/j.fertnstert.2006.11.196. [DOI] [PubMed] [Google Scholar]
- 19.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 20.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 21.Ariyaratne HB, Chamindrani Mendis-Handagama S. Changes in the testis interstitium of Sprague Dawley rats from birth to sexual maturity. Biol Reprod. 2000;62:680–90. doi: 10.1095/biolreprod62.3.680. [DOI] [PubMed] [Google Scholar]
- 22.Pranprawit A, Wolber FM, Heyes JA, Molan AL, Kruger MC. Short-term and long-term effects of excessive consumption of saturated fats and/or sucrose on metabolic variables in Sprague Dawley rats: a pilot study. J Sci Food Agric. 2013;93:3191–7. doi: 10.1002/jsfa.6240. [DOI] [PubMed] [Google Scholar]
- 23.Higa TS, Spinola AV, Fonseca-Alaniz MH, Evangelista FS. Comparison between cafeteria and high-fat diets in the induction of metabolic dysfunction in mice. Int J Physiol Pathophysiol Pharmacol. 2014;6:47–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Castro H, Pomar CA, Sánchez J, Palou A. Cafeteria diet overfeeding in young male rats impairs the adaptive response to fed/fasted conditions and increases adiposity independent of body weight. Int J Obes. 2015;39:430–7. doi: 10.1038/ijo.2014.125. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Smith M, Karthikeyan S, Jeffers MS, Janik R, Thomason LA, et al. A physiological characterization of the Cafeteria diet model of metabolic syndrome in the rat. Physiol Behav. 2016;167:382–91. doi: 10.1016/j.physbeh.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Pons Z, Margalef M, Bravo FI, Arola-Arnal A, Muguerza B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br J Nutr. 2017;117:200–8. doi: 10.1017/S0007114516004426. [DOI] [PubMed] [Google Scholar]
- 27.Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–63. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Zorita S, Fernandez-Quintela A, Macarulla MT, Aguirre L, Hijona E, et al. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107:202–10. doi: 10.1017/S0007114511002753. [DOI] [PubMed] [Google Scholar]
- 30.Bazzano MV, Torelli C, Pustovrh MC, Paz DA, Elia EM. Obesity induced by cafeteria diet disrupts fertility in the rat by affecting multiple ovarian targets. Reprod Biomed Online. 2015;31:655–67. doi: 10.1016/j.rbmo.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, Zhou R, Wang B, Mi MT. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014;99:1510–9. doi: 10.3945/ajcn.113.082024. [DOI] [PubMed] [Google Scholar]
- 32.Tan Z, Zhou LJ, Mu PW, Liu SP, Chen SJ, et al. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J Nutr Biochem. 2012;23:1716–24. doi: 10.1016/j.jnutbio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145–54. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Chen LL, Zhang HH, Zheng J, Hu X, Kong W, et al. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation. Metabolism. 2011;60:1598–609. doi: 10.1016/j.metabol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Burgess TA, Robich MP, Chu LM, Bianchi C, Sellke FW. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg. 2011;146:556–64. doi: 10.1001/archsurg.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–6. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 37.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–10. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 38.Johnson AR, Wilkerson MD, Sampey BP, Troester MA, Hayes DN, et al. Cafeteria diet-induced obesity causes oxidative damage in white adipose. Biochim Biophys Res Commun. 2016;473:545–50. doi: 10.1016/j.bbrc.2016.03.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigueras-Villaseñor RM, Rojas-Castañeda JC, Chávez-Saldaña M, Gutiérrez-Pérez O, García-Cruz ME, et al. Alterations in the spermatic function generated by obesity in rats. Acta Histochem. 2011;113:214–20. doi: 10.1016/j.acthis.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function in sickness and in health. J Androl. 2012;33:1096–106. doi: 10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- 41.Agarwal A, Mulgund A, Sharma R, Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014;60:206–16. doi: 10.3109/19396368.2014.918675. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Zhao HX, Huang XF, Chen GW, Yang ZX, et al. Does high load of oxidants in human semen contribute to male factor infertility? Antioxid Redox Signal. 2012;16:754–9. doi: 10.1089/ars.2011.4461. [DOI] [PubMed] [Google Scholar]
- 43.Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, et al. Differences in blood and sêmen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300–6. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. A randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104:318–24. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Shrilatha B, Muralidhara Occurrence of oxidative impairments, response of antioxidant defences and associated biochemical perturbations in male reproductive milieu in the streptozotocin-diabetic rat. Int J Androl. 2007;30:508–18. doi: 10.1111/j.1365-2605.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 47.Shrilatha B, Muralidhara Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007;23:578–87. doi: 10.1016/j.reprotox.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Mallidis C, Agbaje I, Rogers D, Glenn J, McCullough S, et al. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: prevalence in men with diabetes mellitus. Hum Reprod. 2007;22:2169–77. doi: 10.1093/humrep/dem156. [DOI] [PubMed] [Google Scholar]
- 49.Agbaje IM, McVicar CM, Schock BC, McClure N, Atkinson AB, et al. Increased concentrations of the oxidative DNA adduct 7,8-dihydro-8-oxo-2-deoxyguanosine in the germ-line of men with type 1 diabetes. Reprod Biomed Online. 2008;16:401–9. doi: 10.1016/s1472-6483(10)60602-5. [DOI] [PubMed] [Google Scholar]
- 50.Abdelali A, Al-Bader M, Kilarkaje N. Effects of trans-resveratrol on hyperglycemia-induced abnormal spermatogenesis, DNA damage and alterations in poly (ADP-ribose) polymerase signaling in rat testis. Toxicol Appl Pharmacol. 2016;311:61–73. doi: 10.1016/j.taap.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Fullston T, Palmer NO, Owens JA, Mitchell M, Bakos HW, et al. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27:1391–400. doi: 10.1093/humrep/des030. [DOI] [PubMed] [Google Scholar]
- 52.Fullston T, McPherson NO, Owens JA, Kang WX, Sandeman LY, et al. Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep. 2015;3:e12336. doi: 10.14814/phy2.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sexton WJ, Jarow JP. Effect of diabetes mellitus upon male reproductive function. Urology. 1997;49:508–13. doi: 10.1016/s0090-4295(96)00573-0. [DOI] [PubMed] [Google Scholar]
- 54.Ourique GM, Finamor IA, Saccol EM, Riffel AP, Pes TS, et al. Resveratrol improves sperm motility, prevents lipid peroxidation and enhances antioxidant defences in the testes of hyperthyroid rats. Reprod Toxicol. 2013;37:31–9. doi: 10.1016/j.reprotox.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Faid I, Al-Hussaini H, Kilarkaje N. Resveratrol alleviates diabetes-induced testicular dysfunction by inhibiting oxidative stress and c-Jun N-terminal kinase signaling in rats. Toxicol Appl Pharmacol. 2015;3:482–94. doi: 10.1016/j.taap.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Javkhedkar AA, Quiroz Y, Rodoriguez-Iturbe B, Vaziri ND, Lokhandwala MF, et al. Resveratrol restored Nrf2 function, reduced renal inflammation and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2015;10:R840–6. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagul PK, Banerjee SK. Application of resveratrol in diabetes: rationale, strategies and challenges. Curr Mol Med. 2015;15:312–30. doi: 10.2174/1566524015666150505155702. [DOI] [PubMed] [Google Scholar]
- 58.Curtis SK, Amann RP. Testicular development and establishment of spermatogenesis in Holstein bulls. J Anim Sci. 1981;6:1645–57. doi: 10.2527/jas1982.5361645x. [DOI] [PubMed] [Google Scholar]
- 59.França LR, Russell LD. Madrid: Churchill Communications Europe España; 1998. The testis of domestic mammals. Male reproduction: a multidisciplinary overview; pp. 197–219. [Google Scholar]
- 60.Russell LD, Ettlin RA, Sinha AP, Clegg ED. Bolesta: Cache River Press; 1990. Mammalian spermatogenesis. Histological and histopathological evaluation of the testis; pp. p155–62. [Google Scholar]
- 61.Jacobs S, Teixeira DS, Guilherme C, da Rocha CF, Aranda BC, et al. The impact of maternal consumption of cafeteria diet on reproductive function in the offspring. Physiol Behav. 2014;129:280–6. doi: 10.1016/j.physbeh.2014.03.003. [DOI] [PubMed] [Google Scholar]


