Abstract
Background
Diabetic nephropathy (DN), which is one of the primary causes of end-stage renal disease (ESRD), is increasingly diagnosed in patients due to the continuous increase in the prevalence of diabetic mellitus (DM). Astragali Radix, a traditional Chinese herb, is widely administrated to ameliorate the symptoms of diabetes and diabetic nephropathy, but its mechanism is still not yet fully defined. Calycosin (C16H12O5) is the major active component of Astragali Radix. In this study, we analyzed the role of calycosin in diabetic nephropathy and explored its underlying mechanism.
Material/Methods
Cell activation, inflammatory cytokines expression and secretion, and protein levels were analyzed in cultured mouse tubular epithelial cells (mTEC). db/db mice were intraperitoneally injected with 10 mg/(kg·d) calycosin or control saline for 4 weeks, followed by analysis of structure injury, inflammation, and NF-κB signaling activity.
Results
Our results indicated that TNF-α and IL-1β were significantly induced by advanced glycation end-products (AGEs), but calycosin remarkably reduced the expression of TNF-α and IL-1β in the cultured mouse tubular epithelial cells (mTEC). Calycosin effectively alleviated kidney injury in diabetic kidneys of db/db mice during the progression of diabetic renal injury, indicated by the reduction of histological injury and immunohistochemical of inflammatory cytokines. Mechanistically, we identified calycosin inhibited diabetes-induced inflammation in kidneys by suppressing the phosphorylation of IKBα and NF-κB p65 in vitro and in vivo.
Conclusions
Calycosin significantly ameliorated diabetes-induced renal inflammation in diabetic renal injury by inhibition of the NF-κB-dependent signaling pathway in vivo and in vitro.
MeSH Keywords: Diabetic Nephropathies; Inflammation; Medicine, Chinese Traditional
Background
Type 2 diabetes mellitus (T2DM) is a major metabolic disorder that causes a huge socioeconomic burden worldwide. Notably, T2DM is associated with a series of complications in multiple organ systems, and diabetic nephropathy (DN) is the most severe complication [1]. Currently, DN causes approximately 40% of chronic kidney disease (CKD) patients to develop end-stage renal disease (ESRD) per year [2]. Unfortunately, an effective therapy targeting DN is still not available.
Traditional Chinese medicine has been widely used for the treatment of diabetes and diabetic nephropathy. Several studies reported that Chinese herbal medicines were used to improve the conditions of patients with diabetic nephropathy, such as Tangshen formula (TSF). Further mechanistic studies revealed that TSF protects the kidneys by ABCA1-mediated renal cholesterol efflux [3,4]. Many studies have highlighted the benefits of herbal formulas containing natural flavonoids in the management of diabetes, as evidenced by the astragaloside IV-mediated inhibition of the cell apoptosis and inflammatory response induced by high glucose levels [5]. In the present study, we determined the therapeutic effects of calycosin, another Chinese herbal extract from the plant species Radix astragali (RA, also known as Huang Qi), on diabetic nephropathy. Radix astragali is widely administered to ameliorate the symptoms of diabetes as well as diabetic nephropathy, but its mechanism of action is not yet fully defined [6,7]. Calycosin (C16H12O5) is the major active component of Radix astragali. Previous studies reported that calycosin can rebalance advanced glycation end-products (AGEs)-induced glucose uptake dysfunction in vitro [8]. Our study found that calycosin suppressed renal inflammation by downregulating the phosphorylation of IKBα and NF-κB p65. We found that calycosin inhibited the phosphorylation of IKBα before inhibiting the phosphorylation of p65, which suggests that calycosin mainly inhibits the IKBα phosphorylation site. More importantly, a 4-week treatment with intraperitoneal injections of calycosin effectively ameliorated both renal inflammation and the kidney function of the db/db mice in vivo.
Material and Methods
Cell culture
Mouse tubular epithelial cells (mTEC) were grown in DMEM/F12 containing 10% FBS and maintained in an incubator supplied with 95% air and 5% CO2 at 37°C [9]. Cells were starved with 0.5% FBS for 12 h before stimulation. The herbal monomer was a gift from Dr. Peng Liu, obtained from Institute of Clinical Medical Sciences (Beijing, China) [3]. Calycosin was dissolved in a mixture of the solvents dimethyl sulfoxide (DMSO) and Tween-80 (at a ratio of 1: 4), and AGEs (50 μg/ml) was added to mTEC following calycosin treatment for 2 h. The preparation of the herbal drugs was authenticated and standardized according to the established guidelines in the Chinese Pharmacopoeia 2010.
MTT assay
A total of 2000 cells were seeded per well of a 96-well plate. The next day, the cells were treated with calycosin-containing medium for the indicated times. Then, MTT dye solutions (Affymetrix, USA) were applied for 4 h at 37°C, followed by the addition of 150 μl DMSO (Sigma-Aldrich, USA) to dissolve the purple formazan. The ODs of the lysates were measured at 570 nm, with a reference absorbance at 630 nm [10].
RNA extraction and quantitative real-time PCR
Total RNA was extracted from mTEC. Real-time PCR was performed with a machine (Option 2, Bio-Rad, Hercules, CA, USA) by using the IQ SYBR Green Supermix reagent (Bio-Rad). The primers used in this study were as follows: mouse IL-1β, forward 5′-TGCCACCTTTTGACAGTGATG-3′ and reverse 5′-AAGGTCCACGGGAAAGACAC-3′; TNF-α, forward 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ and reverse 5′-TGGGAGTAGACAAGGTACAACCC-3′ [11,12]. The housekeeping gene β-actin was used as an internal control. The ratio of the specific mRNA: β-actin mRNA was calculated using the 2−ΔCt method.
Enzyme-linked immunosorbent assay
Medium from stimulated mTEC cells was collected to detect cytokine production using an enzyme-linked immunosorbent assay kit. IL-1β and TNF-α were measured with a Quantikine ELISA Kit (R&D Systems) according to the product protocols [13].
Immunofluorescence staining
mTECs were plated on glass sections, and 4% paraformaldehyde was used to fix the cell. The primary antibodies used for immunofluorescence staining included IL-1β (sc-7884, Santa Cruz) and TNF-α (sc-1351, Santa Cruz), followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit and anti-goat secondary antibodies (81–6111, Invitrogen). All slides were mounted with DAPI-containing mounting medium and then analyzed with a fluorescence microscope (Leica Microsystems, Wetzlar, Germany) [14].
Animal models
The male db/db mice (16 weeks of age and 50–60 g body weight) were classified into 2 groups: the saline- and calycosin-treatment groups, with 8 mice per group. The normal littermates (db/m) were used as the control group. All mice were fed a standard diet and were housed under standard conditions, a 12-h/12-h light/dark cycle. Mice were sacrificed by intraperitoneal injection of ketamine/xylene. db/db mice in the calycosin treatment group received i.p. calycosin (10 mg/kg/day) or saline beginning at the age of 17 weeks, and all mice were sacrificed at the age of 20 weeks. All animal experiments were performed according to the guidelines approved by the Animal Ethics Committee of Southwest Medical University [13].
Histology and Immunohistochemistry
Kidney sections were fixed in 4% paraformaldehyde and then dewaxed and rehydrated. Afterwards, histology staining of the samples was conducted with the periodic acid-Schiff (PAS), periodic acid-silver methenamine (PASM), and Masson’s trichrome staining methods.
Immunohistochemistry was performed by using a microwave-based antigen retrieval technique. The primary antibodies used in this study included IL-1β (sc-7884, Santa Cruz), TNF-α (sc-1351, Santa Cruz), and phosphorylated NF-κB P65 (sc-33039, Santa Cruz). The nuclei were counterstained with hematoxylin [15].
Western blot analysis
Protein from the renal cortex and mTEC was extracted using the radio-immunoprecipitation assay (RIPA) lysis buffer. The antibodies used were phospho-NK-kB p65 (Ser536) (#3033, CST), NF-κB p65 (#3034, CST), phospho-IKBα (Ser32) (#2859, CST), and IkBα (#9242, CST). IRDye800-conjugated secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA) were used as secondary antibodies. Signals were detected using the LiCor/Odyssey infrared image system (LI-COR Biosciences, Lincoln, NE), and statistical analysis was performed using the Image J program.
Statistical analysis
All data are expressed as the mean ±SEM. The statistical analyses performed were one-way analysis of variance (ANOVA), followed by Newman-Keuls multiple comparison test using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Results
The chemical structure and safe dose of calycosin in vitro
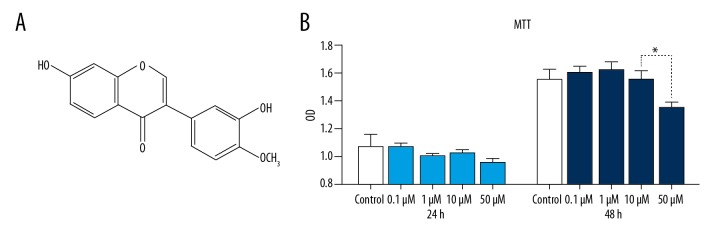
Calycosin (C16H12O5) was obtained from the Institute of Clinical Medical Sciences (Beijing, China). Calycosin (3′7-dihydroxy-4′-methoxyflavone) is an O-methylated isoflavone with a molecular formula of C16H12O5 and an average mass of 284.263 Da (Figure 1A) [16]. To avoid a toxic dose, an MTT assay was used to assess the cytotoxicity of calycosin in mouse tubular epithelial cells (mTEC). A significant decrease in cell viability was observed when the cells were treated with calycosin at doses above 10 μM (Figure 1B).
Figure 1.
The chemical formula and safe dosage of calycosin. (A) The chemical structure of calycosin. (B) Effects of calycosin on the proliferation of mouse tubular epithelial cells (mTEC) using an MTT assay. Each bar represents the mean ±SEM of at least 3 independent experiments. * p<0.05.
Calycosin inhibits the AGEs-induced inflammatory response in mTEC
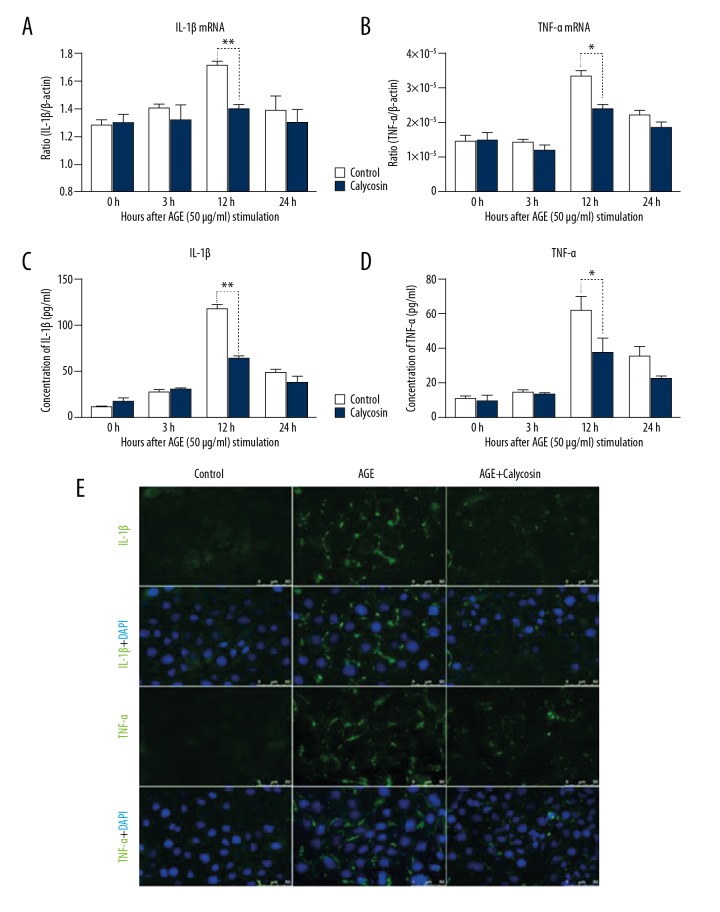
Increasing evidence demonstrates that the accumulation of AGEs contributes to the progression of DN through various pathways, including by promoting inflammation, oxidative stress damage, and cell apoptosis [17]. In this study, we chose AGEs as the stimulatory treatment for the mTEC. The results showed that inflammatory factors (i.e., IL-1β and TNF-α) were largely upregulated following AGEs (50 μg/ml) stimulation, as detected by real-time PCR (Figure 2A, 2B) and immunofluorescence (Figure 2E). Furthermore, we detected the secretion of these factors in the supernatant and found that AGEs significantly increased the section of IL-1β and TNF-α in mTEC (Figure 2C, 2D).
Figure 2.
Calycosin treatment inhibits AGEs-induced inflammatory responses in mTEC. (A, B) Real-time PCR quantified IL-1β and TNF-α expression after AGEs induction in mTEC. (C, D) Enzyme-linked immunosorbent assay (ELISA) detected IL-1β and TNF-α protein expression in the cell culture medium. (E) Protein levels of IL-1β and TNF-α in mTEC detected by immunofluorescence. Magnifications, ×400. Each bar represents the mean ±SEM of at least 3 independent experiments. * p<0.05, ** p<0.01.
Calycosin protects against diabetic renal injury in db/db mice
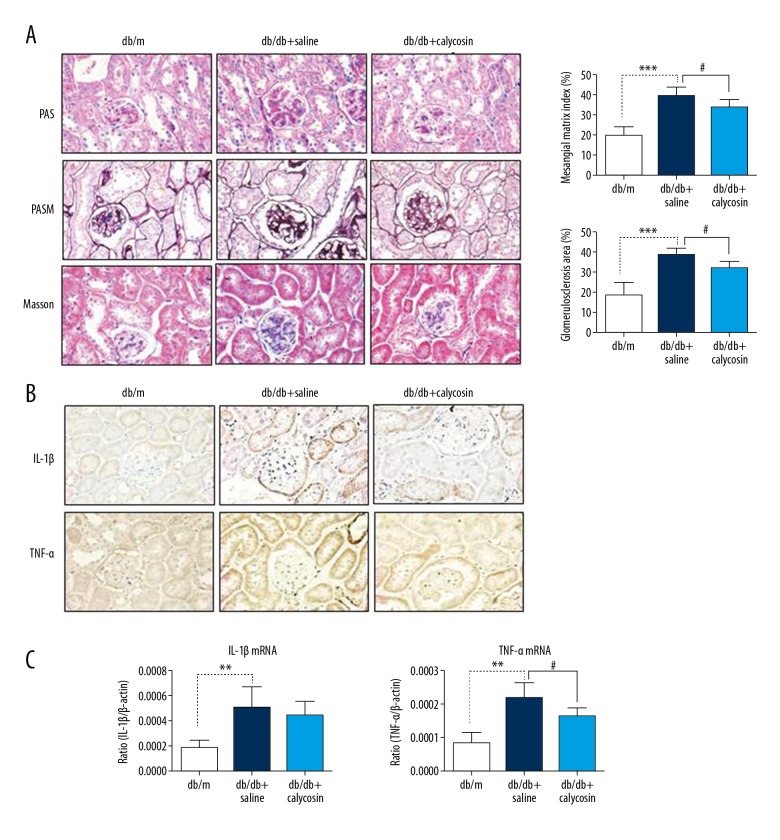
db/db mice are a well-known T2DM animal model characterized by an autosomal-recessive Leprdb mutation on chromosome 4. The therapeutic efficacy of calycosin was further determined in male db/db mice. Calycosin at 10 mg/(kg·d) was given to the drug group (n=8) through intraperitoneal injection from the age of 17 weeks to 20 weeks. The drug was dissolved in a solvent mixture of DMSO and Tween-80 (at a ratio of 1: 4), which was further diluted 20 times with saline as a working solution. Another 8 male db/db mice received an equal volume of saline. After 4 weeks of therapy, we found that calycosin significantly suppressed renal histological injury, including glomerular basement thickening, matrix deposition, and glomerular sclerosis, as determined by PAS, PASM, and Masson’s trichrome staining (Figure 3A). More importantly, immunohistochemistry and real-time PCR revealed a significant reduction in inflammatory markers (TNF-α and IL-1β) in the kidneys of 20-week-old db/db mice with calycosin treatment compared with the saline-treated db/db controls (Figure 3B, 3C).
Figure 3.
Calycosin treatment improves renal histology and inflammatory response in db/db mice. (A) PAS staining, PASM staining and Masson’s trichrome staining show changes in renal histology after calycosin treatment in the db/db kidney. Magnifications, ×400. (B) Immunohistochemistry and quantitative analysis of IL-1β and TNF-α expression. Magnifications, ×400. (C) Real-time PCR analysis of IL-1β and TNF-α mRNA expression in mouse kidneys at the age of 20 weeks. Each bar represents the mean ±SEM of 8 mice. ** p<0.01, *** p<0.001, # p<0.05.
Calycosin treatment effectively inhibits AGEs-driven inflammation via the NF-κB pathway in vivo and in vitro
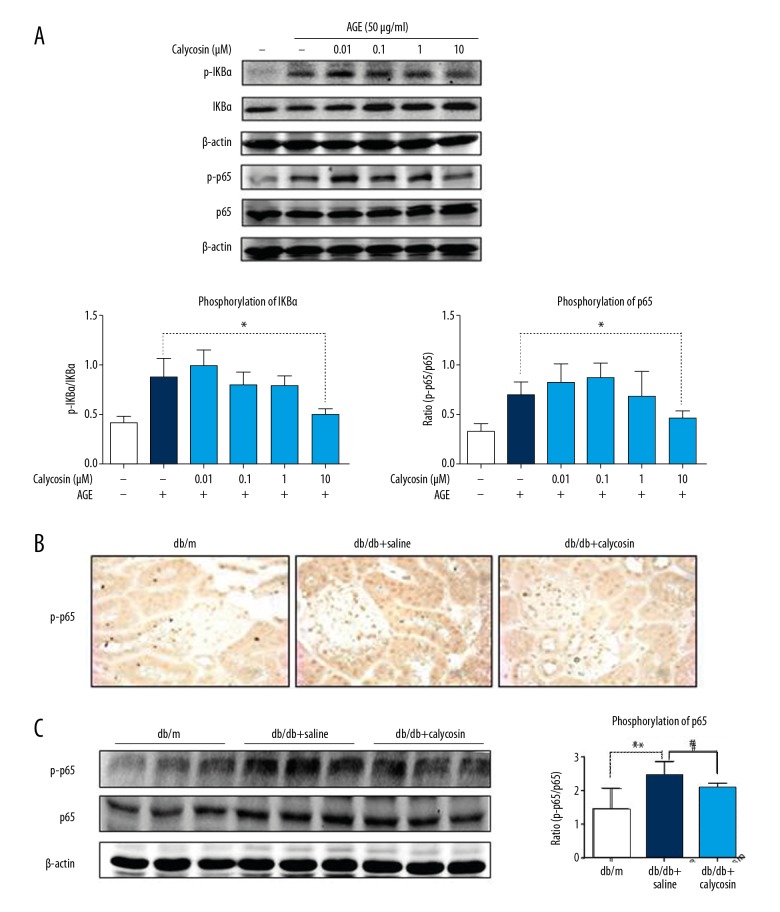
It is well known that DM is a low-grade inflammatory disease. Among the DM signalling pathways, the NF-κB pathway has been extensively reported to be involved in the inflammatory response [18–20]. Thus, we examined whether the protective effect of calycosin in db/db mice is associated with the NF-κB pathway. In the inflammatory response evaluation, Western blot analysis showed that calycosin (10 μM) inhibited the phosphorylation of IKBα and NF-κB p65 induced by AGEs in mTEC (Figure 4A). Additionally, we observed that calycosin inhibited the phosphorylation of IKBα (15 min after AGEs stimulation) more rapidly than it phosphorylated P65 (30 min after AGEs stimulation), which suggests that calycosin mainly inhibits the IKBα phosphorylation site. Using in vivo experiments, we confirmed these findings. Calycosin treatment downregulated the expression of phosphorylated NF-κB p65 by using immunohistochemical and Western blot analyses (Figure 4B, 4C).
Figure 4.
Calycosin treatment markedly inhibits NF-κB signalling in vivo and in vitro. (A) Western blot and quantitative analyses show the phosphorylation levels of IKBα and NF-κB in response to AGEs after calycosin treatment in mTEC. (B, C) Immunohistochemistry and Western blot analysis shows the phosphorylation level of NF-κB in db/db kidneys at 20 weeks of age. Each bar represents the mean ±SEM of at least 3 independent experiments or 8 mice. * p<0.05, # p<0.05.
Discussion
Diabetic nephropathy (DN), due to lack of efficient therapy, has become a major cause of ESRD worldwide. The main approaches to treating DN are ACEI/ARB [21,22]; however, these drugs are of limited use when patients progress to ESRD. Recently, new anti-diabetic agents have been sought to diminish diabetic complications, such as glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP4) inhibitors, and sodium-glucose co-transporter 2 inhibitors. Further studies and clinical trials are currently underway to uncover the potential mechanism. Chinese traditional medicine, deriving substances from plants, might become a useful choice combined with established conventional drugs (e.g., ACEI/ARB), as described above. Astragaloside IV, curcumin, and tanshinone have been reported to protect kidney injury under diabetic conditions [5,23–26]. In this study, we detected a monomer, named calycosin, in a Chinese medicine and found that this monomer ameliorated renal injury in db/db mice. Mechanistically, we determined that calycosin exerts its biological function via the inhibition of inflammation.
First, we identified the effect of calycosin under AGEs-rich conditions, and found that TNF-α and IL-1β were significantly induced by these metabolic by-products (AGEs) on cultured mTEC, and calycosin inhibited inflammatory cytokine secretion. These results suggest that calycosin blunts the inflammatory response that occurs during the progression of DN. The pathogenesis of DN is complex and involves hemodynamics, glycation metabolism, polyol pathway/hexosamine signalling, oxidative stress, and low-grade inflammation [27]. Increasing evidence suggests that inflammation is a critical process in the development of diabetes complications, as evidenced by the commonly elevated levels of serum interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and C-reactive protein (CRP) in patients with T2DM [20]. Additionally, we found that calycosin specifically affects the diabetic kidney and may become a novel therapy for T2DN. Intraperitoneal injection of calycosin for 4 weeks largely blunted renal inflammation (e.g., TNF-α and IL-1β), thereby resulting in improved renal function in the db/db mice.
More importantly, we further uncovered the potential mechanisms. NF-κB activation is a critical mechanism of the inflammatory cascade in the development of diabetic kidney disease [28]. The phosphorylation of the NF-κB inhibitor releases the NF-κBp50/p65 subunits, resulting in the nuclear accumulation and transcriptional regulation of the target genes [29]. IKBα, which is upstream of NK-κB, degrades and prevents the activation of NF-κB signalling. Unexpectedly, we found that calycosin inhibits the inflammatory response not only by inhibiting IKBα, but also by inhibiting the NF-κB pathway, as evidenced by calycosin treatment, which inhibits the phosphorylation of IKBα and NF-κB in vivo and in vitro. We found that calycosin inhibited the phosphorylation of IKBα earlier than it phosphorylated P65, suggesting that calycosin mainly inhibits the IKBα phosphorylation site. Calycosin reduced the degradation of IKBα, decreasing the exposed P65 phosphorylation site, which ultimately blunted the downstream inflammation induced by AGEs.
The present study shows that inflammation is crucial in the pathogenesis of DN. Calycosin can recover diabetic renal injury via an NF-κB-dependent inflammatory mechanism. Our findings suggest that calycosin might be an efficient therapy for diabetic kidney diseases.
Conclusions
Calycosin protected kidneys against inflammation injury through inhibition of NF-κB signaling. Our findings suggest that calycosin, as the major active component, is promising in treatment of diabetic nephropathy.
Footnotes
Source of support: This study is supported by the National Natural Science Foundation of China (No. 81873609, 81600523, and 81700617)
Conflict of interest
None.
References
- 1.Kato M, Natarajan R. Diabetic nephropathy – emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–30. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan CM, Nee R, Ceckowski KA, et al. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin Kidney J. 2017;10(2):257–62. doi: 10.1093/ckj/sfw112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P, Peng L, Zhang H, et al. Tangshen formula attenuates diabetic nephropathy by promoting ABCA1-mediated renal cholesterol efflux in db/db mice. Front Physiol. 2018;9:343. doi: 10.3389/fphys.2018.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao T, Sun S, Zhang H, et al. Therapeutic effects of tangshen formula on diabetic nephropathy in rats. PLoS One. 2016;11(1):e0147693. doi: 10.1371/journal.pone.0147693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Shao X, Xu W, et al. Astragalosides IV inhibits high glucose-induced cell apoptosis through HGF activation in cultured human tubular epithelial cells. Ren Fail. 2014;36(3):400–6. doi: 10.3109/0886022X.2013.867798. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Zhang RR, Li JH, et al. Radix Astragali lowers kidney oxidative stress in diabetic rats treated with insulin. Endocrine. 2012;42(3):592–98. doi: 10.1007/s12020-012-9670-7. [DOI] [PubMed] [Google Scholar]
- 7.Lu ZM, Yu YR, Tang H, Zhang XX. [The protective effects of Radix astragali and Rhizoma ligustici chuanxiong on endothelial dysfunction in type 2 diabetic patients with microalbuminuria]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2005;36(4):529–32. [in Chinese] [PubMed] [Google Scholar]
- 8.Xu Y, Xiong J, Zhao Y, et al. Calycosin rebalances advanced glycation end products-induced glucose uptake dysfunction of hepatocyte in vitro. Am J Chin Med. 2015;43(6):1191–210. doi: 10.1142/S0192415X15500688. [DOI] [PubMed] [Google Scholar]
- 9.Feng M, Tang PM, Huang XR, et al. TGF-beta mediates renal fibrosis via the Smad3-Erbb4-IR long noncoding RNA axis. Mol Ther. 2018;26(1):148–61. doi: 10.1016/j.ymthe.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai F, Makino T, Kono K, et al. Calycosin and formononetin from astragalus root enhance dimethylarginine dimethylaminohydrolase 2 and nitric oxide synthase expressions in Madin Darby Canine Kidney II cells. J Nat Med. 2013;67(4):782–89. doi: 10.1007/s11418-013-0749-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Huang XR, Yu J, et al. Long Noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther. 2015;23(6):1034–43. doi: 10.1038/mt.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv LL, Tang PM, Li CJ, et al. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017;91(3):587–602. doi: 10.1016/j.kint.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Sun SF, Tang PMK, Feng M, et al. Novel lncRNA Erbb4-IR promotes diabetic kidney injury in db/db mice by targeting miR-29b. Diabetes. 2018;67(4):731–44. doi: 10.2337/db17-0816. [DOI] [PubMed] [Google Scholar]
- 14.Lian GY, Wang QM, Tang PM, et al. Combination of asiatic acid and naringenin modulates NK cell anti-cancer immunity by rebalancing Smad3/Smad7 signaling. Mol Ther. 2018;26(9):2255–66. doi: 10.1016/j.ymthe.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You YK, Huang XR, Chen HY, et al. C-reactive protein promotes diabetic kidney disease in db/db mice via the CD32b-Smad3-mTOR signaling pathway. Sci Rep. 2016;6:26740. doi: 10.1038/srep26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Feng L, Wang S, et al. Calycosin protects HUVECs from advanced glycation end products-induced macrophage infiltration. J Ethnopharmacol. 2011;137(1):359–70. doi: 10.1016/j.jep.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Ott C, Jacobs K, Haucke E, et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/146154. 146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124(3):139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 20.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–40. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 21.Strippoli GF, Craig M, Deeks JJ, et al. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: Systematic review. BMJ. 2004;329(7470):828. doi: 10.1136/bmj.38237.585000.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren F, Tang L, Cai Y, et al. Meta-analysis: the efficacy and safety of combined treatment with ARB and ACEI on diabetic nephropathy. Ren Fail. 2015;37(4):548–61. doi: 10.3109/0886022X.2015.1012995. [DOI] [PubMed] [Google Scholar]
- 23.Tu X, Ye X, Xie C, et al. Combination therapy with chinese medicine and ACEI/ARB for the management of diabetic nephropathy: The promise in research fragments. Curr Vasc Pharmacol. 2015;13(4):526–39. doi: 10.2174/1570161112666141014153410. [DOI] [PubMed] [Google Scholar]
- 24.Sun LN, Yang ZY, Lv SS, et al. Curcumin prevents diabetic nephropathy against inflammatory response via reversing caveolin-1 Tyr14 phosphorylation influenced TLR4 activation. Int Immunopharmacol. 2014;23(1):236–46. doi: 10.1016/j.intimp.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Parsamanesh N, Moossavi M, Bahrami A, et al. Therapeutic potential of curcumin in diabetic complications. Pharmacol Res. 2018;136(10):181–93. doi: 10.1016/j.phrs.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wu R, Kong Y, et al. Tanshinone IIA attenuates renal damage in STZ-induced diabetic rats via inhibiting oxidative stress and inflammation. Oncotarget. 2017;8(19):31915–22. doi: 10.18632/oncotarget.16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan HY. Transforming growth factor-beta/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2012;39(8):731–38. doi: 10.1111/j.1440-1681.2011.05663.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen HY, Huang XR, Wang W, et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60(2):590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christian F, Smith EL, Carmody RJ. The regulation of NF-kappaB subunits by phosphorylation. Cells. 2016;5(1) doi: 10.3390/cells5010012. pii: E12. [DOI] [PMC free article] [PubMed] [Google Scholar]