Abstract
Childhood tuberculosis (TB) has a high incidence and prevalence in developing countries like India with tubercular meningitis (TBM) being the most common cause of death. Most cases of TBM are diagnosed late when despite adequate therapy; morbidity and mortality continue to remain high. This review aims to provide a pragmatic approach at dealing with cases of tubercular meningitis in children including clinical features, laboratory and radiological criteria, treatment options and prognostic implications. The objective of this review is to assist in early identification, proper investigation and timely treatment of TBM in children in order to reduce neurological morbidity and mortality associated with it.
KEYWORDS: Antitubercular therapy, tubercular meningitis, tuberculosis, ventriculoperitoneal shunt
Childhood tuberculosis (TB) constitutes approximately 10%–20% of all TB cases in India, causing almost 8%–20% of TB-related deaths. Twenty-five percent of the pediatric tubercular cases are extrapulmonary, with tubercular meningitis (TBM) being the most common cause of death because of TB.[1] It commonly affects children between 6 months and 4 years of age. TBM is a medical emergency. The delay in diagnosing and adequately treating this condition is associated with significant morbidity and mortality.
Q: WHEN TO SUSPECT TBM?
TBM is a subacute meningitic illness, which presents in various stages. The first stage consists of nonspecific symptoms of low-grade fever, headache, irritability, drowsiness, malaise, vomiting, photophobia, listlessness, and poor weight gain/weight loss. In infants, stagnation/loss of developmental milestones, bulging anterior fontanel, fever, cough, altered consciousness, and seizures may be present. Neck stiffness is characteristically absent. This lasts for approximately 1–2 weeks. The presence of nonspecific signs and symptoms makes it difficult to suspect and diagnose TBM in the first stage. A history of contact with an active patient of TB in children (~50% cases) can also be elicited.[1] The second stage is usually abrupt in onset. It is characterized by lethargy, neck rigidity, positive meningeal signs, hypertonia, seizures, vomiting, and focal neurological deficit(s). Development of hydrocephalus, raised intracranial pressure, encephalitis with disorientation/movement disorders/speech impairment, cranial nerve involvement (30%–50% cases) with the sixth nerve being the most common, and vision loss are also seen. Most patients are clinically diagnosed in this stage. This is followed by the third stage, which might be associated with decerebrate/decorticate posturing, hemiplegia, coma, and eventually death.
Q: HOW TO DIAGNOSE TBM?
In the absence of standardized diagnostic criteria, in 2010, a consensus case definition for TBM was proposed for use in future clinical research. These criteria are applicable irrespective of the patients’ age, human immunodeficiency virus (HIV) infection status, or resources available in the research setting. Patients are stratified as definite, probable, and possible diagnosis of TBM as per these criteria.[2] These are detailed as follows:
Clinical criteria
These include symptom duration >5 days (score: 4), systemic symptoms suggestive of TB (>1 of the following): weight loss/poor weight gain, night sweats, cough >2weeks (score: 2), history of recent (past 1 year) close contact with pulmonary TB or positive tuberculin sensitivity test or interferon (IFN) gamma release assays, only in children <10 years (score 2), focal neurological deficit (excluding cranial nerve palsies) (score: 1), cranial nerve palsy (score: 1), and altered consciousness (score: 1). The maximum category score is 6.
Cerebrospinal fluid criteria
These include clear appearance (score: 1), cells 10–500/µL (score: 1), lymphocytic predominance >50% (score: 1), protein concentration >1g/L (score: 1), cerebrospinal fluid (CSF) to plasma glucose ratio <50% or absolute CSF glucose <2.2 mmol/L (score: 1). The maximum category score is 4.
Cerebral imaging criteria
These include hydrocephalus (score: 1), basal meningeal enhancement (score: 2), tuberculoma (score: 2), infarct (score: 1), and pre-contrast: basal hyperdensity (score: 2). The maximum category score is 6.
Evidence of tuberculosis elsewhere
This includes chest radiograph suggestive of signs of active TB = 2, miliary TB = 4 (score: 2/4), computed tomography (CT)/magnetic resonance imaging (MRI)/ultrasound evidence for TB outside the central nervous system (CNS) (score: 2), acid-fast bacilli identified or Mycobacterium tuberculosis cultured from another source (i.e., sputum, lymph node, gastric washing, urine, and blood culture) (score: 4), and positive commercial M. tuberculosis nucleic acid amplification technique (NAAT) from extra neural specimen (score: 4). The maximum category score is 4.
The patient is labeled as a case of definitive TBM when the clinical criteria plus one or more of the following criteria are met: AFB seen in the CSF, M. tuberculosis cultured from CSF, or a CSF M. tuberculosis positive commercial NAAT from a patient who presents with symptoms or signs suggestive of meningitis; acid-fast bacilli seen in the context of histological changes consistent with TB in the brain or spinal cord together with suggestive symptoms or signs and CSF changes; or visible meningitis (on autopsy). A case of probable TBM is labeled when the clinical entry criteria plus a total diagnostic score is of 10 or more points (when cerebral imaging is not available) or 12 or more points (when cerebral imaging is available) plus exclusion of alternative diagnoses. At least 2 points should either come from CSF or from cerebral imaging criteria. Clinical entry criteria plus a total diagnostic score of 6–9 points (when cerebral imaging is not available) or 6–11 points (when cerebral imaging is available) plus exclusion of alternative diagnoses is used to label a case of possible TBM. Hence, a possible TBM cannot be diagnosed or excluded without performing a lumbar puncture or cerebral imaging.
This consensus case definition provides a uniform approach to TBM with the available resources, to ensure a diagnostic certainty of the highest possible standard and to standardize definitions and establish uniformity for direct comparison in future studies.[3]
Q: WHAT IS THE SIGNIFICANCE OF CSF ANALYSIS IN TBM?
A classic case of TBM usually presents with a CSF of 10–500 cells/µL that are polymorphs initially and lymphocytes later. A low glucose <40mg/dL (rarely <20mg/dL) or a CSF/plasma glucose ratio <50% or a high-protein content (400–5000mg/dL) is suggestive of the diagnosis of TBM. The CSF lactate levels are usually raised to 5–10 mmol/L (normal range, 1.2–2.1 mmol/L).[4]
Ziehl–Neelsen (ZN) staining for the smear examination has a sensitivity of approximately 50%, whereas a bacterial culture of 60%–70%.[5] The aforementioned CSF findings are not specific for TBM and can be seen in other conditions including non-mycobacterial tuberculosis (MTB) bacterial meningitis, fungal meningitis, carcinomatous meningitis, and subarachnoid hemorrhage. For all patients with suspected TBM, CSF samples should be examined by ZN staining for acid-fast bacilli, Gram staining for bacteria, India ink preparations for fungi, and antigen testing for Cryptococcus neoformans as other causes may be present with a similar CSF picture or because of the presence of a dual infection. The yield of the CSF examination can be increased by some simple measures such as taking at least 10mL CSF sample, carrying out repeated sample examination, and performing a lumbar puncture before or shortly after starting the treatment. Also, it needs to be centrifuged at a high centrifugal force for 20min followed by a careful examination for at least 20min.
In a recent study conducted, the best performing biochemical parameter for “ruling in” TBM was adenosine deaminase (ADA), with a specificity of 95% at a cutoff point of >6U/L; however, the sensitivity of ADA was low (55%).[6] The mean CSF ADA activity in patients with TBM was significantly higher than patients of non-TBM group with P < 0.01. A cutoff value of 10 U/L for patients with TBM gave a sensitivity and specificity of 90.62% and 95.65%, respectively; thus proving that CSF ADA activity is a rapid and affordable adjunct in differentiating TBM from non-TBM.[7] CSF ADA measurements have been found to be useful in predicting poor neurological outcomes among pediatric TBM cases.[8]
A recent diagnostic advance is Xpert MTB/ rifampicin (RIF), an automated rapid nucleic acid amplification test for MTB endorsed by the World Health Organization (WHO) in December 2010. It employs three specific primers and five unique molecular probes to detect MTB and RIF resistance simultaneously in almost less than 2h. However, the role of Xpert MTB/RIF in diagnosing TBM remains limited. This is because even though a positive result confirms the diagnosis of TBM but a negative result cannot be used to “rule out” TBM.
These molecular methods are more useful when anti tuberculosis drugs have already been started.[9]
The routinely measured CSF parameters are almost similar in HIV positive and negative patients with TBM.[10] A few studies report lower CSF leukocyte count and protein level in HIV-positive patients. CSF examination may even be normal in 5% of HIV-positive patients with TBM.
Q: WHAT ARE OTHER DIAGNOSTIC TESTS IN TBM?
These include the ancillary tests that help us to reach to the diagnosis of TBM.
Tuberculin skin test (Mantoux test): It may be nonreactive in 50% cases of CNS TB. Hence, it is helpful in supporting the diagnosis of TBM when positive, but an isolated positive Mantoux cannot be used to label a case of TBM as false positive/false negative reactions are commonly known.[11,12]
Chest X-ray: It helps to localize the signs of active TB but it may be normal in 20%–50% of the cases of TBM.
Measurement of IFN-γ released by lymphocytes: It is a specific (70%–90%) test but with a low sensitivity (50%–70%). It is available as enzyme-linked immunospot (ELISpot) and QuantiFERON Gold for the diagnosis of latent TB. But currently, the use of these tests is restricted in the developing countries because of the high cost.
Xpert MTB/RIF assay[13]: With a sensitivity of 67%–85% and a specificity of 94%–98%, it is a definitive diagnostic test that uses real-time polymerase chain reaction to amplify and detect M. tuberculosis and identifies drug resistance. A meta-analysis of studies reported up to October 2011 estimated that Xpert MTB/RIF was 80.4% sensitive compared with culture. A study of Xpert MTB/RIF in India for the diagnosis of extrapulmonary TB included 142 CSF samples and reported that the assay was nearly 12 times more sensitive than microscopy. The cost of processing one Xpert MTB/RIF test, however, was 82 times higher than the cost of microscopy. Larger studies to assess Xpert MTB/RIF for the diagnosis of TBM are urgently needed. It may be used as an adjunctive test for TBM.
Q: WHICH IS THE PREFERRED NEUROIMAGING IN TBM: CT VERSUS MRI?
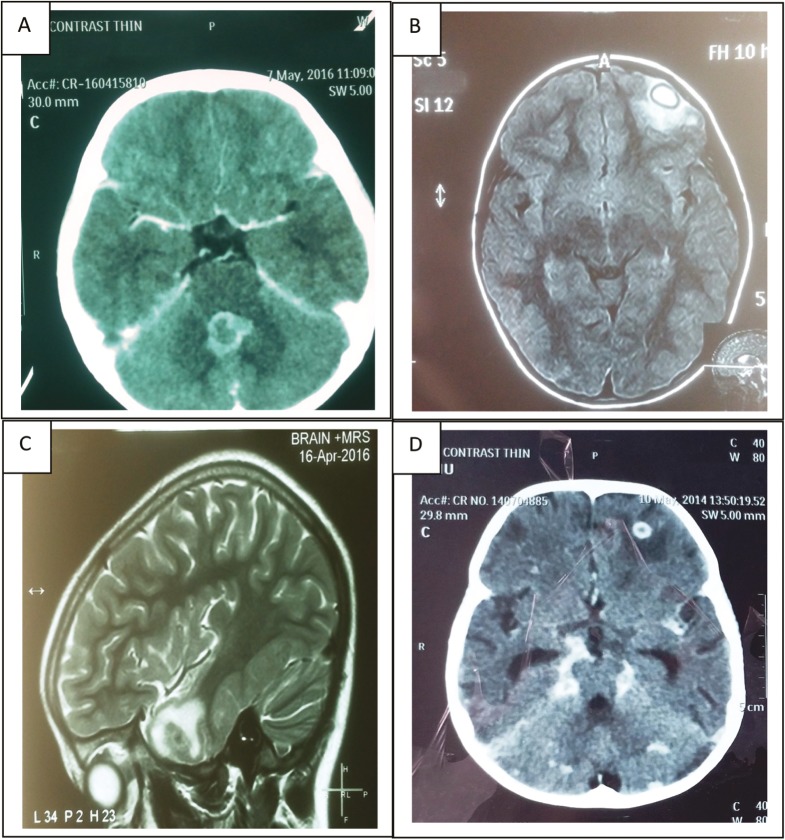
In the various studies conducted over a period, it has been proved that MRI has a higher sensitivity than a CT scan. A CT scan may itself be normal initially in almost 30% of the cases. Hence, a normal neuroimaging initially does not rule out the possibility of TBM. The usual findings on neuroimaging seen in TBM are depicted in [Figure 1] and enumerated in [Table 1].[14,15,16]
Figure 1.
(A) CECT Head (Axial) showing conglomerate peripherally enhancing lesions in the region of the vermis, largest measuring 14.7 X 16.2 mm in size and causing mass effect on the fourth ventricle suggestive of tuberculomas (B) CEMRI Brain (Axial) showing a well defined smooth walled oval lesion measuring 15x 13 x 12 mm in size, appearing hyperintense on FLAIR with hypointense rim in left frontal lobe. The lesion showed restricted diffusion in its peripheral part with thick ring enhancement on post contrast scans. Moderate perilesional edema was also noted suggestive of tubercular granulomatous lesion with abscess formation (C) CEMRI brain (sagittal) showing few small T2 hypointense conglomerate lesions in left temporal lobe with post-contrast ring enhancement and moderate perilesional edema. No restricted diffusion noted on diffusion restriction imaging and subtle blooming on gradient recalled echo images. On magnetic resonance spectroscopy, choline/creatinine 2.38 with mildly elevated lipid peak suggestive of tuberculomas. (D) CECT head (axial) showing basal exudates with ring-enhancing lesion (13×12mm in size) in right frontal region along with noncommunicating hydrocephalus suggestive of tubercular meningitis with tuberculoma in right frontal region
Table 1.
| Basal meningeal enhancement | 75% |
| Infarcts | 8%–44% |
| Communicating hydrocephalus | 80% |
| Tuberculomas | 8%–31% |
Other findings on contrast-enhanced MRI include early infarcts, border zone encephalitis, and cranial neuropathies (optochiasmatic arachnoiditis) with raised lipid peaks on magnetic resonance spectroscopy.
Q: WHICH STEROID TO USE IN TBM: DEXAMETHASONE VERSUS PREDNISOLONE?
Prednisolone 4mg/kg/day for almost 4 weeks initially, gradually tapering over the next 2–3 weeks, and dexamethasone 1–6mg/kg/day initially and then reducing each week to stop over a total of 6–8 weeks have traditionally been used. No data exist to compare the relative efficacy of dexamethasone with prednisolone, but they are widely regarded as equivalent for the treatment of TBM. Either of them can be used based on the choice of ease of administration and availability.
Q: WHAT IS PREFERRED ANTI TUBERCULAR DRUGS (ATT) REGIME: DAILY ATT OR DIRECTLY OBSERVED TREATMENT, SHORT COURSE?
As per the recent Revised National TB Control Program (RNTCP) guidelines, 2016,[17] a daily regimen with fixed-dose combination of the first-line antitubercular drugs has been proposed. For the newly diagnosed TB cases, treatment in the intensive phase will consist of 8 weeks of isoniazid, RIF, pyrazinamide, and ethambutol in daily dosages as per the four weight band categories. There will be no need for extension of the intensive phase. Only pyrazinamide will be stopped in the continuation phase, whereas the rest will be continued as daily dosages.
Q: HOW LONG SHOULD ONE GIVE ANTITUBERCULAR THERAPY IN TBM?
The duration of treatment has always remained controversial. According to the American Thoracic Society/Center for Disease Control and Prevention (CDC)/Infectious Diseases Society of America (2003) recommendations, a 9–12 month regimen is recommended. The British Infection Society (2009) recommendations suggest a minimum of 12 months, whereas the WHO (2010) recommendations warrant 9–12 months of treatment to decrease the risk of disability and mortality.
Q: WHAT IS ATT DOSAGE RECOMMENDATION, SIDE EFFECTS, AND MONITORING PROTOCOL?
The following are the doses to be prescribed in a case of TBM[18]:
Rifampicin: 10–15mg/kg/day (max. 600mg)
Isoniazid: 10–15mg/kg/day (max. 300mg)
Pyrazinamide: 30–35mg/kg/day (max. 2g)
Ethambutol: 20mg/kg/day (max. 1g)
Prednisolone: 2–4mg/kg/day for 4–6 weeks followed by tapering over next 2–3 weeks
Clinical follow-up
A clinical follow-up18 must be carried out at least monthly. Improvement of symptoms, weight gain, and monitoring for any adverse reaction to the ATT must be carried out.
Laboratory investigation
For associated pulmonary tubercular cases, a sputum smear examination at the end of the intensive phase and at the end of the treatment must be carried out. However, in case of any clinical deterioration, it can be carried out anytime even during the continuation phase. A chest X-ray should be offered whenever required and available. Liver function tests should be obtained at baseline, 2, 4, 6, and 8 weeks and then monthly for the first several months of treatment. HIV testing in all cases of TB must be conducted.
Long-term follow-up
A long-term follow-up at the end of 6, 12, 18, and 24 months is advised.
While on ATT, the child should be monitored for side effects as detailed in Table 2.
Table 2.
Side effects of ATT (original)
| Drugs | Hepatotoxicity | Other side effects |
|---|---|---|
| Isoniazid | + | Peripheral neuritis, hypersensitive reactions, drug-induced lupus, psychotic changes |
| Rifampicin | + | Gastrointestinal, autoimmune reactions including flu-like syndrome, thrombocytopenia, purpura, respiratory shock syndrome, ARF, acute hemolytic anemia |
| Pyrazinamide | + | Arthralgia, hyperuricemia, gastrointestinal, allergic reactions |
| Ethambutol | Optic neuritis, color blindness, gastrointestinal, allergic reactions, hyperuricemia | |
| Streptomycin | Vestibular dysfunction, deafness, nephrotoxicity, neuromuscular blockade, peripheral neuritis |
Q: WHAT IS RNTCP GUIDELINES UPDATE (INDIAN GOVERNMENT)?
The salient features of RNTCP guidelines and its updated version have been compared in Table 3.
Table 3.
Comparison of salient features of RNTCP guidelines and its updated version (original)
| RNTCP guidelines update | RNTCP guidelines |
|---|---|
| Daily regimen | Intermittent regimen |
| Ethambutol in continuation phase (category I and II) | Ethambutol in continuation phase of category II only |
| Fixed-dose combination as per the weight band | No fixed-dose combination |
| No need of extension of the intensive phase | Extension of the intensive phase for 1 month if sputum is positive |
| Follow-up clinical and laboratory | Follow-up laboratory only |
| Long-term follow-up for 2 years | No long-term follow-up |
RECENT MODIFICATION IN NATIONAL TUBERCULOSIS CONTROL PROGRAM INDIA
As per the recent communication by Director General Health TB Division, India the current regimen for previously treated TB patients (known as Category II (CAT II) has been suspended with effect from 20th December 2018. All previously treated TB patients are to be started on standard first line Anti TB Regimen (2HRZE/4HRE) as prescribed for new TB patients. However, categorization of ‘Previously treated TB patient’ and sub-categorization of ‘Relapse, Failure, Treatment after lost to follow up, Other Previously Treated’ shall continue to be practiced.
The following reasons have been postulated for this change:
Poor outcomes among patients on category II therapy
High rates of streptomycin resistance among previously treated patients
Lack of evidence to support category II
Widespread access to both drug susceptibility esting and treatment for drug-resistant TB.
Drug susceptibility testing (DST) should be conducted for at least rifampicin (R) for all notified previously treated TB patients at the start of treatment. Patients with a history of previous treatment who have a pan-susceptible disease should receive HRZE (first-line drugs) and those with documented resistance (including mono-resistance) should receive a regimen based on the drug susceptibility test results.
However, the results of DST should not be awaited for starting the first line anti TB treatment. If no resistance is detected, continue a standard 6 months first line treatment regimen (2HRZE/HRE) and if resistance is detected, further treatment should be decided as per Programmatic Management of Drug resistant Tuberculosis (PMDT) guidelines.
Q: WHAT ARE FREQUENTLY ENCOUNTERED COMPLICATIONS DURING TREATMENT?
Because of the presence of nonspecific signs and symptoms initially, lack of certain diagnostic criteria, and low sensitivity of the various tests, most cases of TBM are picked up very late. As a result, they have been associated with an increased risk of the following complications.
Immediate
Electrolyte disturbances: Hyponatremia is the most common (35%–65% cases). This can be due to cerebral salt wasting/syndrome of inappropriate secretion of anti-diuretic hormone (ADH)/increased renal sensitivity to ADH.
Seizures: Almost 50% of children present with seizures. They may be focal or generalized tonic–clonic depending on the underlying CNS involvement. Phenytoin remains the most common antiepileptic drug of choice. Others including valproate should be avoided due to increased risk of hepatotoxicity. Other antiepileptic drugs may affect the metabolism of ATT drugs as well.
Raised intracranial tension: Should be closely monitored for the same, with anti-raised intracranial pressure measures.
Vasculitis: May develop due to cerebral vasospasm or inflammation of meninges or obliterative vasculopathy. The distal internal carotid artery, proximal middle cerebral artery, and its perforating branches remain the most common sites involved. Corticosteroids because of their anti-inflammatory role have been used widely. But recent studies suggested that corticosteroids do not significantly affect the number of new infarcts or the extent of residual hemiplegia in children or adults. Only aspirin might reduce the incidence of stroke, but this effect still needs to be confirmed in larger studies.[19,20]
Short term
Hydrocephalus: Almost 80% of cases of TBM present with hydrocephalus, communicating being more common than the noncommunicating type. Most cases can be managed medically using acetazolamide and furosemide. But a failed medical treatment warrants surgery and ventriculoperitoneal (VP) shunting. As hydrocephalus can be present in a case of pyogenic meningitis as well, the possibility of that must also be considered
Cranial nerve palsy
Diabetes insipidus
Raised intracranial tension
Long term
Cognitive disability
Epilepsy
Stroke
Hydrocephalus
Myeloradiculopathy
Hypothalamic involvement: obesity, precocious puberty, diabetes insipidus, Frohlich syndrome, and growth retardation
Sequelae
Cognitive defects/intellectual disability
Emotional effects: unhappy and moody, short-tempered, obstinate, and aggressive
Physical limitations
Headache
Epilepsy
Neurological deficit
Behavior problems
Drooling
Blindness
Deafness
Nonspecific[21]
Q: WHAT ARE THE PROGNOSTIC MARKERS IN TBM?
The clinical stage and age at which treatment is initiated is the most important determinant for survival and sequelae. Young age, malnutrition, hydrocephalus, presence of focal neurological deficits, miliary disease, underlying debilitating disease, or HIV infection are associated with poor prognosis. Almost 10%–85% of all cases develop sequelae to the maximum in stage 3.
Q: WHAT ARE PARADOXICAL REACTIONS IN PATIENTS ON ATT?
Recurrence or appearance of fresh symptoms, physical, and radiological signs (unusual expansion or formation of a new tuberculous lesion) in a patient who had previously shown improvement with appropriate ATT is called as paradoxical reaction. More commonly seen in people coinfected with HIV, it can also affect 2%–15% of HIV-negative population. It has been reported as early as 2 weeks and as late as 18 months after the initiation of ATT.
Pathophysiology
Killing of bacilli by ATT leads to the release of a large amount of tuberculoprotein and cell wall products, which create a hypersensitivity reaction and recruit lymphocytes and macrophages at the site of the previously inactive tuberculous foci. As a result, foci enlarge and become evident.
Clinical presentation
It presents with development of fresh intracranial tuberculoma(s), progression of the previous lesion, increase in size of lymph nodes, or development of new lymphadenopathy.
With the lack of any clear-cut guidelines, it becomes difficult to differentiate a paradoxical reaction/relapse of the disease/secondary resistance/incorrect primary diagnosis. Hence, this remains a diagnosis of exclusion. The treatment is to continue ATT. Some studies have shown benefit with addition of steroids, increasing the dose of ATT, or addition of ofloxacin to the ongoing treatment. However, no such guidelines have been proposed. This condition is commonly misdiagnosed and hence needs more research for better management.
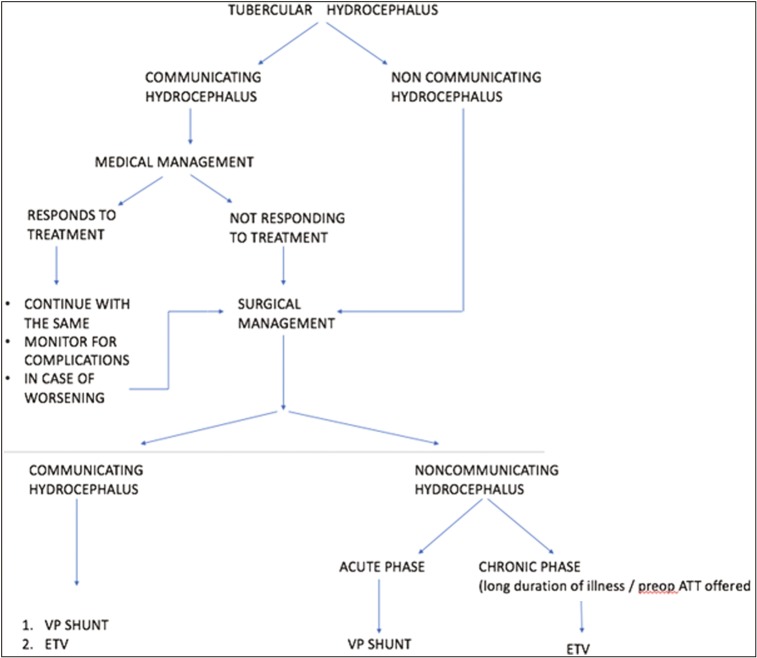
Q: WHAT ARE DIFFERENT SURGICAL INTERVENTIONS IN TBM?
VP shunt
Hydrocephalus is one of the most common complications associated with TBM. A total of 80% cases can be resolved with medical management only. The complications of shunt surgery are higher in patients with TBM than in patients with other conditions. The reasons for this are the poor general condition of the patient, presence of higher protein content, and increased cellular content in the CSF leading to more frequent shunt obstruction.
A shunt should be promptly offered in case of failure of medical management, noncommunicating hydrocephalus, or neurological deterioration as prolonging medical therapy may lead to irreversible brain damage. Placement of a ventricular drain or VP or ventriculoatrial shunt will help improve the outcome in such patients, particularly in patients presenting with minimal neurological deficit.[22]
Endoscopic third ventriculostomy
It offers an added advantage over VP shunting because of the following:
Avoidance of a foreign body implantation
Establishment of a “physiological” CSF circulation
Both of these are particularly important in infants because of the relative high rate of some intraoperative (i.e., abdominal) and late (secondary craniosynostosis, slit-ventricle syndrome) shunt complications. But the main factor against endoscopic third ventriculostomy (ETV) is the relatively high risk of immediate mortality and neurological complications because of the more relevant effect of parenchymal and vascular damage in this age group. Infections are possible in both, but the incidence of infective complications is significantly lower in case of ETV (1%–5% vs. 1%–20%).[23,24]
Hence, both the immediate mortality and neurological damage risk of ETV procedures should be weighed against the long-term mortality and the late neurological damage, which are not infrequently described as a consequence of shunt malfunction and proximal shunt revision procedures [Figure 2].
Figure 2.
Q: WHAT ARE COMPLICATIONS OF VP SHUNT SURGERY?
These include the following:
Mechanical
Obstruction
Overdrainage/underdrainage
Disconnection
Migration of any components of a shunt system either at the ventricular or peritoneal end
Infective
Shunt tract abscess
Skin necrosis overlying the shunt device
Ventriculitis
Other complications
Seizures
Subdural collection
Craniosynostosis
Inguinal hernia and hydrocele
Ascites
Pseudocyst formation
Perforation of a viscus or extrusion of the shunt
Intestinal volvulus and obstruction
Q: WHAT IS NEW TB VACCINE?
The preferred characteristics of new TB vaccine include an efficacy equal to or greater than 80% or a superior efficacy as compared to bacillus calmette gurein in preventing TB disease, including severe, disseminated TB, TB meningitis, and pulmonary TB in infants and young children. Ten or more years of protection should be conferred after primary immunization while offering an improved safety profile, especially in HIV-infected population. Currently the vaccines in Phase IIb trial include MVA85A (viral vectored) and M72/AS01E (protein/adjuvant) and in Phase III include M. vaccae (mycobacterial whole cell or extract) for prime, booster, and postinfectious doses.
Q: HOW IS TBM AND HIV COINFECTION TREATED?
TB is the most common opportunistic infection in persons infected with HIV, and HIV infection is an independent risk factor for extrapulmonary TB including meningitis.[25]
HIV causes an increased risk of activation of latent infection and rapid progression of primary infection without an intervening period of latency.
Without HIV infection, individuals with latent infection have a lifetime risk of 10%–20% of developing TB as compared to the individuals infected with HIV having a 10% annual risk of progression to active infection.[26] Treatment includes free drug therapy HRZE for 2 months, HRE for the remaining 7 months. The CDC guidelines recommend a treatment for 9–12 months for TBM.
Choice of rifamycin versus rifampin
Rifampin has significant interactions with antiretroviral agents because of its inductive effects on drug metabolism through cytochrome P450 (CYP) 3A, which leads to severely reduced concentrations of antiretroviral drugs. Hence, when rifampin-based antituberculous therapy is used in resource-limited settings, the CDC recommends the use of efavirenz-based antiretroviral therapy (ART). Rifabutin is a rifamycin with significantly less induction of P450 enzymes.[27] More clinical research is needed to determine the optimal dosing of antituberculous therapy and ART for a patient with TB and HIV coinfection.
Risk of acquired resistance
Risk of developing resistance on antituberculous therapy is proportional to the bacillary burden, with cavitary pulmonary disease carrying the highest risk. A twice-weekly rifamycin-dosing schedule during the continuation phase has been linked to acquired rifamycin resistance, both with the use of rifampin and rifabutin, and patients with advanced HIV infection (CD4 count <100) are at greatest risk. Rifapentine, a once-weekly rifamycin, is contraindicated for the treatment of TB in the patient infected with HIV because of the risk of relapse with rifamycin mono-resistance.
Timing of ART
In view of the known drug interactions, simultaneous initiation of ATT and ART leads to overlapping drug toxicities. Initiation of ATT in the absence of ART may also be associated with a transient worsening of the signs and symptoms of infection, which is known as the paradoxical reaction. The paradoxical reaction during the treatment of TBM may be exacerbated by immune restoration with ART. However, the delayed initiation of ART carries an increased risk of additional opportunistic infections. Given these competing risks, the optimal timing of initiation of ART in relation to antituberculous therapy has not been established. Until better evidence is available, the CDC recommends individualizing this treatment decision with the possibility of a 4–8 weeks of ATT before initiation of ART.[28]
The SAPIT trial[29] (Starting Antiretroviral Therapy in Tuberculosis) compared the timing of ART relative to antituberculous therapy. Subjects were randomized to one of the three arms: initiation of ART simultaneous with antituberculous therapy, initiation of ART following the intensive phase of antituberculous therapy, or initiation of ART at the completion of antituberculous therapy. The first two arms were considered integrated treatment and the third was considered sequential treatment. Enrollment in the sequential arm of the trial was halted after a hazard ratio of death in the integrated arms was observed to be 0.44 compared with the sequential arm. This mortality difference was observed despite similar TB outcomes. The trial to compare the two arms of integrated treatment is ongoing. Given these findings, earlier initiation of ART may be the trend in the future.
Response to steroids
Corticosteroid could reduce mortality caused by TBM in the short term in children and adults who are HIV negative, but its effect in person who is HIV positive is uncertain and not well studied.[30] No trial has been performed to compare the efficacy of different corticosteroid regimens. Therefore, the choice depends on the regimen used in different trials. Dexamethasone/prednisolone both have been used with good efficacy.[31]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Israni AV, Dave DA, Mandal A, Singh A, Sahi PK, Das RR, et al. Tubercular meningitis in children: clinical, pathological, and radiological profile and factors associated with mortality. J Neurosci Rural Pract. 2016;7:400–4. doi: 10.4103/0976-3147.181475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marais S, Thwaites G, Schoeman JF, Torok ME, Misra UK, Prasad K, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10:803–12. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 3.Kurien R, Sudarsanam TD, Thomas K, Samantha S. Tuberculous meningitis: a comparison of scoring systems for diagnosis. Oman Med J. 2013;28:163–6. doi: 10.5001/omj.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook I, Beetham R, Cruickshank A, Egner W, Fahie-Wilson M, Keir G, et al. National audit of cerebrospinal fluid testing. Ann Clin Biochem. 2007;44:443–8. doi: 10.1258/000456307781646085. [DOI] [PubMed] [Google Scholar]
- 5.Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol. 2004;42:378–9. doi: 10.1128/JCM.42.1.378-379.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solari L, Soto A, Agapito JC, Acurio V, Vargas D, Battaglioli T, et al. The validity of cerebrospinal fluid parameters for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2013;17:e1111–5. doi: 10.1016/j.ijid.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Bindu TH, Reddy RM. Role of cerebrospinal fluid adenosine deaminase activity in the diagnosis of tuberculous meningitis in children. Int J Contemp Pediatr. 2017;4:411–4. [Google Scholar]
- 8.Jakka S, Veena S, Rao AR, Eisenhut M. Cerebrospinal fluid adenosine deaminase levels and adverse neurological outcome in pediatric tuberculous meningitis. Infection. 2005;33:264–6. doi: 10.1007/s15010-005-5005-4. [DOI] [PubMed] [Google Scholar]
- 9.Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. Journal of Neurology. 2011;258:3–13. doi: 10.1007/s00415-010-5744-8. [DOI] [PubMed] [Google Scholar]
- 10.Thwaites GE, Tran TH. Tuberculous meningitis: Many questions, too few answers. Lancet Neurology. 2005;4:160–70. doi: 10.1016/S1474-4422(05)01013-6. [DOI] [PubMed] [Google Scholar]
- 11.Farinha NJ, Razali KA, Holzel H, Morgan G, Novelli VM. Tuberculosis of the central nervous system in children: a 20-year survey. J Infect. 2000;41:61–8. doi: 10.1053/jinf.2000.0692. [DOI] [PubMed] [Google Scholar]
- 12.Yaramiş A, Gurkan F, Elevli M, Söker M, Haspolat K, Kirbaş G, et al. Central nervous system tuberculosis in children: a review of 214 cases. Pediatrics. 1998;102:E49. doi: 10.1542/peds.102.5.e49. [DOI] [PubMed] [Google Scholar]
- 13.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64:580–8. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Ozateş M, Kemaloglu S, Gürkan F, Ozkan U, Hoşoglu S, Simşek MM. CT of the brain in tuberculous meningitis. A review of 289 patients. Acta Radiol. 2000;41:13–7. doi: 10.1034/j.1600-0455.2000.041001013.x. [DOI] [PubMed] [Google Scholar]
- 15.Schoeman JF, Van Zyl LE, Laubscher JA, Donald PR. Serial CT scanning in childhood tuberculous meningitis: prognostic features in 198 cases. J Child Neurol. 1995;10:320–9. doi: 10.1177/088307389501000417. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Kohli N, Thavnani H, Kumar A, Sharma B. Value of CT scan in the diagnosis of meningitis. Indian Pediatr. 1996;33:465–8. [PubMed] [Google Scholar]
- 17.RNTCP 2016. Revised National Tuberculosis Programme NSP-2012-2017. Available at https://www.tbfacts.org .
- 18.Chaudhuri AD. Recent changes in technical and operational guidelines for tuberculosis control programme in India-2016: A paradigm shift in tuberculosis control. J Assoc Chest Physicians. 2017;5:1–9. [Google Scholar]
- 19.Thwaites GE, Macmullen-Price J, Tran TH, Pham PM, Nguyen TD, Simmons CP, et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 2007;6:230–6. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoeman JF, Janse van Rensburg A, Laubscher JA, Springer P. The role of aspirin in childhood tuberculous meningitis. J Child Neurol. 2011;26:956–62. doi: 10.1177/0883073811398132. [DOI] [PubMed] [Google Scholar]
- 21.Schoeman J, Wait J, Burger M, van Zyl F, Fertig G, van Rensburg AJ, et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol. 2002;44:522–6. doi: 10.1017/s0012162201002493. [DOI] [PubMed] [Google Scholar]
- 22.Di Rocco C, Massimi L, Tamburrini G. Shunts vs. endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Childs Nerv Syst. 2006;22:1573–89. doi: 10.1007/s00381-006-0194-4. [DOI] [PubMed] [Google Scholar]
- 23.Yadav YR, Parihar V, Sinha M. Lumbar peritoneal shunt. Neurol India. 2010;58:179–84. doi: 10.4103/0028-3886.63778. [DOI] [PubMed] [Google Scholar]
- 24.Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57:368–74. doi: 10.4103/0028-3886.55572. [DOI] [PubMed] [Google Scholar]
- 25.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 26.Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6:e20077. doi: 10.1371/journal.pone.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep. 2009;6:139–45. doi: 10.1007/s11904-009-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Thoracic Society, Centers for Disease Control, and Infectious Diseases Society of America, Treatment of tuberculosis, Morbidity and Mortality Weekly Report. 2005;53:1203. [Google Scholar]
- 29.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4:CD002244. doi: 10.1002/14651858.CD002244.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167–87. doi: 10.1016/j.jinf.2009.06.011. [DOI] [PubMed] [Google Scholar]