Abstract
Background:
Beta (β)-endorphins are endogenous neuropeptides found in the plasma and cerebrospinal fluid (CSF) of humans but there have been reports of the relationship between the plasma and CSF β-endorphin levels in different clinical conditions. However, the relationship between β-endorphin levels in the plasma and CSF of children with cerebral malaria (CM) has not been reported.
Aim:
To determine the relationship between β-endorphin levels in the CSF and plasma of children with CM.
Settings and Design:
This cross-sectional study involved 40 children, aged between 6 months and 14 years, admitted with a diagnosis of CM at the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria.
Materials and Methods:
One milliliter (mL) of venous blood and 1mL of CSF obtained from each subject at admission were used to determine the β-endorphin levels using enzyme-linked immunosorbent assay (ELISA) method.
Statistical Analysis:
Bivariate linear regression was used to determine the association between plasma and CSF β-endorphin levels using the correlation coefficient (r), coefficient of determination (R2), and P values.
Results:
The plasma β-endorphin levels significantly positively correlated with CSF β-endorphin (r = 0.568, P = 0.001) such that for every unit rise in plasma β-endorphin, CSF β-endorphin rose by 0.252 pmol/L (confidence interval: 0.132–0.371 pmol/L).
Conclusion:
The finding of positive correlation between plasma and CSF β-endorphin levels in this study suggests a possible direct link between plasma and CSF in CM, probably from the disruption of the blood–brain barrier that has been reported in CM.
KEYWORDS: β-Endorphin, cerebral malaria, cerebrospinal fluid, plasma
BACKGROUND
β-endorphin is recognized as one of the most significant endogenous neuropeptides.[1] It is present abundantly in the hypothalamus and pituitary gland and released when the body encounters any sort of stress or pain.[2,3,4] There are two functionally different systems for the release of β-endorphins, one for the peripheral effects via the systemic circulation and one directed at the central nervous system (CNS).[5] Numerous immunocytochemical as well as in situ hybridization studies have confirmed the existence of a main population of β-endorphins-immunoreactive neurons, described as pro-opiomelanocortin (POMC) neurons in the mediobasal hypothalamic region, most of them located in the arcuate hypothalamic nucleus.[6,7,8,9,10,11,12] In addition to the POMC neurons in the hypothalamus, the pituitary contains large numbers of POMC-producing cells located in the intermediate as well as in the anterior lobes.[13] β-endorphins produced in the pituitary is for release into the peripheral systemic circulation, whereas that produced in the hypothalamic POMC neurons is for release inside the CNS.[5] Thus, β-endorphin is found in both plasma and cerebrospinal fluid (CSF).
Previous studies have suggested that an intact blood–brain barrier (BBB) prevents the free exchange of β-endorphin between plasma and CSF,[14,15] thus CSF levels of β-endorphins were believed not to be a reflection of the peripheral levels, but to be controlled and regulated by separate inputs and by specific mechanisms that have separate functions from the pituitary release mechanisms involved in the plasma levels.[5] However, it was later found that peripheral β-endorphins may be able to reach the CNS from the periphery via the circumventricular organs (which lack the BBB) as well as the choroid plexus.[16,17] Conversely, CSF peptides such as β-endorphins can gain access from brain to blood at the brain capillaries through the transporter mechanism by P-glycoprotein.[18,19,20]
It is also known that endorphin levels rise during stress[2,3,4] and inflammation.[4,21,22,23] Cytokines such as tumor necrosis factor-α have been postulated to contribute to the production of β-endorphin in inflamed tissues.[24] Many studies have reported a lack of correlation between the plasma and CSF β-endorphin levels in different clinical states,[25,26,27,28,29,30,31,32] whereas a few others reported positive correlation.[5,33,34] However, no previous report is available on the relationship between β-endorphin levels in the CSF and plasma of children with cerebral malaria (CM). This article is a report on a part of a study on β-endorphin in children with CM carried out at Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile-Ife, Nigeria.
MATERIALS AND METHODS
Participants
This was a cross-sectional study involving 40 children, aged between 6 months and 14 years, admitted with a diagnosis of CM. The diagnosis of CM was based on the World Health Organization (WHO)[35] criteria as follows: unarousable coma (Blantyre Coma Score ≤2) for more than 30min, presence of asexual forms of Plasmodium falciparum parasitemia in peripheral blood film, and exclusion of other common causes of loss of consciousness (such as meningitis and head trauma).
Exclusion criteria include history suggestive of underlying chronic medical conditions such as chronic kidney disease, sickle cell anemia, cerebral palsy, or seizure disorder. The participants were recruited consecutively into the study following admission to the children emergency ward of the OAUTHC.
Ethical approval for the study was obtained from the OAUTHC Ethics and Research Committee. Informed consent was obtained from the parents or guardian of each participant.
The bio-data collected included the age, sex, and anthropometric data of the participants as well as the socioeconomic status of the parents or guardians. Social class was determined using the method described by Oyedeji,[36] which makes use of the occupation and highest education of both the parents.
Weight (in kilograms) was measured to the nearest 0.05kg using the Waymaster weighing scale (made in England) for children weighing below 13kg. Weight for older children was measured to the nearest 0.5kg using RGZ-160 weighing scale by Leaidal Medical, United Kingdom. Length was measured to the nearest 0.1 cm using an infantometer for infants or with an inelastic tape measure on a rigid board with head and feet plates for older children. We measured length instead of height in the older patients because they were unconscious. The length was measured after ensuring that the child was lying flat with the occiput, buttocks, and heel touching the board and knees gently pressed down to straighten out the legs.
Nutritional status was assessed from the obtained anthropometric measurements using the National Centre for Health Statistics/WHO reference growth charts for weight-for-age, weight-for-height, and height-for-age, and body mass index (BMI). Values less than two standard deviations (SDs) below the mean were taken as underweight (weight-for-age criterion), wasting (weight-for-height criterion), and stunting (height-for-age criterion). The Wellcome classification was also used to classify the nutritional state of the children aged 5 years and below.[37] For children older than 5 years, BMI was used. The 5th to 84th percentile range is normal weight, below 5th percentile is underweight, 85th to 95th percentile is at risk for overweight, above 95th percentile is overweight, and below 5th percentile is underweight.[38]
Investigation
Blood sample was collected from the participants at admission using a 21G needle after sterilizing the site thoroughly. This was used for the measurement of β-endorphin levels. Lumbar puncture was also carried out as a routine procedure to exclude meningitis. One milliliter of the CSF was put in a eppendorf tubes for the determination of β-endorphin level. The blood sample was collected in ethylenediaminetetraacetic acid bottles and centrifuged at 1500 revolutions per minute for 15min. The plasma was separated and transported with CSF samples in ice packs until analyzed and then stored together with the CSF sample at –80°C before analysis.
CSF and plasma β-endorphin levels were determined at the research laboratory located at the Department of Biochemistry, Obafemi Awolowo University (OAU), Ile-Ife, Nigeria, using the enzyme-linked immunosorbent assay (ELISA) kit E90806Hu obtained from USCN Life Sciences, China. The kit uses a competitive enzyme inhibition immunoassay technique for the in vitro quantitative measurement of β-endorphin in human serum, plasma, CSF, and other biological fluids with the inter- and intra-assay coefficients of variation of <12% for serum and <10% for CSF. A monoclonal antibody specific for human β-endorphin was pre-coated onto a microplate. A competitive inhibition reaction was launched between biotin-labeled human β-endorphin and unlabeled human β-endorphin (standards or samples) with the pre-coated antibody specific for human β-endorphin. After incubation, the unbound conjugate was washed off. Next, avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. The amount of bound HRP conjugate was proportional to the intensity of color developed, whereas both were inversely proportional to the concentration of β-endorphin in the sample. An average of the duplicate readings for each standard, control, and samples was taken. Thereafter, standard curve was constructed on a graph paper by plotting the log of concentration of β-endorphin against the absorbance. The concentration of β-endorphin of the CSF and plasma samples was determined by drawing a line of best fit through the points of intersection on the graph.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) software for Windows, version 16.0 (SPSS Inc., Chicago, IL). Descriptive statistics including age, gender, socioeconomic characteristics, and anthropometry of the subjects were analyzed and presented using frequency tables. Measures of central tendency were used as appropriate to present continuous variables such as the β-endorphin levels of the study population. Bivariate linear regression was used to determine the degree and direction of association between plasma and CSF β-endorphin levels. This was carried out by determining the correlation coefficient (r), coefficient of determination (R2), and P values. The level of statistical significance was set at P value of less than 0.05.
RESULTS
Demographic characteristics
The age range of the study participants was between 8 and 84 months with a mean of 32.9 (18.6) months. Ninety percent of these participants were 5 years old or younger. The age group with the highest number of subjects was the 13–24 months age group, which accounted for 40% of all the subjects studied. The frequency of CM peaked in this age range. The age and gender distribution of the participants are shown in Table 1.
Table 1.
Age and gender distribution of the participants
| Variable | Participants n (%) |
|---|---|
| Age distribution | |
| 6–12 months | 4 (10.0) |
| 13–24 months | 16 (40.0) |
| 25–36 months | 10 (25.0) |
| 37–60 months | 6 (15.0) |
| >60 months | 4 (10.0) |
| Gender distribution | |
| Male | 25 (62.5) |
| Female | 15 (37.5) |
N = number (frequency)
Social class distribution of the study participants and the relationship with β-endorphin levels
Using the social class classification method described by Oyedeji[36] (Appendix IV); it was found that none of the subjects with CM were from social class I, 4 (10.0%) were from class II, whereas 16 (40.0%) were from social class III, and 18 (45.0%) were from social class IV, respectively. Only two (5.0%) were from social class V. Therefore, 20 (50.0%) of the children with CM were from the lower social classes (IV and V), whereas the remaining half were from higher social classes (II and III). However, no significant relationship was found between the social classes of the subjects and the β-endorphin levels in either their CSF (F = 0.496, P = 0.687) or their plasma (F = 0.440, P = 0.726).
Correlation between plasma and CSF β-endorphin levels at admission
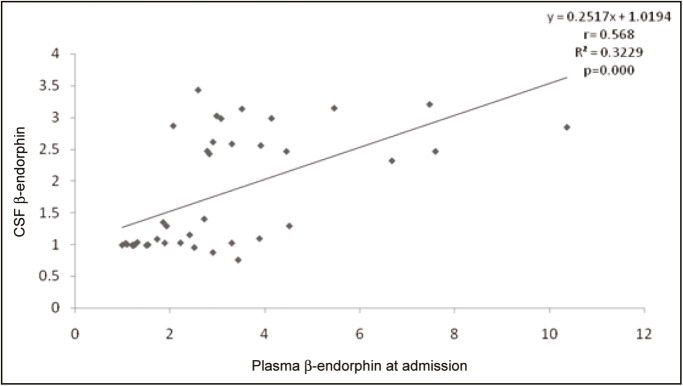
The mean ± SD plasma β-endorphin level for the subjects was 3.1±2.0 pmol/L (range: 1.0–10.4 pmol/L), whereas the mean CSF β-endorphin level was 1.8±0.9 pmol/L (range: 0.8–3.4 pmol/ L). Figure 1 shows the correlation between plasma and CSF β-endorphin levels at admission. The plasma β-endorphin levels significantly positively correlated with CSF β-endorphin (r = 0.568, P ≤ 0.001) such that for every unit rise in plasma β-endorphin, CSF β-endorphin rises by 0.252 pmol/L (confidence interval: 0.132–0.371 pmol/ L). This was carried out by determining the r, (R2), and P values.
Figure 1.
Correlation between plasma and CSF β-endorphin levels at admission
Anthropometric parameters of the participants
The anthropometry of the subjects is shown in Table 2. Over 75% of the children with CM, aged 60 months and below, had normal values of weight for age (77.8%) and height for age (86.1%), whereas only 60.0% had normal weight for height z scores. Wasting was the most common type of undernutrition, whereas stunting was the least common. Using the Wellcome classification, only one child had marasmus. Three (75.0%) of the four children, older than 60 months, had normal BMI (5th–84th percentile), whereas the remaining one was at risk of overweight (85th–95th percentile).
Table 2.
Anthropometry of the participants
| Anthropometric parameters | No. of children | Percentage 100.0% |
|---|---|---|
| Weight-for-age z score | (n = 36)* | |
| Normal | 28 | 77.8 |
| Underweight | 5 | 13.9 |
| Severe underweight | 3 | 8.3 |
| Height-for-age z score | (n = 36)* | |
| Normal | 31 | 86.1 |
| Mild to moderate stunting | 4 | 11.1 |
| Stunting | 1 | 2.8 |
| Weight-for-height z score | (n = 36)* | |
| Normal | 24 | 60.0 |
| Mild to moderate wasting | 6 | 15.0 |
| Severe wasting | 6 | 15.0 |
| Wellcome classification | (n = 36)* | |
| Normal | 30 | 83.3 |
| Underweight | 5 | 13.9 |
| Marasmus | 1 | 2.8 |
| BMI percentile | (n = 4)** | |
| Normal | 3 | 75.0 |
| At risk of overweight | 1 | 25.0 |
*Children aged 60 months and below, **children older than 60 months
The relationships between CSF and plasma β-endorphin levels and anthropometric parameters of the participants are as shown in [Tables 3 and 4], respectively. No statistically significant relationship was found between CSF or plasma β-endorphin levels and the nutritional status of the subjects (P > 0.05).
Table 3.
Relationship between CSF β-endorphin levels and anthropometric parameters of the participants
| Anthropometric parameters | No of children n (%) | Mean CSF β-endorphin level | F value | P value |
|---|---|---|---|---|
| Weight-for-age z score | (n = 36) | |||
| Normal | 28 (77.8) | 1.8 ± 0.9 | 0.601 | 0.558 |
| Underweight | 5 (13.9) | 1.5 ± 1.0 | ||
| Severe underweight | 3 (8.3) | 1.4 ± 0.9 | ||
| Height-for-age z score | (n = 36) | |||
| Normal | 31 (86.1) | 1.8 ± 0.9 | 0.435 | 0.651 |
| Stunting | 5 (13.9) | 1.5 ± 1.1 | ||
| Weight-for-height z score | (n = 36) | |||
| Normal | 24 (60.0) | 2.0 ± 0.9 | 1.566 | 0.224 |
| Mild to moderate wasting | 6 (15.0) | 1.3 ± 0.6 | ||
| Severe wasting | 6 (15.0) | 1.5 ± 1.0 | ||
| BMI percentile | (n = 4)** | |||
| Normal (5th–85th percentile) | 3 (75.0) | 1.7 ± 1.0 | -0.645 | 0.585 |
| At risk of overweight (85th–95th) | 1 (25.0) | 2.5 |
*Children aged 60 months and below, **children older than 60 months
Table 4.
Relationship between plasma β-endorphin levels and anthropometric parameters of the participants
| Anthropometric parameters | No. of children n (%) | Mean plasma β-endorphin level | F value | P value |
|---|---|---|---|---|
| Weight-for-age z score | (n = 36)* | |||
| Normal | 28 (77.8) | 3.1 ± 2.2 | 0.110 | 0.896 |
| Underweight | 5 (13.9) | 3.6 ± 2.3 | ||
| Severe underweight | 3 (8.3) | 3.2 ± 1.4 | ||
| Height-for-age z score | (n = 36)* | |||
| Normal | 31 (86.1) | 3.1 ± 2.1 | 0.471 | 0.628 |
| Stunting | 5 (13.9) | 4.0 ± 2.4 | ||
| Weight-for-height z score | (n = 36)* | |||
| Normal | 27 (75.0) | 3.4 ± 2.4 | 0.549 | 0.583 |
| Mild to moderate wasting | 6 (16.7) | 2.5 ± 1.2 | ||
| Severe wasting | 3 (8.3) | 2.9 ± 1.0 | ||
| BMI percentile | (n = 4)** | |||
| Normal (5th–85th percentile) | 3 (75.0) | 1.8 ± 1.0 | -2.190 | 0.160 |
| At risk of overweight (85th–95th) | 1 (25.0) | 2.8 |
*Children aged 60 months and below, **children older than 60 months
DISCUSSION
The age distribution of the children with CM in this study is similar to what has been found in previous studies where the incidence of CM was found to be higher in children below 5 years.[39,40,41,42,43,44] The age group with the highest incidence of CM in this study (13–24 months) is also similar to previous findings.[41,42,44] This is the period when children are most susceptible to infections, especially severe forms of malaria. The loss of passive immunity acquired from the mother, decrease in concentration of fetal hemoglobin to adult values, as well as the increased activities and exposure of these children to mosquito bites are adduced to be responsible for this susceptibility. The fact that four children in the study population were above 5 years of age suggests that CM is still found in older children, probably as a result of the rebound effect of malaria prevention methods on the immunity in older children, resulting in delayed acquisition of or reduction in previously acquired malaria immunity.[45,46,47]
In this study, the mean plasma β-endorphin levels seen in children with CM were higher than CSF levels. It has been reported that an intact BBB prevents the free exchange of β-endorphin between plasma and CSF. However, little transport of β-endorphin between CSF and plasma may occur through circumventricular organs and P-glycoprotein. This may be contributory to the higher plasma β-endorphin levels in CM.
The finding of a positive correlation between plasma and CSF β-endorphin levels is contrary to what has been reported in many previous studies,[25,26,27,28,29,30,31,32] where no correlation was found between them. However, a few studies reported positive correlation between plasma β-endorphin and CSF β-endorphin levels.[5,33,34] The positive correlation between plasma and CSF β-endorphin levels in this study may suggest a possible communication between the plasma and CSF in CM. This can be as a result of a disruption of the BBB, which has been reported in CM.[48,49] A simultaneous response from the peripheral and central systems for β-endorphin production is also possible.
The rarity of severe malnutrition observed in this study both with WHO z scores as well as with Wellcome classification is similar to the finding by Olumese et al.[50] in Ibadan who reported only one case of marasmus among 55 children with CM studied. Researchers have different opinions on the role of undernutrition in malarial susceptibility and severity. Some have suggested that severe malaria is less likely in undernourished children compared to well-nourished ones because of the deficiency of nutrients, which the malaria parasite thrives on, in these malnourished children.[50,51] Some other researchers postulated that improved nutrition reduces the likelihood of severe malaria including CM because micronutrient deficiencies in undernourished children suppress their immunity against malarial parasite by impairing complement function and antibody production by thymic and splenic lymphocytes.[50,51,52] However, no significant association is present between nutritional status of the subjects in this study and the β-endorphin levels, suggesting that nutritional status may not be contributory to the β-endorphin levels in CM. Also, no significant relationship was reported between the social classes of the subjects and the β-endorphin levels in CSF and plasma, suggesting that β-endorphin level is not affected by the socioeconomic variation of the subjects.
An objective way of ascertaining the positive correlation found between the CSF and plasma β-endorphin levels in children with CM is to carry out the serial measurements of the CSF and plasma β-endorphins. However, this may be unethical in many clinical settings. Thus, it may suffice to analyze plasma β-endorphin levels only in studies on children with CM where repeated lumbar punctures may be unethical. The findings of this study therefore suggest that in CM, CSF β-endorphin levels rise as the plasma β-endorphin levels increase, possibly as a result of a direct link between the plasma and CSF in CM because of the disruption of BBB by the inflammatory process.
Financial support and sponsorship
No financial support was obtained from any source to execute this project. The research was self-funded by the authors.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Herath HM, Cabot PJ, Shaw PN, Hewavitharana AK. Study of beta endorphin metabolism in inflamed tissue, serum and trypsin solution by liquid chromatography-tandem mass spectrometric analysis. Anal Bioanal Chem. 2012;402:2089–100. doi: 10.1007/s00216-011-5686-8. [DOI] [PubMed] [Google Scholar]
- 2.Koneru A, Satyanarayana S, Rizwan S. Endogenous opioids: their physiological role and receptors. Global J Pharmacol. 2009;3:149–53. [Google Scholar]
- 3.Molina PE. Opioids and opiates: analgesia with cardiovascular, haemodynamic and immune implications in critical illness. J Intern Med. 2006;259:138–54. doi: 10.1111/j.1365-2796.2005.01569.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM. 2000;93:323–33. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 5.Veening JG, Gerrits PO, Barendregt HP. Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS. 2012;9:16. doi: 10.1186/2045-8118-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knigge KM, Joseph SA. Anatomy of the opioid-systems of the brain. Can J Neurol Sci. 1984;11:14–23. doi: 10.1017/s0317167100045261. [DOI] [PubMed] [Google Scholar]
- 7.Bloom F, Battenberg E, Rossier J, Ling N, Guillemin R. Neurons containing beta-endorphin in rat brain exist separately from those containing enkephalin: immunocytochemical studies. Proc Natl Acad Sci U S A. 1978;75:1591–5. doi: 10.1073/pnas.75.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson SJ, Akil H. Alpha-MSH in rat brain: occurrence within and outside of beta-endorphin neurons. Brain Res. 1980;182:217–23. doi: 10.1016/0006-8993(80)90849-5. [DOI] [PubMed] [Google Scholar]
- 9.Mezey E, Kiss JZ, Mueller GP, Eskay R, O’Donohue TL, Palkovits M. Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, alpha-melanocyte-stimulating hormone and beta-endorphin (ACTH, alpha-MSH, beta-END) in the rat hypothalamus. Brain Res. 1985;328:341–7. doi: 10.1016/0006-8993(85)91046-7. [DOI] [PubMed] [Google Scholar]
- 10.Tranchand-Bunel D, Delbende C, Guy J, Jegou S, Jenks BJ, Mocaër E, et al. [Pro-opiomelanocortin neuronal systems] Rev Neurol (Paris) 1987;143:471–89. [PubMed] [Google Scholar]
- 11.McGinty JF, Bloom FE. Double immunostaining reveals distinctions among opioid peptidergic neurons in the medial basal hypothalamus. Brain Res. 1983;278:145–53. doi: 10.1016/0006-8993(83)90233-0. [DOI] [PubMed] [Google Scholar]
- 12.Ibata Y, Kawakami F, Okamura H, Obata-Tsuto HL, Morimoto N, Zimmerman EA. Light and electron microscopic immunocytochemistry of beta-endorphin/beta-LPH-like immunoreactive neurons in the arcuate nucleus and surrounding areas of the rat hypothalamus. Brain Res. 1985;341:233–42. doi: 10.1016/0006-8993(85)91062-5. [DOI] [PubMed] [Google Scholar]
- 13.Przewocki R. Some aspects of physiology and pharmacology of endogenous opioid peptides. Polish J Pharmacol Pharmacy. 1984;36:137–58. [PubMed] [Google Scholar]
- 14.Rossier J, Vargo TM, Minick S, Ling N, Bloom FE, Guillemin R. Regional dissociation of beta-endorphin and enkephalin contents in rat brain and pituitary. Proc Natl Acad Sci U S A. 1977;74:5162–5. doi: 10.1073/pnas.74.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houghten RA, Swann RW, Li CH. Beta-endorphin: stability, clearance behavior, and entry into the central nervous system after intravenous injection of the tritiated peptide in rats and rabbits. Proc Natl Acad Sci U S A. 1980;77:4588–91. doi: 10.1073/pnas.77.8.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Brain and spinal cord distribution of biphalin: correlation with opioid receptor density and mechanism of CNS entry. J Neurochem. 1997;69:1236–45. doi: 10.1046/j.1471-4159.1997.69031236.x. [DOI] [PubMed] [Google Scholar]
- 17.Skipor J, Thiery JC. The choroid plexus–cerebrospinal fluid system: undervaluated pathway of neuroendocrine signaling into the brain. Acta Neurobiol Exp (Wars) 2008;68:414–28. doi: 10.55782/ane-2008-1708. [DOI] [PubMed] [Google Scholar]
- 18.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21:79–96. [PubMed] [Google Scholar]
- 19.Stoodley MA, Jones NR, Brown CJ. Evidence for rapid fluid flow from the subarachnoid space into the spinal cord central canal in the rat. Brain Res. 1996;707:155–64. doi: 10.1016/0006-8993(95)01228-1. [DOI] [PubMed] [Google Scholar]
- 20.Stoodley MA, Brown SA, Brown CJ, Jones NR. Arterial pulsation-dependent perivascular cerebrospinal fluid flow into the central canal in the sheep spinal cord. J Neurosurg. 1997;86:686–93. doi: 10.3171/jns.1997.86.4.0686. [DOI] [PubMed] [Google Scholar]
- 21.Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257:126–38. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 22.Machelska H, Stein C. Pain control by immune-derived opioids. Clin Exp Pharmacol Physiol. 2000;27:533–6. doi: 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 23.Brack A, Rittner HL, Machelska H, Beschmann K, Sitte N, Schäfer M, et al. Mobilization of opioid-containing polymorphonuclear cells by hematopoietic growth factors and influence on inflammatory pain. Anesthesiology. 2004;100:149–57. doi: 10.1097/00000542-200401000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Sacerdote P, Brini AT, Locatelli L, Radulovic J, Panerai AE. Tumor necrosis factor alpha differentially regulates beta-endorphin concentrations and proopiomelanocortin RNA in the anterior and neurointermediate pituitary in vivo. Neuroimmunomodulation. 1994;1:357–60. doi: 10.1159/000097188. [DOI] [PubMed] [Google Scholar]
- 25.Jeffcoate WJ, Rees LH, McLoughlin L, Ratter SJ, Hope J, Lowry PJ, et al. Beta-endorphin in human cerebrospinal fluid. Lancet. 1978;2:119–21. doi: 10.1016/s0140-6736(78)91506-4. [DOI] [PubMed] [Google Scholar]
- 26.Nakao K, Nakai Y, Oki S, Matsubara S, Konishi T, Nishitani H, et al. Immunoreactive beta-endorphin in human cerebrospinal fluid. J Clin Endocrinol Metab. 1980;50:230–3. doi: 10.1210/jcem-50-2-230. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Owens PC, Lovelock M, Chan EC, Falconer J. Acute hemorrhagic stress in conscious sheep elevates immunoreactive beta-endorphin in plasma but not in cerebrospinal fluid. Endocrinology. 1986;118:2572–6. doi: 10.1210/endo-118-6-2572. [DOI] [PubMed] [Google Scholar]
- 28.Shealy NC, Cady RK, Culver-Veehoff D, Cox R, Liss S. Cerebrospinal fluid and plasma neurochemicals: response to cranial electrical stimulation. J Neurol Orthop Med Surg. 1998;18:94–7. [Google Scholar]
- 29.Bach FW, Langemark M, Secher NH, Olesen J. Plasma and cerebrospinal fluid beta-endorphin in chronic tension-type headache. Pain. 1992;51:163–8. doi: 10.1016/0304-3959(92)90257-C. [DOI] [PubMed] [Google Scholar]
- 30.McKay RJ, O’Donnell M, Sankaran K, Hindmarsh KW, Wallace SM. Cerebrospinal fluid and plasma beta-endorphin concentrations in prolonged infant apnea (near-miss sudden infant death syndrome) Dev Pharmacol Ther. 1986;9:224–30. doi: 10.1159/000457097. [DOI] [PubMed] [Google Scholar]
- 31.Facchinetti F, Petraglia F, Sances G, Garuti C, Tosca P, Nappi G, et al. Dissociation between CSF and plasma B-endorphin in major depressive disorders: evidence for a different regulation. J Endocrinol Invest. 1986;9:11–4. doi: 10.1007/BF03348054. [DOI] [PubMed] [Google Scholar]
- 32.Barna I, Sweep CG, Veldhuis HD, Wiegant VM. Differential effects of cisterna magna cannulation on beta-endorphin levels in rat plasma and cerebrospinal fluid. Acta Endocrinol (Copenh) 1988;117:517–24. doi: 10.1530/acta.0.1170517. [DOI] [PubMed] [Google Scholar]
- 33.Kosten TR, Kreek MJ, Swift C, Carney MK, Ferdinands L. Beta endorphin levels in CSF during methadone maintenance. Life Sci. 1987;41:1071–6. doi: 10.1016/0024-3205(87)90623-0. [DOI] [PubMed] [Google Scholar]
- 34.Paşaoglu H, Inci Karaküçük E, Kurtşoy A, Paşaoğlu A. Endogenous neuropeptides in patients with acute traumatic head injury, I: cerebrospinal fluid beta-endorphin levels are increased within 24 hours following the trauma. Neuropeptides. 1996;30:47–51. doi: 10.1016/s0143-4179(96)90054-2. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Severe and complicated malaria. 2nd ed. Trans R Soc Trop Med Hyg. 1990;84:1–65. [PubMed] [Google Scholar]
- 36.Oyedeji GA. Socioeconomic and cultural background of hospitalized children in Ilesa. Nig J Pediatr. 1985;12:111–7. [Google Scholar]
- 37.Wellcome Trust International Working Party. Classification of infantile malnutrition. Lancet. 1972;ii:143–6. [Google Scholar]
- 38.Skelton JA, Rudolph CD. Overweight and obesity. In: Berhman RE, Kleigman RM, Jenson HB, editors. Nelson textbook of paediatrics. 18th ed. Philadelphia, PA: WB Saunders; 2007. pp. 232–41. [Google Scholar]
- 39.Orimadegun AE, Fawole O, Okereke JO, Akinbami FO, Sodeinde O. Increasing burden of childhood severe malaria in a Nigerian tertiary hospital: implication for control. J Trop Pediatr. 2007;53:185–9. doi: 10.1093/tropej/fmm002. [DOI] [PubMed] [Google Scholar]
- 40.Phillips RE, Solomon T. Cerebral malaria in children. Lancet. 1990;336:1355–60. doi: 10.1016/0140-6736(90)92903-u. [DOI] [PubMed] [Google Scholar]
- 41.Hendrickse RG. Parasitic diseases: malaria. In: Hendrickse RG, Barr DGD, Matthews TS, editors. Paediatrics in the tropics. Oxford, UK: Blackwell Scientific Publications; 1991. pp. 695–710. [Google Scholar]
- 42.Elusiyan JBE, Obiajunwa PO, Adejuyigbe EA, Olowu WA, Adeodu OO, et al. Pattern of morbidity and mortality among children hospitalized at the Obafemi Awolowo University Teaching Hospital, Ile-Ife. Nig J Paediatri. 2009;36:22–8. [Google Scholar]
- 43.Monebenimp F, Bisong CE, Chiabi A, Chelo D, Moyo-Somo R. Clinical and biological factors associated with treatment outcome of cerebral malaria in children under five in Yaounde. J Neuroparasitol. 2010;1:1–5. [Google Scholar]
- 44.Oguche S, Omokhodion SI, Adeyemo AA, Olumese PE. Low plasma bicarbonate predicts poor outcome of cerebral malaria in Nigerian children. West Afr J Med. 2002;21:276–9. doi: 10.4314/wajm.v21i4.27996. [DOI] [PubMed] [Google Scholar]
- 45.Coleman PG, Goodman CA, Mills A. Rebound mortality and the cost-effectiveness of malaria control: potential impact of increased mortality in late childhood following the introduction of insecticide treated nets. Trop Med Int Health. 1999;4:175–86. doi: 10.1046/j.1365-3156.1999.43382.x. [DOI] [PubMed] [Google Scholar]
- 46.Greenwood BM. The impact of malaria chemoprophylaxis on the immune status of Africans. Bull World Health Organ. 1984;62:69–75. [PMC free article] [PubMed] [Google Scholar]
- 47.Stanfield P, Brueton M, Chan M, Parkin M, Waterson J. Disease of children in the subtropics and tropics. 4th ed. London, UK: Edward-Arnold; 1991. [Google Scholar]
- 48.Murray MJ, Murray AB, Murray NJ, Murray MB. Diet and cerebral malaria: the effect of famine and refeeding. Am J Clin Nutr. 1978;31:57–61. doi: 10.1093/ajcn/31.1.57. [DOI] [PubMed] [Google Scholar]
- 49.Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555–68. doi: 10.1016/j.ijpara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Olumese PE, Sodeinde O, Ademowo OG, Walker O. Protein energy malnutrition and cerebral malaria in Nigerian children. J Trop Pediatr. 1997;43:217–9. doi: 10.1093/tropej/43.4.217. [DOI] [PubMed] [Google Scholar]
- 51.Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, et al. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–13. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- 52.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464–77S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]