INTRODUCTION
Brain tumors constitute the most common solid neoplasms in children and the second most common cancer after leukemia. Astrocytomas are the most frequent, followed by medulloblastomas (MB), the most aggressive tumor, and ependymomas.[1,2] In a previous study, we performed cell cycle analysis by propidium iodine staining of CD56+ (gated) cells by flow cytometry and found that this method could accurately differentiate between neoplastic and nonneoplastic tissue, as well as high-grade from low-grade tumors.[3] An important observation during that study has been the great variability in the expression of CD56, a neural cell adhesion molecule (NCAM), among tumors. On the basis of this observation, we set out to quantify the expression of CD56 in pediatric brain tumors and to investigate its correlation with clinicopathologic parameters.
MATERIALS AND METHODS
Herein, we quantified CD56 expression in tissues obtained from 46 pediatric brain tumor cases. The study population has been as reported previously,[2] and it included 17 MB, 12 anaplastic ependymomas, 9 atypical teratoid/rhabdoid tumors, 1 primitive neuroectodermal tumor, 1 glioblastoma, 2 low-grade astrocytomas, 1 atypical meningioma, and 2 atypical papillomas. We have used samples of brain tissue from three patients as controls, obtained during surgery for epilepsy (mean age, 2.8 years).
CD56 QUANTIFICATION PROTOCOL AND IMMUNOHISTOCHEMICAL ANALYSIS
In all samples, quantitative assessment of the cell surface expression of CD56 was performed. Tumor samples (0.5–2mm2) were minced (Medimachine System, BD Bioscience, San Jose, USA) for 1min in phosphate-buffered saline (Ca2+ and Mg2+ free, with 0.5mg/mL RNase) and a cell suspension was obtained. The cell suspension was then filtered (Consult No. 10; Medicons, BD Bioscience, San Jose, USA). In order to get a final concentration of 1.0 × 106 cells/mL the cells were counted using an automated hematology analyzer. Then, 20 μL of CD56 FITC antibody, which recognizes an extracellular immunoglobulin-like domain common to three molecular weight forms—20, 140, and 180 kDa—of the NCAM, were added to 100 μL of the cell suspension and incubated for 15 min at room temperature (in the dark). Flow cytometric analysis was then performed. All the stained samples were analyzed within 1h on a FACSCalibur (Becton-Dickinson) flow cytometer, using CellQuest software (Becton-Dickinson), for at least 10,000 cells/sample. From the histograms, the geometrical mean was calculated and used for quantification. Quantitative measurement of bound anti-CD56 antibodies was achieved using the flow cytometry-based QIFIKIT assay (Dako, Glostrup, Denmark) according to the manufacturer’s instructions. Immunohistochemical staining of Ki-67 and P-53 was performed using the Bond-Max Autostainer (Leica Microsystems, Buffalo Grove, Illinois) as reported previously.[3]
STATISTICAL ANALYSIS
The CD56 molecules/cells between grade I/II, grade III, and grade IV tumors were compared using the two-sided, nonparametric Mann–Whitney U test. Correlation between Ki-67, P-53, and CD56 molecules/cell were analyzed using Spearman’s rho test. A two-sided P-value <0.05 was considered statistically significant.
RESULTS
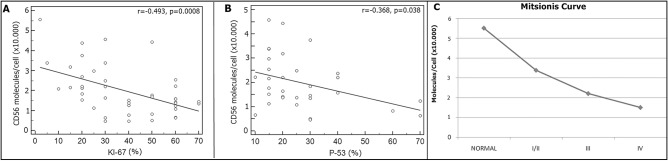
We found a significant negative correlation between Ki-67 index and CD56 molecules/cells (r = –0.493, P = 0.0008) and a significant negative correlation between P-53 index and CD56 molecules/cells (r = –0.368, P = 0.038) [Figure 1]. Using the Mann–Whitney U test, normal brain tissue could be differentiated from all tumor groups based on CD56 molecules/cell. Grade I/II tumors could be differentiated from grade III tumors (39.004±4.584 vs. 24.721±12.638 molecules/cell, P = 0.035) and grade III from grade IV tumors (24.721±12.638 vs. 16.380±7833 molecules/cell, P = 0.037).
Figure 1.
Correlation between number of CD56 molecules/cell and Ki-67 (A) and P-53 (B) expression. (C) Relationship between histological grade and number of CD56 molecules/cell.
DISCUSSION
This study showed that quantification of CD56 expression in tumor cells might be a novel indicator of pediatric brain tumor’s grade and aggressiveness and could be an adjunct to the standard histopathological evaluation of tumor samples. To the best of our knowledge, no previous study quantified CD56 expression in pediatric brain tumors by flow cytometry.
The NCAM, also known as CD56, is involved in the intercellular junctions of neurons and glial cells and is also expressed on the surface of a subset of lymphocytes, the natural killer cells.[4] CD56 has three main isoforms (NCAM-120, NCAM-140, and NCAM-180).[5] Furthermore, several brain tumors, such as gliomas, MB, and ependymomas, express CD56.[6,7,8] As a cell surface glycoprotein, CD56 is involved in cell-to-cell adhesion. Furthermore, there is evidence that CD56 plays the additional role of a signaling receptor having an impact on cells’ several functions such as proliferation, differentiation, migration, and survival.[6,7,8,9] On the basis of immunohistochemical methods, Todaro et al.[4] reported NCAM immunopositivity, in tissues from brain tumors, being inversely correlated with the histological grade of malignancy. In glioblastoma, CD56 expression was correlated with tumor chemoresistance.[10] In a rat model, transfected glioma tumor cells with the 140-kDa isoform of the NCAM became less invasive and destructive than control cells.[11] Blaheta et al.,[12] after studying 11 neuroblastoma cell lines, showed an inverse correlation between CD56 expression and cell adhesion, thus promoting tumor metastases. Contrary to immunohistochemistry, flow cytometry is able to provide objective and quantitative results, even on very small samples, within minutes. Furthermore, for the diagnosis, staging, classification, and monitoring response to the therapy of hematologic malignancies, it has been proven to be superior to immunohistochemistry.[13]
As quantification of CD56 in brain tumors can be performed within 20min, this technique could be also used during intraoperative consultation. To date, frozen section analysis is the gold standard; however, this procedure has several shortcomings (for e.g., there might be freezing artifacts, morphology of cells might be changed by the freezing process, and sections might be of poor quality).[14,15] Thus, a technique that could provide additional information to pathologists would be of great value.
In this study, we found that tumor aggressiveness inversely correlated with CD56 expression. On the basis of the results so far, assessment of CD56 molecules/cell by flow cytometry in pediatric brain tumors provides important information for the assessment of tumor grade and aggressiveness. Thus, this method could be a novel adjunct to the standard histopathological evaluation of tumor samples. Further studies with larger number of cases are needed to verify our preliminary results and investigate possible prognostic significance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alexiou GA, Moschovi M, Stefanaki K, Sfakianos G, Prodromou N. Epidemiology of pediatric brain tumors in Greece (1991–2008). Experience from the Agia Sofia Children’s Hospital. Cent Eur Neurosurg. 2011;72:1–4. doi: 10.1055/s-0030-1268495. [DOI] [PubMed] [Google Scholar]
- 2.Nalita N, Ratanalert S, Kanjanapradit K, Chotsampancharoen T, Tunthanathip T. Survival and prognostic factors in pediatric patients with medulloblastoma in Southern Thailand. J Pediatr Neurosci. 2018;13:150–7. doi: 10.4103/jpn.JPN_111_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vartholomatos G, Alexiou GA, Stefanaki K, Lykoudis EG, Tseka G, Tzoufi M, et al. The value of cell cycle analysis by propidium-iodine staining of CD56+ cells in pediatric brain tumors. Clin Neurol Neurosurg. 2015;133:70–4. doi: 10.1016/j.clineuro.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Todaro L, Christiansen S, Varela M, Campodónico P, Pallotta MG, Lastiri J, et al. Alteration of serum and tumoral neural cell adhesion molecule (NCAM) isoforms in patients with brain tumors. J Neurooncol. 2007;83:135–44. doi: 10.1007/s11060-006-9312-0. [DOI] [PubMed] [Google Scholar]
- 5.Ditlevsen DK, Povlsen GK, Berezin V, Bock E. NCAM-induced intracellular signaling revisited. J Neurosci Res. 2008;86:727–43. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- 6.Etzell JE, Keet C, McDonald W, Banerjee A. Medulloblastoma simulating acute myeloid leukemia: case report with a review of “myeloid antigen” expression in nonhematopoietic tissues and tumors. J Pediatr Hematol Oncol. 2006;28:703–10. doi: 10.1097/01.mph.0000243647.66734.0f. [DOI] [PubMed] [Google Scholar]
- 7.Ditlevsen DK, Køhler LB, Pedersen MV, Risell M, Kolkova K, Meyer M, et al. The role of phosphatidylinositol 3-kinase in neural cell adhesion molecule-mediated neuronal differentiation and survival. J Neurochem. 2003;84:546–56. doi: 10.1046/j.1471-4159.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- 8.Kiryushko D, Kofoed T, Skladchikova G, Holm A, Berezin V, Bock E. A synthetic peptide ligand of neural cell adhesion molecule (NCAM), C3d, promotes neuritogenesis and synaptogenesis and modulates presynaptic function in primwwwary cultures of rat hippocampal neurons. J Biol Chem. 2003;278:12325–34. doi: 10.1074/jbc.M211628200. [DOI] [PubMed] [Google Scholar]
- 9.Gattenlöhner S, Stühmer T, Leich E, Reinhard M, Etschmann B, Völker HU, et al. Specific detection of CD56 (NCAM) isoforms for the identification of aggressive malignant neoplasms with progressive development. Am J Pathol. 2009;174:1160–71. doi: 10.2353/ajpath.2009.080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balik V, Mirossay P, Bohus P, Sulla I, Mirossay L, Sarissky M. Flow cytometry analysis of neural differentiation markers expression in human glioblastomas may predict their response to chemotherapy. Cell Mol Neurobiol. 2009;29:845–58. doi: 10.1007/s10571-009-9366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edvardsen K, Pedersen PH, Bjerkvig R, Hermann GG, Zeuthen J, Laerum OD, et al. Transfection of glioma cells with the neural-cell adhesion molecule NCAM: effect on glioma-cell invasion and growth in vivo. Int J Cancer. 1994;58:116–22. doi: 10.1002/ijc.2910580119. [DOI] [PubMed] [Google Scholar]
- 12.Blaheta RA, Hundemer M, Mayer G, Vogel JU, Kornhuber B, Cinatl J, et al. Expression level of neural cell adhesion molecule (NCAM) inversely correlates with the ability of neuroblastoma cells to adhere to endothelium in vitro. Cell Commun Adhes. 2002;9:131–47. doi: 10.1080/15419060214520. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira-Facio CS, Milito C, Botafogo V, Fontana M, Thiago LS, Oliveira E, et al. Contribution of multiparameter flow cytometry immunophenotyping to the diagnostic screening and classification of pediatric cancer. PLoS One. 2013;8:e55534. doi: 10.1371/journal.pone.0055534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaafar H. Intra-operative frozen section consultation: concepts, applications and limitations. Malays J Med Sci. 2006;13:4–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Chand P, Amit S, Gupta R, Agarwal A. Errors, limitations, and pitfalls in the diagnosis of central and peripheral nervous system lesions in intraoperative cytology and frozen sections. J Cytol. 2016;33:93–7. doi: 10.4103/0970-9371.182530. [DOI] [PMC free article] [PubMed] [Google Scholar]