Abstract
Background
The resistance to EGF receptor (EGFR) tyrosine kinase inhibitors (TKI) is a major challenge in the treatment of non-small cell lung cancer (NSCLC). Understanding the molecular mechanisms behind resistance is therefore an important issue. Here we assessed the role of EGFR pathway substrate 8 (EPS8) and Forkhead box O 3a (FoxO3a) as potentially valuable targets in the resistance of NSCLC .
Methods
The expression levels of EPS8 and FoxO3a in patients with NSCLC (n = 75) were examined by immunohistochemistry staining, while in cells were detected by qPCR and western blot. The effects of EPS8 and FoxO3a on resistance, migration and invasion, cell cycle arrest were detected by MTT, transwell and flow cytometry, respectively. Chromatin immunoprecipitation and luciferase reporter assays were performed to determine the mechanisms of EPS8 expression and FoxO3a regulation.
Findings
We observed that the expression of EPS8 inversely correlated with FoxO3a in NSCLC cell lines and NSCLC patients. FoxO3a levels were significantly decreased in tumor tissues compared with para-carcinoma tissues, while EPS8 is opposite. Besides, they play reverse roles in the resistance to gefitinib, the migration and invasion abilities, the cell cycle arrest in vitro and the tumor growth in vivo. Mechanistically, FoxO3a inhibits EPS8 levels by directly binding its gene promoter and they form a negative loop in EGFR pathway.
Interpretation
Targeting FoxO3a and EPS8 in EGFR signaling pathway prevents the progression of NSCLC, which implied that the negative loop they formed could served as a therapeutic target for overcoming resistance in NSCLC.
Funds
National Natural Science Foundation of China, Science and Technology Project of Henan, Outstanding Young Talent Research Fund of Zhengzhou University and the National Scholarship Fund.
Keywords: Forkhead box O 3a (FoxO3a), Epidermal growth factor receptor (EGFR), EGFR pathway substrate 8 (EPS8), Gefitinib, Tumor resistance, Non-small cell lung cancer (NSCLC)
Research in context.
Evidence before this study
EGFR pathway substrate 8 (EPS8) is a crucial molecule that mediates EGFR-induced activation of Akt and ERK. Previous studies have described that EPS8 was overexpressed in leukemia and solid tumors. EPS8 promotes the proliferation and migration of tumor cells in vivo and reduces the sensitivity to chemotherapeutic drugs in vitro. It has been reported as a new target for anticancer therapy. Forkhead box O 3a (FoxO3a) is a central transcription factor and is reported to coordinate a wide range of functions through binding with its target genes involved in apoptosis, proliferation, cell cycle progression, survival, and DNA damage. Emerging evidences indicate that FoxO3a acts as a tumor suppressor in many cancers and dephosphorylated FoxO3a can inhibit progression of tumor growth in NSCLC. However, the precise regulation mechanism between FoxO3a and EPS8 is not yet clear.
Added value of this study
In the present study, we demonstrated the opposite effects of EPS8 and FoxO3a on the resistance to gefitinib, the migration and invasion abilities, the cell cycle arrest in PC9 cells and the tumor growth in BALB/c nude mice. In addition, the expression of EPS8 and FoxO3a were reverse in NSCLC cell lines and clinical samples. Moreover, we proved that FoxO3a inhibits EPS8 by binding to the activity center of EPS8 gene promoter directly and they could form a negative loop in EGFR induced cascade.
Implications of all the available evidence
Our data suggest that the direct inhibition of FoxO3a on EPS8 expression formed a negative loop in EGFR induced cascade. More importantly, the tumor proliferation and growth inhibition induced by EGFR and FoxO3a respectively suggest a dual-core mode in the regulation of cellular life-mechanism. The dual-core mode, two opposing factors negatively regulate each other in the same pathway, will bring us new ideas for drug development, clinical treatment and ERFR-TKI resistance overcoming in NSCLC.
Alt-text: Unlabelled Box
1. Introduction
Lung cancer is the leading cause of cancer-related death worldwide, with non-small cell lung cancer (NSCLC) being the most common type [28]. Despite surgery, approximately 30% to 40% of patients with NSCLC die of recurrent diseases, possibly due to occult micrometastases beyond the margins of original surgical resection [4]. Tumor cell proliferation and metastasis are closely controlled by growth factor signaling pathways. In particular, the well-known PI3K/Akt cascade [17,37] is activated by binding of epidermal growth factor (EGF) to its receptor to suppress cell apoptosis and promote migration and invasion in cancers [5,33,53,59]. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are popular cancer treatment agents that target this pathway, but they are often rendered ineffective due to mutations in EGFR [40,56].
EGFR pathway substrate 8 (EPS8) is a crucial molecule that mediates EGFR-induced activation of Akt and ERK [27]. EPS8 significantly increases the expression of local focal adhesion kinase (FAK) and promotes the proliferation and migration of tumor cells in vivo [29,51,66]. EPS8 is strongly associated with tumor progression and metastasis [18,51]. Furthermore, it has been reported that EPS8 reduces the sensitivity to chemotherapeutic drugs in cancer cell lines [9].
Forkhead box O 3a (FoxO3a) is a central transcription factor and is reported to coordinate a wide range of functions through binding with its target genes involved in apoptosis [11], proliferation [48], cell cycle progression [49], survival [30], and DNA damage [19]. FoxO3a is also associated with longevity [69], autophagy process [47] and oxidative stress [41]. Emerging evidences indicate that FoxO3a acts as a tumor suppressor in many cancers, such as gastric [71], ovarian [16] and prostate [61] cancers. FoxO3a is also an important downstream target of PI3K/Akt pathway [60]. Activated Akt phosphorylates FoxO3a, causing it to migrate from the nucleus to the cytoplasm and prevent it from binding to the target genes [2]. Studies have shown that FoxO3a, depending on phosphorylation, is associated with both cell proliferation and apoptosis in multiple cancers. In particular, dephosphorylated FoxO3a can inhibit progression of tumor growth in NSCLC [15,45,50,54,60]. However, the precise regulation mechanism between FoxO3a and EPS8 is not yet clear.
EPS8 mediates EGFR-induced activation of Akt [27] and FoxO3a is a downstream transcription factor of PI3K/Akt pathway [13]. Therefore, we speculate that EPS8 maybe an upstream substrate that controls the activation of FoxO3a. Furthermore, when we transfected PC9 cells (an NSCLC cell line) with FoxO3a, the transcription of EPS8 is decreased, suggesting the presence of a negative control loop. Here, we investigated the impact of FoxO3a on EPS8 and studied the biological functions of FoxO3a and EPS8 on chemo-resistance both in vitro and in vivo.
2. Materials and methods
2.1. Cell lines, plasmid generation, and infection
The lung cancer cell lines PC9, A549, H1975, H1299 and H358 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone, Thermo Fisher, USA) containing 0.1 mM sodium pyruvate, 10% FBS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a chamber (5% CO2 atmosphere). Cells (2 × 106/per well) were seeded in 6-well plates and transfected with 4 μg of the pEGFP-N1 (control), pEGFP-FoxO3a or pEGFP-EPS8, 5 μl of si-FoxO3a or si-EPS8 (Ribobio, China) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) in antibiotic-free medium. After 48 h, the transient cells were collected for further experiments.
2.2. Human NSCLC samples
This study was performed according to an established protocol approved by the Ethics Committee of Zhengzhou University School. The data does not contain any information that may lead to the identification of the patients. Lung adenocarcinoma tissue microarrays (HLug-Ade150Sur-02; Shanghai Outdo Biotech) were constructed with 75 formalin fixed, paraffin-embedded lung adenocarcinoma tissues and their corresponding adjacent lung tissues. Immunohistochemical staining was performed to detect the expression of FoxO3a and EPS8 in NSCLC tissues and matched non-cancerous tissues. Scoring was measured by the percentage of positive cells with the following staining intensities: <25% scored “0”; 25–50% scored “1”; 51–75% scored “2”; and >75% scored “3”.
2.3. Reverse transcription and real-time PCR
Primers specific for FoxO3a and EPS8 were obtained from Sango Biotech (Shanghai, China). Real-time PCR was performed using a StepOnePlus Real-time PCR system with FoxO3a or EPS8 primers and DNA templates and ROX Reference Dye (Takara, Japan). Values represent the average of three independent experiments, normalized to the endogenous control gene β-actin.
2.4. Cell proliferation assay
Lung cancer cells (2 × 103 cells) were cultured in 96-well flat-bottomed microtiter plates supplemented with DMEM containing 10% heat-inactivated FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin in a humidified atmosphere with 95% air and 5% CO2 at 37 °C. Cell viability was determined with the MTT (methyl thiazolyl tetrazolium) assay (absorbance read at 570 nm), and cell viability is expressed as a percentage of viability measured for the relevant control cells.
2.5. Western blotting
The detailed procedure for western blot has been well described [7]. The dilution of all the human antibodies that purchased from Abcam (Abcam, UK) were listed as followed: anti-Akt antibody (1:2000; Cat. ab179463), anti-FoxO3a antibody (1:2000; Cat. ab53287), anti-ERK1/2 antibody (1:2000; Cat. ab184699), anti-EPS8 antibody (1:2000; Cat. ab 124,882), anti-GAPDH antibody (1:5000; Cat. ab181602), anti-p-Akt antibody (1:2000; ab183758), anti-p-Erk1/2 antibody (1:5000; Cat. ab76299), anti-p-FoxO3a antibody (1:3000; ab154786), anti-CyclinD1 antibody (1:10000; ab134175), anti-Bcl-2 antibody (1:1000; ab32124), anti-CD44 antibody (1:5000; ab51037), anti-CD31 antibody (1:10000; ab76533). Proteins were separated by SDS-PAGE transferred to a transfer membrane (Immobilon, Germany) that was then subjected to western blot with an appropriate primary antibody. Anti-rabbit IgG conjugated to horseradish peroxidase was used as the secondary antibody for detection using an ECL western blot detection system (GE Healthcare, Little Chalfont, UK).
2.6. Flow cytometry
Cells (5 × 105) were seeded onto 6 cm dishes and cultured at 37 °C for 24 h. The cell pellets were washed twice with PBS and fixed overnight with 70% ethanol at 4 °C. After centrifugation at 200 ×g for 5 min, the cell pellets were washed with PBS to remove any residual ethanol. Finally, the cells were resuspended in 420 μl of the solution containing 20 μl RNase A and incubated at 37 °C for 30 min. The cells were filtered through a 40 μm nylon mesh before flow cytometry analysis of cell-cycle distribution using a MACS Quant Analyzer 10 (Miltenyi Biotec, Germany).
2.7. Migration/invasion assay
Cells were trypsinized and collected from culture dishes. 5 × 104 cells were seeded on 24 well modified Boyden chambers coated with Matrigel (Corning, New York, U.S. 1 mg/ml) without serum for invasion or without Matrigel for migration. The chambers were then put on 24-well plate contained DMEM plus 20% FBS for 12 h at 37 °C in a humidified atmosphere containing 5% CO2. The migrated or invaded cells on the lower surface of membrane were fixed, stained, and counted under a microscope.
2.8. Xenograft tumor formations
All mice were supplied by the animal facility at the Beijing Vital River Laboratory Animal Technology, Beijing, China. Ethics approval was obtained for the use of animals, and all experiments were performed in accordance with the guidelines for animal care of the Institutional Animal Care and Use Committee of Zhengzhou University. Six-week-old female immunodeficient nude mice (BALB/c, nu/nu) were injected with PC9/pEGFP-N1 (control), PC9/pEGFP-FoxO3a, PC9/pEGFP-EPS8, PC9/si-FoxO3a or PC9/si-EPS8 cells at the right axilla (2 × 106 cells in 0.1 ml of PBS). The sizes of tumors of each mouse were measured every 3 days. After 21 days, mice were sacrificed by CO2 asphyxiation. The volume and weight of tumors of each mouse were measured.
2.9. Dual luciferase reporter assays
The 2000 bp EPS8 promoter region was found on the website e!Ensembl and was verified on NCBI. The putative binding sites of FoxO3a on the promoter of EPS8 were predicted by http://jaspar.genereg.net/. The EPS8 promoter region (−1336~ − 20), (−837~ − 20) or (−382~ − 20) was cloned into plasmid pEZX-PG04 (GeneCopoeia, USA) to produce the recombinant vector, which contains the Gaussia Luciferase (GLuc) open reading frame under the control of the SV40 promoter. The second reporter gene is Secreted Alkaline Phosphatase (SEAP) as the negative control, which could standardize transfection. The GLuc/SEAP activity ratio of each sample was measured in the Secrete-Pair Dual Luminescence Assay system (GeneCopoeia, USA).
2.10. ChIP
ChIP assay was performed using the kit from Thermo Fisher Scientific following the manufacturer's procedure. In brief, cells were fixed with 1% formaldehyde, washed, and lysed. These cell lysates were diluted with immunoprecipitation buffer and then share DNA to an average size of 500 bp with micrococcal nuclease. Protein–DNA complexes were precipitated with either nonimmune IgG or target protein FoxO3a (1:100; Abcam) overnight at 4 °C with rotation. After reverse cross-link of protein–DNA complexes to free DNA, real-time RT-PCR was performed to analyze. The primer sequence is shown below:
EPS8 primer (Forward primer:5’GCAGTTGGGAGACTCTCAGG3’, 1553–1572 bp; Reverse primer: 5’AGAGGAGGAAGACGAAGGCT3’, 1848–1867 bp).
2.11. Nuclear translocation
PC9 cells, transfected with GFP-FoxO3a were cultured in DMEM supplemented with 5% FBS for 24 h. Then the medium was changed to serum-free DMEM and cultured for 2 h before adding EGF. Images were captured by inverted fluorescence microscope under excitation light (490 nm for GFP-FoxO3a) before and 2 h after serum-starvation. Two hours after EGF (100 ng/ml) stimulation, cells were stained with Hoechst 33342 and captured again under different excitation light (490 nm for GFP-FoxO3a and 360 nm for nucleus stained by Hoechst), respectively. The merged images are shown on the right column (original magnification, 400×).
2.12. Statistical analyses
Statistical significance of the experimental data grouped by one variable was assessed by the unpaired two-tailed Student's t-test, one-way ANOVA, or Pearson's χ2 test as appropriate. All statistical analyses were performed using Prism 6 (GraphPad Software, Inc., CA, USA). A value of P < .05 was considered to indicate statistical significance.
3. Results
3.1. Expression of FoxO3a is associated with gefitinib resistance in NSCLC
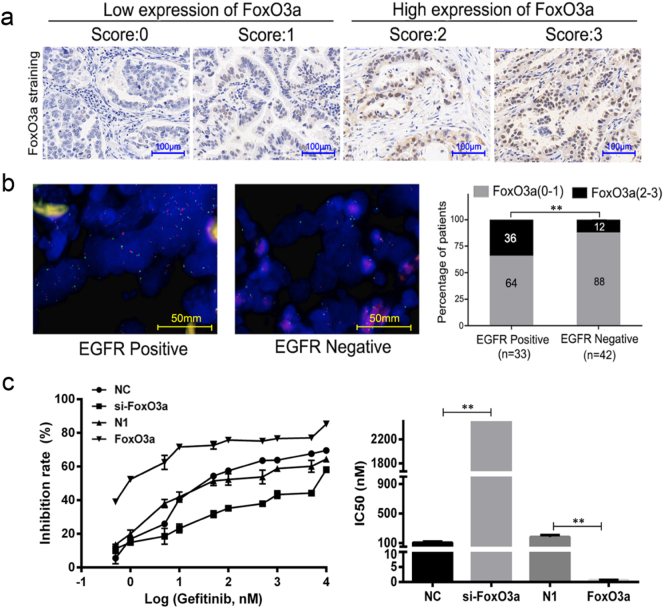
High EGFR gene copy number is an effective molecular predictive marker for gefitinib sensitivity in patients with advanced NSCLC [74]. To study the relationship between FoxO3a expression and gefitinib resistance, we examined FoxO3a expression by immunohistochemical (IHC) staining and detected the amplification of EGFR by fluorescence in situ hybridization (FISH) in tumor tissues from 75 patients with NSCLC. A high EGFR gene copy number is defined as ≥4 copies in ≥40% of cells [64]. The patients with high EGFR gene copy number were defined as EGFR positive group, and the others were defined as EGFR negative group.
The staining intensity of FoxO3a in the nucleus was stronger than in cytoplasm in tumor tissues (Fig. 1a). In this study, the proportion of patients with low expression of FoxO3a in the EGFR negative group (88%, n = 42) was more than that in the EGFR positive group (64%, n = 33) (Student's t-test, P < .01) (Fig. 1b). For the high EGFR gene copy number, an effective biomarker of EGFR-TKI sensitivity [14], we speculate that the low expression of FoxO3a is associated with EGFR-TKI resistance. To verify this hypothesis, we tested the inhibitory profile of gefitinib in vitro using PC9 cells [3]. As shown in Fig. 1c, following transfection of pEGFP-FoxO3a vector or si-FoxO3a, the small interfering RNA (siRNA) for FoxO3a in PC9 cells, we tested the response of PC9 cells to gefitinib. Compared with the empty vector group (IC50 = 182.40 ± 25.89 nM), FoxO3a overexpression significantly enhanced the concentration-dependent inhibition of gefitinib on PC9 cells (IC50 = 0.62 ± 0.10 nM) (Student's t-test, P < .01), while FoxO3a siRNA dramatically reduced the inhibition rate by increasing the IC50 value of gefitinib from 107.87 ± 13.87 nM to 4331.67 ± 992.56 nM (Student's t-test, P < .01). These results reminded us that the lower FoxO3a expression may be related to EGFR-TKI resistance in vivo and in vitro.
Fig. 1.
The lower FoxO3a expression is associated with gefitinib resistance in NSCLC in vivo and in vitro. (a) IHC staining scores ranged from 0 to 3, divided into four orders of magnitude, representing the positive expression level of FoxO3a in NSCLC patients. 0–1 represents FoxO3a low expression, 2–3 represents FoxO3a high expression, scale = 100 μm. (b) The patients were divided into two groups according to the EGFR amplification detected by FISH (EGFR positive, n = 33; EGFR negative, n = 42) and statistics the FoxO3a low expression (gray bars) and high expression (black bars) patients in each group accounted for the proportion. P-values were calculated using the independent Student's t-test. **P < .01, scale = 50 mm. (c) PC9 cells were treated with indicated concentrations of gefitinib; cell viability was measured by MTT assay. Data is expressed as a percentage of the corresponding control value, which was set at 100%. Data is presented as the mean ± SD of three independent experiments. P-values were calculated using the independent Student's t-test. **P < .01.
3.2. Phosphorylation and translocation of FoxO3a induced by EGF
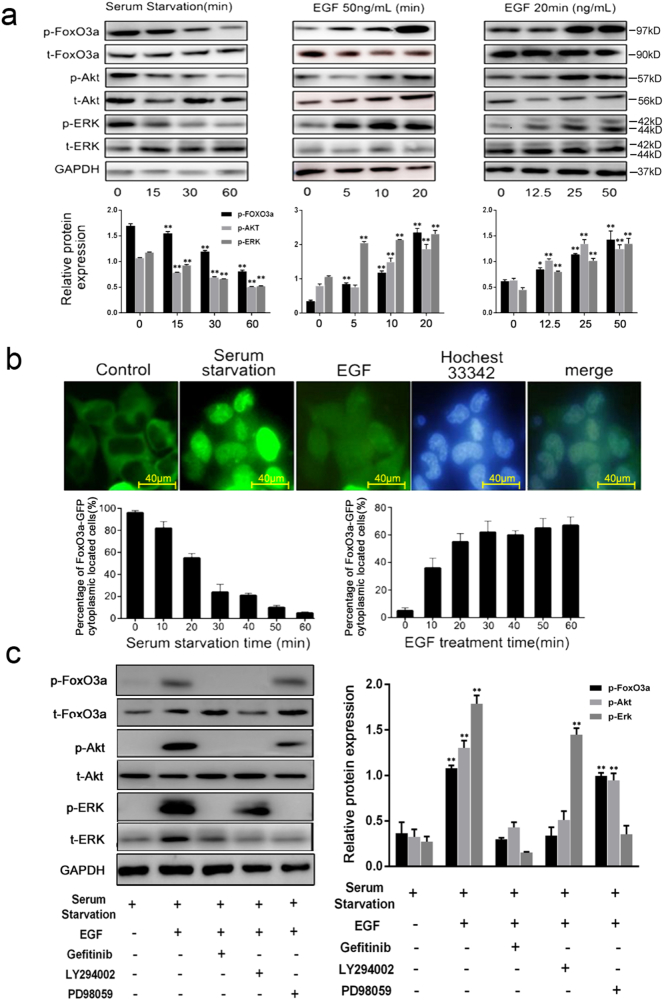
To understand the role of EGF and FoxO3a phosphorylation in the PI3K/Akt pathway, we treated PC9 cells with different concentrations of EGF (0, 12.5, 25, 50 ng/ml) and for different amounts of time (0, 5, 10, 20 min). The phosphorylated proteins were determined by western blot. As shown in Fig. 2a, serum starvation reduced the phosphorylation of Akt, ERK and FoxO3a to baseline in 60 min in a time-dependent manner. EGF (50 ng/ml) induced the phosphorylation of Akt, ERK and FoxO3a in time- and dose-dependent manner. In PC9 cells, serum starvation dephosphorylates FoxO3a, causing its translocation into the nucleus, while EGF induced phosphorylated FoxO3a to translocate from nucleus to cytoplasm (Fig. 2b). We also showed that the phosphorylation of FoxO3a induced by EGF (50 ng/ml, 20 min) could be blocked by gefitinib (1000 nM, 10 min) and LY294002 (50 μM, 30 min), an inhibitor of phosphoinositide 3-kinase (PI3K), but not PD98059 (50 μM, 30 min), an ERK inhibitor (Fig. 2c). These results suggested that the phosphorylation and translocation of FoxO3a could be induced by EGF.
Fig. 2.
EGF induces FoxO3a phosphorylation and translocation. (a) Serum starvation reduced the phosphorylation of Akt, ERK and FoxO3a in a time-dependent manner. EGF induced the phosphorylation of Akt, ERK and FoxO3a in time- and dose-dependent manner, and to peak in 20 min with 50 ng/ml. Data is presented as the mean ± SD of three independent experiments. P-values were calculated using the one-way ANOVA. *P < .05, **P < .01. (b) Serum starvation reduced the percentage of FoxO3a-GFP located in the cytoplasm in a time-dependent manner. EGF induced the percentage of FoxO3a-GFP located in the cytoplasm in a time-dependent manner, scale = 40 μm. (c) The phosphorylation of FoxO3a induced by EGF could be blocked by gefitinib and PI3K/Akt inhibitor LY294002 but ERK inhibitor PD98059. Data is presented as the mean ± SD of three independent experiments. P-values were calculated using the one-way ANOVA. *P < .05, **P < .01 versus control.
3.3. FoxO3a inhibits EPS8 expression in NSCLC cell lines
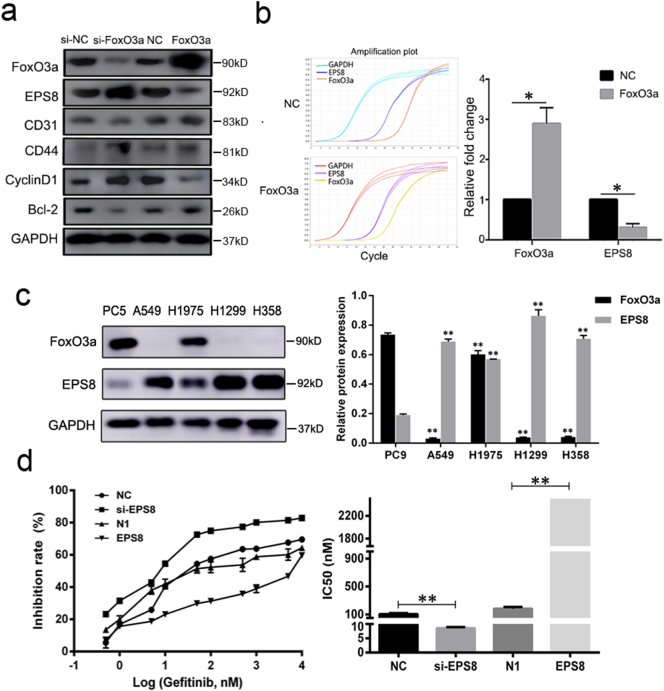
In PC9 cells with overexpressed or silenced FoxO3a, we detected the expression of CD31, CD44, CyclinD1, Bcl-2 and EPS8, which are closely related to the development of the tumor [24,42,52,63,73]. It is noteworthy that when we transfected si-FoxO3a, the expression of EPS8 increased; while the expression of EPS8 was down-regulated when FoxO3a was overexpressed (Fig. 3a). Furthermore, the level of EPS8 mRNA was significantly lower in PC9 cells with FoxO3a overexpression than that in cells with p-EGFP-N1 transfected as the negative control (Fig. 3b) (Student's t-test, P < .05). These results suggest that the inhibition of FoxO3a on EPS8 occurred during the process of transcription.
Fig. 3.
FoxO3a reduces EPS8 expression in PC9 cells. (a) Western blot assays revealed that FoxO3a overexpression decreased the expression of EPS8 in the PC9 cell. The expression of EPS8 is up-regulated when transfected with FoxO3a siRNA. GAPDH is an internal reference. (b) When FoxO3a overexpressed, EPS8 mRNA changes were detected by the RT-PCR method, using the formula 2-ΔΔCt to calculate the relative multiple changes. P-values were calculated using the independent Student's t-test. *P < .05. (c) Western blot analysis showed that the protein content of FoxO3a in PC9 cells was higher than that in NSCLC resistant cell lines (A549, H1975, H1299, H358), while the EPS8 protein was higher in NSCLC resistant cell lines than PC9 cells. P-values were calculated using the one-way ANOVA. *P < .05, **P < .01 versus PC9. (d) Subsequent high expression and silencing of EPS8 revealed that EPS8 promoted the tolerance of PC9 cells to gefitinib by MTT method. Data is presented as the mean ± SD of three independent experiments. P-values were calculated using the independent Student's t-test. **P < .01 versus control.
A549, H1975, H1299, H358 are all NSCLC cell lines that resistant to EGFR-TKIs. Results of western blotting detection showed the levels of EPS8 expression in A549, H1975, H1299 and H358 cells were significantly higher than that in PC9 cells (Fig. 3c) (ANOVA, P < .01) while the expression of FoxO3a is exactly the opposite in the several cells compared with PC9 cells (ANOVA, P < .01). Subsequently, results of MTT assay showed that compared with empty vector group (IC50 = 182.40 ± 25.89 nM) overexpression of EPS8 significantly decreased the sensitivity (IC50 = 4472.0 ± 261.51 nM) (P < .01), while EPS8 siRNA transfection increased the sensitivity to gefitinib in PC9 cells (IC50 = 8.53 ± 0.40 nM), compared with NC group (IC50 = 107.87 ± 13.87 nM) (Student's t-test, P < .01) (Fig. 3d). These results suggest that EPS8 plays an important role in resistance to EGFR TKIs and is regulated by FoxO3a in the transcription process.
3.4. Effects of FoxO3a and EPS8 on cell migration, invasion and cycle arrest
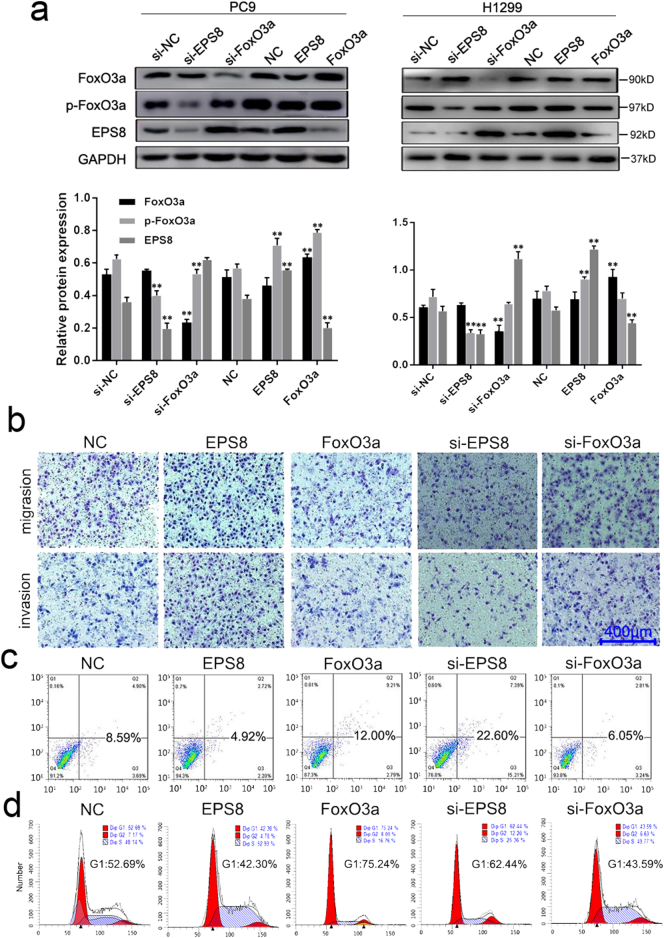
By western blotting, we examined the effect of FoxO3a and EPS8 on each other's expression in PC9 and H1299 cells. The expression of FoxO3a was not changed by up- or down-regulation of EPS8, while the EPS8 expression changed significantly compared with si-NC and NC vector transfection respectively when we silenced or overexpressed FoxO3a. The results showed that FoxO3a negatively regulated the expression of EPS8. Besides, the expression of phosphorylated FoxO3a was affected significantly by EPS8, which indicated that EPS8 could regulate the activation of FoxO3a and acts though it. (Fig. 4a).
Fig. 4.
FoxO3a and EPS8 affect the biological functions of PC9 cells. (a) The expression of FoxO3a and EPS8 was detected by western blot in PC9 and H1299 cells. P-values were calculated using the one-way ANOVA. *P < .05, **P < .01. (b) Transwell assay was used to detect the migration and invasion of cells when transfected with different genes. (c) AnnexinV/PE data suggests that FoxO3a significantly induced PC9 cells apoptosis while EPS8 has the effect of inhibiting apoptosis by Flow Cytometry. 8000 cells were counted for each sample detection. The lower right quadrant (Q3) and the upper right quadrant (Q2) represent the rates of early apoptosis and late apoptotic, respectively. (d) The percentages of cells in the G0/G1, S and G2/M phases of the cell cycle were counted and analyzed using Modifit. Data is presented as the mean ± SD of three independent experiments. P-values were calculated using the independent Student's t-test. *P < .05, **P < .01 versus NC/si-NC.
We used transwell assay and flow cytometry to detect changes in migration, invasion, apoptosis, and cycle arrest of PC9 cells during altered FoxO3a and EPS8 expression. The results showed that the migration and invasion abilities were decreased in PC9 cells with either pEGFP-FoxO3a or siRNA-EPS8 transfection, while both siRNA-FoxO3a and pEGFP-EPS8 transfections enhanced these abilities in PC9 cells (Fig. 4b). In addition, the apoptotic rates of PC9 cells after overexpressing FoxO3a or silencing EPS8 increased from 8.59% to 12% and 22.6% respectively, while those rates decreased to 6.05% and 4.92% respectively compared with the control group when silencing FoxO3a or overexpressing EPS8 (Fig. 4c).
Moreover, cell cycle analysis demonstrated that silencing EPS8 or overexpressing FoxO3a induced cell cycle arrest at the G1 phase and enhanced the proportions of cells in G1 phase from 52.69% (control group cells were transfected with the empty vector,pEGFP-N1) to 62.44% and 75.24% respectively. While silencing FoxO3a or overexpressing EPS8 reduced the cells proportions in G1 phase to 43.59% and 42.30% respectively compared with 52.69% of the control group (Fig. 4d). The results suggest that FoxO3a inhibits tumor motility, promotes apoptosis, and induces cell cycle arrest. These functions are the opposite to those of EPS8.
3.5. Roles of FoxO3a and EPS8 in tumor growth in vivo
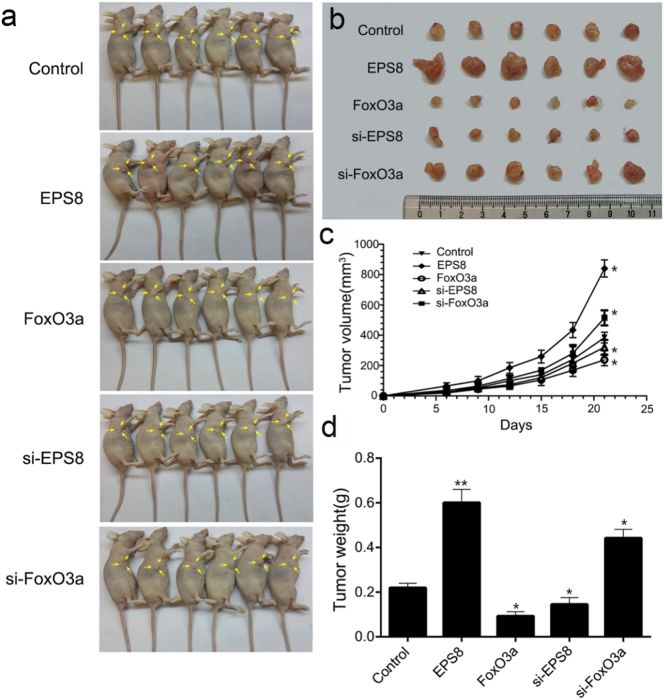
To explore the roles of FoxO3a and EPS8 in tumor growth in vivo, PC9 cells transfected with pEGFP-FoxO3a, siRNA–FoxO3a, pEGFP-EPS8 and siRNA-EPS8 were injected into six weeks old BALB/c female nude mice (Fig. 5a). Mice in the negative control group were injected PC9 cells transfected with pEGFP-N1.
Fig. 5.
FoxO3a and EPS8 affect tumor growth in BALB/c nude mice. Six-week-old female BALB/c nude mice were used to examine tumorigenicity. PC9 cells were highly expressed by transfection of FoxO3a and EPS8 genes and silenced by siRNA, transfected into empty plasmid PC9 cells as the control. 1 × 106 cells in 0.1 ml normal saline were injected subcutaneously into the right flanks of nude mice followed by measurements tumor volumes with the slide caliper at 3-day intervals by the formula: volume = (short diameter)2 × (long diameter)/2. At 21 days after inoculation, the mice were killed and the tumor weights were weighted. (a) and (b) Typical features of tumoral nodules in nude mice. (c) Tumor growth curve. (d) Tumor weight. Data is expressed as the mean ± SD. P-values were calculated using the one-way ANOVA. (* P < .05, ** P < .01 versus Control group, n = 6/group).
Compared with the mean volume of tumors in the control group (380 ± 25 mm3), the tumor average mean volumes in si-FoxO3a and EPS8 groups were increased (541 ± 62 mm3, P < .05; 826 ± 82 mm3, P < .01, ANOVA) while those in FoxO3a and siRNA-EPS8 groups were decreased significantly (216 ± 18 mm3, P < .05; 249 ± 35 mm3, P < .01, ANOVA) (Fig. 5b and c). As shown in Fig. 5d, compared with the control group (0.28 ± 0.04 g), the tumor mean weights of EPS8 group and si-FoxO3a group were increased significantly (0.44 ± 0.06 g, P < .01; 0.61 ± 0.11 g, P < .01, ANOVA); the tumor mean weights of FoxO3a group and si-EPS8 group were decreased (0.10 ± 0.02 g, P < .05; 0.16 ± 0.03 g, P < .05, ANOVA). Therefore, these results showed that FoxO3a overexpression and EPS8 downregulation could inhibit the tumor growth in vivo remarkably.
3.6. The relationship between FoxO3a and EPS8 in clinical samples
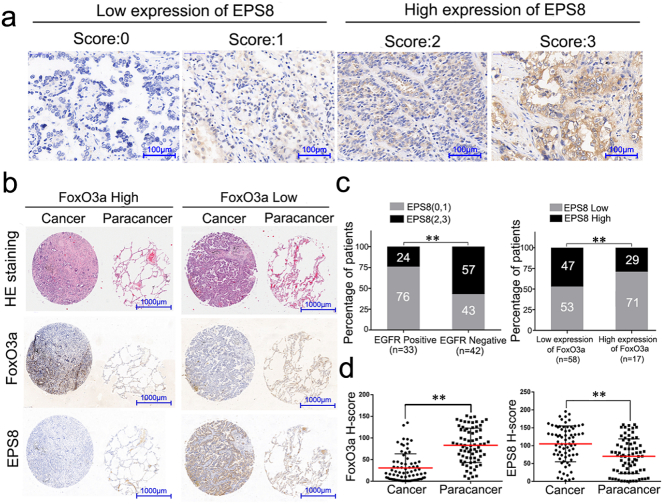
To investigate the relationship between FoxO3a and EPS8 in tissues from NSCLC patients, we measured the expression levels of FoxO3a and EPS8 by IHC staining. The degree of EPS8 expression was graded as shown in Fig. 6a. The ratio of patients with high EPS8 expression in EGFR positive group is significantly lower than that in EGFR negative group (24%, n = 33 vs. 57%, n = 42). When we separated these patients into two groups according to FoxO3a expression, we found there were 27 patients who had high-expression EPS8 in the low-FoxO3a group (n = 58). The ratio was significantly higher than that in the high-FoxO3a group (47% vs. 29%, P < .01, Student's t-test, Fig. 6c). The average value of H-scores of FoxO3a in tumor tissues (H-scores = 32) was significantly lower than that in para-carcinoma tissues (H-scores = 88) (Student's t-test, P < .01). While the H-scores of EPS8 in tumor tissues (H-scores = 104) was higher than that in para-carcinoma tissues (H-scores = 69) (Student's t-test, P < .01) (Fig. 6d). These results suggest that the expression of EPS8 is negatively correlated with FoxO3a in both tumor and para-carcinoma tissues.
Fig. 6.
Expression of FoxO3a and EPS8 in clinical tissue samples. (a) IHC staining scores ranged from 0 to 3, divided into four orders of magnitude, representing the positive expression level of EPS8 in lung cancer samples. 0–1 represents EPS8 low expression, 2–3 represents EPS8 high expression, scale = 100 μm. (b) Comparison the differences of H&E staining, FoxO3a and EPS8 expression between cancer and para-carcinoma tissue of the same patient, scale = 1000 μm. (c) The left histogram represents the proportion of EPS8 overexpression (black bars) and low expression (gray bars) in EGFR positive group (n = 33) and EGFR negative group (n = 42). The right histogram represents the proportion of EPS8 in FoxO3a low expression group (n = 58) and high expression group (n = 17). Data is presented as the mean ± SD. P-values were calculated using the independent Student's t-test. **P < .01. (d) EPS8 and FoxO3a in patients with cancer and para-carcinoma to the H-score situation. Data is presented as the mean ± SD. P-values were calculated using the independent Student's t-test. **P < .01.
3.7. FoxO3a inhibits EPS8 expression directly
Promoter sequence analysis in EPS8 gene revealed 3 consensus binding sequences of FoxO3a within 1500 bp upstream of the transcription start site (sequence I: 5′-TTGTTTGC-3′, location: −289 to −281; sequence II: 5’-CAGTTTAC-3′, location: −401 to −394; sequence III: 5′-GTAAATAT-3′, location: −1042 to −1035) (Fig. 7a). To further confirm the inhibition of FoxO3a on EPS8, we employed the luciferase reporter assay system to determine the activity center in the EPS8 promoter region. The results showed that when the inserted promoter fragment changed from −837 to −382, the GLuc/SEAP activity ratio decreased significantly (Student's t-test, P < .05). We suspected that this area might be the activity center of the EPS8 promoter (Fig. 7a).
Fig. 7.
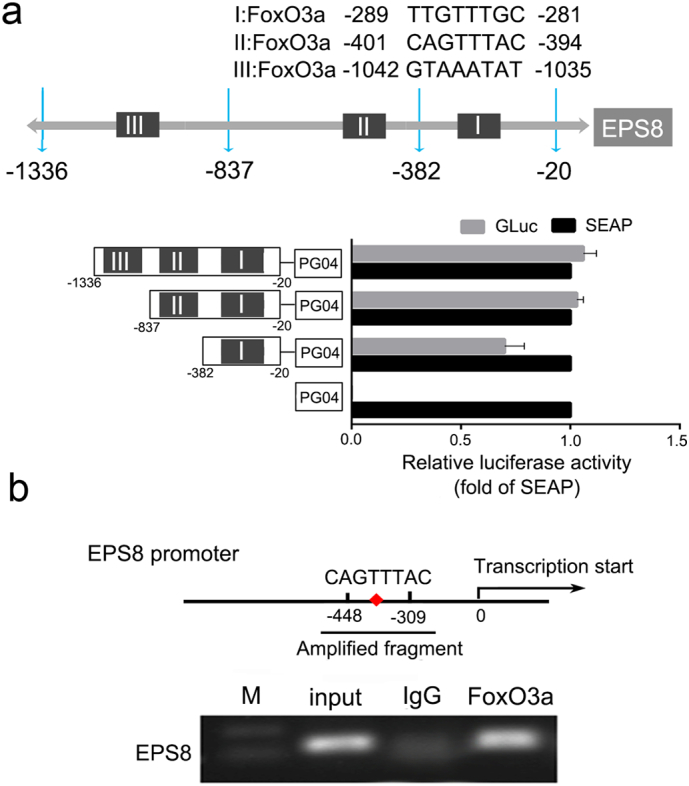
FoxO3a directly binds to the promoter region of EPS8. (a) Predicted binding sites and sequences to FoxO3a in EPS8 promoter region. Schematic description of serial deletion reporter constructs of the EPS8 promoter cloned into the pEZX-PG04 vector and the luciferase activity was measured by the dual luciferase reporter assay. (b) ChIP was used to capture the downstream target gene. Normal rabbit IgG was used as a negative IP control, data is presented as fold enrichment of the FoxO3a antibody signal vs. the negative control IgG, calculated using the 2% × 2(C[T]Input Sample– C[T]IP Sample) method. The results were also analyzed by PCR and 1% agarose gel electrophoresis. P-values were calculated using the independent Student's t-test. *P < .05, **P < .01.
Then we performed the chromatin immunoprecipitation (ChIP) assay to further confirm whether FoxO3a can bind to this area directly. Quantitative PCR reactions were performed and the respective Ct values were obtained. ChIP enrichment efficiency was calculated according to the following formula: Percent Input = 2% × 2 (C [T] Input Sample-C [T] IP Sample) and compared with the enrichment factor of FoxO3a in the same primer amplification group. As shown in Fig. 7b, anti-FoxO3a antibody, but not the control IgG, specifically immunoprecipitated the promoter region (−448 to −309, containing the sequence II: 5’-CAGTTTAC-3′, location: −401 to −394) of the EPS8 gene. The results demonstrate that FoxO3a binds to the activity center of EPS8 gene promoter directly.
4. Discussion
In this work, our results show the opposite effects between FoxO3a and EPS8 in tumorigenesis, metastasis and chemoresistance in NSCLC. We have observed the expression trend of EPS8 is inversely related to FoxO3a in clinical samples and cell lines. Moreover, we have verified FoxO3a inhibited the transcription of EPS8 by binding to its promoter directly.
EPS8 is a skeletal protein that accumulates in F-actin microfilaments and filopodia of cells. As a substrate of EGFR, EPS8 activates Akt, ERK and other signaling proteins and regulates the functions involved in proliferation and migration, in vivo and in vitro [36,43,44,73]. FoxO3a, one of the downstream targets of Akt, plays roles in tumorigenesis, metastasis and chemotherapy resistance in multiple cancers, particularly in NSCLC, through its target genes, such as TRAIL, PUMA, FasL and BIM [13,25,26,39,60,62,72]. EPS8 and FoxO3a are both crucial molecules in EGFR signaling and play roles in transmitting signals and regulating transcription [10,21,36,57,65,68].
In the present study, we verified EPS8 is a new target gene of FoxO3a and demonstrated the opposite effects of EPS8 and FoxO3a on the resistance to gefitinib, the migration and invasion abilities, the cell cycle arrest in PC9 cells and the tumor growth in BALB/c nude mice. In addition, we explored the relationship between EPS8 and FoxO3a in clinical samples. Unlike other targets, EPS8 transcription is inhibited by the binding of FoxO3a to the activity center in its promoter region. Due to the strong oncogenic role of EPS8, we speculate that the suppression of FoxO3a in cancer is at least partially achieved by reducing EPS8 expression.
It is generally believed that transcription factors promote or inhibit target gene transcription by binding to the promoter and studies show that FoxO3a acts the same way. For example, FoxO3a directly activates the bim promoter via two conserved FoxO binding sites and mutation of these sites abolishes bim promoter activation after NGF withdrawal in sympathetic neurons [22]. Besides, it is also reported that histone deacetylase 2 (HDAC2) can form a complex with FoxO3a and inhibit the transcription of a certain target gene of FoxO3a [55]. Our future work will examine whether FoxO3a regulates EPS8 expression by binding to deacetylases or methylases or by other mechanisms. However, what deserves our more attention is that the negative loop formed by FoxO3a and its upstream molecule EPS8 and the significance of this negative loop in tumorigenesis and antitumor drug development.
There is no doubt that EGFR is the key point to activate the entire PI3K/Akt signaling pathway, the important tumorigenesis and chemoresistance cascade. Therefore it is not surprising that dozens of monoclonal antibodies (mAbs) and small-molecule tyrosine kinase inhibitors (TKIs) have been designed and developed over the past few decades and a few of them are being used in clinical targeted therapies [23,31]. Compared with traditional chemotherapy drugs, the EGFR-target therapy broadens treatment options especially in NSCLC and improves the survival and safety. EGFR remains the “core” in the study of tumor growth and chemotherapy due to its downstream activation of multiple signal pathway including mTOR/STAT3 and Ras/ERK, although gene mutation eventually leads to resistance to every generation of agents [67].
Researchers have classified the resistance to EGFR TKI as “on target” and “off-target”. “On target” means the resistance is mainly caused by the primary drug target variation. “Off-target” resistance refers to the activation of parallel signaling pathways [58]. PI3K/Akt, which is involved in both “on target” and “off-target” resistance mechanisms, is frequently hyperactivated in human cancers and is targeted for cancer therapy. The activation of PI3K/Akt pathway promotes tumor growth at least partly by inhibiting the role of FoxO3a. FoxO3a is well known as a transcription factor playing roles in differentiation [1], apoptosis [34], and DNA damage repair [6]. In the present study, we showed that FoxO3a, the downstream molecule of PI3K/Akt pathway, inhibits the expression of EPS8, the upstream signaling protein by directly binding to the EPS8 gene promoter. Through EPS8, the signal pathways of inhibition and promotion are connected to form a negative regulatory loop. This negative loop bypasses EGFR and reduces the activities of PI3K/Akt pathway. So, it might affect the EGFR-TKI resistance in both “on target” and “off-target”. Therefore, FoxO3a may be the other “core” in signaling net of resistance to EGFR TKIs. The “dual cores”, EGFR and FoxO3a, negatively regulate each other in growth factor signaling network to maintain the physiological and biochemical functions of cells.
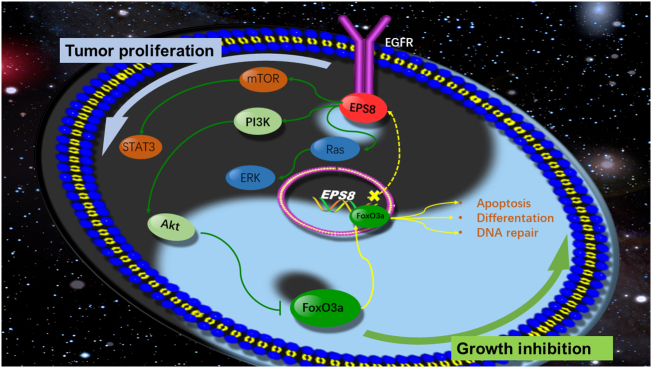
The Yin-Yang balance theory, which is used in many life science fields to explain the relationship between two opposing factors in the same system [20,38], is also suited to the relationship between EGFR and FoxO3a. EGFR initiates the signal of “Yang” such as proliferation and growth, and FoxO3a mediates the signal of “Yin” such as differentiation and growth inhibition. The “dual cores” regulate the balance of the normal survival and apoptosis of lives through the balance of “Yin” and “Yang”. “Yin” and “Yang” oppose each other but are also interdependent [70]. Therefore, through the function of FoxO3a, we can perhaps find a new solution for resistance to EGFR TKIs in NSCLC (Fig. 8).
Fig. 8.
Negative regulation between FoxO3a and EPS8 in EGF/PI3K/Akt pathway. EGF and its receptor conduct signal into the cell and EPS8 active PI3K/Akt pathway to phosphorylate FoxO3a. The phosphorylated FoxO3a translocate from the nucleus into the cytoplasm of the cell and loses its regulation of apoptosis, differentiation and DNA repair. In the nucleus, FoxO3a combines with the core promoter region of EPS8 and directly inhibits EPS8 expression, which forms a new negative loop of EGFR signaling pathway.
Many researchers have verified FoxO3a as an inhibiting factor playing negative feedback roles in various tumors and other diseases [8,12,13,32,35,46]. In most of their conclusions, FoxO3a plays indirect roles, usually by regulating the target genes and micro RNA. In this study, we verified FoxO3a directly inhibited the production of EPS8, the key protein in carcinogenesis signaling. In other words, the promotion of EGFR and the inhibition of FoxO3a are achieved through the same signaling loop. Compared with EGFR, we have not paid enough attention to FoxO3a, although many advances have been made in the research of FoxO3a as an anti-tumor target.
Cell membrane receptors acting as the initiating factors and transcription factors acting as terminal elements are widespread in cell signaling networks. Whether there are other “dual cores” models in other signaling pathways, in addition to EGFR and FoxO3a, remains to be investigated by further research. We will further examine the role of FoxO3a in overcoming the resistance to EGFR TKI in the “on target” and “off-target” resistant models of NSCLC. It is foreseeable that more investigation in the balance between different “cores” in the signaling network, especially in EGFR signaling cascade, will be necessary to understand the mechanisms of tumorigenesis, chemoresistance and oncotherapy in NSCLC.
Acknowledgments
Acknowledgements
Not applicable.
Funding sources
This work was partly supported by the National Natural Science Foundation of China (No. U1304815 and No. 81703759), Science and Technology Project of Henan (13210230097), Outstanding Young Talent Research Fund of Zhengzhou University (No.1521328003) and the National Scholarship Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interests
The authors declare no competing interests.
Author contributions
Conceptualization, H.Q, S.T. and Q.W.; Methodology, Q.W., X.J. and F.K.; Investigation, Q.W., X.J., F.K., B.H. and Y.Z.; Resources, Q.W., G.S, Y.B. and D.Y.; Writing – Original Draft, Q.W. and X.J.; Writing –Review & Editing, S.T., G.S, Y.B., D.Y. D.W. and F.Z.; Visualization, X.J., F.K. H. C.Q. and S.W.; Supervision, H.Q.; Funding Acquisition S.T., Q.W. and H.Q.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.053.
Contributor Information
Shu Tang, Email: tangshu2008@163.com.
Hailing Qiao, Email: qiaohl@zzu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Accili D., Arden K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 2.Allen J.E., Krigsfeld G., Mayes P.A., Patel L., Dicker D.T., Patel A.S. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013;5(171):171ra17. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard P., Yates J.W., Yang Z., Kim D.W., Yang J.C., Cantarini M. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 2016;22(20):5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 4.Brock M.V., Hooker C.M., Ota-Machida E., Han Y., Guo M., Ames S. DNA methylation markers and early recurrence in stage I lung cancer. N. Engl. J. Med. 2008;358(11):1118–1128. doi: 10.1056/NEJMoa0706550. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z., Zhou Y., Lei T., Chiu J.F., He Q.Y. Mammary serine protease inhibitor inhibits epithelial growth factor-induced epithelial-mesenchymal transition of esophageal carcinoma cells. Cancer. 2009;115(1):36–48. doi: 10.1002/cncr.23991. [DOI] [PubMed] [Google Scholar]
- 6.Carter M.E., Brunet A. FOXO transcription factors. Curr. Biol. 2007;17(4):R113–R114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Castel P., Ellis H., Bago R., Toska E., Razavi P., Carmona F.J. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell. 2016;30(2):229–242. doi: 10.1016/j.ccell.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandarlapaty S., Sawai A., Scaltriti M., Rodrik-Outmezguine V., Grbovic-Huezo O., Serra V. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.J., Shen M.R., Chen Y.J., Maa M.C., Leu T.H. Eps8 decreases chemosensitivity and affects survival of cervical cancer patients. Mol. Cancer Ther. 2008;7(6):1376–1385. doi: 10.1158/1535-7163.MCT-07-2388. [DOI] [PubMed] [Google Scholar]
- 10.Chen H., Wu X., Pan Z.K., Huang S. Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer metastasis. Cancer Res. 2010;70(23):9979–9990. doi: 10.1158/0008-5472.CAN-10-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.F., Pandey S., Day C.H., Chen Y.F., Jiang A.Z., Ho T.J. Synergistic effect of HIF-1alpha and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell. Physiol. 2018;233(4):3660–3671. doi: 10.1002/jcp.26235. [DOI] [PubMed] [Google Scholar]
- 12.Cheong J.K., Zhang F., Chua P.J., Bay B.H., Thorburn A., Virshup D.M. Casein kinase 1alpha-dependent feedback loop controls autophagy in RAS-driven cancers. J. Clin. Invest. 2015;125(4):1401–1418. doi: 10.1172/JCI78018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu C.F., Chang Y.W., Kuo K.T., Shen Y.S., Liu C.Y., Yu Y.H. NF-kappaB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance. Proc. Natl. Acad. Sci. U. S. A. 2016;113(18):E2526–E2535. doi: 10.1073/pnas.1522612113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahabreh I.J., Linardou H., Kosmidis P., Bafaloukos D., Murray S. EGFR gene copy number as a predictive biomarker for patients receiving tyrosine kinase inhibitor treatment: a systematic review and meta-analysis in non-small-cell lung cancer. Ann. Oncol. 2011;22(3):545–552. doi: 10.1093/annonc/mdq432. [DOI] [PubMed] [Google Scholar]
- 15.Das T.P., Suman S., Alatassi H., Ankem M.K., Damodaran C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Q., Chen Y., Zhang Q., Guo Y., Huang Z., Dai L. 8bromo7methoxychrysin induces apoptosis by regulating Akt/FOXO3a pathway in cisplatinsensitive and resistant ovarian cancer cells. Mol. Med. Rep. 2015;12(4):5100–5108. doi: 10.3892/mmr.2015.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Er E.E., Mendoza M.C., Mackey A.M., Rameh L.E., Blenis J. AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Sci. Signal. 2013;6(279):ra45. doi: 10.1126/scisignal.2004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang D., Chen H., Zhu J.Y., Wang W., Teng Y., Ding H.F. Epithelial-mesenchymal transition of ovarian cancer cells is sustained by Rac1 through simultaneous activation of MEK1/2 and Src signaling pathways. Oncogene. 2017;36(11):1546–1558. doi: 10.1038/onc.2016.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fluteau A., Ince P.G., Minett T., Matthews F.E., Brayne C., Garwood C.J. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci. Lett. 2015;609:11–17. doi: 10.1016/j.neulet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiser D.M. Antifungal design: the toxicity-resistance yin-yang. Nat. Chem. Biol. 2015;11(7):453–454. doi: 10.1038/nchembio.1838. [DOI] [PubMed] [Google Scholar]
- 21.Giampietro C., Disanza A., Bravi L., Barrios-Rodiles M., Corada M., Frittoli E. The actin-binding protein EPS8 binds VE-cadherin and modulates YAP localization and signaling. J. Cell Biol. 2015;211(6):1177–1192. doi: 10.1083/jcb.201501089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilley J., Coffer P.J., Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell Biol. 2003;162(4):613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harari P.M., Allen G.W., Bonner J.A. Biology of interactions: antiepidermal growth factor receptor agents. J. Clin. Oncol. 2007;25(26):4057–4065. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 24.Hiraga T., Ito S., Nakamura H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013;73(13):4112–4122. doi: 10.1158/0008-5472.CAN-12-3801. [DOI] [PubMed] [Google Scholar]
- 25.Hu M.C., Lee D.F., Xia W., Golfman L.S., Ou-Yang F., Yang J.Y. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 26.Hu L., Liang S., Chen H., Lv T., Wu J., Chen D. DeltaNp63alpha is a common inhibitory target in oncogenic PI3K/Ras/Her2-induced cell motility and tumor metastasis. Proc. Natl. Acad. Sci. U. S. A. 2017;114(20) doi: 10.1073/pnas.1617816114. (E3964-E73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Innocenti M., Frittoli E., Ponzanelli I., Falck J.R., Brachmann S.M., Di Fiore P.P. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J. Cell Biol. 2003;160(1):17–23. doi: 10.1083/jcb.200206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamal-Hanjani M., Wilson G.A., McGranahan N., Birkbak N.J., Watkins T.B.K., Veeriah S. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 29.Jeganathan N., Predescu D., Zhang J., Sha F., Bardita C., Patel M. Rac1-mediated cytoskeleton rearrangements induced by intersectin-1s deficiency promotes lung cancer cell proliferation, migration and metastasis. Mol. Cancer. 2016;15(1):59. doi: 10.1186/s12943-016-0543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph J., Ametepe E.S., Haribabu N., Agbayani G., Krishnan L., Blais A. Inhibition of ROS and upregulation of inflammatory cytokines by FoxO3a promotes survival against Salmonella typhimurium. Nat. Commun. 2016;7:12748. doi: 10.1038/ncomms12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jotte R.M., Spigel D.R. Advances in molecular-based personalized non-small-cell lung cancer therapy: targeting epidermal growth factor receptor and mechanisms of resistance. Cancer Med. 2015;4(11):1621–1632. doi: 10.1002/cam4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannike K., Sepp M., Zuccato C., Cattaneo E., Timmusk T. Forkhead transcription factor FOXO3a levels are increased in Huntington disease because of overactivated positive autofeedback loop. J. Biol. Chem. 2014;289(47):32845–32857. doi: 10.1074/jbc.M114.612424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedmi M., Ben-Chetrit N., Korner C., Mancini M., Ben-Moshe N.B., Lauriola M. EGF induces microRNAs that target suppressors of cell migration: miR-15b targets MTSS1 in breast cancer. Sci. Signal. 2015;8(368):ra29. doi: 10.1126/scisignal.2005866. [DOI] [PubMed] [Google Scholar]
- 34.Knowlton W.M., Jin Y. Ground control to major tom: the cell body signals axon degeneration. Cell. 2016;164(5):842–844. doi: 10.1016/j.cell.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Kress T.R., Cannell I.G., Brenkman A.B., Samans B., Gaestel M., Roepman P. The MK5/PRAK kinase and Myc form a negative feedback loop that is disrupted during colorectal tumorigenesis. Mol. Cell. 2011;41(4):445–457. doi: 10.1016/j.molcel.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Lanzetti L., Rybin V., Malabarba M.G., Christoforidis S., Scita G., Zerial M. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408(6810):374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 37.Li C.J., Chang J.K., Chou C.H., Wang G.J., Ho M.L. The PI3K/Akt/FOXO3a/p27Kip1 signaling contributes to anti-inflammatory drug-suppressed proliferation of human osteoblasts. Biochem. Pharmacol. 2010;79(6):926–937. doi: 10.1016/j.bcp.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Li W., Herrera-Estrella L., Tran L.S. The yin-yang of cytokinin homeostasis and drought acclimation/adaptation. Trends Plant Sci. 2016;21(7):548–550. doi: 10.1016/j.tplants.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Liang Z., Wang X., Xu X., Xie B., Ji A., Meng S. MicroRNA-608 inhibits proliferation of bladder cancer via AKT/FOXO3a signaling pathway. Mol. Cancer. 2017;16(1):96. doi: 10.1186/s12943-017-0664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y.H., Chiang K.H., Shieh J.M., Huang C.R., Shen C.J., Huang W.C. Epidermal growth factor-induced ANGPTL4 enhances anoikis resistance and tumour metastasis in head and neck squamous cell carcinoma. Oncogene. 2017;36(16):2228–2242. doi: 10.1038/onc.2016.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim S.W., Jin L., Luo K., Jin J., Shin Y.J., Hong S.Y. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 2017;8(8) doi: 10.1038/cddis.2017.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling H., Jolicoeur P. Notch-1 signaling promotes the cyclinD1-dependent generation of mammary tumor-initiating cells that can revert to bi-potential progenitors from which they arise. Oncogene. 2013;32(29):3410–3419. doi: 10.1038/onc.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maa M.C., Hsieh C.Y., Leu T.H. Overexpression of p97Eps8 leads to cellular transformation: implication of pleckstrin homology domain in p97Eps8-mediated ERK activation. Oncogene. 2001;20(1):106–112. doi: 10.1038/sj.onc.1204069. [DOI] [PubMed] [Google Scholar]
- 44.Maa M.C., Lee J.C., Chen Y.J., Chen Y.J., Lee Y.C., Wang S.T. Eps8 facilitates cellular growth and motility of colon cancer cells by increasing the expression and activity of focal adhesion kinase. J. Biol. Chem. 2007;282(27):19399–19409. doi: 10.1074/jbc.M610280200. [DOI] [PubMed] [Google Scholar]
- 45.Marzi L., Combes E., Vie N., Ayrolles-Torro A., Tosi D., Desigaud D. FOXO3a and the MAPK p38 are activated by cetuximab to induce cell death and inhibit cell proliferation and their expression predicts cetuximab efficacy in colorectal cancer. Br. J. Cancer. 2016;115(10):1223–1233. doi: 10.1038/bjc.2016.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matkar S., An C., Hua X. Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop. Am. J. Cancer Res. 2017;7(7):1476–1485. [PMC free article] [PubMed] [Google Scholar]
- 47.Matrone A., Grossi V., Chiacchiera F., Fina E., Cappellari M., Caringella A.M. p38alpha is required for ovarian cancer cell metabolism and survival. Int. J. Gynecol. Cancer. 2010;20(2):203–211. doi: 10.1111/igc.0b013e3181c8ca12. [DOI] [PubMed] [Google Scholar]
- 48.McClelland Descalzo D.L., Satoorian T.S., Walker L.M., Sparks N.R., Pulyanina P.Y., Zur Nieden N.I. Glucose-induced oxidative stress reduces proliferation in embryonic stem cells via FOXO3A/beta-catenin-dependent transcription of p21(cip1) Stem Cell Rep. 2016;7(1):55–68. doi: 10.1016/j.stemcr.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan S.E., McCoy D.M. Platelet-derived growth factor-a regulates lung fibroblast S-phase entry through p27(kip1) and FoxO3a. Respir. Res. 2013;14:68. doi: 10.1186/1465-9921-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikse O.R., Blake D.C., Jr., Jones N.R., Sun Y.W., Amin S., Gallagher C.J. FOXO3 encodes a carcinogen-activated transcription factor frequently deleted in early-stage lung adenocarcinoma. Cancer Res. 2010;70(15):6205–6215. doi: 10.1158/0008-5472.CAN-09-4008. [DOI] [PubMed] [Google Scholar]
- 51.Mitra S., Lee J.S., Cantrell M., Van den Berg C.L. C-Jun N-terminal kinase 2 (JNK2) enhances cell migration through epidermal growth factor substrate 8 (EPS8) J. Biol. Chem. 2011;286(17):15287–15297. doi: 10.1074/jbc.M109.094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musumeci G., Castorina A., Magro G., Cardile V., Castorina S., Ribatti D. Enhanced expression of CD31/platelet endothelial cell adhesion molecule 1 (PECAM1) correlates with hypoxia inducible factor-1 alpha (HIF-1alpha) in human glioblastoma multiforme. Exp. Cell Res. 2015;339(2):407–416. doi: 10.1016/j.yexcr.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Pan D., Jiang C., Ma Z., Blonska M., You M.J., Lin X. MALT1 is required for EGFR-induced NF-kappaB activation and contributes to EGFR-driven lung cancer progression. Oncogene. 2016;35(7):919–928. doi: 10.1038/onc.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pellicano F., Scott M.T., Helgason G.V., Hopcroft L.E., Allan E.K., Aspinall-O'Dea M. The antiproliferative activity of kinase inhibitors in chronic myeloid leukemia cells is mediated by FOXO transcription factors. Stem Cells. 2014;32(9):2324–2337. doi: 10.1002/stem.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng S., Zhao S., Yan F., Cheng J., Huang L., Chen H. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J. Neurosci. 2015;35(3):1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phuchareon J., McCormick F., Eisele D.W., Tetsu O. EGFR inhibition evokes innate drug resistance in lung cancer cells by preventing Akt activity and thus inactivating Ets-1 function. Proc. Natl. Acad. Sci. U. S. A. 2015;112(29):E3855–E3863. doi: 10.1073/pnas.1510733112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Politi K., Ayeni D., Lynch T. The next wave of EGFR tyrosine kinase inhibitors enter the clinic. Cancer Cell. 2015;27(6):751–753. doi: 10.1016/j.ccell.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Rotow J., Bivona T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer. 2017;17(11):637–658. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 59.Sakuma K., Aoki M., Kannagi R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. U. S. A. 2012;109(20):7776–7781. doi: 10.1073/pnas.1111135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santo E.E., Stroeken P., Sluis P.V., Koster J., Versteeg R., Westerhout E.M. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013;73(7):2189–2198. doi: 10.1158/0008-5472.CAN-12-3767. [DOI] [PubMed] [Google Scholar]
- 61.Shukla S., Bhaskaran N., Maclennan G.T., Gupta S. Deregulation of FoxO3a accelerates prostate cancer progression in TRAMP mice. Prostate. 2013;73(14):1507–1517. doi: 10.1002/pros.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukla S., Rizvi F., Raisuddin S., Kakkar P. FoxO proteins' nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells. Free Radic. Biol. Med. 2014;76:185–199. doi: 10.1016/j.freeradbiomed.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 63.Sun T., Sun B.C., Zhao X.L., Zhao N., Dong X.Y., Che N. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54(5):1690–1706. doi: 10.1002/hep.24543. [DOI] [PubMed] [Google Scholar]
- 64.Szutowicz-Zielinska E., Konopa K., Kowalczyk A., Suszko-Kazarnowicz M., Duchnowska R., Szczesna A. An open label phase II study evaluating first-line EGFR tyrosine kinase inhibitor erlotinib in non-small cell lung cancer patients with tumors showing high EGFR gene copy number. Oncotarget. 2017;8(10):17270–17278. doi: 10.18632/oncotarget.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trotman L.C., Alimonti A., Scaglioni P.P., Koutcher J.A., Cordon-Cardo C., Pandolfi P.P. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441(7092):523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H., Patel V., Miyazaki H., Gutkind J.S., Yeudall W.A. Role for EPS8 in squamous carcinogenesis. Carcinogenesis. 2009;30(1):165–174. doi: 10.1093/carcin/bgn252. [DOI] [PubMed] [Google Scholar]
- 67.Wang S., Tsui S.T., Liu C., Song Y., Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016;9(1):59. doi: 10.1186/s13045-016-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Y., Zou Z., Becker N., Anderson M., Sumpter R., Xiao G. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154(6):1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willcox B.J., Donlon T.A., He Q., Chen R., Grove J.S., Yano K. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. U. S. A. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S.G., He L., Wang Q., Tang Y.J. An ancient Chinese wisdom for metabolic engineering. Microb. Cell Fact. 2015;14:39. doi: 10.1186/s12934-015-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamura Y., Lee W.L., Inoue K., Ida H., Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J. Biol. Chem. 2006;281(8):5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- 72.Yang J.Y., Hung M.C. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin. Cancer Res. 2009;15(3):752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yap L.F., Jenei V., Robinson C.M., Moutasim K., Benn T.M., Threadgold S.P. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene. 2009;28(27):2524–2534. doi: 10.1038/onc.2009.105. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X., Zhang Y., Tang H., He J. EGFR gene copy number as a predictive/biomarker for patients with non-small-cell lung cancer receiving tyrosine kinase inhibitor treatment: a systematic review and meta-analysis. J. Invest. Med. 2017;65(1):72–81. doi: 10.1136/jim-2016-000252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material