Abstract
Background
Atherosclerosis is a hyperlipidemia-induced condition affecting the arterial wall that damages healthy endothelial cell (EC) function, leading to enhanced risk of atherothrombotic events. Certain microRNAs regulate EC dysfunction in response to hyperlipidemia and may be suitable therapeutic targets to combat atherosclerosis.
Methods
miRNA expression in human ECs was analyzed under various conditions to identify key microRNAs. High-cholesterol diet (HCD)-fed Mir652−/−Apoe−/− (Mir652−/−) mice and matching Mir652+/+Apoe−/− (Mir652+/+) mice were subjected to carotid injury to analyze the effects of miR-652 knockdown on endothelial repair. In silico analysis followed by in vitro and in vivo experiments were applied to identify miR-652's target gene Ccnd2 and investigate the pair's effects on ECs. miR-652-5p and miR-652-3p antagomir therapies were tested in Mir652+/+ mice under normal and HCD diet to assess their effect on endothelial repair.
Findings
miR-652-3p, which is upregulated in human and murine atherosclerotic plaques, suppresses expression of the endothelial repair gene Ccnd2, thereby enhancing atherosclerotic lesion formation. Post-denudation recovery of ECs was promoted in Mir652−/− mice due to enhanced EC proliferation attributable to de-repression of miR-652-3p's (but not miR-652-5p's) regulation of Ccnd2 expression. Under hyperlipidemic conditions at non-predilection sites, miR-652-3p produces anti-proliferative effects in ECs, such that Mir652−/− mice display reduced atherosclerotic progression. In contrast, neither miR-652-3p nor Ccnd2 displayed significant effects on the endothelium at predilection sites or under disturbed flow conditions. Administration of a miR-652-3p antagomir rescued the proliferation of ECs in vivo, thereby limiting atherosclerotic development.
Interpretation
miR-652-3p blockade may be a potential therapeutic strategy against atherosclerosis.
Abbreviations: AGO2, Argonaute 2; ANOVA, One-way analysis of variance; BM, Bone marrow; Ccnd2, Cyclin D2; HCD, High-cholesterol diet; HUVEC, human umbilical vein endothelial cell; IP, Immunoprecipitation; LDL, Low density lipoprotein; LNA, Locked nucleic acid; miRNA, microRNA; moxLDL, Mildly oxidized LDL; NF-κB, Nuclear factor kappa B; nLDL, Native LDL; siRNA, Small interfering RNA/short interfering RNA/silencing RNA; SMA, smooth muscle actin; SMC, Smooth muscle cell; TNF-α, Tumor necrosis factor alpha; vWF, von Willebrand factor
Keywords: Atherosclerosis, Endothelium, Cyclin D2, CCND2, miR-652
Highlights
-
•
miR-652-3p is upregulated in human and murine atherosclerotic plaques.
-
•
miR-652 knockdown promotes endothelial repair in injured arteries.
-
•
miR-652-3p suppresses expression of the endothelial repair gene Ccnd2.
-
•
miR-652-3p antagomir rescues EC proliferation under hyperlipidemic stress.
Research in context.
Evidence before this study
Atherosclerotic diseases are one of the most prominent causes of morbidity and mortality worldwide. microRNAs – small non-coding RNAs that post-transcriptionally regulate gene expression – are involved in regulating endothelial cell (EC) characteristics associated with atherosclerotic progression. For example, microRNAs have been shown to regulate the inflammatory EC phenotype observed in atherosclerotic plaques as well as the EC proliferative reserve, which drives endothelial regeneration in response to stressors such as oxidative stress and hyperlipidemia. Thus, controlling the expression levels of specific microRNAs may become a more effective treatment approach for atherosclerosis.
Added value of this study
The objective of the current work was to investigate the effects of silencing the microRNA miR-652 on EC characteristics in vitro, as well as to assess the therapeutic potential of miR-652 knockdown in a murine model of atherosclerosis. We show that miR-652-3p, which is upregulated in human and murine atherosclerotic plaques, suppresses expression of the endothelial repair gene Ccnd2, thereby enhancing the formation of atherosclerotic lesions. We also show that post-denudation recovery of ECs was promoted in Mir652−/−Apoe−/− mice due to enhanced EC proliferation attributable to de-repression of miR-652-3p's (but not miR-652-5p's) regulation of Ccnd2 expression. Under hyperlipidemic conditions at non-predilection sites, we demonstrate that miR-652-3p produces anti-proliferative effects in ECs, such that Mir652−/−Apoe−/− mice display reduced levels of atherosclerosis. Our administration of an engineered miR-652-3p antagomir rescued the proliferation of ECs in vivo, thereby limiting atherosclerotic development.
Implications of all the available evidence
We advance a model of impaired endothelial regeneration during atherosclerosis, where the increase in EC miR-652-3p expression downregulates Ccnd2 expression, thereby negatively regulating cell proliferation under hyperlipidemic stress. Our results suggest that miR-652-3p blockade in the endothelium may offer a potentially novel strategy to combat atherosclerosis.
Alt-text: Unlabelled Box
1. Introduction
Atherosclerotic diseases are one of the most prominent causes of morbidity and mortality worldwide [1]. Inflammation leads to atherosclerotic lesion formation through a host of cell types [2]. A defect in the resolution of vascular inflammation may lead to advanced atherosclerotic lesions with overt clinical manifestations [3].
miRNAs are small non-coding RNAs that post-transcriptionally regulate gene expression [4]. Binding of miRNAs to target messenger RNAs (mRNAs) may lead to mRNA degradation, gene silencing, or translational repression [4]. Individual miRNAs are potent regulators of biological activity, as they can negatively regulate the expression of several hundred target mRNAs and profoundly affect multiple biological networks [5].
With respect to atherosclerosis, miRNAs are involved in regulating endothelial cell (EC) characteristics associated with atherosclerotic progression [6]. For example, miRNAs have been shown to regulate the inflammatory EC phenotype observed in atherosclerotic plaques [7,8]. Moreover, miRNAs have been shown to regulate the EC proliferative reserve, which drives endothelial regeneration in response to stressors such as oxidative stress and hyperlipidemia [9].
In view of the above observations, controlling the expression levels of specific miRNAs may become a more effective treatment approach for atherosclerosis [10]. miRNA animal models, miRNA therapeutic development, and clinical applications of miRNAs in cardiovascular diseases have been reviewed elsewhere [[11], [12], [13]]. The objective of the current work was to investigate the effects of silencing miR-652 on EC characteristics in vitro, as well as to assess the therapeutic potential of miR-652 knockdown in a murine model of atherosclerosis. Our results suggest that miR-652 blockade in the endothelium may offer a potentially novel strategy to combat atherosclerosis.
2. Results
2.1. miR-652 abundantly expressed in human ECs, upregulated in response to moxLDL
Reduced EC expression of the miR-processing endonuclease Dicer suppresses EC proliferation and migration [14,15]. Hartmann et al. previously identified four miRNAs (miR-103, miR-301b, miR-433, and miR-652) that are significantly downregulated in endothelial Dicer-silenced Apoe−/− mice after three months of high-fat feeding; these same four miRNAs are also downregulated in Dicer-silenced human aortic ECs [16]. This previous evidence suggests that these four miRNAs may play a role in EC proliferation and atherosclerosis [16].
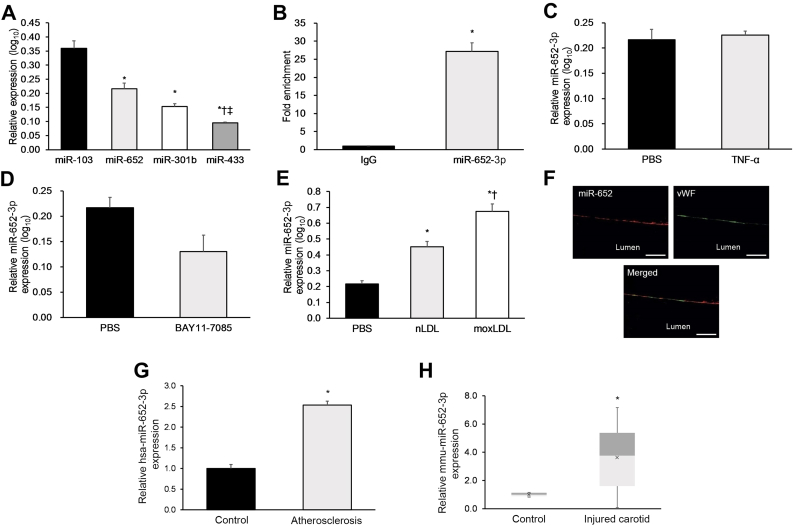
Therefore, here we initially analyzed the expression of these four miRNAs in cultured HUVECs under various conditions to identify ones that may be suitable targets or further investigation. Of the four candidate miRNAs, miR-652 was the second-most highly abundant miRNA in non-stimulated HUVECs after miR-103, whose role in atherosclerosis has been previously investigated (Fig. 1a) [16]. miR-652-3p is significantly enriched in the miRNA-induced silencing complex (RISC) of HUVECs as determined by AGO2-IP (Fig. 1b), suggesting a putative role for miR-652-3p in regulating EC function [16]. There was no significant difference in miR-652-3p expression in HUVECs in the presence of pro-inflammatory TNF-α or the NF-κB inhibitor BAY11-7085 (Fig. 1c, d), suggesting that HUVEC miR-652-3p expression does not significantly change in response to classical pro-inflammatory or anti-inflammatory stimulus [17]. However, miR-652-3p expression was significantly increased after treatment with normal low-density lipoprotein (nLDL) and even more so after treatment with mildly-oxidized low-density lipoprotein (moxLDL) (Fig. 1e).
Fig. 1.
miR-652 is abundantly expressed in human ECs, upregulated in response to moxLDL.
(a–e) Various experiments on miRNA expression were conducted in cultured non-stimulated HUVECs. (a) Expression of the four most abundant miRNAs in cultured non-stimulated HUVECs. (b) Enrichment of miR-652 in the AGO2-IP fraction. (c) Effect of TNF-α treatment on HUVEC miR-652 expression. (d) Effect of treatment with the NF-κB-inhibitor BAY11–7085 on HUVEC miR-652 expression. (e) Effect of nLDL or moxLDL exposure on miR-652 expression. (f) In situ PCR and vWF immunostaining used to assess EC miR-652 expression in human carotid plaque sections (n = 12). Scale bar = 25 mm. (g) Analysis of hsa-miR-652-3p expression in intimal ECs from human carotid lesions (n = 30 samples), with post-mortem normal human carotid intimal ECs used as controls (n = 20 samples). (h) Analysis of mmu-miR-652-3p expression in injured carotid and contralateral normal carotid tissue from high cholesterol diet-fed Mir652+/+ mice on day 28 post-injury. Data reported as means ± SEMs. *P < .05 vs. first group, †P < .05 vs. second group.
To specify the subcellular localization of miR-652-3p within HUVECs, the cells were transfected with a Cy3-labeled miR-652-3p. After 24 h, Cy3-miR-652-3p fluorescence was detected within the HUVEC cytoplasm (Supp. Fig. 1). This preliminary evidence suggests that cytoplasmic miR-652-3p may be linked to LDL-induced EC dysfunction and requires further study.
2.2. miR-652-3p is upregulated in human and murine atherosclerotic plaques
As our evidence suggests that miR-652-3p may be linked to LDL-induced EC dysfunction, we next analyzed miR-652-3p expression in carotid atherosclerotic lesions. We discovered ex vivo evidence of prominent EC miR-652-3p expression in human atherosclerotic plaques using in situ PCR combined with von Willebrand factor (vWF) staining (Fig. 1f). We also discovered significantly elevated intimal EC hsa-miR-652-3p expression in human carotid lesions relative to post-mortem normal human carotid tissue (Fig. 1g). Furthermore, we found significantly elevated mmu-miR-652-3p expression in murine carotid lesions from high cholesterol diet (HCD)-fed Mir652+/+Apoe−/− mice 28 days post-wire injury relative to contralateral normal carotid tissue (Fig. 1h). This evidence suggests that endothelial miR-652-3p upregulation may play a pro-atherogenic role in humans and mice.
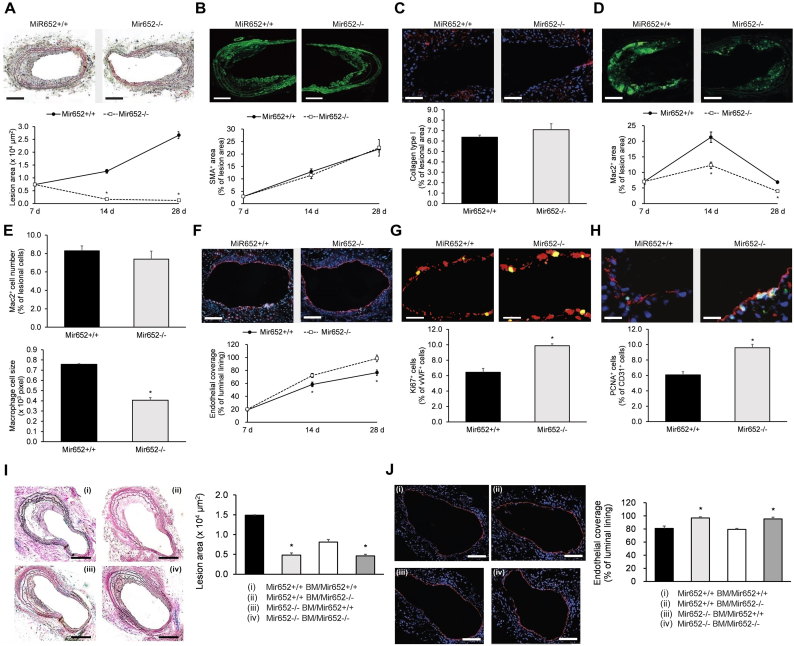
2.3. Endothelial miR-652 knockdown promotes endothelial repair in injured arteries
Endothelial denudation of carotid arteries in HCD-fed Mir652−/−Apoe−/− (Mir652−/−) mice and matching Mir652+/+Apoe−/− (Mir652+/+) mice (12–14 months old) were used to investigate the role of miR-652 in endothelial repair. Lesion formation was much smaller in HCD-fed Mir652−/− mice than in matching Mir652+/+ mice (Fig. 2a). Lesional accumulation of SMC (Fig. 2b) and collagen (Fig. 2c) was not significantly different in Mir652−/− and Mir652+/+ mice. However, we observed a significant decrease of Mac2+ area in Mir652−/− mice compared to Mir652+/+ mice (Fig. 2d). Specifically, although the number of lesional Mac2+ macrophages was not significantly different (Fig. 2e), we observed a significant decrease in their size in Mir652−/− mice compared to Mir652+/+ mice (Fig. 2e). Notably, endothelial repair after carotid injury was significantly greater in Mir652−/− mice compared to Mir652+/+ mice (Fig. 2f). Moreover, proliferation of carotid ECs, as assessed by immunostaining for vWF/Ki67 (Fig. 2g) or CD31/PCNA (Fig. 2h) 28-day post injury, was significantly greater in Mir652−/− mice relative to Mir652+/+ mice.
Fig. 2.
miR-652 knockout promotes endothelial repair post-injury.
Repair of carotid injury in high cholesterol diet-fed Mir652+/+ and Mir652−/− mice (12–14 months old). Measurements of (a) lesion area, (b) SMC accumulation, (c) collagen content, (d) Mac2+ macrophage area, as well as (e) number and size of Mac2+ macrophages in lesions at 28 days post-injury. Representative images are from lesions at 28 days post-injury. Scale bars = (a, c) 100 μm and (b, d) 200 μm. Assessment of (f) post-carotid injury repair of endothelium via immunostaining for vWF (red) and (g, h) EC proliferation by vWF (red) and Ki67 (green) immunostaining or CD31 (red) and PCNA (green) immunostaining in carotid sections at 28 days post-injury. Representative images are from carotid sections at 28 days post-injury. Scale bars = (f) 200 μm, (g) 10 μm, and (h) 20 μm. (i, j) Measurement of (i) plaque area and (j) endothelial coverage via CD31 immunostaining in Mir652+/+ and Mir652−/− mice transplanted with Mir652+/+ or Mir652−/− BM cells (Mir652+/+ BM group and Mir652−/− BM group, respectively) 28-days post-injury. Nuclei stained with DAPI (blue). Scale bars = (i) 200 μm and (j) 100 μm. n = 12–18 mice per experimental group. Data reported as means ± SEMs. *P < .05 vs. first group, †P < .05 vs. second group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Given that bone marrow (BM)-derived cells also express miR-652 [18], we next utilized a murine BM chimera model to elucidate the roles of endothelial-resident miR-652 versus BM-derived miR-652 in endothelial repair. We found that knockdown of endothelial-resident miR-652 significantly reduced lesion size and improved EC coverage 28-days post injury (Fig. 2i, j). However, knockdown of BM-derived miR-652 did not have statistically significant effects on lesion size or EC coverage in recipient mice (Fig. 2i, j). These results indicate that endothelial-resident mir-652 knockdown (not BM-derived miR-652 knockdown) impairs endothelial recovery.
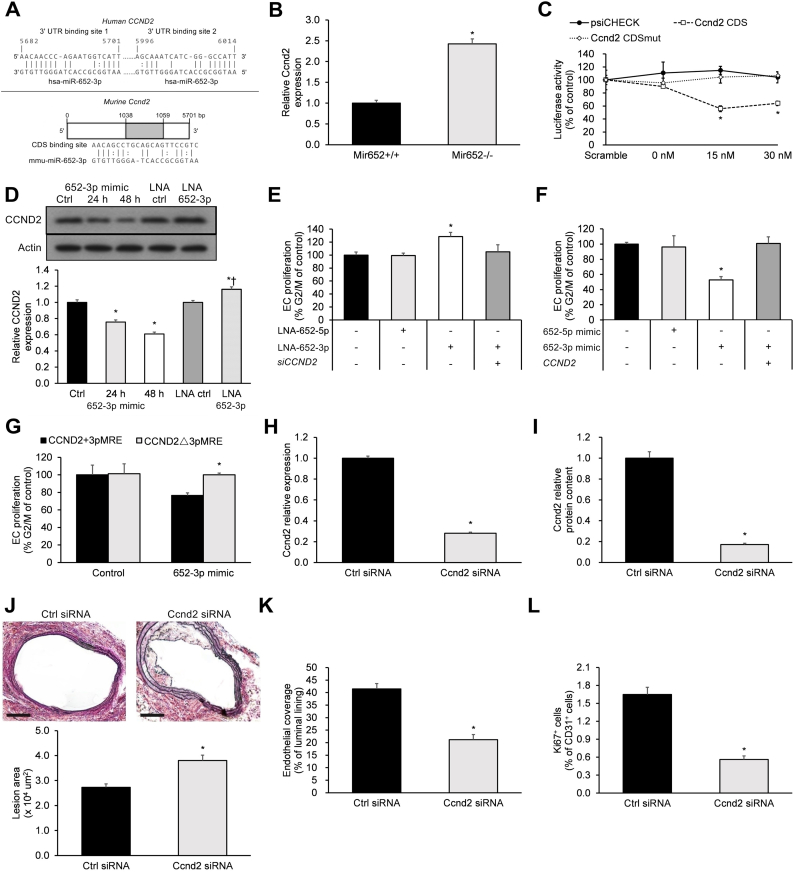
2.4. miR-652-3p negatively regulates the endothelial repair gene Ccnd2
Normal endothelial repair is dependent upon pro-proliferative genes that stimulate EC recovery following injury [19,20]. In order to identify putative miR-652-3p targets that may serve to stimulate EC recovery post-injury, we conducted a bioinformatics analysis of a proliferation-associated gene dataset (n = 3025 genes; GeneCards) integrated with the miRNA target predictions for miR-652-3p (n = 39 putative target genes; RNA22 version 2.0 and DIANA-TarBase version 8). This bioinformatics analysis identified seven proliferation-associated putative target genes of miR-652-3p: CAV1, CCND2, CSNK1A1, G3BP1, PRKCI, SFPQ, and SOX4. Of these seven candidate genes, only CCND2 has been shown to be a key promoter of EC proliferation and has also been shown to be downregulated in atherosclerotic plaques [21]. Therefore, CCND2 was selected for further investigation.
Bioinformatics algorithms predicted two miR-652-3p binding sites (5682–5701 bp and 5996–6014 bp) on the human Ccnd2 3′UTR and one binding site (1038–1059 bp) on the murine Ccdn2 coding sequence (CDS, Fig. 3a). Several lines of evidence support miR-652-3p's targeting of CCND2/Ccnd2. First, the expression level of carotid Ccnd2 mRNA in Mir652−/− mice was significantly greater compared to Mir652+/+ mice 28 days post-injury (Fig. 3b). Second, miR-652-3p significantly reduced luciferase activity in HEK293 cells carrying the wild-type CDS of murine Ccnd2 in a dose-dependent manner, an effect abolished by the corresponding mutated CDS (CDSmut) (Fig. 3c). Third, HUVECs treated with a miR-652-3p mimic revealed significant downregulation of CCND2 expression in a time-dependent manner, while those treated with a locked nucleic acid (LNA) inhibitor of miR-652-3p (LNA-652-3p) displayed significant upregulation of CCND2 expression (Fig. 3d).
Fig. 3.
miR-652-3p negatively regulates the endothelial repair gene Ccnd2.
(a) Bioinformatics algorithms predicted two miR-652 binding spots in the 3′UTR of human CCND2 and one binding spot in the CDS of mouse Ccnd2. (b) Intimal EC expression of Ccnd2 mRNA at 28 days post-injury. (c) The effect of miR-652-3p on luciferase activity of psiCHECK-2 Ccnd2 promoter constructs. Empty psiCHECK-2 (psiCHECK), WT Ccnd2 CDS (wild-type murine Ccnd2), and Ccnd2 CDSmut (mutated murine Ccnd2) constructs were co-transfected with scrambled pre-miRNA (30 nM) or synthetic miR-652-3p (0 nM, 15 nM, or 30 nM). (d) Western blot analysis of CCND2 expression in HUVECs. Band intensities were normalized with respect to β-actin band intensity. *P < .05 vs. Ctrl, †P < .05 vs. LNA ctrl. (e-g) Flow cytometric HUVEC proliferation assays under shear flow conditions. (e) HUVECs were treated with LNA-652-5p and/or LNA-652-3p, or siCCND2 (CCND2-specific siRNA). (f) HUVECs were treated with 652-5p mimic and/or 652-3p mimic (miR-652-5p mimic and miR-652-3p mimic, respectively), with (+) or without (−) overexpression of CCND2. (g) HUVECs, with (CCND2+5pMRE) or lacking (CCND2Δ5pMRE) the miR-652-3p recognition element, were treated by a miR-652-3p mimic. (h-l) Carotid-injured Mir652−/− mice were perivascularly administered a single 4-nmol dose of scrambled control or a Ccnd2-specific siRNA on days 14 and 21 post-carotid injury. On day 28 post-injury, mice were sacrificed, and intimal EC expression of (h) Ccnd2 mRNA and (i) Ccnd2 protein, (j) plaque area, as well as (k) endothelial recovery and (l) proliferation were analyzed. Scale bar = 100 μm. n = 12–18 mice per experimental group. Data reported as means ± SEMs. *P < .05 vs. first group, †P < .05 vs. second group.
Flow cytometry was then applied to study the effects of miR-652-3p and CCND2 on HUVEC proliferation under Ibidi apparatus-induced shear flow conditions. HUVEC proliferation was significantly enhanced with LNA-652-3p (but not LNA-652-5p), an effect that was abolished with CCND2-silencing (siCCND2) (Fig. 3e). HUVEC proliferation was significantly attenuated with a miR-652-3p mimic (but not the miR-652-5p mimic), an effect that was abolished with CCND2 overexpression (Fig. 3f). Following miR-652-3p mimic exposure, HUVECs with the miR-652-3p recognition element (CCND2+3pMRE) showed less proliferation than those without the miR-652-3p recognition element (CCND2Δ3pMRE) (Fig. 3g). Given the relatively high constitutive levels of miR-652 in HUVECs, we expected that HUVECs transfected with CCND2Δ3pMRE would display higher proliferation levels under control conditions. Therefore, we assessed the relative expression of endogenous versus transfected CCND2 protein and found transfected CCND2 protein levels to be significantly lower than the endogenous CCND2 protein levels (Supp. Fig. 2). This may explain why CCND2Δ3pMRE had no significant effect upon HUVEC proliferation under control conditions.
Mir652−/− mice were then employed to the effects of Ccnd2 expression on miR-652-knockout endothelium in vivo. Treatment of carotid arteries of Mir652−/− mice with Ccnd2-specific siRNA (siCcnd2) starting on day 14 post-injury resulted in decreased Ccnd2 mRNA and protein expression (Fig. 3h, i), enhanced lesion area (Fig. 3j), reduced EC recovery (Fig. 3k), and reduced EC proliferation (Fig. 3l) at day 28 post-injury.
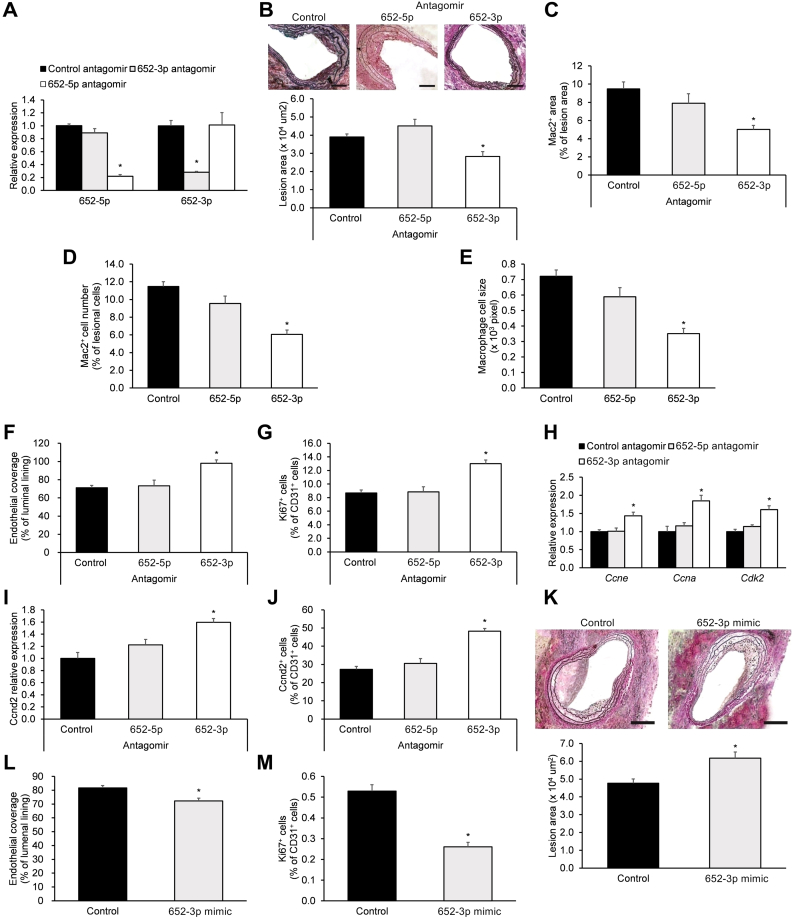
2.5. Engineered miR-652-3p antagomir promotes endothelial repair in vivo and ex vivo
The roles of the two miR-652 strands (miR-652-3p and − 652-5p) were comparatively investigated by studying the effects of their antagomirs on denuded arteries from HCD-fed Mir652+/+ mice 28-days post-carotid injury. As expected, the respective antagomirs reduced miR-652-3p and miR-652-5p expression (Fig. 4a). However, only the miR-652-3p antagomir decreased lesion formation (Fig. 4b), reduced lesional Mac2+ macrophage area (Fig. 4c), number (Fig. 4d), and size (Fig. 4e), while increasing EC coverage (Fig. 4f) and EC proliferation (Fig. 4g). With respect to effects on Ccnd2, only the miR-652-3p antagomir enhanced Ccne, Ccna, and Cdk2 transcript expression (Fig. 4h), enhanced Ccnd2 transcript expression (Fig. 4i), and enhanced Ccnd2 protein expression in ECs (Fig. 4j).
Fig. 4.
Promotion of endothelial repair by miR-652-3p antagomir.
Carotid-injured high cholesterol diet-fed Mir652+/+ mice were perivascularly administered a single 160-μg dose of control (non-specific antagomir), miR-652-5p antagomir, or miR-652-3p antagomir on days 7, 14, and 21 post-carotid injury. On day 28 post-injury, mice were sacrificed, and (a) intimal EC expression of miR-652-5p and miR-652-3p, (b) plaque area (scale bar = 200 μm), (c) Mac2+ macrophage area, (d) number and (e) size of Mac2+ macrophages, (f) endothelial coverage area, and (g) EC proliferation, as well as (h) Ccne, Ccna and Cdk2 transcript expression, and (i) Ccnd2 transcript expression and (j) Ccnd2 protein expression in intimal ECs were analyzed. (k-m) Carotid-injured high cholesterol diet-fed Mir652+/+ mice were perivascularly administered a single 5-nmol dose of carrier-packaged miR-652-3p mimic or scrambled control mimic on days 7, 14, and 21 post-carotid injury. On day 28 post-injury, mice were sacrificed, and (k) lesion formation (scale bar = 100 μm), (l) endothelial coverage, and (m) EC proliferation were analyzed. n = 12–18 mice per experimental group. Data reported as means ± SEMs. *P < .05 vs. first group, †P < .05 vs. second group.
To further investigate the effects of miR-652-3p ex vivo, the carotid arteries from HCD-fed Mir652+/+ mice after injury was subjected to perivascular treatment with a miR-652-3p mimic for 28 days. The miR-652-3p mimic enhanced lesion formation (Fig. 4k), decreased luminal EC coverage (Fig. 4l), and reduced EC proliferation (Fig. 4m). Taken together, these results demonstrate that the miR-652-3p antagomir therapy promotes endothelial repair.
2.6. miR-652-3p antagomir therapy improves EC proliferation under hyperlipidemic conditions
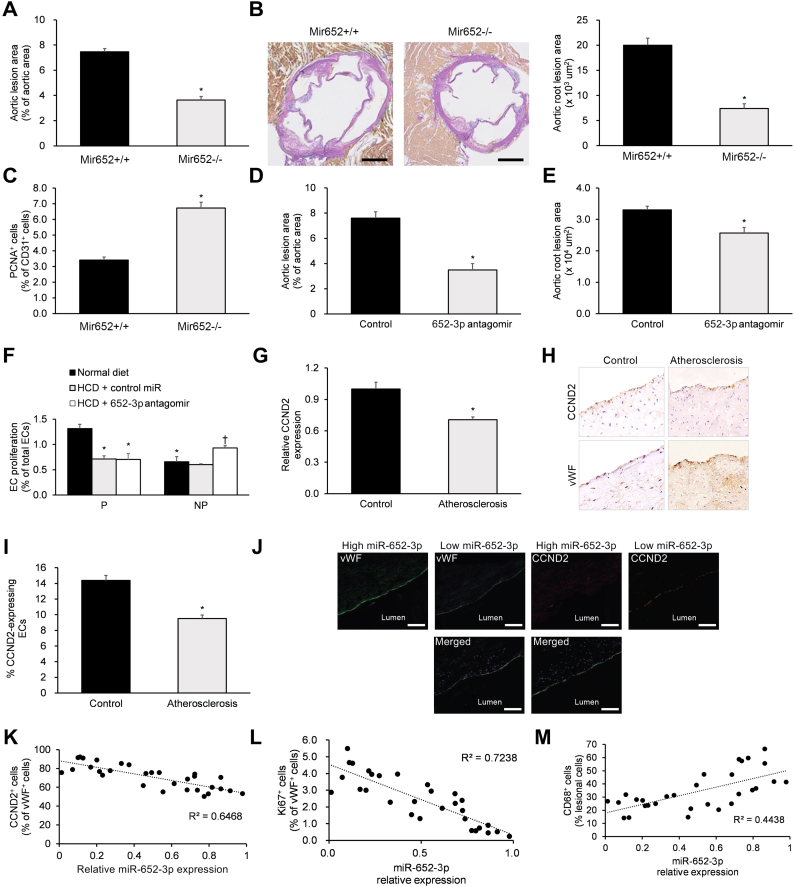
To assess the effects of miR-652 under hyperlipidemic conditions in vivo, we investigated the effects of genetic miR-652 knockdown under HCD conditions using Mir652+/+ and Mir652−/− mice. After a 12-week HCD regimen, Mir652−/− mice displayed reduced lesion formation in aortas (Fig. 5a) and aortic roots (Fig. 5b) as well as enhanced EC proliferation in aortic root sections (Fig. 5c).
Fig. 5.
Rescue of EC proliferation during hyperlipidemia by miR-652-3p antagomir.
(a–c) Non-injured Mir652+/+ and Mir652−/− mice were placed on 12-week high cholesterol feeding regimen. Then, (a) en face preparations of the aortic lesion area and (b) aortic root lesion area (scale bar = 200 μm), as well as (c) aortic root EC proliferation were analyzed. (d, e) Non-injured Mir652+/+ mice were systemically administered a miR-652-3p antagomir or negative control (100 μg/dose, i.v.) every 3 days over the last four weeks of the 12-week high cholesterol feeding regimen to analyze lesion areas in the (d) thoracoabdominal aorta and (e) aortic root. (f) Non-injured Mir652+/+ mice consuming either regular chow or high cholesterol feed were systemically administered a miR-652-3p antagomir or negative control (100 μg/dose, i.v.) every 3 days over the last four weeks of the 12-week feeding regimen. EC proliferation was assayed via CD31/EdU immunostaining in en face prepared aortic arch at predilection (P) sites and non-predilection (NP) sites. *P < .05 vs. predilection (P) normal diet, †P < .05 vs. non-predilection (NP) normal diet. Analysis of (g) CCND2 transcript expression and (h, i) CCND2 protein expression in intimal ECs from human carotid lesions (n = 30 samples), with post-mortem normal human carotid intimal ECs used as controls (n = 20 samples). (j) Immunofluorescent staining images of vWF and CCND2 in human carotid lesions (scale bar = 200 μm). High miR-652-3p expression was defined as greater than or equal to the median value, while low miR-652-3p expression was defined as lower than the median value. Correlations were observed between intimal EC miR-652-3p expression and (k) intimal EC CCND2 abundance, (l) EC proliferation, and (m) lesion macrophages (n = 30 samples). n = 12–18 mice per experimental group. Data reported as means ± SEMs. *P < .05 vs. first group, †P < .05 vs. second group.
Having shown the positive effects of genetic miR-652-3p knockdown under hyperlipidemic conditions in vivo, we next investigated the effects of miR-652-3p antagomir administration in Mir652+/+ mice under HCD and normal diet conditions. HCD-fed Mir652+/+ mice were systemically administered a miR-652-3p antagomir for the last four weeks of a 12-week HCD regimen. We validated miR-652-3p knockdown and associated Ccnd2 upregulation in HCD-fed murine aortas after four weeks of miR-652-3p antagomir therapy (Supp. Fig. 3). Mir652+/+ mice systemically administered a miR-652-3p antagomir showed decreased lesion formation in the thoracoabdominal aortas (Fig. 5d) and aortic roots (Fig. 5e). We also assessed the effects of miR-652-3p antagomir therapy on EC proliferation at predilection (P) and non-predilection (NP) sites in aortic arches of normal diet-fed and HCD-fed Mir652+/+ mice. Notably, we found that miR-652-3p antagomir therapy improved EC proliferation only at NP sites (Fig. 5f).
We next analyzed CCND2 expression levels in human carotid lesions (using post-mortem normal human carotid arterial specimens as controls) and discovered significantly decreased CCND2 mRNA and protein expression in human carotid lesions (Fig. 5g–i). We next analyzed correlations of miR-652-3p and CCND2 in human carotid lesions (Fig. 5j). Relative expression levels of miR-652-3p in human carotid lesions were negatively correlated with endothelial CCND2 abundance (Fig. 5k) and EC proliferation (Fig. 5l) but positively correlated with the macrophage percentage (Fig. 5m). Based on this evidence, we propose a model of faulty endothelial regeneration in atherosclerosis, where miR-652-3p-mediated Ccnd2 downregulation counteracts the pro-proliferative response to hyperlipidemic EC injury.
2.7. Neither miR-652-3p nor Ccnd2 affect ECs under disturbed flow conditions
We finally analyzed the effects of miR-652 and Ccnd2 under disturbed flow conditions using Mir652+/+ and Mir652−/− mice following partial carotid ligation of the left carotid (LC) artery, with the unperturbed right carotid (RC) artery used as a control. We found no significant changes in miR-652-3p, miR-652-5p, or Ccnd2 levels when comparing LC to RC arteries within one week post-ligation (Supp. Fig. 4a), indicating that disturbed flow does not significantly affect miR-652 levels or Ccnd2 gene expression. Mir652−/− mice displayed higher intimal EC Ccnd2 protein expression than Mir652+/+ regardless of disturbed flow conditions (Supp. Fig. 4b). Notably, control RC arteries of HCD-fed Mir652−/− mice displayed decreased lesion formation (Supp. Fig. 4c) and higher EC proliferation (Supp. Fig. 4d) relative to matching Mir652+/+ mice, effects not observed in disturbed-flow LC arteries. Similarly, control RC arteries of HCD-fed Mir652+/+ mice after a four-week treatment with a miR-652-3p antagomir displayed decreased lesion size (Supp. Fig. 4e) and greater EC proliferation (Supp. Fig. 4f), effects not observed in disturbed-flow LC arteries. In addition, control RC arteries of HCD-fed Mir652+/+ mice after siCcnd2 administration displayed larger lesion sizes (Supp. Fig. 4g) and decreased EC proliferation (Supp. Fig. 4h), effects not observed in disturbed-flow LC arteries. In aggregate, these results support that disturbed flow conditions do not affect miR-652-3p or Ccnd2 expression and that neither miR-652-3p nor Ccnd2 affect EC characteristics under disturbed flow conditions.
3. Discussion
Endothelial inflammation is a key indicator of atherosclerotic progression [22]. Elucidating the critical roles of miRNAs in regulating inflammation, proliferation, and regeneration of ECs is a key area of research [23]. Thus, discovering the cellular and molecular mechanisms through which miRNAs regulate these EC processes is likely to aid in the design and development of novel therapeutic agents useful in managing atherosclerotic diseases.
Endothelial miRNAs are recognized to be involved in modulating vascular inflammation [24]. In human ECs, the most abundant miRNA miR-103 has been shown to induce inflammation [16]. Our results show that miRNA-652 is the second most abundant miRNA expressed in HUVECs following miR-103 (Fig. 1a). Previous reports have identified intracellular miRNAs that make use of RNA-binding proteins, such as AGO2, to protect themselves against degradation [25,26]. Our results show that miR-652 is significantly enriched in the AGO2-IP fraction from human ECs, suggesting that the observed abundance of miR-652 within the cytoplasm of human ECs may be due to an AGO2-mediated anti-degradation mechanism (Fig. 1, Supp. Fig. 1).
Oxidized LDLs (oxLDL and moxLDL), which are products of oxidative modification of circulating LDL molecules (particularly under hyperlidemic conditions [27,28]), enhance endothelial activation and monocyte adhesion at an early stage of atherosclerotic plaque development [29,30]. Here, we found that HUVEC expression of miR-652 was significantly enhanced as a result of nLDL exposure, with a more profound effect observed after moxLDL exposure (Fig. 1). The observed increase in the expression of miR-652 after nLDL exposure may result from the presence of some products of oxidative modification present in the nLDL preparation [31]. Naturally, the levels of these oxidation products would be much higher in the moxLDL preparation. This initial evidence suggested that hyperlipidemia/oxLDL exposure promotes miR-652 expression in the human endothelium, making miR-652 an exciting target for further investigation.
Dysfunction in EC repair is critical to the pathogenesis of atherosclerosis [32]. We show that, in our murine model of atherosclerosis, the absence of miR-652 promotes endothelial repair (Fig. 2). We further show that atherosclerotic lesion formation is much smaller in HCD-fed Mir652−/− mice relative to matching Mir652+/+ mice (Fig. 2). This is consistent with the accelerated endothelial repair following carotid denudation in the absence of miR-652 (Fig. 2). In addition, changes in macrophage phenotype are of paramount importance for the progression of atherosclerotic diseases [33]. Specifically, increases in macrophage size indicate enhanced pro-atherogenic foam cell formation within atherosclerotic plaques [34]. Although the number of lesional Mac2+ macrophages after injury was not affected by miR-652 knockout, macrophage size was smaller in Mir652−/− mice compared to Mir652+/+ mice (Fig. 2). This evidence suggests the involvement of miR-652 in promoting endothelial dysfunction and increasing foam cell formation in atherosclerosis.
Bioinformatics is an established tool for identifying miRNA-target gene couples in silico [35]. Here, our bioinformatics algorithms predicted two miR-652 binding sites in the human CCND2 3′UTR and one binding site in the CDS of mouse Ccnd2 (Fig. 3). This target gene was of particular interest, as upregulated activity/expression of the Ccnd2 gene (which encodes the cell cycle checkpoint protein Cyclin D2 [36]) is critically involved in promoting EC proliferation [37,38]. Consistent with our in vitro findings, we found that carotid Ccnd2 mRNA levels in Mir652−/− mice were significantly greater than those in Mir652+/+ mice (Fig. 3). Using several experimental approaches, we also revealed that miR-652-3p negatively regulates Ccnd2 expression (Fig. 3). Consistent with previous work [37,38], we also found that enhanced Ccnd2 expression promotes EC proliferation and endothelial repair (Fig. 3).
When investigating the roles of miRNA in disease processes, it is crucial that the -3p and -5p strands be analytically distinguished due to the significant differences in sequences and target transcripts that may be present. For example, in vitro studies in the lung cancer cell line A549 showed that miR-652-5p displays an anti-proliferative effect, while the miR-652-3p strand produces the completely opposite effect [38,39]. Therefore, here we distinguished the effects of miR-652-5p and miR-652-3p in endothelial repair using a wide variety of experimental approaches and demonstrated that the miR-652-3p antagomir enhances EC proliferation and endothelial repair ex vivo (Fig. 4) and in vivo (Fig. 5). Furthermore, we show that miR-652-3p levels in human carotid artery lesions are negatively associated with EC CCND2 expression, negatively associated with EC proliferation, but positively correlated with the macrophage percentage (Fig. 5). This combined evidence from our murine model supports the application of miR-652-3p antagomir therapy in preventing atherosclerotic progression.
ECs are best adapted to the high pulsatile shear conditions in unbranched vessel segments (termed non-predilection sites), with high pulsatile shear producing a healthy, quiescent EC phenotype [[40], [41], [42]]. At predilection sites of vessel branching, disturbed blood flow results in lower pulsatile shear levels and higher oscillatory shear levels, which increases pro-inflammatory activity and EC proliferation [40,[42], [43], [44]]. Moreover, the miRNA profile in laminar flow and disturbed flow-exposed ECs is significantly different, with disturbed flow supporting pro-atherogenic miRNA profiles [45]. Therefore, here we analyzed the effects of miR-652-3p or Ccnd2 on lesion size and EC proliferation under disturbed flow conditions using a murine partial carotid ligation model. We demonstrated that disturbed flow does not significantly affect miR-652 levels or Ccnd2 gene expression and that miR-652-3p or Ccnd2 do not affect lesion size or EC proliferation under disturbed flow conditions (Supp. Fig. 4). This is consistent with previous research showing that disturbed flow does not impact miR-652-3p or Ccnd2 expression in ECs [45,46]. We speculate that the proliferative EC phenotype present under disturbed flow, but not under laminar flow conditions, may not be susceptible to regulation by the miR-652-3p-Ccnd2 axis due to upregulation of other key pro-proliferative cyclins, such as CCND1 [46]. Targeting the miR-652-3p-Ccnd2 axis still remains a promising strategy for controlling atherosclerotic progression at non-predilection sites.
The foregoing findings raised an additional question of why miR-652-3p was dysregulated under hyperlipidemic stress conditions but not under disturbed flow conditions. It is known that hyperlipidemic stress influences distinct molecular pathways from those influenced by disturbed flow conditions in ECs [47]. According to Schober et al.'s two-hit model of atherosclerosis, disturbed flow at susceptible arterial sites initially induces pathways primarily associated with low-grade, chronic EC apoptosis and activation [47]. Then, hyperlipidemia promotes alternative pathways primarily associated with EC inflammation and inhibition of EC proliferation, thereby further hampering EC regeneration and promoting atherosclerosis [47]. This two-hit model is consistent with our current findings, as hyperlipidemia (but not disturbed flow) promotes inhibition of EC proliferation via the miR-652-3p/Ccnd2 axis.
Based on our experimental findings, we advance a model of impaired endothelial regeneration during atherosclerosis, where the increase in EC miR-652-3p expression downregulates Ccnd2, thereby negatively regulating cell proliferation under hyperlipidemic stress (Fig. 5). On this basis, our results suggest that miR-652 blockade in the endothelium may offer a potentially novel strategy to combat atherosclerosis.
4. Materials and methods
The methods are fully detailed in the Supplementary Information.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number: 31300137). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
None.
Author contributions
Conceived and designed the study: LHJ and RZH.
Performed the experimental procedures: ZCH, YC, HRL, HZ WHS, and LWL.
Analyzed the data: YX and RZH.
Drafted the manuscript: RZH and NDM.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.01.032.
Contributor Information
N.D. Melgiri, Email: nd.melgiri@impactys.com.
Lihong Jiang, Email: Lihong-jiang@hotmail.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Libby P., Bornfeldt K.E., Tall A.R. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118(4):531–534. doi: 10.1161/CIRCRESAHA.116.308334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geovanini G.R., Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci. 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinberg M.W., Moore K.J. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118(4):703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones Buie J.N., Goodwin A.J., Cook J.A., Halushka P.V., Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. 2016;254:271–281. doi: 10.1016/j.atherosclerosis.2016.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma S., Tian X.Y., Mu C., Shen H., Bismuth J., Wong W.T. Targeted delivery of microRNAs by microparticles to inflamed endothelium ameliorates endothelial inflammation and atherosclerosis. FASEB J. 2015;29(1_supplement):LB580. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma S., Tian X.Y., Mu C., Shen H., Bismuth J., Wong W.T. E-selectin-targeting microparticle-mediated delivery of MicroRNAs for the Treatment of Atherosclerosis. Am Heart Assoc. 2015:A232. [Google Scholar]
- 9.Schober A., Nazari-Jahantigh M., Weber C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat Rev Cardiol. 2015;12(6):361–374. doi: 10.1038/nrcardio.2015.38. [DOI] [PubMed] [Google Scholar]
- 10.Gadde S., Rayner K.J. Nanomedicine meets microRNA: current advances in RNA-based nanotherapies for atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(9):e73–e79. doi: 10.1161/ATVBAHA.116.307481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangwal S., Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol. 2014;54:185–203. doi: 10.1146/annurev-pharmtox-011613-135957. [DOI] [PubMed] [Google Scholar]
- 12.van Rooij E., Olson E.N. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11(11):860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernardo B.C., Charchar F.J., Lin R.C., McMullen J.R. A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ. 2012;21(3):131–142. doi: 10.1016/j.hlc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Suárez Y., Fernández-Hernando C., Pober J.S., Sessa W.C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100(8):1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 15.Kuehbacher A., Urbich C., Zeiher A.M., Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann P., Zhou Z., Natarelli L. Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nat Commun. 2016;7 doi: 10.1038/ncomms10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrot-Applanat M., Vacher S., Toullec A. Similar NF-κB gene signatures in TNF-α treated human endothelial cells and breast tumor biopsies. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams B.D., Guo S., Bai H. An in vivo functional screen uncovers miR-150-mediated regulation of hematopoietic injury response. Cell Rep. 2012;2(4):1048–1060. doi: 10.1016/j.celrep.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv J., Zeng J., Guo F. Endothelial Cdc42 deficiency impairs endothelial regeneration and vascular repair after inflammatory vascular injury. Respir Res. 2018;19(1):27. doi: 10.1186/s12931-018-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y.-Y., Gao X.-P., Zhao Y.D. Endothelial cell–restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116(9):2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan X., Zhang X., Pan L., Tian X., Dong P. Identification of Key Pathways and Genes in Advanced Coronary Atherosclerosis using Bioinformatics Analysis. Biomed Res Int. 2017;2017 doi: 10.1155/2017/4323496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biessen E.A.L., Wouters K. Macrophage complexity in human atherosclerosis: opportunities for treatment? Curr Opin Lipidol. 2017;28(5):419–426. doi: 10.1097/MOL.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y., Zhu M., Schober A. Macrophage MicroRNAs as Therapeutic Targets for Atherosclerosis, Metabolic Syndrome, and Cancer. Int J Mol Sci. 2018;19(6) doi: 10.3390/ijms19061756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neth P., Nazari-Jahantigh M., Schober A., Weber C. MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res. 2013;99(2):294–303. doi: 10.1093/cvr/cvt096. [DOI] [PubMed] [Google Scholar]
- 25.Arroyo J.D., Chevillet J.R., Kroh E.M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J., Li Y.S., Nguyen P. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113(1):40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holvoet P., Theilmeier G., Shivalkar B., Flameng W., Collen D. LDL hypercholesterolemia is associated with accumulation of oxidized LDL, atherosclerotic plaque growth, and compensatory vessel enlargement in coronary arteries of miniature pigs. Arterioscler Thromb Vasc Biol. 1998;18(3):415–422. doi: 10.1161/01.atv.18.3.415. [DOI] [PubMed] [Google Scholar]
- 28.Heitzer T., Ylä-Herttuala S., Luoma J. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia: role of oxidized LDL. Circulation. 1996;93(7):1346–1353. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- 29.Yurdagul A., Pattillo C.B., Schlaepfer D.D., Orr W. Oxidized LDL Promotes Endothelial NF-kappaB Activation and Inflammation through Focal Adhesion Kinase-dependent RSK Signaling. Am Heart Assoc. 2016:A563. [Google Scholar]
- 30.Yurdagul A., Sulzmaier F.J., Chen X.L., Pattillo C.B., Schlaepfer D.D., Orr A.W. Oxidized LDL induces FAK-dependent RSK signaling to drive NF-κB activation and VCAM-1 expression. J Cell Sci. 2016;129(8):1580–1591. doi: 10.1242/jcs.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z., Subramanian P., Sevilmis G. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13(5):592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Davies P.F., Civelek M., Fang Y., Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res. 2013;99(2):315–327. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeksema M.A., Gijbels M.J., Van den Bossche J. Targeting macrophage Histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol Med. 2014;6(9):1124–1132. doi: 10.15252/emmm.201404170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backofen R., Engelhardt J., Erxleben A. RNA-bioinformatics: tools, services and databases for the analysis of RNA-based regulation. J Biotechnol. 2017;261:76–84. doi: 10.1016/j.jbiotec.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu R., Tang S., Wang M., Xu X., Yao C., Wang S. MicroRNA-497 induces apoptosis and suppresses proliferation via the Bcl-2/Bax-caspase9-caspase3 pathway and cyclin D2 protein in HUVECs. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X.X., Liu Y.M., Li Y.J. High glucose concentration induces endothelial cell proliferation by regulating cyclin-D2-related miR-98. J Cell Mol Med. 2016;20(6):1159–1169. doi: 10.1111/jcmm.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B., Lv F., Zhao L. MicroRNA-652 inhibits proliferation and induces apoptosis of non-small cell lung cancer A549 cells. Int J Clin Exp Pathol. 2017;10(6):6719–6726. [Google Scholar]
- 40.Kwak B.R., Bäck M., Bochaton-Piallat M.-L. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35(43):3013–3020. doi: 10.1093/eurheartj/ehu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain M.K., Sangwung P., Hamik A. Regulation of an inflammatory disease: Krüppel-like factors and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.301925. ATVBAHA-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Effects of shear stress on endothelial cells: go with the flow. Acta Physiologica. 2017;219(2):382–408. doi: 10.1111/apha.12725. [DOI] [PubMed] [Google Scholar]
- 43.Hajra L., Evans A.I., Chen M., Hyduk S.J., Collins T., Cybulsky M.I. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci. 2000;97(16):9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schober A., Nazari-Jahantigh M., Wei Y. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20(4):368–376. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S., Kim C.W., Simmons R.D., Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303425. ATVBAHA-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajami N.E., Gupta S., Maurya M.R. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc Natl Acad Sci. 2017;114(41):10990–10995. doi: 10.1073/pnas.1707517114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schober A., Nazari-Jahantigh M., Weber C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat Rev Cardiol. 2015;12(6):361. doi: 10.1038/nrcardio.2015.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material