Abstract
This study aimed to investigate the situation in which interpersonal brain synchronization (IBS) occurs during a collaborative task and examined its trajectory over time by developing a novel functional near-infrared spectroscopy (fNIRS)-based hyperscanning paradigm. Participants were asked to perform a collaborative task in three-person groups where two of the members are real participants and one is a confederate. Compared to dyads between real participants and confederates, real-participant pairings showed greater cooperation behavior and IBS between bilateral dorsolateral prefrontal cortex. And, IBS and cooperation increased over time in real-participant pairings, whereas they remained low and constant in dyads with the confederate. These findings indicate that IBS occurs between individuals engaging in interpersonal interaction during a collaborative task, during which both IBS and cooperatively interpersonal interaction tend to increase over time.
Keywords: Interpersonal interaction, hyperscanning, interpersonal brain synchronization, fNIRS
Introduction
The evolution of human society necessitates social interaction among individuals. The complex and large-scale social interaction in human society was suggested as one of the special features that distinguishes our mankind from other species (Adolphs, 2003; Dunbar, 2009). Considering the importance of social interaction, plenty of studies have been conducted to unveil the underlying mechanism of social interaction, especially the neural mechanism. Most of the neuroimaging studies in this field investigated the neural correlates related to social cognition by measuring brain activity of one individual per time (Montague et al., 2002). Although this kind of typical paradigm could help identify and characterized neural activities related to social cognition, the valuable information of the dynamic interaction among multiple brains was neglected.
To unveil the dynamic neural interaction among multiple individuals, the multi-brain neuroimaging technique was resurrected and renamed as ‘hyperscanning technique’ (Montague et al., 2002). Thereafter, increasing hyperscanning studies emerged in the field of social interaction (Funane et al., 2011; Cui et al., 2012; Holper et al., 2012; Jiang et al., 2012; Dikker et al., 2017; Dai et al., 2018; Goldstein et al., 2018; Lu et al., 2018; Xue et al., 2018; Lu et al., 2019). Most of these studies revealed enhanced interpersonal brain synchronization (IBS) between individuals while they were engaged in social interaction contexts. To explain the emergence of the IBS between individuals in interpersonal interaction situations, two typical hypotheses were proposed: cooperative interaction hypothesis and similar task hypothesis. The cooperative interaction hypothesis suggests that the IBS indicates that individuals are engaged in cooperatively interaction, whereas the similar task hypothesis suggests that the IBS merely indicates that individuals are working on the similar task.
Some studies provided evidence for the cooperative interaction hypothesis by showing that IBS emerged during cooperatively interpersonal interaction, such as group humming (Osaka et al., 2014), guitar playing (Lindenberger et al., 2009; Müller et al., 2013), cooperative button press (Cui et al., 2012; Pan et al., 2017), coordinated walking (Ikeda et al., 2017), group communication (Jiang et al., 2012; Nozawa et al., 2016; Liu et al., 2017) and group creativity problem solving (Lu et al., 2018; Xue et al., 2018). Besides, researchers have also successfully tracked the IBS underlies teaching–learning interactions which was associated with teaching–learning performance (Dikker et al., 2017; Pan et al., 2018; Zheng et al., 2018). All of this indicated that the IBS between individuals might reflect that individuals were cooperatively interacting with each other. However, several studies supported the similar task hypothesis (Nummenmaa et al., 2012; Abrams et al., 2013; Kawasaki et al., 2013). In each of these studies, although individuals performed the similar tasks solely, enhanced IBS between individuals was still observed. For instance, the participants were instructed to watch several films depicting unpleasant, neutral and pleasant emotions in a fixed order individually while being scanned with fMRI. Although there was no interaction between participants, enhanced IBS was still observed (Nummenmaa et al., 2012). Since interpersonal interaction between individuals scarcely occurred, the enhanced IBS could not be attributed to the interpersonal interaction between individuals. Alternatively, it was supposed that the enhanced IBS might reflect that individuals were just engaged in the similar tasks.
In the current study, we aimed to explore the situation in which IBS occurs during a collaborative task to seek evidence that supports the cooperative interaction hypothesis over the similar task hypothesis and examine its trajectory over time. We developed a novel paradigm in which three people—two of them are participants, one is a confederate—interact while using functional near-infrared spectroscopy (fNIRS)-based hyperscanning technique, which allows for the comparison of IBS between two real participants pairings and control dyads. During the collaborative task, the confederate pretended to perform the task. In fact, the confederate was asked to recall and report prepared task-related ideas merely. In other words, although the confederate was working on the same task as other partners, he was not engaged in any cooperatively interpersonal interaction with others. Hence, according to the cooperative interaction hypothesis, we hypothesized that the IBS between the two real participants should be higher than that of the control dyads in the three-person group (dyads with confederate). However, according to the similar task hypothesis, we hypothesized that there might be no significant differences in IBS among different dyads in the group. Besides, although without any precise hypothesis, we expected to examine the trajectory of the IBS over time, which might help understand the relationship between IBS and interpersonal interaction process more thoroughly.
Methods
Participants
Forty four college students (40 females, age: 20.66 ± 2.29 years old) took part in this study. Participants are all right-handed, with normal or corrected-to-normal vision. They were randomly assigned as dyads to solve one collaborated task with one fake participant (male, the confederate). Here, a collaborated brainstorming task was used. Consequently, a total of 22 three-person groups (4 ‘female–male–male’ groups) were created. Participants in each group were typically unknown to each other. Informed consent was obtained from each participant prior to the experiment. Each participant was paid ¥30 for the participation. The study procedure was approved by the University Committee on Human Research Protection of East China Normal University.
Experimental designs and procedure
The role of fake participant (F), namely the ‘confederate’, was played by one of our experimental assistants. The experimental assistant is a 25-year-old male student majoring in psychology. He played the role of fake participant among all groups. Participants were not informed of the real identity of the fake participant before the experiment was completed. Immediately after the experiment, participants were debriefed about the identity of the fake participant. No participant had realized the real identity of the experimental assistant during the experiment.
Upon arrival, participants were asked to sit in a triangle. The distances between any two participants were equal (see Figure 1A). Hence, the three members of each group worked together in the same room and they could see and hear what the other two partners did and said. In each group, an initial 2 min resting-state session served as a baseline (see Figure 1C). During this session, participants were required to remain as still as possible, with their eyes closed, and mind relaxed (Lu et al., 2010). Next, the task instruction was clarified and the rules of brainstorming (i.e. quantity breeds quality, free-wheeling is encouraged and combination and improvement are sought) were emphasized (Osborn, 1957). Participants were asked to discuss on the following topic for 5 min: ‘Your friend Pat sits next to you in class. Pat really likes to talk to you and often bothers you while you are doing your work. Sometimes he distracts you and you miss an important part of the lecture, and many times you don’t finish your work because he is bothering you. What should you do? How would you solve this problem?’ This is a typical realistic presented problem (RPP), which is used to assess individual creativity in solving open-ended realistic problems (Runco et al., 2016).
Fig. 1.
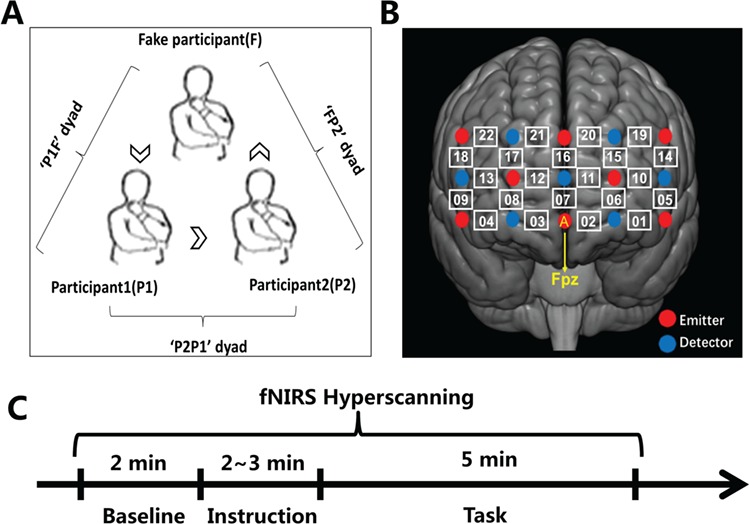
Experimental design. (A) Experimental setup. ‘P1’ reported firstly, ‘P2’ reported secondly and ‘F’ answered lastly. (B) Optode probe set. The probe patch was placed on the prefrontal cortex. (C) Hyperscanning design. Baseline: 2 min resting state session; Instruction: (2–3) min instructions introduction; Brainstorming: 5 min creativity task session.
During the task, the participants were instructed to answer while taking turns and report only one idea at a time. They were asked to answer in a clockwise order. The participant who was required to report firstly/secondly was marked as participant no.1 (P1) or participant no.2 (P2), respectively (see Figure 1A). The fake participant answered lastly and reported prepared ideas (common ideas prepared by the experimenter) in each cycle. Participants were allowed to say ‘pass’ when they failed to generate an idea during their respective turns. The confederate just behaved like a real participant: listened to others’ reporting, waited for his turns to report, retrieved task-related information from the memory system (although these ideas were prepared) and reported task-related ideas orally, nodded or iterated the previous idea reported by others sometimes to show that he was attending to others’ ideas, said ‘pass’ deliberately. In this case, we considered that the confederate was engaging in the similar task with the other two partners.
Assessment of performance on the RPP
Participants’ performance on the RPP was evaluated using the fluency and originality of their ideas (Runco, 1991). Fluency was based on the amount of ideas each participant presented. The fluency score for each group was obtained by combining the fluency scores of ‘P1’ and ‘P2’ in the group. Originality was assessed using a subjective method. Four trained raters independently rated the originality of each idea on a 5-point Likert scale ranging from 1 (‘not original at all’) to 5 (‘highly original). The inter-rater agreement (internal consistency coefficient, ICC = 0.78) was satisfactory. Individual ratings for each idea from all the raters were averaged into a single originality score for each idea. The final originality score for each participant was calculated by averaging the originality scores of all generated ideas. The originality score for each group was obtained by averaging the originality scores of ‘P1’ and ‘P2’ in the group.
Behavioral index of cooperation for dyads in each group
To assess the extent to which individuals cooperated with each other in each dyad, the behavioral index of cooperation ‘combination of ideas’ was used (Lu et al., 2018; Xue et al., 2018). The assumption behind this index is that the more individual in the dyad cooperates with one another, the more improvement and combination of ideas might occur. These improved (or combinative) ideas can be recognized as the responses within the same category. Accordingly, such an index may help reflect the degree to which individuals in the dyad cooperated with each other. To calculate this index, two trained raters were asked to assess the collective flexibility of each dyad in the group independently (‘P1P2’ dyad, ‘P1F’ dyad, ‘FP2’ dyad). For instance, with respect to the collective flexibility of ‘P1P2’ dyad, raters were asked to neglect the ideas from F and rated the amount of idea category for ideas from P1 and P2. The inter-rater agreement was satisfactory (ICC = 0.96) in the study. Next, the collective flexibility scores from two raters were averaged to obtain the final collective flexibility score for each dyad in the group. Finally, ‘combination of ideas’ for each dyad was calculated by the following equation: ‘Combination of ideas = Dyad fluency/Collective flexibility’. For instance, the ‘combination of ideas’ of ‘P1P2’ dyad was calculated by dividing ‘the sum of the fluency scores of P1 and P2’ by ‘the collective flexibility of P1P2 dyad’
fNIRS data acquisition
A near-infrared spectroscopy (NIRS) system (ETG-7100, Hitachi Medical Corporation, Japan) was used for the continuous measure of the concentrations of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR). The absorption of near infrared light (wavelengths: 695 and 830 nm) was measured at a sampling rate of 10 Hz. Three 3 × 5 optode probe sets (eight emitters and seven detectors, 3 cm optode separation) including 22 recording channels (CH) were used. Since previous studies have shown the prefrontal region is associated with creativity cognition and interpersonal cooperative interaction, the optode probe set was placed over the forehead of each participant (Cui et al., 2012; Wu et al., 2015; Beaty et al., 2016; see Figure 1B).
The placement of the optode probe set followed the International 10–20 system. The lowest probe was aligned with the horizontal reference curve, with the middle optode placed on the frontal pole midline point. Meanwhile, the middle probe of the patch was aligned exactly along the sagittal reference curve (see Figure 1B). To determine the correspondence between the recording channels and the measurement points on the cerebral cortex, the virtual registration method was used (Singh et al., 2005; Tsuzuki et al., 2007).
Interpersonal brain synchronization (IBS)
According to the modified Beer–Lambert Law, variations of the HbO and HbR concentrations were assessed by measuring the absorption changes of fNIRS light after its transmission through the cerebral cortical tissue. Given that previous studies have shown that the HbO signal is more sensitive to the changes in cerebral blood flow, this study mainly focused on the HbO signal (Hoshi, 2007; Cui et al., 2012; Jiang et al., 2012).
Due to the low signal/noise ratio, the fNIRS data of one group was excluded from the analysis. The raw fNIRS data of each participant were preprocessed using the hrf low-pass filtering and wavelet minimum description length (Wavelet-MDL) detrending algorithm in NIRS-Statistical Parametric Mapping (SPM)(Jang et al., 2009; Ye et al., 2009; see a representative example demonstrating the change of signals in the Supplement). The low-pass filtering was used to attenuate the high-frequency non-neuronal components in the fNIRS data. Moreover, the Wavelet-MDL detrending algorithm could remove the unknown global trend due to breathing, cardiac, vaso-motion or other experimental errors. The data from the resting-state session and task session were entered into analyses. Meanwhile, data in the initial and ending 30 s periods of the task session were removed to obtain data from the period of steady state, leaving 240 s data for the task session. Further, wavelet transform coherence was performed to assess the relationship between HbO time series of the two participants in each dyad (IBS; Grinsted et al., 2004). In each group, we calculated the IBS between ‘P2’ and ‘P1’ (‘P2P1’ dyad), the IBS between ‘F’ and ‘P2’ (‘FP2’ dyad), as well as the IBS between ‘P1’ and ‘F’ (‘P1F’ dyad) for each CH combination (a total of 484 channel combinations: 22CHs × 22CHs). The time-averaged IBS of the resting-state session was subtracted from that of the task session. The difference served as the index of IBS increment. For further analysis, IBS increments in the frequency band of interest (FOI) were converted to Fisher z-statistics (Chang and Glover, 2010; Cui et al., 2012).
To identify the FOI in the study, we conducted one-way ANOVA using DYAD as the between-subject factor on IBS increments of each CH combination along the full frequency range (0.01–0.7 Hz; Nozawa et al., 2016; Zheng et al., 2018). Data above 0.7 Hz were excluded for aliasing of higher frequency physiological noise such as cardiac activity (0.8–2.5 Hz) (Tong et al., 2011; Barrett et al., 2015). Besides, to remove very low-frequency fluctuations, the data below 0.01 Hz were not considered as well. The results were thresholded at P < 0.0005. Since the analysis was merely used to determine the FOI rather than to obtain the final results, no correction was further performed (Lu et al., 2018; Zheng et al., 2018). The results showed that frequencies between 0.0337–0.0401 Hz as well as 0.0450–0.0505 Hz had CH combinations whose P-values survived the thresholding. However, no further significant difference in IBS increment among conditions was observed in the frequency band ranging from 0.0337–0.0401 Hz. Consequently, the frequency band between 0.0450–0.0505 Hz was determined as the FOI in the study. Next, another one-way ANOVA using DYAD as the between-subject factor (Lu et al., 2019) was performed on IBS increments in the FOI across all CH combinations. Results were corrected with the false discovery rate (FDR) method for all CH combinations at P < 0.05. In addition, Bonferroni correction was used to account for the post-hoc multiple comparisons. Further, two-way mixed design ANOVA using DYAD as the between-subject factor and EPOCH (the remaining 240 s task period were equally divided into three epochs: EPOCH1, EPOCH2, EPOCH3) as the within-subject factor was performed on IBS increments of CH combinations that showed a significant main effect of DYAD. Follow-up simple effect analysis with Bonferroni corrections was performed. Finally, bivariate Pearson correlations between IBS and behavioral indices (i.e. fluency, originality and behavioral index of cooperation) were estimated to reveal brain–behavior relationship.
Results
IBS of different DYADS in the group
It should be noted that the following analyses focused on the different dyads within the three-person group hereafter.
One-way ANOVA using DYAD as the between-subject factor was performed on IBS increments across all CH combinations. Results were corrected using the FDR method at P < 0.05. The results demonstrated a significant main effect of DYAD on the IBS increments of CH17–CH19, F (2, 60) = 11.65, P < 0.001, ηp2 = 0.28 (see Figures 2 and Fig. 3A). The post hoc test revealed that the IBS of ‘P2P1’ (M = 0.17, s.d. = 0.16) was significantly higher than that of ‘P1F’ (M = −0.10, s.d. = 0.17; P < 0.001, Bonferroni corrected) and ‘FP2’ (M = 0.03, s.d. = 0.21; P = 0.043, Bonferroni corrected) (see Fig. 3B).
Fig. 2.
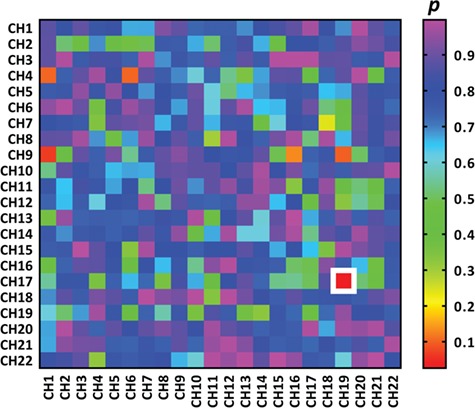
Heatmaps of the P-values (FDR corrected) for the one-way ANOVA using DYAD as the between-subject factor on the IBS increment of different CH combinations. The colors reflect P-values for the main effect of DYAD on the IBS increment of CH combinations. The white rectangle indicates that the main effect of DYAD on the IBS increment of the CH combination is less than 0.05. The vertical axis and horizontal axis represents CHs of different participants. It should be noted that significant difference in IBS increment among conditions was observed in the CH combination of CH17–CH19. The color bars denote the P-values.
Fig. 3.
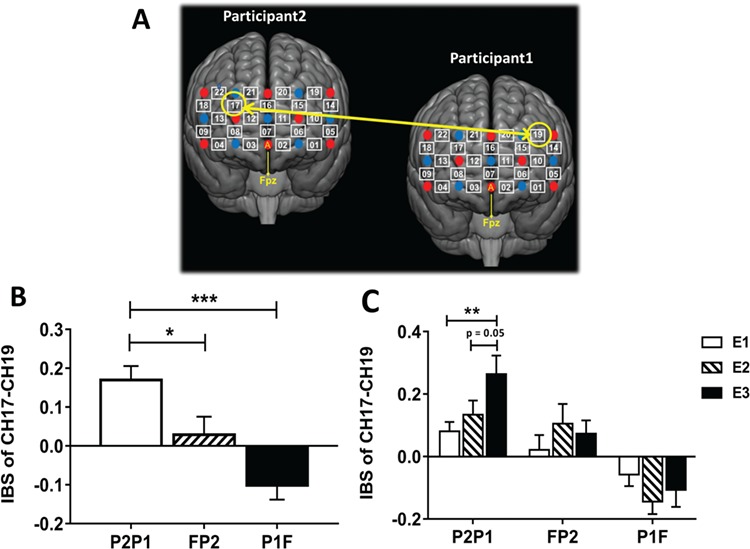
The CH combination showed significant difference in IBS increment among conditions. (A) One-way ANOVA showed a significant main effect of DYAD on the IBS increment of CH17–CH19, which survived the FDR correction (P = 0.05). (B) The amplitude of IBS increment of CH17–CH19. The IBS increment of CH17–CH19 from ‘P2P1’ indicates the IBS increment between the CH17 of ‘P2’ and the CH19 of ‘P1’. The IBS increment of CH17–CH19 from ‘FP2’ indicates the IBS increment between the CH17 of ‘F’ and the CH19 of ‘P2’. The IBS increment of CH17–CH19 from ‘P1F’ indicates the IBS increment between the CH17 of ‘P1’ and the CH19 of ‘F’. (C) The fluctuation of IBS increment of CH17–CH19 from different dyads over time. E1/E2/E3 indicates EPOCH1/EPOCH2/EPOCH3, respectively. The duration of each epoch is 80 s. *P < 0.05, **P < 0.01, ***P < 0.001.
Further, one-sample t-test with FDR correction (P < 0.05) was performed on the IBS increments of all CH combinations from different dyads, respectively. With respect to ‘P2P1’, results showed that the IBS increment of CH17–CH19 was significant and survived the FDR correction, t (20) = 4.77, P < 0.001, Cohen's d = 2.13. In contrast, the IBS increment of CH17–CH19 from ‘FP2’ or ‘P1F’ was not significant (Ps > 0.05). This may indicate that the IBS of ‘P2P1’ was significantly enhanced when compared with the baseline.
IBS in different EPOCHS
To further explore how IBS increments of CH17–CH19 from different dyads fluctuated over time, two-way mixed design ANOVA with DYAD as the between-subject factor and EPOCH as the within-subject factor was performed on the IBS increments of CH17–CH19. The results showed a significant interaction effect of DYAD × EPOCH on IBS of CH17–CH19, F (4, 120) = 3.36, P = 0.012, ηp2 = 0.10. Further simple effect analysis showed that the IBS increments of ‘P2P1’ in the EPOCH3 (M = 0.26, s.d. = 0.27) was significantly higher than that in the EPOCH1 (M = 0.08, s.d. = 0.14; P = 0.006, Bonferroni corrected) and marginally higher than EPOCH2 (M = 0.13, s.d. = 0.21; P = 0.050, Bonferroni corrected) (see Fig. 3C). In contrast, no significant difference among three EPOCHs was observed in the IBS of ‘FP2’ or ‘P1F’ (Ps > 0.05). In addition, no main effect was observed.
To further examine the trajectory of IBS increment of CH17–CH19 over time, we also treated IBS increment as continuous variables changing over time. Since there were 240 s task period left in the analysis procedure and the sampling rate of fNIRS is 10 Hz, the IBS increment in each frame from the 240 s task period (2400 frames in total) was estimated. Next, the IBS increments in each frame from 21 groups were averaged for each dyad in the group (see Fig. 4A). Subsequently, the bivariate Pearson correlation was performed on the IBS increments of CH17–CH19 and frames. The results showed that the IBS increments of CH17–CH19 from ‘P2P1’ dyad was significantly, positively correlated with frames (r = 0.71, P < 0.0001; see Fig. 4B). Besides, the IBS increments of CH17–CH19 from ‘FP2’ dyad was significantly, negatively correlated with frames (r = −0.10, P < 0.0001; see Fig. 4C). The IBS increments of CH17–CH19 from ‘P1F’ dyad was significantly, negatively correlated with frames (r = −0.42, P < 0.0001; see Fig. 4D).
Fig. 4.
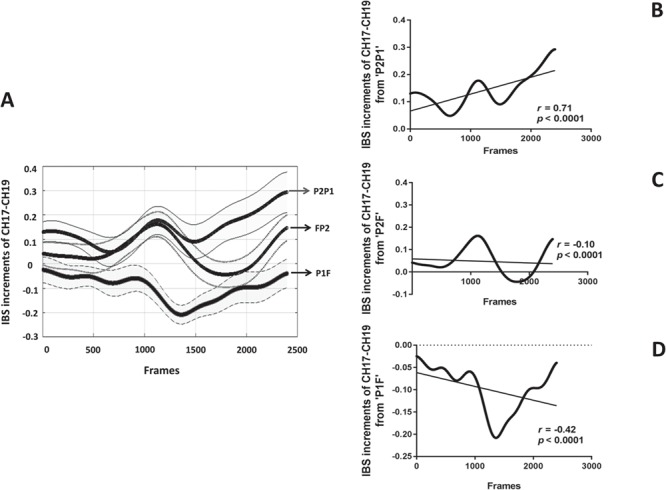
The trajectory of IBS increment of CH17–CH19 over time. (A) The IBS increments between participants (y-axis) is plotted against the frames (x-axis) for different dyads in the group (shaded areas: 95% confidence interval). (B) The correlation between the IBS increment of ‘P2P1’ and frames. (C) The correlation between the IBS increment of ‘FP2’ and frames. (D) The correlation between the IBS increment of ‘P1F’ and frames.
Group performance on RPP in different EPOCHS
One-way repeated measures ANOVA using EPOCH as the within-subject factor was performed on RPP fluency of ‘P2P1’. The results showed a significant main effect of EPOCH on RPP fluency, F (2, 42) = 7.07, P = 0.002, ηp2 = 0.25. The post-hoc test revealed that the fluency in the EPOCH1 (M = 5.32, s.d. = 1.52) was significantly higher than that in the EPOCH2 (M = 4.67, s.d. = 1.08; P = 0.041, Cohen's d = 0.49) and EPOCH3 (M = 4.19, s.d. = 1.05; P = 0.001, Cohen's d = 0.86; see Fig. 5A).
Fig. 5.
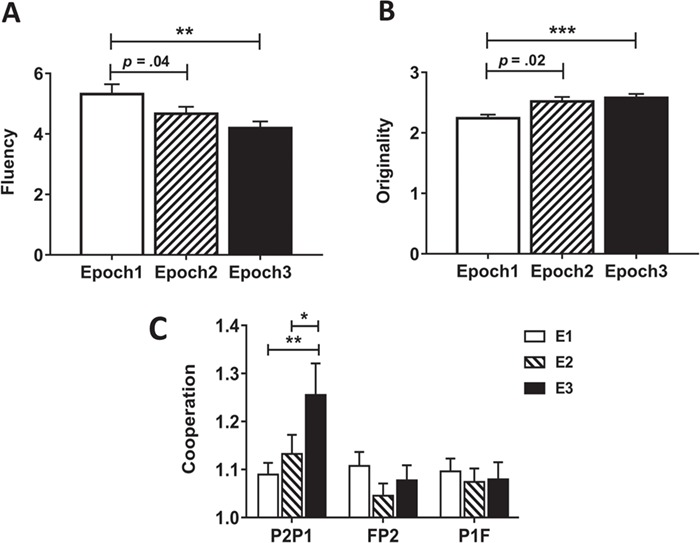
Performance on the RPP and cooperation behavior. (A) Group RPP fluency in different EPOCHs. (B) Group RPP originality in different EPOCHs. (C) The fluctuation of cooperation behavior of different dyads over time. Error bars indicate standard errors of the mean. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition, one-way repeated measures ANOVA using EPOCH as the within-subject factor was performed on RPP originality of ‘P2P1’. The results showed a significant main effect of EPOCH on RPP originality, F (2, 42) = 7.07, P = 0.002, ηp2 = 0.25. The post hoc test revealed that the originality in the EPOCH1 (M = 2.24, s.d. = 0.28) was significantly lower than that in the EPOCH2 (M = 2.52, s.d. = 0.35; P = 0.02, Cohen's d = 0.88) and EPOCH3 (M = 2.58, s.d. = 0.30; P < 0.001, Cohen's d = 1.17; see Fig. 5B).
Behavioral index of cooperation in the group
Two-way mixed design ANOVA with DYAD as the between-subject factor and EPOCH as the within-subject factor was performed on the behavioral index of cooperation. The results demonstrated a significant main effect of DYAD on the cooperation, F (2, 63) = 4.37, P = 0.017, ηp2 = 0.12. The post hoc test revealed that the cooperation of ‘P2P1’ (M = 1.16, s.d. = 0.22) was significantly higher than that of ‘FP2’ (M = 1.08, s.d. = 0.13; P = 0.006, Cohen's d = 0.44) and ‘P1F’ (M = 1.08, SD = 0.14; P = 0.012, Cohen's d = 0.43). Moreover, there was also a significant interaction effect of DYAD × EPOCH on cooperation, F (4, 126) = 2.52, P = 0.044, ηp2 = 0.07. Further simple effect analysis showed that, with respect to the cooperation of ‘P2P1’, the cooperation in the EPOCH3 (M = 1.26, s.d. = 0.31) was significantly higher than that in the EPOCH1 (M = 1.09, s.d. = 0.12; P = 0.003, Cohen's d = 0.72) and EPOCH2 (M = 1.13, s.d. = 0.19; P = 0.022, Cohen's d = 0.50) (see Fig. 5C). In contrast, no significant difference among three EPOCHs was observed in the behavioral index of cooperation of ‘FP2’ or ‘P1F’ (Ps > 0.05). In addition, no main effect of EPOCH was observed.
The IBS-behavior relations
Bivariate Pearson correlations were performed on the IBS increments of CH17–CH19 from ‘P2P1’ and group creative performance (i.e. fluency and originality), as well as behavioral index of cooperation. Results showed no significant correlation between one another (Ps > 0.05). Bivariate Pearson correlations were performed on the above IBS increments and group creative performance, as well as behavioral index of cooperation in different EPOCHs, respectively. Results showed no significant correlation in each epoch (Ps > 0.05).
Similar Pearson correlations were performed on the IBS increments of CH17–CH19 from ‘FP2’ or ‘P1F’ and group creative performance (i.e. fluency and originality), as well as behavioral index of cooperation. Results showed no significant correlation between one another (Ps > 0.05).
Discussion
In this study, we investigated the situation in which IBS occurs during a collaborative task and examined its trajectory over time by using a novel fNIRS-based hyperscanning paradigm. Participants were asked to perform a collaborative task (RPP) in three-person groups where two of the members are real participants and one is a confederate. Results revealed that the two real participants pairing (i.e. P2P1) showed higher IBS increment of CH17–CH19 than the other dyads in the group (i.e. FP2, P1F). Besides, while the IBS increment of CH17–CH19 from ‘P2P1’ increased over time, no significant difference in the IBS increment from ‘FP2’ or ‘P1F’ was observed among epochs. Moreover, results also showed that ‘P2P1’ showed higher level of cooperation behavior when compared with the other dyads in the group. The cooperation behavior of ‘P2P1’ increased over time. However, there was no significant difference in the cooperation behavior of ‘FP2’ or ‘P1F’ among different epochs.
More specifically, the fNIRS results revealed that the IBS increment between the right dorsolateral prefrontal cortex (r-DLPFC) and left dorsolateral prefrontal cortex (l-DLPFC) was significantly higher in the dyad ‘P2P1’ (i.e. the IBS increment between the r-DLPFC of P2 and the l-DLPFC of P1) than in the dyad ‘FP2’ and ‘P1F’ (see Fig. 3A and B). Since the confederate did not interact with other partners during the whole task period, we supposed that this finding supported the cooperative interaction hypothesis, namely IBS only emerges during cooperatively interpersonal interaction. That is to say, IBS will not emerge between individuals without engaging in cooperatively mutual interaction even they were in the same room, working on the similar task, hearing each other talk.
Previous studies have shown that IBS increment is an interpersonal neural marker for the cooperatively interpersonal interaction process (Jiang et al., 2012; Pan et al., 2018; Zheng et al., 2018). Further, several studies suggested that both l-DLPFC and r-DLPFC are recruited in literally semantic processing (Mitchell et al., 2016; Klaus and Schutter, 2018). The r-DLPFC is also known to be involved with creativity-related cognitive functions, including cognitive control, working memory and goal maintenance (Macdonald et al., 2000; Miller and Cohen, 2001; Sanfey et al., 2003; Knoch et al., 2009; Silton et al., 2010; Sai et al., 2014) and interpersonal interaction between individuals engaging in group creativity tasks (Lu et al., 2018; Xue et al., 2018). The significant IBS increment of dyad ‘P2P1’ observed in the study might indicate they actively attended to and processed the semantic meaning of the ideas generated by one another. Meanwhile, they built on the ideas from one another as well as combined the ideas from one another with their own. In other words, they were interacting with each other cooperatively and effectively. Consequently, the IBS increment observed in the dyad ‘P2P1’ might reflect ‘P2’ and ‘P1’ were interacted cooperatively and effectively with one another, which was also supported by the finding that dyad ‘P2P1’ showed highest level of cooperation behavior. However, the bivariate Pearson correlations showed no significant correlation between the IBS increment and cooperation behavior. We suggest that the small sample size in the study might lead to the consequence.
Besides, the IBS increment of dyad ‘P2P1’ increased over time, whereas no significant difference in IBS increments of the other dyads was observed among different epochs (see Fig. 3C and Fig. 4A). This may indicate that IBS not only emerges when cooperatively interaction occurs between individuals, it also increased over time. Intriguingly, similar results pattern was observed in the cooperation behavior of dyad ‘P2P1’, which increased over time as well. This might suggest that individuals were more willing to interact with each other cooperatively, namely build on others’ ideas or combine others’ ideas with their own in the RPP task, in the later stage. It could be interpreted that individuals would exhaust most of their own ideas over time, which might be partly reflected by the decrease of RPP fluency over time. Consequently, they began to seek inspiration by attending to and process partners’ ideas. Meanwhile, we also observed higher group originality in the later stage. This partly supported the suggestion that group creativity is only likely to flourish when team members interacted with their partners effectively (Gilson and Shalley, 2004; Vera and Crossan, 2005; Hargadon and Bechky, 2006). In addition, the finding that IBS increased over time may also indicate that the two real participants were more and more proficient at interacting with each other cooperatively. Just as the saying goes, ‘A great team is forged by time.’
Intriguingly, although significant IBS increment was observed between the r-DLPFC (CH17) of ‘P2’ and the l-DLPFC (CH19) of ‘P1’, no similar IBS increment was found between the r-DLPFC (CH17) of ‘P1’ and the l-DLPFC (CH19) of ‘P2’. We supposed that the sequence of the idea report might offer some explanations. Since ‘P2’ reported immediately after ‘P1’, ‘P2’ was more likely and willing to process the ideas from ‘P1’. We suggested it could be much easier and convenient for ‘P2’ to process the recent idea from ‘P1’ than an earlier idea from ‘F’. For instance, when ‘F’ reported idea A and ‘P1’ reported idea B immediately after ‘F’, the recent or temporally closer idea B might catch much more attention from ‘P2’. Similarly, ‘P1’ might be more likely to process the ideas from ‘F’. Besides, the l-DLPFC and r-DLPFC are recruited in language production and interpersonal interaction, respectively (Klaus and Schutter, 2018; Lu et al., 2018). All of this partly indicated that ‘P2’ was processing or even predicting the ideas from ‘P1’, which might also explain why no IBS increment was observed between the r-DLPFC (CH17) of ‘P1’and the l-DLPFC (CH19) of ‘P2’.
The study has several additional limitations. Primarily, Baker et al. (2016) have observed the effect of gender composition on the interpersonal neural correlates underlying cooperative interpersonal interaction. Although participants in the study were mostly female, there were a few male participants. Hence, whether the findings were affected by the gender composition should be further investigated. Besides, considering that the ‘confederate’ was always a male and the real participants were mostly female in this study, the difference in IBS increment between the two real participants pairing and other dyads in the group might be due to the non-matched genders. Namely, the two real participants pairing showed higher IBS increment because the gender is matched. To rule out this potentially contaminant effect, we firstly calculated the mean value of IBS increments (CH17–CH19) from dyads ‘P2P1’ in the groups with one male real participant, which is 0.2382. Next, one-sample t-test using 0.2382 as the test value was performed on the IBS increments (CH17–CH19) from dyads ‘P2P1’ in the groups with two female real participants (i.e. female–female ‘P2P1’). The results showed that the IBS increments of the female–female ‘P2P1’ (M = 0.15, s.d. = 0.16) was significantly lower than the testing value, t (16) = −2.13, P = 0.049. If our finding was due to the non-matched genders, the IBS increment of female–female ‘P2P1’ should be significantly higher than that of the female–male ‘P2P1’. Hence, this might partly rule out the potentially contaminant effect of gender match on our findings. However, further studies should be conducted to examine the potential effect of non-matched genders. Moreover, although the confederate was working on the same task as other partners, we could not rule out the possibility that the IBS increment in real participant–confederate pairings was lower due to the fact that the confederate was saying scripted lines (reporting prepared task-related ideas) and likely not in the same psychological state as the real participants but not without engaging in cooperatively interpersonal interaction. Further studies should be conducted to rule out such confounding. For instance, researchers can replace the ‘confederate’ with one real participant whose ears were blocked from the other two real participants during the task. Eventually, due to the device limitation, the study only focused on the prefrontal cortex. Given that previous studies have shown the IBS increment in the temporal-parietal cortex is also associated with interpersonal interaction (Xue et al., 2018; Zheng et al., 2018), more brain areas should be explored in future studies.
Funding
This work was sponsored by the ‘Shuguang Program’ supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (16SG25), the Philosophy and Social Science Foundation of Shanghai (2017BSH008) and the Humanity and Social Science foundation of Ministry of Education of China (17YJA190007 to N.H.).
Author Contributions
K.L. and N.H. conceived the experiment. K.L. performed the research and analyzed the data. K.L. and N.H. wrote the paper.
Conflict of interest. None declared.
Supplementary Material
References
- Adolphs R. (2003). Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience, 4, 165–178. doi: 10.1038/nrn1056 [DOI] [PubMed] [Google Scholar]
- Abrams D.A., Ryali S., Chen T., et al. (2013). Inter-subject synchronization of brain responses during natural music listening. European Journal of Neuroscience, 37, 1458–1469. doi: 10.1111/ejn.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.M., Liu N., Cui X., et al. (2016). Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Scientific Reports, 6, 26492. doi: 10.1038/srep26492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K.E., Barman S.M., Boitano S., Brooks H. (2015). Ganong’s review of medical physiology 25th. New York: McGraw-Hill Education. [Google Scholar]
- Beaty R.E., Benedek M., Silvia P.J., Schacter D.L. (2016). Creative cognition and brain network dynamics. Trends in Cognitive Science, 20, 87–95. doi: 10.1016/j.tics.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., and Glover G.H. (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage, 50, 81–98. doi: 10.1016/j.neuroimage.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Bryant D.M., and Reiss A.L. (2012). NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage, 59, 2430–2437. doi: 10.1016/j.neuroimage.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B., Chen C., Long Y., et al. (2018). Neural mechanisms for selectively tuning in to the target speaker in a naturalistic noisy situation. Nature Communication, 9, 2405. doi: 10.1038/s41467-018-04819-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikker S., Wan L., Davidesco I., et al. (2017). Brain-to-brain synchrony tracks real-world dynamic group interactions in the classroom. Current Biology, 27, 1375–1380. doi: 10.1016/j.cub.2017.04.002 [DOI] [PubMed] [Google Scholar]
- Dunbar R.I. (2009). The social brain hypothesis and its implications for social evolution. Annals of Human Biology, 36, 562–572. doi: 10.1080/03014460902960289 [DOI] [PubMed] [Google Scholar]
- Funane T., Kiguchi M., Atsumori H., Sato H., Kubota K., Koizumi H. (2011). Synchronous activity of two people’s prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. Journal of Biomedical Optics, 16, 077011. doi: 10.1117/1.3602853 [DOI] [PubMed] [Google Scholar]
- Gilson L.L., and Shalley C.E. (2004). A little creativity goes a long way: An examination of teams’ engagement in creative processes. Journal of Management, 30, 453–470. doi: 10.1016/j.jm.2003.07.001 [DOI] [Google Scholar]
- Goldstein P., Weissman-Fogel I., Dumas G., Shamay-Tsoory S.G. (2018). Brain-to-brain coupling during handholding is associated with pain reduction. Proceedings of the National Academy of Sciences, 115, E2528–E2537. doi: 10.1073/pnas.1703643115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted A., Moore J.C., Jevrejeva S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics, 11, 561–566. doi: hal-00302394 [Google Scholar]
- Hargadon A.B., Bechky B.A. (2006). When collections of creatives become creative collectives: a field study of problem solving at work. Organization Science, 4, 484–500. doi: 10.1287/orsc.1060.0200 [DOI] [Google Scholar]
- Holper L., Scholkmann F., Wolf M. (2012). Between-brain connectivity during imitation measured by fNIRS. NeuroImage, 63, 212–222. doi: 10.1016/j.neuroimage.2012.06.028 [DOI] [PubMed] [Google Scholar]
- Hoshi Y. (2007). Functional near-infrared spectroscopy: current status and future prospects. Journal of Biomedical Optics, 12, 062106. doi: 10.1117/1.2804911 [DOI] [PubMed] [Google Scholar]
- Ikeda S., Nozawa T., Yokoyama R., et al. (2017). Steady beat sound facilitates both coordinated group walking and inter-subject neural synchrony. Frontiers in Human Neuroscience, 11, 147. doi: 10.3389/fnhum.2017.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K.E., Tak S., Jung J., Jang J., Jeong Y., Ye J.C.(2009). Wavelet minimum description length detrending for near-infrared spectroscopy. Journal of Biomedical Optics, 14, 034004–13. doi: 10.1117/1.3127204 [DOI] [PubMed] [Google Scholar]
- Jiang J., Dai B., Peng D., Zhu C., Liu L., Lu C. (2012). Neural synchronization during face-to-face communication. Journal of Neuroscience, 32, 16064–16069. doi: 10.1523/JNEUROSCI.2926-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M., Yamada Y., Ushiku Y., Miyauchi E., Yamaguchi Y. (2013). Inter-brain synchronization during coordination of speech rhythm in human-to-human social interaction. Scientific Reports, 3, 1692. doi: 10.1038/srep01692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus J., Schutter D. (2018). The role of left dorsolateral prefrontal cortex in language processing. Neuroscience, 377, 197–205. doi: 10.1016/j.neuroscience.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Knoch D., Schneider F., Schunk D., Hohmann M., Fehr E. (2009). Disrupting the prefrontal cortex diminishes the human ability to build a good reputation. Proceedings of the National Academy of Sciences, 106, 20895–20899. doi: 10.1073/pnas.0911619106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U., Li S.C., Gruber W., Muller V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BioMed Central Neuroscience, 10, 22. doi: 10.1186/1471-2202-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Piazza E. A., Simony E., et al. (2017). Measuring speaker–listener neural coupling with functional near infrared spectroscopy. Scientific Reports, 7, 43293. doi: 10.1038/srep43293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.M., Zhang Y.J., Biswal B.B., Zang Y.F., Peng D.L., Zhu C.Z. (2010). Use of fNIRS to assess resting state functional connectivity. Jorunal of Neuroscience Methods, 186, 242–249. doi: 10.1016/j.jneumeth.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Lu K., Qiao X., Hao N. (2019). Praising or keeping silent on partner’s ideas: leading brainstorming in particular ways. Neuropsychologia, 124, 19–30. doi: 10.1016/j.neuropsychologia.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Lu K., Xue H., Nozawa T., Hao N. (2018). Cooperation makes a group be more creative. Cerebral Cortex. Online publication. doi: 10.1093/cercor/bhy215 [DOI] [PubMed] [Google Scholar]
- Macdonald A.W., Cohen J.D., Stenger V.A., Carter C.S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838. doi: 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- Miller E.K., and Cohen J.D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mitchell R.L., Vidaki K., Lavidor M. (2016). The role of left and right dorsolateral prefrontal cortex in semantic processing: a transcranial direct current stimulation study. Neuropsychologia, 91, 480–489. doi: 10.1016/j.neuropsychologia.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Montague P.R., Berns G.S., Cohen J.D., et al. (2002). Hyperscanning: simultaneous fMRI during linked social interactions. NeuroImage, 16, 1159–1164. doi: 10.1006/nimg.2002.1150 [DOI] [PubMed] [Google Scholar]
- Müller V., Sänger J., Lindenberger U. (2013). Intra- and inter-brain synchronization during musical improvisation on the guitar. Plos One, 8, e73852. doi: 10.1371/journal.pone.0073852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T., Sasaki Y., Sakaki K., Yokoyama R., Kawashima R. (2016). Interpersonal frontopolar neural synchronization in group communication: an exploration toward fNIRS hyperscanning of natural interactions. NeuroImage, 133, 484–497. doi: 10.1016/j.neuroimage.2016.03.059 [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Glerean E., Viinikainen M., Jääskeläinen I.P., Hari R., Sams M. (2012). Emotions promote social interaction by synchronizing brain activity across individuals. Proceedings of the National Academy of Sciences, 109, 9599–9604. doi: 10.1073/pnas.1206095109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka N., Minamoto T., Yaoi K., Azuma M., Osaka M. (2014). Neural synchronization during cooperated humming: a hyperscanning study using fNIRS. Procedia—Social and Behavioral Sciences, 126, 241–243. doi: 10.1016/j.sbspro.2014.02.395 [DOI] [Google Scholar]
- Osborn (1957). Applied Imagination, 1st edn, New York: Scribner's. [Google Scholar]
- Pan Y., Cheng X., Zhang Z., Li X., Hu Y. (2017). Cooperation in lovers: an fNIRS-based hyperscanning study. Human Brain Mapping, 38, 831–841. doi: 10.1002/hbm.23421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Novembre G., Song B., Li X., Hu Y. (2018). Interpersonal synchronization of inferior frontal cortices tracks social interactive learning of a song. Neuroimage, 183, 280–290. doi: 10.1016/j.neuroimage.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Runco M.A. (1991). Divergent Thinking, Norwood, NJ: Ablex. [Google Scholar]
- Runco M.A., Abdulla A.M., Paek S.H., Al-Jasim F.A., Alsuwaidi H.N. (2016). Which test of divergent thinking is best? Creativity. Theories—Research—Applications, 3, 4–18. doi: 10.1515/ctra-2016-0001 [DOI] [Google Scholar]
- Sai L., Zhou X., Ding X.P., Fu G., Sang B. (2014). Detecting concealed information using functional near-infrared spectroscopy. Brain Topography, 27, 652–662. doi: 10.1007/s10548-014-0352-z [DOI] [PubMed] [Google Scholar]
- Sanfey A.G., Rilling J.K., Aronson J.A., Nystrom L.E., Cohen J.D. (2003). The neural basis of economic decision-making in the ultimatum game. Science, 300, 1755–1758. doi: 10.1126/science.1082976 [DOI] [PubMed] [Google Scholar]
- Silton R.L., Heller W., Towers D.N., et al. (2010). The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. NeuroImage, 50, 1292–1302. doi: 10.1016/j.neuroimage.2009.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Okamoto M., Dan H., Jurcak V., Dan I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage, 27, 842–851. doi: 10.1016/j.neuroimage.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Tong Y., Lindsey K.P., Frederick B.D. (2011). Partitioning of physiological noise signals in the brain with concurrent near-infrared spectroscopy and fMRI. Journal of Cerebral Blood Flow Metabolism, 31, 2352–2362. doi: 10.1038/2Fjcbfm.2011.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D., Jurcak V., Singh A.K., Okamoto M., Watanabe E., Dan I. (2007). Virtual spatial registration of stand-alone fNIRS data to MNI space. NeuroImage, 34, 1506–1518. doi: 10.1016/j.neuroimage.2006.10.043 [DOI] [PubMed] [Google Scholar]
- Vera D., Crossan M. (2005). Improvisation and innovative performance in teams. Organization Science, 16, 203–224. doi: 10.1287/orsc.1050.0126 [DOI] [Google Scholar]
- Wu X., Yang W., Tong D., et al. (2015). A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping, 36, 2703–2718. doi: 10.1002/hbm.22801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H., Lu K., Hao N. (2018). Cooperation makes two less-creative individuals turn into a highly-creative pair. NeuroImage, 172, 527–537. doi: 10.1016/j.neuroimage.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Ye J.C., Tak S., Jang K.E., Jung J., Jang J. (2009). NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage, 44, 428–447. doi: 10.1016/j.neuroimage.2008.08.036 [DOI] [PubMed] [Google Scholar]
- Zheng L., Chen C., Liu W., et al. (2018). Enhancement of teaching outcome through neural prediction of the students' knowledge state. Human Brain Mapping, 00, 1–12. doi: 10.1002/hbm.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


