Abstract
Exposure to aristolochic acids (AAs) from Aristolochia plants is one of the major global causes of nephropathy, renal failure and urothelial cancer, including Balkan endemic nephropathy (BEN). The high incidence of BEN on the Balkan Peninsula is assumed to result from consumption of Aristolochia clematitis L. seeds co-harvested with crops. Here we show that AAs are long-lived soil contaminants that enter wheat and maize plants by root uptake, with strong pH-dependence. Soil and crops from Serbian farms in areas endemic for A. clematitis were found to be extensively contaminated with AAs, with contamination strongly correlated with local incidence of BEN. The persistence of AAs as soil contaminants suggests that weed control for A. clematitis plants is needed to reduce the incidence of BEN and aristolochic acid nephropathy, and that systematic surveys of soil and crop AA levels would identify high-risk regions and it is imperative to research soil remediation methods.
Keywords: Aristolochic acid nephropathy, Balkan endemic nephropathy, Aristolochic acids, Aristolochia clematitis L., Soil pollution, Plant uptake
Graphical Abstract
Introduction
Aristolochic acids (AAs) comprise a family of nitrophenanthrene carboxylic acids naturally produced by Aristolochia or “birthwort” plants, with aristolochic acid-I (AA-I), 1, and aristolochic acid-II (AA-II), 2, as the two major homologs (Figure 1).1 Consumption of AAs in herbal medicines is now recognized as a major cause of nephropathy, kidney failure, and upper tract urothelial cancer (UTUC),2 the so-called Chinese herb nephropathy or aristolochic acid nephropathy (AAN).3 For example, the inadvertent substitution of Stephania tetrandra S. Moore with AA-containing Aristolochia fangchi Y.C. Wu ex L.D. Chow & S.M. Hwang in the preparation of slimming pills caused ~100 cases of AAN in Belgium in 1991.4,5 Cases of AAN caused by the misuse of AA-containing herbal medicines have been reported in Australia, China, Hong Kong, Japan, Taiwan, and the U.K..6–8 Because of the potent carcinogenicity observed in both laboratory rodents and humans, AAs are classified as Group I carcinogens by the International Agency for Research on Cancer (IARC).9 Currently, the sale of AA-containing herbs or their use in medicine is banned in many countries,10 with the US FDA requiring verification of lack of AAs in dietary supplements and botanical products.
Figure 1.
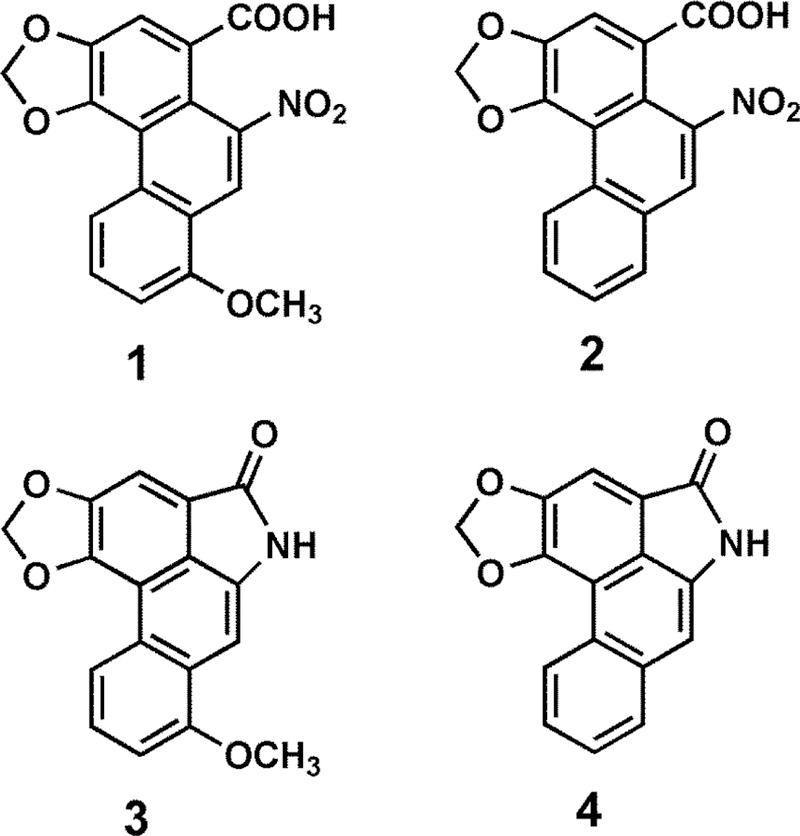
Chemical structures of non-fluorescent aristolochic acids, 1 and 2, and fluorescent aristolactams, 3 and 4.
Chronic exposure to AAs has now been established as one of the major etiologies of Balkan endemic nephropathy (BEN),7,11,12 a unique type of slowly progressive kidney fibrosis that affects hundreds of thousands of farmers in Bosnia and Herzegovina, Bulgaria, Croatia, Romania, and Serbia, the Balkan Peninsula.13–16 The disease was first reported in 1956 as having similar clinical and morphologic features as those observed in AAN patients and is characterized by its long incubation time and gradual progression to renal failure.17,18 While AA exposure is assumed to cause BEN, the mechanism of exposure remains unclear. Ivić19 speculated that the seeds of A. clematitis, an AA-containing weed growing abundantly in the wheat fields of affected areas, become intermingled with grain during the harvesting process, thus contaminating the resulting baking flour.20 However, this exposure pathway was never established, and there is no convincing evidence to support it.18,21
Contrary to this model, we recently demonstrated that decaying A. clematitis can release AAs into soil with subsequent uptake into wheat and maize plants.22 We have now used a simple and cost-effective HPLC method to perform a large-scale comparison of AA levels in farm soil and food crops in regions of Serbia that are endemic and non-endemic for growth of A. clematitis.
Materials and Methods
Chemicals.
All chemicals and reagents used were of the highest purity available and were used without further purification. AAs (a mixture of AA-I and AA-II, 1:1) were purchased at purity of 96% (Acros, Morris Plains, NJ). Deionized water was further purified by a Milli-Q Ultrapure water purification system (Millipore, Billerica, MA) and used in all experiments. Acetonitrile and methanol used were HPLC grade (Mallinckrodt Baker, Phillipsburg, NJ). C18 solid-phase extraction (SPE) columns were packed with 500 mg of sorbents (Waters, Milford, MA).
Soil sample collection.
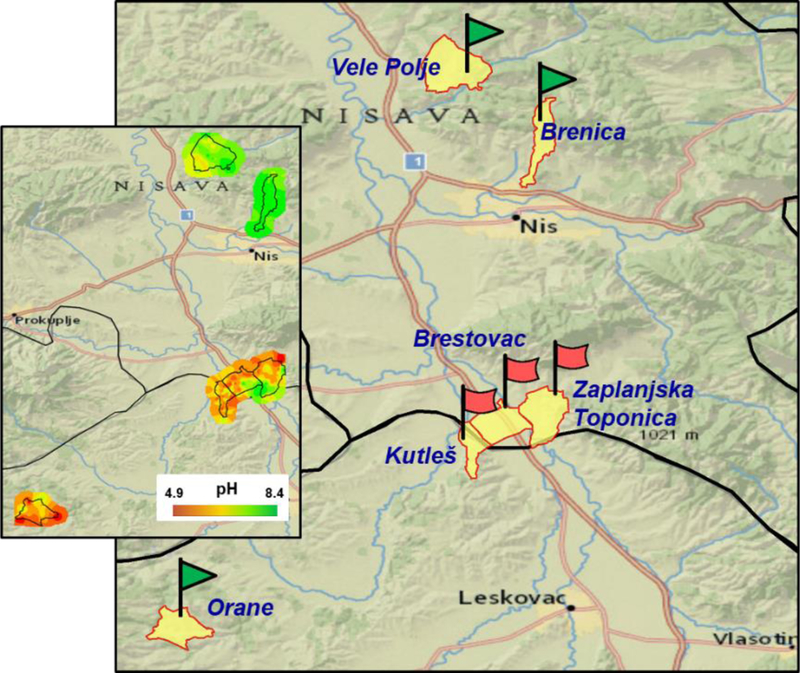
A total of 472 soil samples were taken at soil depths of 5–10 cm from agricultural fields in Serbia. These samples were taken from soil which is turned by plowing once a year up to a depth of 30–50 cm. It is important to note that living, AA-containing A. clematitis plants were observed in the vicinity of many sampling sites. Soil samples (n = 276) were collected randomly from wheat and maize fields in three endemic villages (Kutleš, 43° 8′22.93′′N, 21°51′39.44′′E, elevation 206–214 m; Brestovac, 43°09′09.7′′N, 21°53′15.0′′E, elevation 164 m; and Zaplanjska Toponica, 43°08′57.8′′N, 21°41′16.1′′E, elevation 126 m) in Serbia during the harvest seasons of 2015 and 2016. At the same time, matching wheat (n = 138) and maize (n = 138) grain samples were manually harvested 10 cm away from where the soil samples were collected.
Control soil (n = 196), wheat grain (n = 43), and maize grain (n = 98) samples were collected at a similar time from wheat and maize fields in three remote non-endemic villages (Vele Polje, 43°26′43.86′′N, 21°50′49.32′′E, elevation 233–258 m; Brenica, 43°22′52.75′′N, 21°55′40.58′′E, elevation 403–473 m; and Orane, 42°58′55.3′′N, 21°37′32.9′′E, elevation 457 m). All the collected samples were kept in polypropylene containers and stored in a −20 °C freezer prior to analysis.
Environmental stability of AAs.
The environmental stability of the AAs was investigated by incubating a mixture of 1 and 2 in the soil in the soil samples collected from an endemic area in Serbia. In brief, 2.0 mg of AA-I and AA-II were spiked on 5.0 g of soil and air-dried, and the AA-fortified soil samples were added to 15.0 g of soil sample in 50 mL polypropylene tubes (n = 3), vortex mixed, and stored at room ambient conditions with occasionally mixing by inversion. Then, 300 mg of each soil sample was collected at 0, 30, 60, 80, 100, 120, 150, 180 and 270 days after starting the experiment. The collected samples were processed and analyzed by high-performance liquid chromatography with fluorescence detection (HPLC–FLD) as described below.
Thermal stability of AAs.
The thermal stability of the AAs was investigated by baking dough made from AA-fortified wheat flour in an oven. Specifically, 1 g portions of the AA-fortified dough (100 µg/g, w:w; n = 3) were heated in an oven (180 °C) until they turned golden brown (~ 30 min). The samples were then hardened by freezing at −80 °C before being ground using a mortar and pestle. The powdered samples were then extracted and analyzed using the method described below. Using a similar approach, a control experiment was conducted in which an equal amount of AA-fortified unbaked flour dough was analyzed (n = 3).
Soil sample preparation.
The collected soil samples were processed using our previously developed method.21 In brief, 300 mg of the ground samples were extracted using 3 mL of a methanol/water/acetic acid mixture (70:25:5, v/v/v) through ultrasound-assisted extraction, treated with zinc dust to reduce the non-fluorescent AAs to fluorescent aristolactams, and then cleaned-up and enriched by SPE (Waters). The AA contents in the sample extracts were determined indirectly by detection of the corresponding aristolactams using a previously developed HPLC with fluorescence detection method.23 Homogenized wheat and maize sample were also processed and analyzed using the same protocol.
HPLC–FLD analysis of AAs.
The concentrations of AAs in the aristolactam-containing sample extracts were reduced by Zn/H+ and enriched by SPE before analysis on a model 1260 HPLC (Agilent, Palo Alto, CA) system. The column used was a 50 mm x 2.1 mm i.d., 2.7 μm, Poroshell 120 EC-C8 (Agilent, Palo Alto, CA). The column was eluted at 0.3 mL/min using gradient elution with (A) water/ (B) methanol as the mobile phase. The solvent gradient started from 20% B, programmed to 100% in 15 min, and was held for 5 min before column reconditioning. The eluate was monitored by a fluorescence detector at the excitation and emission wavelengths of 393 nm and 460 nm, respectively.
Statistical analyses.
A daily dietary exposure assessment was conducted as reported previously.24,25 In calculating the human exposure, samples from endemic villages with AAs present but with concentrations below the limit of quantitation (LOQ) were treated as having half the LOQ concentration,25 and as zero from non-endemic villages due to the very low detection frequency.24 The transfer factor (TF) was obtained by dividing the concentrations of AA-I and AA-II in the wheat and maize grains by those in the corresponding soil samples.26,27 The obtained TF values were then plotted against the concentrations of AAs in the soil to investigate the relationship between the plant uptake and the accumulation of AAs. Heat maps illustrating pH of cultivation soil and AA concentrations in farmland soil and food crops were generated using ArcGIS 10.3 (ESRI, Redlands, CA).
Results and Discussion
Quantitation of AAs in soil samples collected from endemic areas in Serbia.
We first established the levels of the two major AAs (AA-I, AA-II) in soil samples from farms in three villages associated with high BEN incidence (Kutleš, Brestovac, Zaplanjska Toponica), which are also considered to be endemic for growth of A. clematitis, and in three villages considered to be non-endemic (Brenica, Orane, Vele Polje) (Figure 2). In total, we analyzed 276 soil samples from 138 wheat fields and 138 maize fields in endemic areas and 98 wheat fields and 98 maize fields in non-endemic areas (Tables 1 and 2). As shown in Figure 3, to quantitate 1 and 2 in both soil and plant material, we developed a straightforward approach of HPLC with fluorescence detection, which contain a highly efficient nitro-reduction step (>99% yield) to convert the non-fluorescing AAs to their strongly fluorescing aristolactams,22 3 and 4 (Figure 1), and is readily implemented in resource-constrained settings. This method, with good recovery (>90%) of both AA-I and AA-II in the extraction and SPE cleanup processes, was validated against the less cost-effective chromatography-coupled tandem quadrupole mass spectrometry method that we developed previously.22
Figure 2.
Map of the Serbian villages studied. Villages endemic for Aristolochia clematitis plants and with high incidence of nephropathy are labeled with red rectangular flags; non-endemic villages are labeled with green triangular flags. The inset shows a heat map representing the soil pH in the different villages under investigation.
Table 1.
Concentrations of Aristolochic Acids in Soil, Wheat, and Maize Grain Samples Collected from the Endemic Villages of Kutleš, Brestovac and Zaplanjska Toponica, Serbia.
| Location | Wheat field |
Maize field |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kutleš | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | 80 | 80 | 80 | 80 | ||||||||
| # of positive samplesa | 49 | 45 | 58 | 34 | 26 | 19 | 35 | 19 | ||||
| Concentration range, ng/g | 12.7–777.5 | 12.5–218.1 | 11.2–642.3 | 12.7–102.4 | 11.8–510.4 | 19.7–250.1 | 15.8–336.6 | 16.3–141.7 | ||||
| Mean concentration, ng/gb | 85 ± 120 | 41 ± 45 | 84 ± 133 | 17 ± 20 | 38 ± 82 | 20 ± 39 | 26 ± 51 | 12 ± 22 | ||||
| Transfer factor | - | - | 0.06–11.89 | 0.15–1.65 | - | - | 0.05–3.56 | 0.07–1.63 | ||||
| Mean transfer factor | - | - | 1.4 ± 2.6 | 0.66 ± 0.47 | - | - | 0.77 ± 0.84 | 0.56 ± 0.44 | ||||
| Daily exposure, μg/dayc | - | - | 27.7 | 5.36 | - | - | 1.45 | 0.63 | ||||
| Brestovac | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | 37 | 37 | 37 | 37 | ||||||||
| # of positive samplesa | 25 | 4 | 34 | 12 | 21 | 9 | 29 | 5 | ||||
| Concentration range, ng/g | 11.4–50.8 | 11.0–12.8 | 22.6–2872.1 | 10.5–245.4 | 12.4–56.3 | 10.4–15.5 | 28.5–195.6 | 11.0–13.6 | ||||
| Mean concentration, ng/gb | 22 ± 15 | 5.8 ± 2.1 | 196 ± 515 | 16 ± 40 | 21 ± 16 | 6.8 ± 3.0 | 57 ± 39 | 6.0 ± 2.4 | ||||
| Transfer factor | - | - | 0.89–14.28 | 1.16–2.77 | - | - | 0.67–5.68 | 1.00–1.27 | ||||
| Mean transfer factor | - | - | 3.2 ± 2.8 | 2.0 ±1.1 | - | - | 2.4 ± 1.4 | 1.1 ± 0.15 | ||||
| Daily exposure, μg/dayc | - | - | 66.4 | 4.94 | - | - | 3.20 | 0.31 | ||||
| Zaplanjska Toponica | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | 21 | 21 | 21 | 21 | ||||||||
| # of positive samplesa | 17 | 14 | 10 | 1 | 18 | 19 | 7 | 7 | ||||
| Concentration range, ng/g | 13.8–741.2 | 11.3–416.1 | 12.3–48.8 | 10.3 | 33.1–267.4 | 11.3–15.5 | 10.8–47.4 | 10.5–14.7 | ||||
| Mean concentration, ng/gb | 85 ± 155 | 37 ± 88 | 12 ± 10 | 5.4 ± 1.1 | 68 ± 56 | 20 ± 8.8 | 11 ± 10 | 7.7 ± 3.8 | ||||
| Transfer factor | - | - | 0.15–0.95 | 0.52 | - | - | 0.15–0.74 | 0.52–1.12 | ||||
| Mean transfer factor | - | - | 0.40 ± 0.28 | 0.52 | - | - | 0.34 ± 0.23 | 0.73 ± 0.21 | ||||
| Daily exposure, μg/dayc | - | - | 3.83 | 1.66 | - | - | 0.60 | 0.40 | ||||
Samples with concentrations above the limit of quantitation (LOQ): 10.89 ng/g of AA-I, 10.23 ng/g of AA-II.
Samples with concentrations below the LOQ were assigned a concentration of one-half of the LOQ: 5.44 ng/g of AA-I, 5.12 ng/g of AA-II
Average daily intake based on the daily consumption of 0.5 kg wheat products or 0.1 kg maize grain/products.
Table 2.
Concentrations of Aristolochic Acids in Soil, Wheat, and Maize Grain Samples Collected from the Non-endemic Villages of Brenica, Orane and Vele Polje, Serbia.
| Location | Wheat field |
Maize field |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brenica | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | 85 | 30 | 63 | 63 | ||||||||
| # of positive samplesa | 7 | 3 | 7 | 3 | 1 | 0 | 7 | 1 | ||||
| Concentration range, ng/g | 11.1–55.3 | 12.7–17.2 | 20.1–177 | 11.8–20.3 | 17.8 | - | 10.9–27.9 | 13.6 | ||||
| Mean concentration, ng/gb | 2.3 ± 8.8 | 0.5 ± 2.8 | 14 ± 35 | 1.5 ± 4.7 | 0.3 ± 2.2 | - | 1.8 ± 5.3 | 0.2 ± 1.7 | ||||
| Daily exposure, μg/dayc | - | - | 4.46 | 0.47 | - | - | 0.10 | 0.01 | ||||
| Orane | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | 13 | 13 | 13 | 13 | ||||||||
| # of positive samplesa | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | ||||
| Concentration range, ng/g | 34.3 | - | - | - | 27.9 | - | 23.9 | 10.8 | ||||
| Mean concentration, ng/gb | 2.6 ± 9.5 | - | - | - | 2.1 ± 7.7 | - | 1.8 ± 6.6 | 0.8 ± 3.0 | ||||
| Daily exposure, μg/dayc | - | - | - | - | - | - | 0.10 | 0.04 | ||||
| Vele Polje | Soil | Grain | Soil | Grain | ||||||||
| AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | AA-I | AA-II | |||||
| # of samples analyzed | - | - | 22 | 22 | ||||||||
| # of positive samplesa | - | - | - | - | 2 | 0 | 0 | 0 | ||||
| Concentration range, ng/g | - | - | - | - | 12.4–17.3 | - | - | - | ||||
| Mean concentration, ng/gb | - | - | - | - | 1.3 ± 4.4 | - | - | - | ||||
| Daily exposure, μg/dayc | - | - | - | - | - | - | - | - | ||||
Samples with concentrations above the limit of quantitation (LOQ): 10.89 ng/g of AA-I, 10.23 ng/g of AA-II.
Samples with concentrations below the LOQ were treated as zero.
Average daily intake based on the daily consumption of 0.5 kg wheat products or 0.1 kg maize grain/products
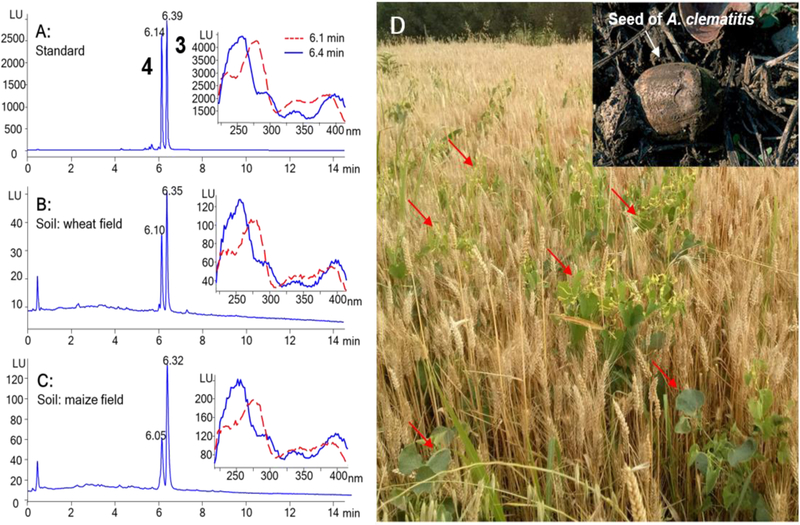
Figure 3.
Typical chromatograms from HPLC–FLD analysis of aristolactam I and aristolactam II, the nitroreduction products of AA-I and AA-II, respectively, (A) in authentic standard solution mixture, and in soil samples collected from (B) wheat and (C) maize fields. Shown in the insets are the fluorescence excitation spectra of the chromatographic peaks at migration times of 6.1 (aristolactam II, 2) and 6.4 min (aristolactam I, 1). (D) Photos showing Aristolochia clematitis (indicated by red arrows) growing in wheat field in the endemic Village, Kutleš. (LU: Luminescence unit).
These analyses revealed that the cultivated fields in the endemic area are extensively contaminated with both AAs (Table 1). For example, AA-I and AA-II were identified in 49 and 45 of the 80 soil samples collected from wheat fields in Kutleš and in 26 and 19 of the 80 soil samples collected from maize fields, respectively (Table 1). In general, the average concentration of AA-I was roughly twice that of AA-II in the soil samples collected from wheat and maize fields (Figure 3, Table 1). This result is in good agreement with those of previous studies, in which higher levels of AA-I than AA-II were detected in Aristolochia plants.22,23 While the proportion of positive samples and the average concentrations were slightly lower for maize than for wheat (Table 1), the concentration ranges in the soil samples were similar for the two crops. This phenomenon can be explained by the practice of crop rotation in the area, whereby the cultivation of wheat and maize is rotated in the same field, which makes an absolute distinction between wheat and maize fields difficult to establish.
Analysis of soil samples from three non-endemic villages revealed significantly lower frequencies of AA-containing samples and ~10-fold lower levels of AAs in the positive samples (Table 2) compared to samples from the endemic areas (Table 1). The levels of AAs in the soil samples raised questions about the relationship to AAs in food crops.
AA levels in wheat and maize crops parallel the soil levels.
In parallel with soil sample analysis, wheat and maize grain samples (276 from endemic areas and 141 from non-endemic areas) (Tables 1 and 2) were also collected and analyzed for AA content. Both AA-I and AA-II in the endemic villages were detected in roughly half of the analyzed crop samples and at slightly lower concentrations than the soil samples (Figure 4, Table 1). Although the soil AA concentrations were lower in the endemic village of Brestovac than those in the samples collected in Kutleš, AAs were detected at higher frequencies in the crop samples from Brestovac than those from Kutleš. Similarly, while AA concentrations in crop samples were lower in the endemic village of Zaplanjska Toponica than in Kutleš (Figure 4), AAs occurred at higher frequencies in soil samples from Zaplanjska Toponica than from Kutleš, with positive detection in most samples from Brestovac and Toponica (Table 1). This inverse relationship between levels of AAs in soil and crops is addressed shortly.
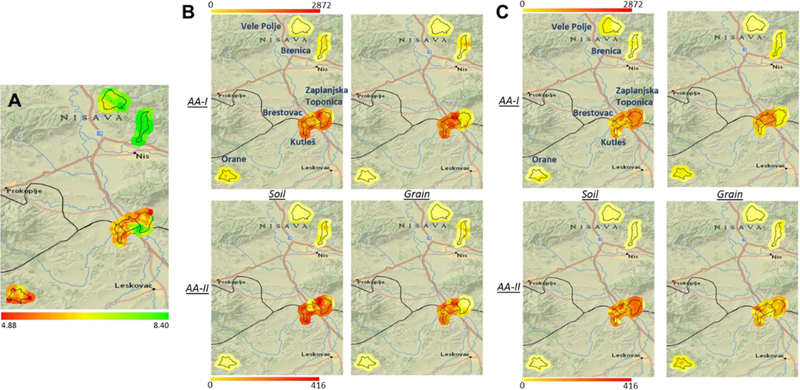
Figure 4.
Relationships among (A) soil pH and AA levels in soil and crops from (B) wheat and (C) maize fields in Serbian villages endemic for Aristolochia plants (Kutleš, Brestovac, Zaplanjska Toponica) and non-endemic villages (Vele Polje, Brenica, Orane). Soil pH and concentrations of spots nearby are estimated using inverse distance weighted interpolation method. Color scales below each map are geometric intervals with eight classes showing the range of values of soil pH (4.88 to 8.40), and AA-I (0 to 2872 ng/g) and AA-II (0 to 416 ng/g) concentrations.
Two observations support the idea that the crops were contaminated with AAs by uptake from the soil. First, AA-I was again detected at higher concentrations than AA-II in both the wheat and maize grain samples (Table 1), which parallels the data for soil levels. Second, we observed a positive correlation between concentrations of AA-I and AA-II in the soil, wheat, and maize samples, which suggests that both AAs originated from the same source. It is thus likely that A. clematitis weeds senesce and decay in the farmland to release AAs in the soil. While the previous proposal of AA exposure through the consumption of wheat flour tainted with Aristolochia seeds was only hypothetical,19,20 the present study demonstrates unequivocally that the food grains themselves are extensively contaminated with AAs in areas endemic for A. clematitis growth, which has significant implications for human exposure to AAs in food.
AAs are persistent pollutants for which plant uptake is determined by soil conditions.
We next sought to define factors that affected the fate of soil AAs and their uptake into plants. The stability of AAs toward heat was demonstrated by subjecting AA-I and AA-II to baking temperatures (180 °C) for 30 min, during which the level of AAs dropped ~40–50%. AAs also proved to be stable in soil for at least 9 months, with an initial drop of ~40–50% in concentration in the first 3 months stabilizing over the remaining 6 months. Parallel experiments performed in darkness showed similar results, indicating AAs are not photosensitive. Both results point to AAs as persistent soil pollutants.
To investigate the relationship between soil levels and crop contamination by AAs, we calculated the transfer (TF) or bioconcentration factor for AAs (Figure 5). As an established index of the efficiency of transfer of elements from soil into plants,26,27 the TF for AA uptake into wheat and maize was calculated by dividing the concentrations of AA-I and AA-II in the wheat and maize grains by the soil levels (Table 1). Remarkably, a plot of TFs versus soil levels revealed an inverse relationship between soil and plant concentrations of AA (Figure 5). This analysis reveals an interesting phenomenon in which a high absorption efficiency and accumulation rate was observed in the food grains harvested from farmlands with low levels of AA pollution and vice versa (Figure 5). Previous studies have demonstrated that AAs are efficiently taken up by food crops, with >99.9% absorption in 48 h by maize and cucumber plants.28 On the other hand, a reduced transfer rate was observed in farmland with high AA concentrations, which could be due to a “saturation effect”. This has been proposed by Dudka and Miller for heavily contaminated soils29 and observed in food crops grown in soils contaminated with radioactive substances, heavy metals, and polycyclic aromatic hydrocarbons.26,27 This phenomenon was also observed in our experiment when wheat plants at the V1 growth stage were exposed to different concentrations of AA (Figure 6).
Figure 5.
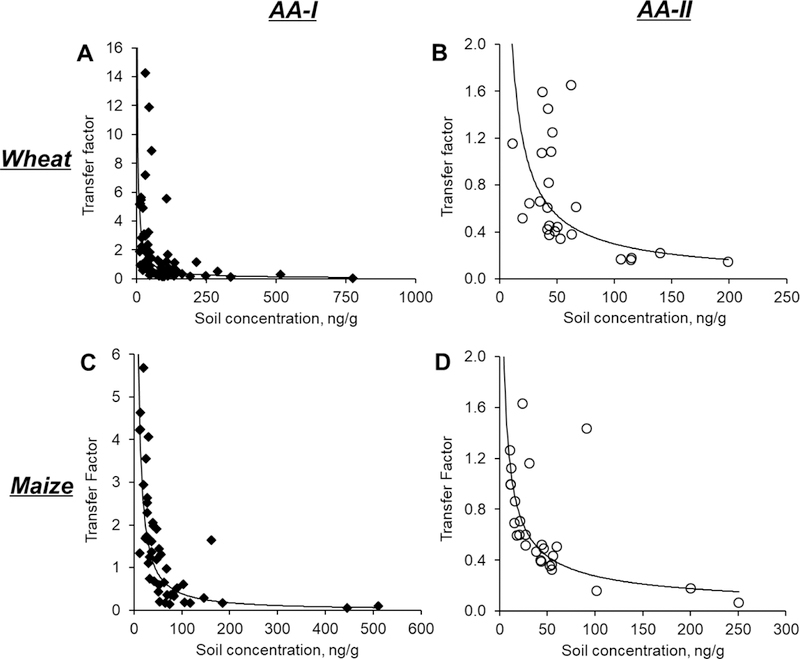
Relationship between transfer factors and soil concentrations of (A,C) AA-I (B,D) AA-II in (A,B) wheat and (C,D) maize in samples collected from farmland in endemic villages.
Figure 6.
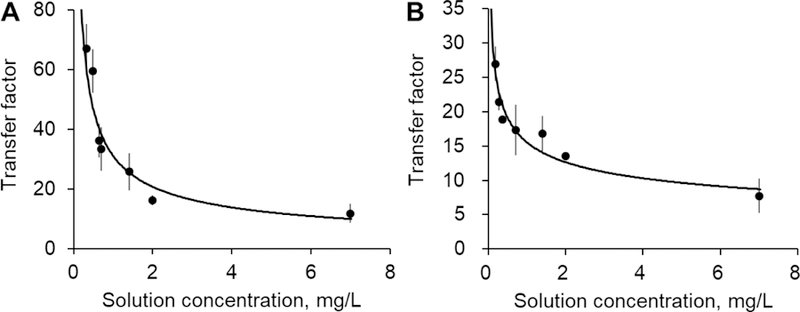
Root uptake efficiency of (A) AA-I and (B) AA-II by wheat plant at V1 growing stage in media with different concentration of AA in pH 6.0 (n = 3).
Given the fact that AAs are acids, we assessed the role of soil pH as a determinant of TF. As shown in Figures 4A and 7, this analysis revealed a surprising difference in pH for soil samples collected from endemic and non-endemic villages, with median pH values of 6.0 and 7.6, respectively. The germination and density of some Aristolochia plants have been observed to be favored in slightly acidic environments,30 which are consistent with the observation that AAs were detected at higher concentrations and frequencies in the lower pH surface soils of farmlands in endemic villages (Table 1) than in the higher pH present in soils in non-endemic villages (Table 2).
Figure 7.
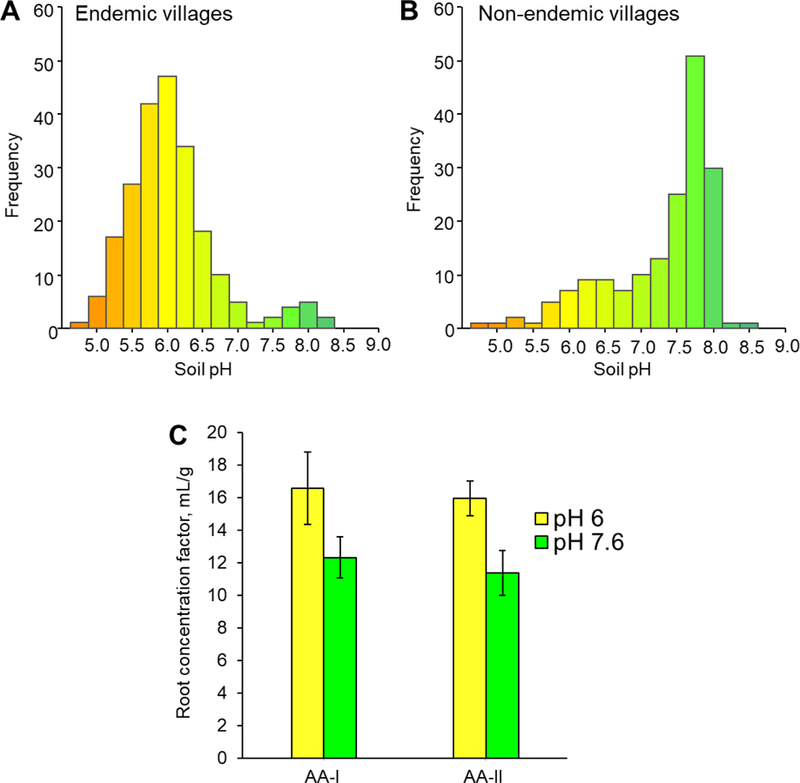
Soil pH as a determinant of AA uptake into crops. The pH of surface soil was determined for samples collected from farmlands in Serbian villages (A, n = 221) endemic and (B, n = 173) non-endemic for Aristolochia plants. Analyses were performed according to the standard procedure in ISO 10390:2005. (C) The root uptake efficiency of AA from pH 6.0 and 7.6 environments was determined by planting wheat plants in V1 growth stage in an aqueous extract of Aristolochia clematitis fruit containing 15 µg/mL and 1.5 µg/mL of AA-I and AA-II, respectively, for two days.
We then investigated the effect of pH on the efficiency of root uptake of AAs, by growing wheat in aqueous extracts of the dried A. clematitis fruit. These results revealed a higher absorption efficiency of both AA-I and AA-II in the slightly acidic medium, as indicated by the higher root concentration factor (RCF) values at pH 6.0 than that at pH 7.6 (Figure 7C). These results are consistent with enhanced absorption of the neutral forms of weakly acidic AAs, perhaps by increased partitioning into the lipophilic root membranes and root sap. Corroborating this observation of pH-dependent root uptake of AAs from the environment, analysis of the acquired data from analyzing AAs in food grain showed significantly higher AA concentrations in food grains collected from farmland with acidic soil (Figure 8). The higher root uptake efficiency of AA by food crops growing in more acidic environments in endemic areas may also have contributed to the observed higher crop concentrations of AAs. Our studies suggest that soil pH contributes to the risk for kidney disease in regions endemic for Aristolochia plants.
Figure 8.
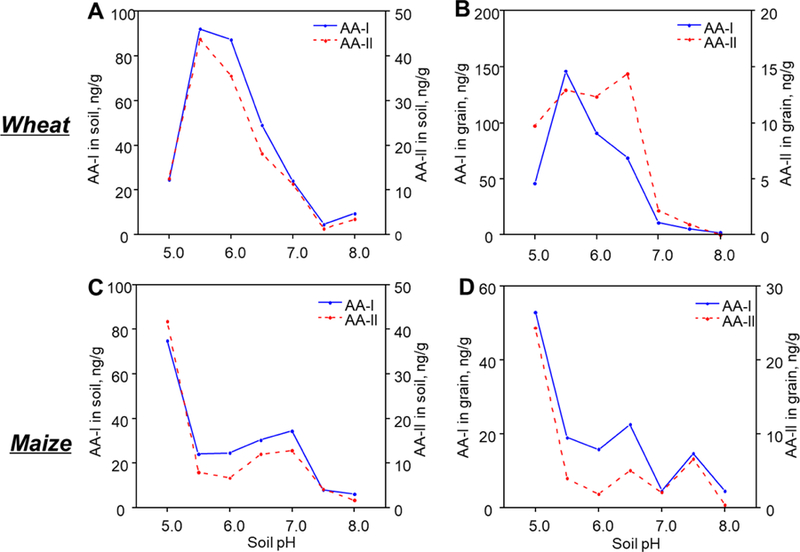
Relationship between AA concentration in (A,C) farmland soil and soil pH, together with the relationship between AA concentration in (B) wheat and (D) maize grains and soil pH in samples collected from farmland in Serbia.
As another potential determinant of AA transport into plants, the total organic carbon (TOC) content in the collected soil samples was also analyzed using the modified Mebius method.31 The results show only a slight difference in the samples collected from endemic and non-endemic villages: 1.3% for endemic villages (n = 147) and 1.5% for non-endemic villages (n = 115). Based on these results, it is possible that soil TOC content do not contributed significantly to the development of an area into a kidney disease hotspot.
Food consumption in endemic regions predicts toxic AA exposures that correlate with BEN risk.
Given the high levels of AAs in food crops in regions endemic for A. clematitis growth and the low levels in non-endemic areas, we next sought to determine the effects of exposure to these AA levels on the risk for toxicity in humans. Based on the estimate that Serbians consume 0.5 kg of wheat and 0.1 kg of maize per day32 and on the fact that AAs are highly persistent during commonly used cooking methods, such as baking, we estimated that residents of endemic villages (e.g. Kutleš) ingest ~30 μg of AA-I per day and ~6 μg of AA-II per day through contaminated wheat and maize products grown on local farms, while residents of non-endemic villages are exposed to levels 10- to 20-fold lower (Tables 1 and 2). These data are consistent with the observation that BEN affects 2.6% of the population of Kutleš33 while BEN incidence is significantly lower in non-endemic villages.21 BEN is a late-onset disease normally observed between the ages of 40 and 60, with ≥30 years of exposure needed for manifestation of the carcinogenic and nephrotoxic effects of AAs.34 Our results thus suggest that a cumulative dose of ~350–400 mg of AAs is needed to trigger BEN. This is in good agreement the conclusion by Hoang et al.35 that cumulative ingestion of ≥250 mg of AAs increases the risk of urothelial carcinomas of the upper urinary tract in Taiwanese patients. It should be pointed out that safe threshold levels of AAs in human food have yet to be established.
While the mechanism linking AA exposure to pathology in humans has not been conclusively established, previous studies demonstrated that reactive intermediates generated from the metabolism of AAs bind to DNA to produce highly persistent DNA adducts.6,36 For example, the highly mutagenic 2′-deoxyadenosine adduct of AA-I exhibited a lifelong persistence in the kidneys of AA-I-dosed rats.37 AA-associated DNA adducts were also detected in the kidneys of patients suffering from BEN.12 Furthermore, AAs were recently demonstrated to induce oxidative stress.38,39 Therefore, despite the relatively low level of ingestion through dietary exposure, it is reasonable to anticipate that prolonged exposure to AAs through AA-contaminated foodstuffs causes kidney fibrosis by yet-to-be defined mechanism(s) and is one of the major causes of the UTUC also observed in patients with BEN.
In the present study, by analyzing agricultural soil and food crops in Serbia, a region with high levels of AAN/BEN, we identified for the first time that AAs released from the decay of A. clematitis weeds form a new class of environmental and food-borne contaminants. The analysis of samples from endemic and non-endemic areas revealed a positive correlation between the AA concentration in soil and food grains and the occurrence of BEN, indicating that AAs are etiological agents for BEN development. Furthermore, the results from this study may have revealed a causal role of soil pH and TOC content in the increased occurrence of BEN in certain farming villages. Because the potent nephrotoxicity and carcinogenicity of AAs are well documented, the results of our studies have important public health implications that merit the attention of regulatory authorities. It is imperative to research possible soil remediation methods for areas contaminated with AAs.
Supplementary Material
Acknowledgments
Financial support from the Research Grant Council of Hong Kong (GRF 16313916 and 16303117) is acknowledged. W. Chan expresses his sincere gratitude to Hong Kong University of Science and Technology for a Startup Funding (Grant R9310). We extend thanks to Prof. Jianzhen Yu and Dr. Stephen Griffith (Dept. of Chemistry, Hong Kong University of Science and Technology) for discussions and insights into data interpretation, to Dr. Jimmy W.M. Chan (Division of Environment & Sustainability, Hong Kong University of Science and Technology) for helping with use of ArcGIS. We also thank Ms. Pui-Yin Chung, Hiu-Hang Kwok, Mr. Dongwei Wang for their assistance with the sample preparation. Special thanks are extended to Mrs. Milan Stojanović, Živan Stamenković, Slaviša Jović, and Dr. Jordan Radojičić for their assistance with sample collection. Any use of trade, firm or product names are for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Supporting Information
Method validation by liquid chromatography-coupled tandem quadrupole mass spectrometry analyses of farmland soil sample from wheat field, wheat grain, and maize grain samples. Correlation of AA-I and AA-II concentrations in soil samples from wheat fields and maize fields in the endemic village Kutleš. Stability of AA-I and AA-II in the bread baking process and in the soil environment. Root uptake efficiency of AA-I and AA-II by wheat plants at the V1 growing stage in media at a pH of 6.0 and 7.6. Total organic carbon content of surface soil samples collected from cultivated wheat and maize fields in Serbian This material is available free of charge via the Internet at http://pubs.acs.org.
Caution
AAs are carcinogenic and should be handled carefully.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- (1).Hashimoto K; Higuchi M; Makino B; Sakakibara I; Kubo M; Komatsu Y; Maruno M; Okada M Quantitative analysis of aristolochic acids, toxic compounds, contained in some medicinal plants. J. Ethnopharmacol 1999, 64, 185–189. [DOI] [PubMed] [Google Scholar]
- (2).Nortier JL; Martinez MC; Schmeiser HH; Arlt VM; Bieler CA; Petein M; Depierreux MF; De Pauw L; Abramowicz D; Vereerstraeten P; et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N. Engl. J. Med 2000, 342, 1686–1692. [DOI] [PubMed] [Google Scholar]
- (3).Schmeiser HH; Kucab JE; Arlt VM; Phillips DH; Hollstein M; Gluhovschi G; Gluhovschi C; Modilca M; Daminescu L; Petrica L; et al. Evidence of exposure to aristolochic acid in patients with urothelial cancer from a Balkan endemic nephropathy region of Romania. Environ. Mol. Mutagen 2012, 53, 636–641. [DOI] [PubMed] [Google Scholar]
- (4).Vanherweghem JL Misuse of herbal remedies: the case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy). J. Altern. Complement. Med 1998, 4, 9–13. [DOI] [PubMed] [Google Scholar]
- (5).Kessler DA Cancer and herbs. N. Engl. J. Med 2000, 342, 1742–1743. [DOI] [PubMed] [Google Scholar]
- (6).Arlt VM; Alunni-Perret V; Quatrehomme G; Ohayon P; Albano L; Gaïd H; Michiels JF; Meyrier A; Cassuto E; Wiessler M; et al. Aristolochic acid (AA)-DNA adduct as marker of AA exposure and risk factor for AA nephropathy-associated cancer. Int. J. Cancer 2004, 111, 977–980. [DOI] [PubMed] [Google Scholar]
- (7).Debelle FD; Vanherweghem JL; Nortier JL Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008, 74, 158–169. [DOI] [PubMed] [Google Scholar]
- (8).Gold LS; Slone TH Aristolochic acid, an herbal carcinogen, sold on the web after FDA alert. N. Engl. J. Med 2003, 349, 1576–1577. [DOI] [PubMed] [Google Scholar]
- (9).IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Press: Lyon, France, 2002. [PMC free article] [PubMed] [Google Scholar]
- (10).Chan W; Hui KM; Poon WT; Lee KC; Cai Z Differentiation of herbs linked to “Chinese herb nephropathy” from the liquid chromatographic determination of aristolochic acids. Anal. Chim. Acta 2006, 576, 112–116. [DOI] [PubMed] [Google Scholar]
- (11).Li W; Hu Q; Chan W Uptake and accumulation of nephrotoxic and carcinogenic aristolochic acids in food crops grown in Aristolochia clematitis-contaminated soil and water. J. Agric. Food Chem 2016, 64, 107–112. [DOI] [PubMed] [Google Scholar]
- (12).Grollman AP; Shibutani S; Moriya M; Miller F; Wu L; Moll U; Suzuki N; Fernandes A; Rosenquist T; Medverec Z; Jakovina K; Brdar B; Slade N; Turesky RJ; Goodenough AK; Rieger R; Vukelić M; Jelaković B Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci 2007, 104, 12129–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Arlt VM; Stiborová M; vom brocke J; Simões ML; Lord GM; Nortier JI; Hollstein M; Phillips DH; Schmeiser HH Aristolochic acid mutagenesis: molecular clues to the aetiology of Balkan endemic nephropathy-associated urothelial cancer. Carcinogenesis 2007, 28, 2253–2261. [DOI] [PubMed] [Google Scholar]
- (14).Cukuranovic R; Petrovic B; Cukuranovic Z; Stefanovic V Balkan endemic nephropathy: a decreasing incidence of the disease. Pathol. Biol 2000, 48, 558–561. [PubMed] [Google Scholar]
- (15).Janković S; Bukvić D; Marinković J; Janković J; Marić I; Djukanović L Time trends in Balkan endemic nephropathy incidence in the most affected region in Serbia, 1977–2009: the disease has not yet disappeared. Nephrol. Dial. Transplant 2011, 26, 3171–3176. [DOI] [PubMed] [Google Scholar]
- (16).Dimitrov PS; Simeonov VA; Stein AD Balkan endemic nephropathy in Vratza, Bulgaria, 1964–1987: an epidemiologic analysis of population-based disease registers. Eur. J. Epidemiol 2001, 17, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cosyns JP; Jadoul M; Squifflet JP; De Plaen JF; Ferluga D; Van Ypersele De Strihou C Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int 1994, 45, 1680–1688. [DOI] [PubMed] [Google Scholar]
- (18).Bamias G; Boletis J Balkan nephropathy: evolution of our knowledge. Am. J. Kidney Dis 2008, 52, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ivić M Etiology of endemic nephropathy. Lijec. Vjesn 1969, 91, 1273–1281. [PubMed] [Google Scholar]
- (20).Hranjec T; Kovač A; Kos J; Mao W; Chen JJ; Grollman AP; Jelaković B Endemic nephropathy: the case for chronic poisoning by Aristolochia. Croat. Med. J 2005, 46, 116–125. [PubMed] [Google Scholar]
- (21).Tatu CA; Orem WH; Finkelman RB; Feder GL The Etiology of Balkan endemic nephropathy: still more questions than answers. Environ. Health Perspect 1998, 106, 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Chan W; Pavlović NM; Li W; Chan CK; Liu J; Deng K; Wang Y; Milosavljević B; Kostić EN Quantitation of aristolochic acids in corn, wheat grain, and soil samples collected in Serbia: identifying a novel exposure pathway in the etiology of Balkan endemic nephropathy. J. Agric. Food Chem 2016, 64, 5928–5934. [DOI] [PubMed] [Google Scholar]
- (23).Chan W; Lee KC; Liu N; Cai Z A sensitivity enhanced high-performance liquid chromatography fluorescence method for the detection of nephrotoxic and carcinogenic aristolochic acid in herbal medicines. J. Chromatogr. A 2007, 1164, 113–119. [DOI] [PubMed] [Google Scholar]
- (24).Song S; Ruan T; Wang T; Liu R; Jiang G Distribution and preliminary exposure assessment of bisphenol af (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol 2012, 46, 13136–13143. [DOI] [PubMed] [Google Scholar]
- (25).Liao C; Liu F; Kannan K Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ. Sci. Technol 2013, 47, 3918–3925. [DOI] [PubMed] [Google Scholar]
- (26).Samsøe-Petersen L; Larsen EH; Larsen PB; Bruun P Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils. Environ. Sci. Technol 2002, 36, 3057–3063. [DOI] [PubMed] [Google Scholar]
- (27).Yan X; Zhang F; Zeng C; Zhang M; Devkota LP; Yao T Relationship between heavy metal concentrations in soils and grasses of roadside farmland in Nepal. Int. J. Environ. Res. Public Health 2012, 9, 3209–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pavlović NM; Maksimović V; Maksimović JD; Orem WH; Tatu CA; Lerch HE; Bunnell JE; Kostić EN; Szilagyi DN; Paunescu V Possible health impacts of naturally occurring uptake of aristolochic acids by maize and cucumber roots: links to the etiology of endemic (Balkan) nephropathy. Environ. Geochem. Health 2013, 35, 215–226. [DOI] [PubMed] [Google Scholar]
- (29).Dudka S; Miller WP Accumulation of potentially toxic elements in plants and their transfer to human food chain. J. Environ. Sci. Heal. Part B 1999, 34, 681–708. [DOI] [PubMed] [Google Scholar]
- (30).Sands DPA; New TR Conservation of the Richmond Birdwing Butterfly in Australia; Springer: Dordrecht, Netherlands, 2013. [Google Scholar]
- (31).Nelson DW; Sommers LE Total Carbon, Organic Carbon, and Organic Matter; SSSA Book Series: Madison, U.S.A., 1996. [Google Scholar]
- (32).Maslac T; USDA. Annual Report on Wheat, Corn and Barley for Serbia; Global Agricultural Information Network Report: New York, U.S.A., 2016. [Google Scholar]
- (33).Komatina M Medical Geology, Volume 2: Effects of Geological Environments on Human Health; Elsevier: Amsterdam, Netherlands, 2004. [Google Scholar]
- (34).Grollman AP Aristolochic acid nephropathy: harbinger of a global iatrogenic disease. Environ. Mol. Mutagen 2013, 54, 1–7. [DOI] [PubMed] [Google Scholar]
- (35).Hoang ML; Chen CH; Chen PC; Roberts NJ; Dickman KG; Yun BH; Turesky RJ; Pu YS; Vogelstein B; Papadopoulos N; Grollman AP; Kinzler KW; Rosenquist TA Aristolochic acid in the etiology of renal cell carcinoma. Cancer Epidemiol. Biomarkers Prev 2016, 25, 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Schmeiser HH; Bieler CA; Wiessler M; van Ypersele de Strihou C; Cosyns JP Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res 1996, 56, 2025–2028. [PubMed] [Google Scholar]
- (37).Nortier J; Pozdzik A; Roumeguere T; Vanherweghem JL Aristolochic acid nephropathy (“Chinese herb nephropathy”). Nephrol. Ther 2015, 11, 574–588. [DOI] [PubMed] [Google Scholar]
- (38).Zhao YY; Wang HL; Cheng XL; Wei F; Bai X; Lin RC; Vaziri ND Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci. Rep 2015, 5, 12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Pozdzik AA; Salmon IJ; Debelle FD; Decaestecker C; Van Den Branden C; Verbeelen D; Deschodt-Lanckman MM; Vanherweghem JL; Nortier JL Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int 2008, 73, 595–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.