Abstract
Wnt signaling is a well‐known molecular pathway in age‐related pathogenesis and therapy of disease. While prior studies have mainly focused on Wnt ligands or Wnt activators, the in vivo functions of naturally secreted Wnt inhibitors are not clear, especially in brain aging. Using BubR1 H/H mice as a novel mouse model of accelerated aging, we report that genetic inhibition of sFRP3 restores the reduced body and brain size observed in BubR1 H/H mice. Furthermore, sFRP3 inhibition ameliorates hypomyelination in the corpus callosum and rescues neural progenitor proliferation in the hippocampal dentate gyrus of BubR1 H/H mice. Taken together, our study identifies sFRP3 as a new molecular factor that cooperates with BubR1 function to regulate brain development, myelination, and hippocampal neurogenesis.
Keywords: BubR1, myelination, neurogenesis, progeria, sFRP3
1. INTRODUCTION
Decline in the mitotic checkpoint kinase BubR1 level occurs with natural aging and induces progeroid features in mice and humans with mosaic variegated aneuploidy syndrome (Baker et al., 2004). Our previous studies show that BubR1 expression levels in WT mice significantly decline with natural aging in the brain. Mutant mice producing low levels of BubR1 (BubR1 H/H mice) exhibit smaller brain sizes and were rescued by constitutive overexpression of BubR1, suggesting that brain development is mediated through BubR1 (Supporting Information Figure S1). In addition, BubR1 H/H mice exhibit reduced hippocampal neurogenesis and impaired myelination (Choi et al., 2016; Yang et al., 2017). Together, these findings suggest that decreased BubR1 expression with aging contributes to brain dysfunction. Therefore, determining a molecular target that can counteract this process is of great interest.
We previously showed that a genetic deletion of secreted frizzled related protein 3 (sFRP3), an endogenous Wnt antagonist, stimulated adult hippocampal neurogenesis (Jang, Bonaguidi, et al., 2013) and promotes antidepressant action in mice and humans (Jang, Kitabatake, et al., 2013). While enhancing Wnt signaling can ameliorate age‐related deficits in cellular and cognitive function (Seib et al., 2013), we sought to determine whether inhibition of sFRP3 also has a neuroprotective role in BubR1‐regulated brain aging.
To address this in vivo, sfrp3 knockout (KO) mice were crossed to BubR1 H/H mice. In comparison with BubR1 H/H;sfrp3 WT mice, which displayed significant decreases in body and brain size, we found that both BubR1 H/H;sfrp3 KO mice and BubR1 H/H;sfrp3 HET mice exhibited restored body (Figure 1a–c) and brain size (Figure 1d,e). These results indicate an importance of sFRP3 in this process.
Figure 1.
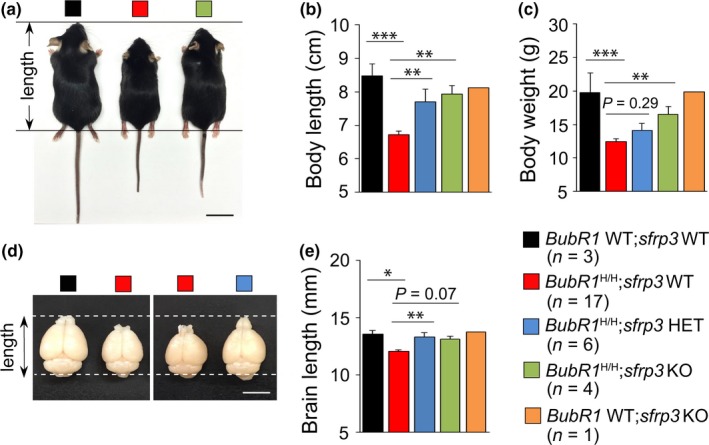
Genetic inhibition of sFRP3 restores body and brain size of BubR1 H/H mice. (a–c) Representative images and quantification of body size and body weight of each group. Scale bar: 1 cm. KO: knockout; HET: heterozygous. (d, e) Representative images and quantification of brain size of each group. Scale bar: 0.5 cm. All values represent mean ± SEM. (* p < 0.05, ** p < 0.01, *** p < 0.001, one‐way ANOVA)
How sFRP3 inhibition restores reduced brain size in BubR1 H/H mice is unknown. In our previous report, we showed that BubR1 H/H mice exhibited smaller brain sizes mainly attributed to hypomyelination, resulting in abnormal corpus callosum formation (Choi et al., 2016). Consequently, we tested whether sFRP3 inhibition could ameliorate impaired myelination in BubR1 H/H mice. Our examination of myelin density (Supporting Information Figure S2) and ultrastructural analysis (Figure 2a,b) revealed that BubR1 H/H;sfrp3 WT mice showed profound hypomyelination in the adult corpus callosum. However, BubR1 H/H;sfrp3 HET or BubR1 H/H;sfrp3 KO mice show significantly improved myelination compared to BubR1 H/H;sfrp3 WT mice, indicating that genetic inhibition of sFRP3 prevents hypomyelination, potentially explaining how sFRP3 inhibition normalizes reduced brain size in BubR1 H/H mice.
Figure 2.
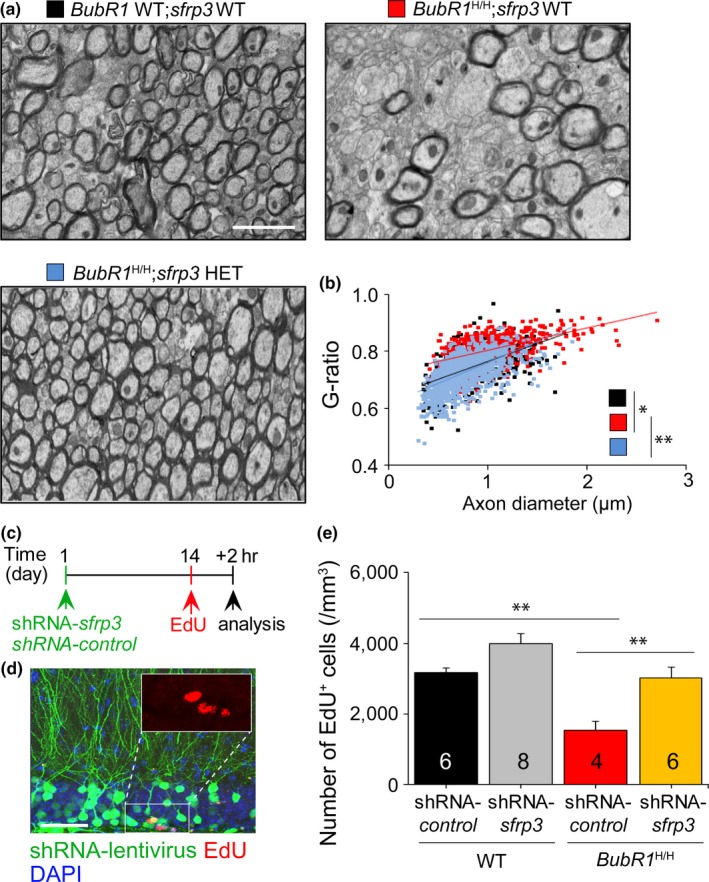
Genetic inhibition of sFRP3 ameliorates cellular abnormalities observed in BubR1 H/H mice. (a) Electron microscopy analysis of the corpus callosum of each genotype. Scale bar: 2 µm. (b) Scatter diagram and quantification of G‐ratio. (c) Experimental design. (d) Representative confocal images demonstrating infected lentiviral tdTomato+ (green), EdU+ (red; inset), and DAPI+ (blue) cells. Scale bar: 20 μm. (e) Quantification of EdU+ cells in each group. All values represent mean ± SEM. (* p < 0.05, ** p < 0.01, *** p < 0.001, two‐way ANOVA). Number associated with bar graphs indicates number of mice tested
Aging is known to result in significant reductions in hippocampal neurogenesis and cognitive dysfunction. We previously reported that BubR1 H/H mice exhibited impaired hippocampal neurogenesis (Yang et al., 2017). Therefore, we examined whether inhibition of sFRP3 could mitigate reduced neurogenesis in BubR1 H/H mice. To test sFRP3’s specific role in the dentate gyrus, we used lentiviruses to acutely knockdown the expression of endogenous sFRP3 with short‐hairpin RNA (shRNA) in a non‐cell autonomous manner (Figure 2c,d). Stereological analysis showed a significant decrease in EdU+ cell density in the subgranular zone (SGZ) of shRNA‐control injected BubR1 H/H mice compared to shRNA‐control injected WT mice. However, shRNA‐sfrp3 injected BubR1 H/H mice showed significantly increased EdU+ cell density compared to shRNA‐control injected BubR1 H/H mice (Figure 2e), suggesting that sFRP3 knockdown ameliorates impaired neural progenitor proliferation in BubR1 H/H mice.
In this study, we were initially surprised by the ability of sFRP3 inhibition to reverse the microcephaly phenotype. Wnt signaling serves a prominent role in the regulation of early brain development, neurogenesis, cell migration, dendrite morphogenesis, and synapse formation, as well as cognitive function. Accordingly, Wnt signaling has been a therapeutic target for a diverse range of neurodevelopmental, neurological and neurodegenerative disorders (Hussaini et al., 2014). While prior studies have mainly focused on Wnt ligands or Wnt activators in disease pathogenesis and therapy, recent discoveries focusing on the naturally secreted Wnt inhibitors have just started to shed light on it. For example, the Wnt antagonists such as Dickkopf‐1 (Dkk1) or Dkk3 are known to increase with age. Conditional deletion of Dkk1 enhances neurogenic function to counteract cognitive deficits (Seib et al., 2013; Zhu et al., 2014). Interestingly, different from these Wnt antagonists, hippocampal sFRP3 mRNA levels were unchanged across the lifespan (Supporting Information Figure S3a), suggesting that sFRP3‐mediated Wnt inhibition is not a contributing factor in age‐related neuropathology. Rather, sFRP3 reduction stimulates adult hippocampal neurogenesis (Jang, Bonaguidi, et al., 2013) and neuroprotective promotion of myelination, as we demonstrate that sFRP3 deletion significantly upregulates multiple genes critical for myelin production including MBP (Supporting Information Figure S3b). Thus, we propose that mechanistically, inhibition of sFRP3 rescues BubR1 H/H‐mediated deficits in neural progenitor proliferation, myelination, and brain growth.
Abnormalities in myelination and neurogenesis are hallmarks of age‐related neurodegeneration. Therefore, the ability to sustain myelin integrity and neurogenesis holds significant implications for aging, age‐related disorders, and future therapeutic strategies. In this regard, identification of sFRP3 function provides a novel perspective on the development of effective therapies preventing cognitive decline associated with neurodevelopmental and age‐related disorders.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
C.H.C., K.H.Y., and M.H.J. designed research; C.H.C., K.H.Y., S.P., S.M.Q.H., and A.O. performed research; J.M.v.D. provided BubR1H/H and BubR1T23 overexpression mice; C.H.C., K.H.Y., S.M.Q.H., A.O., and M.H.J. wrote the paper.
Supporting information
ACKNOWLEDGMENTS
This work was supported by NIA (R01AG058560), Regenerative Medicine Minnesota, Mayo Clinic Center for Regenerative Medicine to M.H.J., and K.H.Y.
Cho CH, Yoo KH, Oliveros A, et al. sFRP3 inhibition improves age‐related cellular changes in BubR1 progeroid mice. Aging Cell. 2019;18:e12899 10.1111/acel.12899
REFERENCES
- Baker, D. J. , Jeganathan, K. B. , Cameron, J. D. , Thompson, M. , Juneja, S. , Kopecka, A. , … van Deursen, J. M. (2004). BubR1 insufficiency causes early onset of aging‐associated phenotypes and infertility in mice. Nature Genetics, 36, 744–749. 10.1038/ng1382 [DOI] [PubMed] [Google Scholar]
- Choi, C. I. , Yoo, K. H. , Hussaini, S. M. , Jeon, B. T. , Welby, J. , Gan, H. , … Jang, M. H. (2016). The progeroid gene BubR1 regulates axon myelination and motor function. Aging (Albany NY), 8, 2667–2688. 10.18632/aging.101032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini, S. M. , Choi, C. I. , Cho, C. H. , Kim, H. J. , Jun, H. , & Jang, M. H. (2014). Wnt signaling in neuropsychiatric disorders: Ties with adult hippocampal neurogenesis and behavior. Neuroscience and Biobehavioral Reviews, 47C, 369–383. 10.1016/j.neubiorev.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, M. H. , Bonaguidi, M. A. , Kitabatake, Y. , Sun, J. , Song, J. , Kang, E. , … Song, H. (2013). Secreted frizzled‐related protein 3 regulates activity‐dependent adult hippocampal neurogenesis. Cell Stem Cell, 12, 215–223. 10.1016/j.stem.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, M. H. , Kitabatake, Y. , Kang, E. , Jun, H. , Pletnikov, M. V. , Christian, K. M. , … Ming, G. I. (2013). Secreted frizzled‐related protein 3 (sFRP3) regulates antidepressant responses in mice and humans. Molecular Psychiatry, 18, 957–958. 10.1038/mp.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib, D. R. , Corsini, N. S. , Ellwanger, K. , Plaas, C. , Mateos, A. , Pitzer, C. , … Martin‐Villalba, A. (2013). Loss of Dickkopf‐1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell, 12, 204–214. 10.1016/j.stem.2012.11.010 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Jun, H. , Choi, C. I. , Yoo, K. H. , Cho, C. H. , Hussaini, S. M. Q. , … Jang, M. H. (2017). Age‐related decline in BubR1 impairs adult hippocampal neurogenesis. Aging Cell, 16, 598–601. 10.1111/acel.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y. , Demidov, O. N. , Goh, A. M. , Virshup, D. M. , Lane, D. P. , & Bulavin, D. V. (2014). Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. Journal of Clinical Investigation, 124, 3263–3273. 10.1172/JCI73015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


