Abstract
It has been reported that 2.5%–30% of human peripheral CD27– B cells are autoreactive and anergic based on unresponsiveness to antigen receptor (BCR) stimulation and autoreactivity of cloned and expressed BCR. The molecular mechanisms that maintain this unresponsiveness are unknown. Here, we showed that in humans anergy is maintained by elevated expression of PTEN, a phosphatidylinositol 3,4,5P-3-phosphatase. Upregulation of PTEN was associated with reduced expression of microRNAs that control its expression. Pharmacologic inhibition of PTEN lead to significant restoration of responsiveness. Consistent with a role in conferring risk of autoimmunity, B cells from type 1 diabetics and autoimmune thyroid disease patients expressed reduced PTEN. Unexpectedly, in healthy individuals PTEN expression was elevated in on average 40% of CD27– B cells, with levels gradually decreasing as IgM levels increase. Our findings suggest that a much higher proportion of the peripheral repertoire is autoreactive than previously thought and that B cells upregulate PTEN in a manner that is proportional to the recognition of autoantigens of increasing avidity, thus tuning BCR signaling to prevent development of autoimmunity while providing a reservoir of cells that can be readily activated to respond when needed.
Keywords: Autoimmunity, Immunology
Keywords: Anergy, Autoimmune diseases, B cells
Increased expression of PTEN helps maintain the hyporesponsivness of the more than 40% of human peripheral blood B cells that are significantly autoreactive.
Introduction
Previous studies have shown that in mice and humans as much as 75% of newly produced unvetted B cells in the bone marrow express B cell receptor (BCR) that is autoreactive (1, 2). While self-reactive cells can exist in an ignorant state when autoantigen concentration and/or avidity of B cell–antigen interactions is low, high avidity interactions activate three known tolerance mechanisms: receptor editing, clonal deletion, or anergy. Particularly hazardous are anergic B cells, as they reach the periphery where they are readily exposed to locally produced inflammatory mediators that could provoke their participation in immune responses, leading ultimately to autoimmunity.
Anergy is characterized by unresponsiveness to antigen stimulation despite retention of the ability to bind antigen. Studies of anergy in mice have shown that 2%–5% of peripheral B cells in the wild-type repertoire are anergic (3). Anergic B cells are identified based on their appearance as a naive subset that lacks expression of surface IgM (mIgM). Duty and colleagues first identified anergic B cells in humans, defining them as CD19+CD27–mIgM–mIgD+. They termed these BND cells (naive IgD+ cells). These cells are hyporesponsive to BCR stimulation, and cloning, expression, and analysis of their BCRs revealed that most, if not all, are autoreactive. BND cells represent approximately 2.5% of peripheral naive B cells (4). Some studies, including our own previous report, have suggested a similar BND cell frequency (5–7). However, others have shown that anergic cells can also be found in the mIgMlo, naive-like population, and suggested that up to 30% of peripheral naive B cells are autoreactive and may be anergic (8, 9). Discrepancies in observed frequencies are due in part to lack of a discrete population of mIgM– but mIgD+ B cells. Rather, mIgM expression varies in a continuum over multiple log decades, possibly reflecting a continuum of autoantigen reactivity in the stochastically determined repertoire. Finally, in studies that did not resolve relative frequency of ignorant and anergic cells, Wardemann et al. determined that at least 20% of peripheral naive cells are autoreactive (1). More studies are needed to resolve the functional relationships of these populations.
The molecular basis of anergic B cell unresponsiveness to BCR stimulation is not well understood. One group has suggested a role for genetic reprograming in maintaining anergy (10). However, anergy is rapidly reversed by removal of antigen from receptors, suggesting maintenance by nondurable biochemical mechanisms rather than genetic reprograming (11, 12). Studies of mouse B cells have implicated the tyrosine phosphatase SHP-1 and the inositol lipid phosphatases SHIP-1 and phosphatase and tensin homolog (PTEN) as mediators of this unresponsiveness (13–17). PTEN is a dual protein and lipid phosphatase, functioning as negative regulator of the PI3-kinase pathway. It was first identified as a tumor suppressor gene that is inactivated in many cancers (18–20). In mice, B cell–targeted deletion of PTEN results in sustained elevation in PIP3, failed induction of tolerance, and autoantibody production (13, 15, 16, 21). PTEN has been shown to be increased in anergic MD4/ML5 B cells and is partially responsible for the hyporesponsiveness of these B cells (15, 22). However, PTEN elevation is not apparent in Ars/A1 anti-DNA anergic B cells whose antigen receptors have lower affinity for autoantigen (14). Thus, relative PTEN upregulation may be determined by avidity of B cell–autoantigen interaction.
To begin to explore the role of PTEN in human B cell anergy, we analyzed the expression of PTEN in peripheral blood B cells. Results demonstrate that PTEN is elevated in the previously defined BND cell population. Unexpectedly, its expression is also increased in many B cells previously thought to be nonautoreactive along a continuum that is inversely correlated with mIgM expression. Further analysis revealed that, along this continuum, cell responsiveness to BCR stimulation is inversely correlated with PTEN expression. When PTEN is inhibited pharmacologically, the responsiveness of anergic B cells is largely restored, demonstrating a requirement for the phosphatase to maintain unresponsiveness. Moreover, in recent-onset autoimmune patients, PTEN expression in B cells was found to be decreased irrespective of mIgM expression level. Thus, we identify PTEN as a mediator of anergic B cell hyporesponsiveness in humans and present evidence that dysregulation of PTEN expression in these cells may contribute to development of autoimmunity. Finally, our findings provide the basis for a conceptual change in our thinking about B cell anergy. They suggest that a much larger than expected proportion of the repertoire has substantial reactivity to self and that responsiveness of these B cells is continuously dampened by increasing PTEN expression in proportion to autoreactivity, thus preventing activation by self-antigens. This mechanism preserves them to serve some as-yet undefined biological function, possibly redemption for participation in protective responses to pathogens (10, 23).
Results
BND cells are hyporesponsive to BCR stimulation and express elevated levels of PTEN.
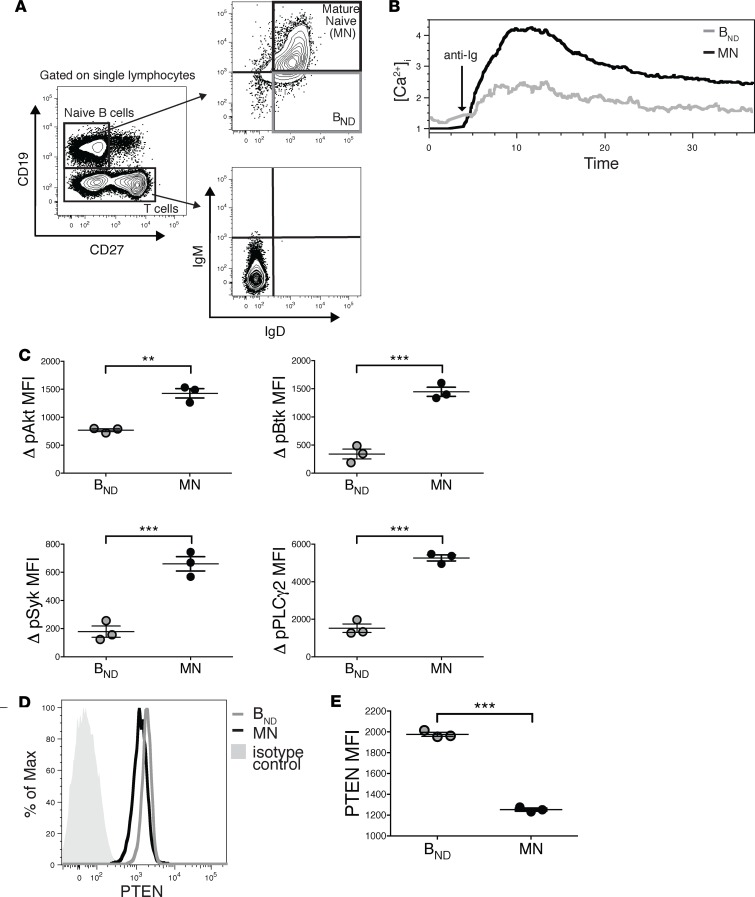
Previous studies have shown that up to 10% of naive-phenotype B cells in peripheral blood of healthy individuals are autoreactive and anergic (4, 9). To confirm and extend findings that BND cells (CD19+CD27–IgD+IgMlo/–) are anergic, we utilized the gating strategy previously described by these authors (Figure 1A) to identify BND cells and their mature naive (MN) counterparts and assessed their response to BCR stimulation based Ca2+ mobilization and phosphorylation of B cell signaling intermediaries (Figure 1, B and C). BND cells exhibited elevated basal [Ca+2]i and greatly reduced BCR-mediated Ca2+ mobilization (Figure 1B). To our knowledge, it has not been previously shown that BND cells mount significantly reduced phosphorylation of Akt, Btk, PLCγ2, and Syk after BCR stimulation, relative to MN B cells (Figure 1C).
Figure 1. Anergic BND cells (representing 2.5% of B cells) are hyporesponsive and have elevated expression of PTEN.
(A) Gating strategy for BND cells and mature naive (MN) B cells in healthy subjects. (B) BND cells have an elevated basal calcium and decreased calcium flux following stimulation with 10 μg/ml F(ab′)2 rabbit anti-human IgG H&L. (C) BND cells have decreased pSyk, pPLCy2, pAkt, and pBtk compared with MN cells following stimulation used as in B. (D) Representative histogram showing PTEN expression is increased in BND cells compared with MN cells and isotype control. (E) PTEN expression is increased in BND cells compared with MN cells. Phosflow samples were run and analyzed in triplicates. Data depict results from triplicate samples from the same individual; data are representative of n = 12 independent experiments. Statistics were determined using Mann-Whitney unpaired t test; **P < 0.01, ***P < 0.001.
The basis of human anergic B cell hyporesponsiveness is unclear. Previous studies in transgenic mice have shown that, in one mouse model, the MD4xML5 HEL-anti-HEL model, anergic B cells express elevated levels of the phosphatase PTEN, a negative regulator of BCR signaling, while, in a second model, the Ars/A1 anti-DNA model, PTEN is not elevated (13–15). To reconcile and extend these findings to naturally occurring anergic human B cells we analyzed expression of PTEN in BND cells (Figure 1, D and E). Similar to the MD4xML5 model, BND cells showed significantly increased expression of PTEN compared with MN cells.
Upregulation of PTEN in anergic cells is associated with reduced expression of microRNAs known to control its expression.
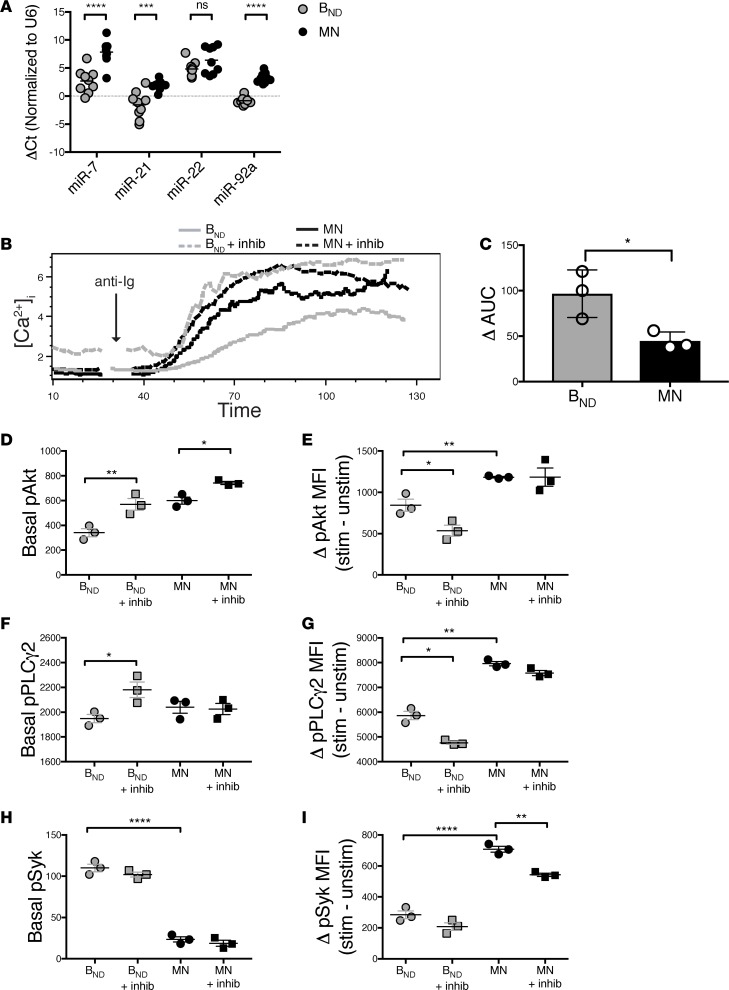
Previous studies in a variety of cellular contexts have shown that PTEN expression can be controlled by microRNAs, including miR7, miR21, miR22, and miR92a (24–29). To begin to explore the role of these microRNAs in determining PTEN levels in autoreactive cells, we analyzed the relative expression in BND cells versus that of the 20% of MN B cells expressing the highest levels of mIgM. Results demonstrate that miR-7, miR-21, and miR-92a expression was significantly decreased in BND cells compared with that in MN B cells (Figure 2A). There was no significant difference in expression of miR-22 (Figure 2A) or in our control miR-15, which is not known to regulate PTEN expression (data not shown). These data provide insights regarding the mechanism operative in BCR-mediated control of PTEN expression.
Figure 2. PTEN limits signaling in anergic B cells.
(A) miRNA-7, -21, and -92a (but not miRNA-22) are decreased in BND cells compared with mature naive (MN) B cells; the miRNA comparison was compiled from n = 5 healthy individuals. Statistics were determined using unpaired Mann-Whitney t test. (B) Inhibition of PTEN with 325 μM SF1670 restores Ca2+ mobilization in BND cells to similar levels to MN B cells. Representative plot shown for n = 5 healthy individuals. (C) The change in the AUC in BND and MN B cells following addition of SF1670, ran in triplicates. Data are representative of n = 5 healthy individuals. (D and F) Inhibition of PTEN with SF1670 causes an elevation in basal pPLCy2 and pAkt in BND cells, (E and G) without an overall increase in change in pPLCy2 or pAkt following stimulation. (H and I) Basal and changes in pSyk following stimulation were unchanged following addition of the PTEN inhibitor in BND cells. Phosflow samples were run and analyzed in triplicates. Data depict results from triplicate samples from the same individual; data is representative of n = 9 independent experiments. Statistics were determined using 1-way ANOVA followed by a Bonferroni’s multiple comparison post-test test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Pharmacologic inhibition of PTEN restores calcium responses to stimulation in anergic B cells.
If PTEN expression determines the responsiveness of anergic B cells, inhibition of PTEN should restore the response of BND cells to stimulation. To test this possibility, we incubated peripheral blood mononuclear cells (PBMCs) from healthy individuals with the selective and reversible PTEN inhibitor SF1670 for 30 minutes before stimulation with anti-BCR and analysis of intracellular calcium mobilization. SF1670 has previously been shown to bind the active site of PTEN and increase cellular PIP3 levels in many cell types, including neutrophils, adipocytes, and cancer cells (30–32). PTEN inhibition normalized the responses of BND cells and MN cells (Figure 2B). Interestingly, following treatment with the inhibitor, BND cells displayed elevated basal calcium, suggesting that PTEN inhibition had allowed their response to the presence of ambient autoantigen in the system. When the calcium mobilization response was calculated based on the total AUC after addition of the PTEN inhibitor, we found that it was significantly greater for BND cells compared with that for MN B cells, consistent with the notion that increased PTEN expression controls the responsiveness of anergic B cells (Figure 2C). Equivalent effects were seen when we used a different PTEN inhibitor, bpV(HOpic) (data not shown). These inhibitors did not affect the BCR-mediated calcium mobilization induced in B cells in which PTEN was inducibly deleted, demonstrating that their effects in this context are PTEN specific (data not shown) (13). Taken together, these results demonstrate that elevated PTEN expression is at least partially responsible for hyporesponsiveness of anergic human B cells to BCR stimulation.
Inhibition of PTEN increases basal phosphorylation of Akt and PLCy2 in anergic B cells.
Based on the above studies, we posited that inhibition of PTEN would restore the responsiveness to stimulation in BND cells, as indicated by enhanced phosphorylation of Akt and PLCy2, whose translocation to the plasma membrane and phosphorylation following BCR stimulation are PIP3 dependent. Syk phosphorylation is thought to be independent of the PI3-kinase pathway, yet it is depressed in anergic cells, possibly reflecting the activity of a second anergy enforcing pathway, such as reduced BCR expression. As shown in Figure 2, D–G, while elevated basal pAkt and pPLCy2 were found in BND cells treated with PTEN inhibitor, the overall change in phosphorylation was decreased (Figure 2, E and G). The decreased delta in pAkt and pPLCy2 following stimulation was due to the elevated basal phosphorylation, with little change in the levels after stimulation (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.123384DS1). These results indicate that the amount of Akt and PLCy2 phosphorylation that can be induced in anergic BND cells may have a ceiling, which may be dictated by reduced expression of mIgM or by other negative regulators of the PI3-kinase pathway, such as SHIP-1. Future studies are needed to explore this possibility. Nevertheless, the increase in basal pPLCy2 and pAkt indicates that inhibition of PTEN likely allows the chronic stimulation by self-antigen to activate the cells to some degree. As expected, inhibition of PTEN did not affect levels of pSyk in BND cells, since phosphorylation of this kinase is independent of the PI3-kinase pathway (Figure 2, H and I). Hence, the elevation in basal pSyk and subsequent decrease in change in pSyk after stimulation in BND cells compared with that in MN B cells indicates that signaling upstream of the PI3-kinase pathway is also impaired in anergic B cells but is independent of PTEN expression.
Elevated B cell PTEN expression indicates greater than expected repertoire autoreactivity.
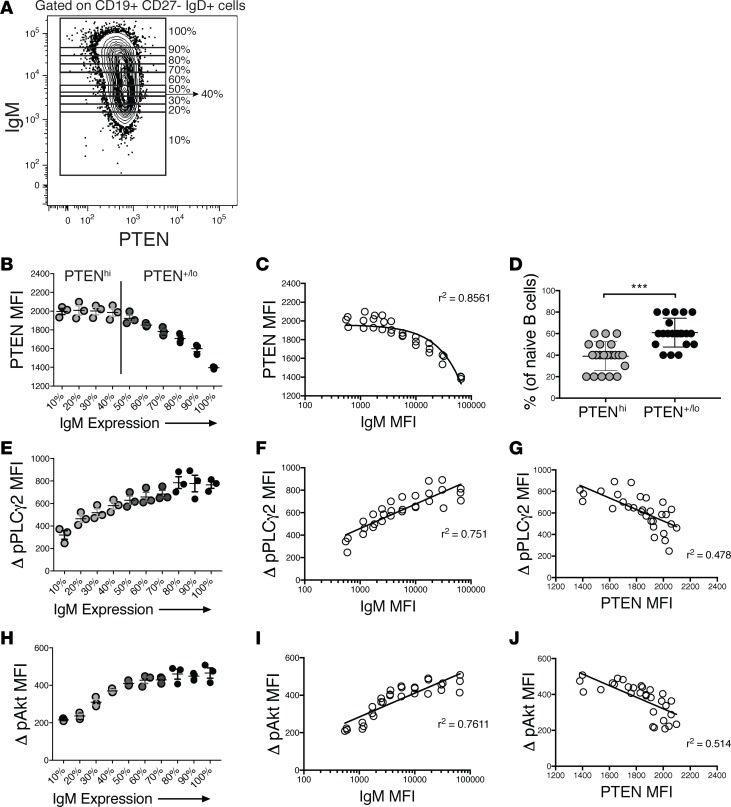
While mIgD expression by naive (CD27–, mIgD+, mIgM+/–) B cells exists in a narrow Gaussian distribution, mIgM expression is much more broadly distributed, spanning 2–3 decades. The breadth of this distribution may be explained by observations in mouse immunoglobulin-transgenic models that anergic B cells downregulate surface mIgM but not mIgD in response to chronic autoantigen exposure (33, 34). If reduced mIgM expression is a high-fidelity indicator of degree of autoreactivity and encounter with autoantigen, the proportion of the repertoire that is autoreactive must greatly exceed the proportion (2.5%–10%) that is reported to be anergic. Indeed, absence of an abrupt change in mIgM expression may indicate the existence of a continuum of autoreactivity. If varied mIgM expression is indicative of a continuum of autoreactivity, it might be expected that PTEN would be upregulated in inverse correlation with mIgM. To begin to explore this notion, we analyzed PTEN expression relative to surface IgM levels by gating on IgM expression in 10% increments and determining the respective PTEN MFI (Figure 3A). If the lowest 10% of IgM-expressing cells are composed of anergic B cells in humans, as suggested by Quach et al. (9), then the prediction would be that PTEN levels are elevated in only the lowest IgM-expressing B cells and then would sharply decrease and be constant in the remaining naive B cells. However, as shown in Figure 3B, PTEN levels were elevated in a larger proportion of CD27– B cells than those previously classified as BND cells and gradually decreased in inverse proportion to IgM expression (Figure 3C). Comparative analysis of 24 healthy individuals indicated that PTEN levels were significantly increased, termed PTENhi, in >40% of mIgD+CD27– peripheral blood B cells and were greatest in those expressing the lowest levels of mIgM (Figure 3, B and D).
Figure 3. PTEN expression is correlated with hyporesponsiveness of anergic B cells.
(A) Gating strategy to determine the geometric mean fluorescence intensity of PTEN in 10% increments of increasing mIgM expression from naive (CD27–) IgD+ B cells in healthy individuals. (B) Representative pattern of PTEN expression versus mIgM expression of CD19+CD27–IgD+ cells from 1 healthy control. Cutoff for determination of PTENhi (gray circles) versus PTEN+/lo (dark gray to black circles) is shown and was determined as the point at which PTEN levels begin to decrease steadily as IgM expression increased. Results depict triplicate samples from the same individual; data are representative of (n = 24) individual experiments. (C) PTEN levels are inversely correlated with IgM expression. A nonlinear regression line is shown. (D) Percentage of PTENhi B cells (gray circles) versus PTEN+/lo B cells (black circles), as determined in B, from 24 healthy controls; the average percentage of PTENhi B cells is approximately 40%. ***P < 0.001 by unpaired Mann-Whitney test. (E) Representative change in pPLCy2 following stimulation (average MFI of unstimulated cells subtracted from individual stimulated MFI) with 10 μg/ml F(ab′)2 rabbit anti-human IgG H&L along the continuum of mIgM expression. (F) Change in pPLCy2 is correlated with IgM expression. A nonlinear regression line is shown. (G) PTEN levels are inversely correlated with change in pPLCy2. A linear regression line is shown. (H) Representative change in pAkt following stimulation, as in F, along the continuum of mIgM expression. (I) Change in pAkt is correlated with IgM expression. A nonlinear regression line is shown. (J) PTEN levels are inversely correlated with change in pAkt. A linear regression line is shown. For E–J, results are representative of at least 5 healthy controls.
PTEN expression is inversely correlated with responsiveness to BCR stimulation across the continuum of mIgM expression.
If PTEN expression levels limit the responsiveness of human B cells as shown above, B cell responsiveness to BCR stimulation should be inversely correlated with PTEN levels throughout the continuum of its expression, rather than being unique to BND cells. Consistent with this possibility, stimulation with cross-linking anti-BCR induced phosphorylation of PLCy2 and Akt, two signaling intermediaries whose activation is dependent on the PI3-kinase pathway, in proportion to mIgM expression (Figure 3, E, F, H, and I) and, thus, in inverse proportion to PTEN expression (Figure 3, G and J), suggesting that PTEN levels determine the hyporesponsiveness of B cells.
PTEN expression and susceptibility to autoimmunity.
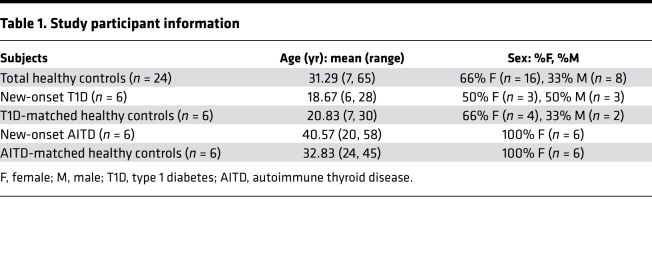
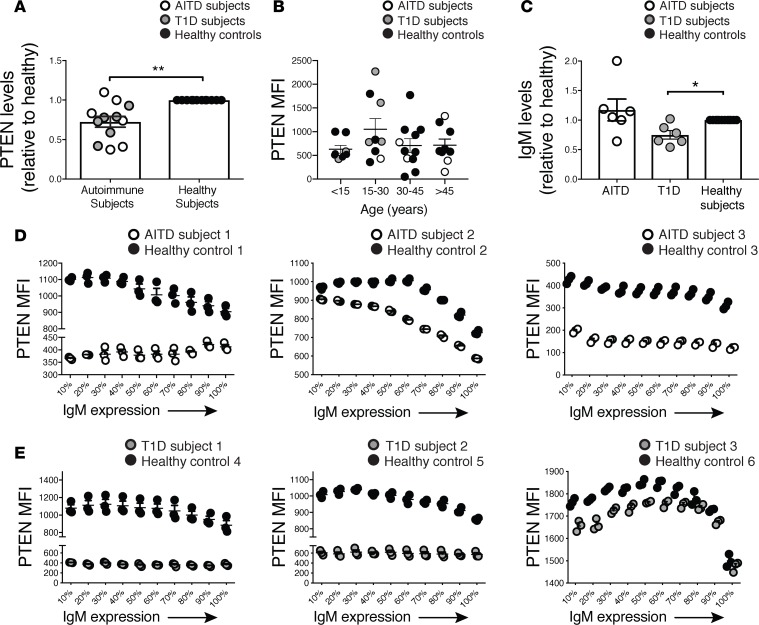
Recent studies have demonstrated that PTEN expression is reduced in B cells from systemic lupus erythematosus (SLE) patients relative to that in healthy controls (24). To extend these observations, we compared the expression of PTEN in total peripheral B cells from patients with autoimmune thyroid disease (AITD) and type 1 diabetic (T1D) to healthy controls (Table 1). Naive B cells (CD19+CD27–) from autoimmune subjects exhibited significantly lower PTEN expression compared with age- and sex-matched healthy controls run concurrently on the same day (Figure 4A). These results extend previous findings in SLE patients to include these additional autoimmune diseases, suggesting decreased PTEN expression is a common phenomenon in autoimmunity and may predispose to development of disease. In order to rule out the possibility that the variations in PTEN expression observed could simply be due to differences in age, we analyzed the level of PTEN expression in all of our autoimmune subjects (n = 12) as well as all of our healthy controls (n = 24). As shown in Figure 4B, the age of the subject did not affect the expression level of PTEN.
Table 1. Study participant information.
Figure 4. PTEN expression is decreased in autoimmune patients.
(A) PTEN is decreased in CD27– B cells from new-onset autoimmune thyroid disease (AITD) (n = 6) and type 1 diabetic (T1D) patients (n = 6) compared with healthy controls (n = 12). (B) PTEN expression is not dependent on the age of the individual. (C) IgM levels were decreased in CD27– B cells from T1D subjects compared with healthy controls, despite also having less PTEN expression. (D and E) Representative expression of PTEN along the continuum of mIgM expression in 3 of 6 AITD and 3 of 6 T1D autoimmune and healthy control samples, run concurrently on the same day. Results are representative of the 12 autoimmune patients. Statistics were determined by unpaired Mann-Whitney t tests; *P < 0.05, **P < 0.01.
Since autoimmune subjects tend to have decreased levels of PTEN in B cells in general compared with controls, we thought they may have increased levels of mIgM, since we have shown that they are inversely correlated in healthy individuals (Figure 3C). Interestingly, when we compared the relative expression levels of mIgM in our autoimmune subjects to those in healthy controls, there was no significant difference in AITD subjects (Figure 4C). However, naive B cells from T1D subjects had significantly decreased levels of mIgM compared with healthy controls, despite having lower levels of PTEN (Figure 4C). These findings indicate that there is no mechanistic linkage between these parameters.
To explore this further we examined these parameters in TID patients, exploring whether they exhibit variations in PTEN expression inversely correlated with IgM levels or whether they exhibit an overall decrease in both PTEN and mIgM expression compared with controls. When we analyzed the pattern of PTEN expression along the continuum of IgM expression in autoimmune subjects, we found that there was an inverse correlation of PTEN and mIgM expression in some patients, as seen in healthy controls, despite their B cells having an overall decrease in PTEN expression (Figure 4, D and E). In other autoimmune patients, PTEN expression was not inversely correlated with mIgM expression and exhibited a steady low level of PTEN expression, even as IgM increased (Figure 4, D and E). Hence, while the patterns of PTEN and mIgM expression varied to some degree among patients, autoimmune patients had an overall decrease in PTEN expression relative to healthy controls, suggesting that dysregulation of PTEN expression in B cells in these individuals may contribute to their development of autoimmunity. When we compared the responsiveness to stimulation of B cells from autoimmune patients to healthy controls, we found that B cells from some patients responded more strongly than those of healthy controls, consistent with lower expression of PTEN (data not shown).
Discussion
This study is the first to our knowledge to demonstrate that PTEN is elevated in and limits the BCR responsiveness of healthy human anergic B cells. As such, levels of PTEN expression may be an effective surrogate indicator of autoreactivity. Based on PTEN expression, >40% of CD27– peripheral blood B cells in healthy individuals possess significant autoreactivity. These findings suggest that a much higher proportion of the peripheral normal B cell repertoire is autoreactive than previously thought. The disparity in our reported frequency of autoreactive B cells in peripheral blood of healthy individuals, based on levels of PTEN (>40%), and the previously reported frequencies (5%–20%), based on cloned immunoglobulins from single sorted B cells (1, 4), likely reflects an inherent limitation of the method previously used to establish autoreactivity. These conclusions have been based on the reactivity of recombinant bivalent IgG antibodies. B cells expressing >105 antigen BCRs undergo a much higher avidity interaction with antigen than these bivalent antibodies, thus the latter measure underestimates autoreactivity.
PTEN expression was found to be inversely correlated with levels of mIgM in healthy subjects. These results suggest that mIgM expression is also actively and inversely tuned based on the level of self reactivity in humans, a concept elegantly demonstrated by Zikherman et al. using a NUR77 reporter mouse in which GFP levels correlated with levels of autoreactivity in mature B cells (35). If PTEN levels are inversely correlated with mIgM expression, it raises the “chicken or egg” question regarding causality. We hypothesize that, in the absence of T cell help, the decreased mIgM expression that follows chronic exposure of naive B cells to cognate antigen leads to increased expression of PTEN.
Hence, we posit that B cells upregulate PTEN in proportion to recognition of autoantigens of increasing avidity, thus tuning BCR signaling to prevent development of autoimmunity. This mechanism would preserve autoreactive B cells in a quiescent state, while providing a reservoir of cells that can be called upon in times of need to mount a response to more avid pathogenic immunogens, a process herein referred to as redemption. Moreover, consistent with a role in conferring risk of autoimmunity, B cells from T1D and autoimmune thyroid disease patients were found to express reduced PTEN, despite having lower levels of IgM in the case of T1D subjects. Thus, dysregulation of controlling miRNAs may be a common risk factor of autoimmunity, a risk factor that might not be recognized by GWAS. Clearly more studies are required to define this relationship. In conclusion, findings from this study demonstrate that PTEN limits BCR responsiveness of anergic human B cells and may be an effective surrogate indicator of autoreactivity. These findings are paradigm shifting in terms of our perceptions of repertoire autoreactivity.
Methods
Subject selection and peripheral blood processing.
Eligible subjects were male or female, had been diagnosed with AITD within the last 6 months or T1D within the last 2 weeks. Only subjects that had not begun treatment or had only had minimal treatment with insulin, thyroid replacement, or antithyroid drugs were enrolled. The presence of antibodies against Tg, TPO, and TSH-R as well as TSH, free T4, and total T3 tests were used to confirm a diagnosis of GD or HT. Eligible T1D subjects were males or females who met the American Diabetes Association criteria for classification of disease. Autoantibodies against insulin, GAD, IA-2, and ZnT8 were assayed using radioimmunoassays, as previously described (36). PBMCs from 6 new-onset AITD, 6 new-onset T1D, and 24 healthy age/sex matched controls were isolated from heparinized blood by Ficoll-Hypaque fractionation (Table 1).
Intracellular PTEN, pSyk, pPLCy2, pAkt, and pBtk flow cytometric analysis.
Approximately 2 million PBMCs (107 cells/ml) in triplicates were used for both PTEN and phosphflow staining. For PTEN staining, cells were resuspended with 500 μl BD Cytofix/Perm and incubated on ice for 30 minutes. Cells were washed twice with BD Perm/Wash buffer and then stained with anti-CD19-BV510 (BioLegend, HIB19), anti-CD27-BUV395 (BD; L128), anti-IgM-PE-Cy7 (BioLegend; MHM-88), anti-IgD-BV421 (BD; HIB19), and anti-PTEN-Alexa 647 (BD; A2B1) or isotype control (BD; MOPC-21) for 1 hour on ice. Cells were washed twice with BD Perm/Wash and then fixed with 2% paraformaldehyde prior to flow cytometry.
For intracellular phosphorylated proteins, cells were incubated at 37°C for 1 hour in serum-free RPMI medium in order to decrease basal phosphorylation. For assays in which PTEN inhibitors were used, either 325 μM SF1670 (R&D Systems) or 1440 μM bpV(HOpic) (MilliporeSigma) were added during the incubation period. Cells were then resuspended in RPMI + 5% FBS and stimulated with 10 μg/ml F(ab′)2 rabbit anti-human IgG (H&L) (Jackson Labs) for 3 minutes, followed by a quick spin (3300 g for 0.5 minute) and immediately resuspended in 500 μl Cytofix/Cytoperm (BD) for 30 minutes on ice. Cells were washed twice with BD Perm/Wash and then stained with anti-CD19-BV510 (BioLegend; HIB19), anti-CD27-BUV395 (BD; L128), anti-IgM-PE-Cy7 (BioLegend; MHM-88), anti-IgD-FITC (BD, IA6-2), and anti-pAkt-Alexa 647 (BD; pS473), anti-pSyk-PerCPCy5.5 (BD, pY352), anti-pPLCy2-PE (BD; pY759), or anti-pBtk-BV421 (BD; pY223) for 1 hour on ice. Cells were washed twice with BD Perm/Wash and fixed with 2% paraformaldehyde prior to flow cytometry.
Flow cytometry was performed on a LSR Fortessa X20 (BD), and data were analyzed with FlowJo software version 9.9.4. Gates for BND cells were drawn based on CD19– T cells, which are negative for IgM and IgD.
B cell calcium flux analysis.
Using a strategy similar to that in previous reports (4, 9), PBMCs freshly isolated from buffy coats were enriched for B cells using a no-touch method (Miltenyi Naive B cell Isolation Kit II). Cells were suspended in warmed 37°C RPMI, 2% BSA, and 1 μM Indo1-AM (Molecular Probes). For assays in which PTEN inhibitors were used, either 325 μM SF1670 (R&D Systems) or 1440 μM bpV(HOpic) (MilliporeSigma) was added. Cells were also stained with antibodies, as previously described (4), for 30 minutes and washed 2 times with warmed RPMI containing 2% BSA. Cells were then placed on a LSR Fortessa X20 flow cytometer, and, after 15 seconds of baseline, readings were stimulated with 10 μg/ml F(ab′)2 rabbit anti-human IgG (H&L) (Jackson Labs) or 1 μl of 1 mg/ml ionomycin as a control. The MN fractions were gated as CD19+CD27–IgG–, while the BND cell fractions were gated as CD19+CD27–IgM–. Side stains for PTEN and IgD were also used to determine gates. Calcium mobilization analysis was conducted using FlowJo software.
miRNA analysis.
Peripheral B cells from healthy individuals were sorted to obtain the populations expressing the top 20% of IgM (MN) and the bottom 20% of IgM (BND) using a BD FACS Aria and resuspended in TRIzol (Invitrogen) and stored at –80°C. Side stains for PTEN expression were used to verify gates. Total RNA was extracted using the RNeasy Mini Kit (Qiagen). RNA concentration and purity were determined using an Agilent 2100 Bioanalyzer (Agilent Technologies). cDNA was obtained using the miScript II RT kit (Qiagen), and qPCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen) and miScript primers (miR-92a-3p, miR-7-5p, miR-21-2-5p, miR-22-3p, and miR-15-5p) (Qiagen). The reaction was run on an Applied Biosciences real-time cycler. Expression of each miRNA was normalized to U6 (Qiagen) and then expression in BND cells was compared with MN B cells.
Statistics.
Data were analyzed using Prism software (GraphPad Software Inc.). Mann-Whitney nonparametric unpaired, 2-tailed t tests were used to determine differences between BND cell and MN cells as well as autoimmune subjects and healthy subjects. One-way ANOVA followed by a Bonferroni’s multiple comparison post-test was used to determine significance of differences among samples of BND cells, BND cells with inhibitor, MN cells, and MN with plus inhibitor. Significance was defined as P < 0.05. Data represent mean ± SEM.
Study approval.
Samples from human subjects were obtained with informed consent prior to inclusion in the study at the University of Colorado Anschutz Medical Center and the Barbara Davis Center for Childhood Diabetes using protocols approved by the University of Colorado Institutional Review Board.
Author contributions
JCC designed the research; JCC and PAG provided funding; JCC, PAG, and AG reviewed and edited the manuscript; MJS, BRF, BMC, and AG performed experiments for Figures 1–4 and analyzed the data; MR was the study coordinator; PAG and VDS recruited autoimmune patients; and MJS and JCC wrote the manuscript.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DP3DK110845, R21AI124488, R01AI124487, F30OD021477). JCC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Version 1. 02/07/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
License: Copyright 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(3):e123384. https://doi.org/10.1172/jci.insight.123384.
Contributor Information
B. Rhodes Ford, Email: brford9@gmail.com.
Marynette Rihanek, Email: marynette.rihanek@ucdenver.edu.
Andrew Getahun, Email: andrew.getahun@ucdenver.edu.
References
- 1.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Grandien A, Fucs R, Nobrega A, Andersson J, Coutinho A. Negative selection of multireactive B cell clones in normal adult mice. Eur J Immunol. 1994;24(6):1345–1352. doi: 10.1002/eji.1830240616. [DOI] [PubMed] [Google Scholar]
- 3.Merrell KT, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25(6):953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Duty JA, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206(1):139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MJ, et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes. 2015;64(5):1703–1712. doi: 10.2337/db13-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habib T, et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J Immunol. 2012;188(1):487–496. doi: 10.4049/jimmunol.1102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MJ, Rihanek M, Coleman BM, Gottlieb PA, Sarapura VD, Cambier JC. Activation of thyroid antigen-reactive B cells in recent onset autoimmune thyroid disease patients. J Autoimmun. 2018;89:82–89. doi: 10.1016/j.jaut.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malkiel S, et al. Checkpoints for autoreactive B cells in peripheral blood of lupus patients assessed by flow cytometry. Arthritis Rheumatol. 2016;68(9):2210–2220. doi: 10.1002/art.39710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quách TD, et al. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186(8):4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabouri Z, et al. IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat Commun. 2016;7:13381. doi: 10.1038/ncomms13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6(11):1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 12.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352(6335):532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 13.Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J Exp Med. 2016;213(5):751–769. doi: 10.1084/jem.20150537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neill SK, et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35(5):746–756. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31(5):749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4(3):287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- 17.Pao LI, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27(1):35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11(4):289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamaspishvili T, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15(4):222–234. doi: 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer. 2008;99(8):1296–1301. doi: 10.1038/sj.bjc.6604680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197(5):657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akerlund J, Getahun A, Cambier JC. B cell expression of the SH2-containing inositol 5-phosphatase (SHIP-1) is required to establish anergy to high affinity, proteinacious autoantigens. J Autoimmun. 2015;62:45–54. doi: 10.1016/j.jaut.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed JH, Jackson J, Christ D, Goodnow CC. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med. 2016;213(7):1255–1265. doi: 10.1084/jem.20151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu XN, et al. Defective PTEN regulation contributes to B cell hyperresponsiveness in systemic lupus erythematosus. Sci Transl Med. 2014;6(246):246ra99. doi: 10.1126/scitranslmed.3009131. [DOI] [PubMed] [Google Scholar]
- 25.Serr I, et al. miRNA92a targets KLF2 and the phosphatase PTEN signaling to promote human T follicular helper precursors in T1D islet autoimmunity. Proc Natl Acad Sci USA. 2016;113(43):E6659–E6668. doi: 10.1073/pnas.1606646113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke TW, Wei PL, Yeh KT, Chen WT, Cheng YW. MiR-92a promotes cell metastasis of colorectal cancer through PTEN-mediated PI3K/AKT pathway. Ann Surg Oncol. 2015;22(8):2649–2655. doi: 10.1245/s10434-014-4305-2. [DOI] [PubMed] [Google Scholar]
- 27.Lu C, Shan Z, Hong J, Yang L. MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis. Int J Oncol. 2017;51(1):235–244. doi: 10.3892/ijo.2017.3999. [DOI] [PubMed] [Google Scholar]
- 28.Xiao J, Yu W, Hu K, Li M, Chen J, Li Z. miR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT signaling pathway. Oncol Rep. 2017;37(4):2513–2521. doi: 10.3892/or.2017.5484. [DOI] [PubMed] [Google Scholar]
- 29.Yu X, et al. miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. Oncol Lett. 2017;14(2):1807–1810. doi: 10.3892/ol.2017.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, et al. Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood. 2011;117(24):6702–6713. doi: 10.1182/blood-2010-09-309864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo H, Yang Y, Duan J, Wu P, Jiang Q, Xu C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013;4:e481. doi: 10.1038/cddis.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kälin S, et al. A Stat6/Pten axis links regulatory T cells with adipose tissue function. Cell Metab. 2017;26(3):475–492.e7. doi: 10.1016/j.cmet.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 34.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14(1):33–43. doi: 10.1016/S1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 35.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steck AK, et al. Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? Diabetes. 2009;58(4):1028–1033. doi: 10.2337/db08-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.