Abstract
Strength training (ST) is known to promote muscle hypertrophy and body composition adaptations. However, only a few studies investigated the effects of ST combined with antioxidant supplementation (AS) on these adaptations. The aim of this study was to investigate chronic effects of ST combined with AS on fat mass (FM) and fat-free mass (FFM) of young women. In a double-blinded design, thirty-three subjects (22.9 ± 2.5 years, 57.7 ± 8.4 kg, 1.6 ± 0.6 m) were allocated into three groups: 1) vitamins (n=12), 2) placebo (n=11) and 3) control (n=10). Vitamins and placebo underwent a ST program for 10 weeks. Vitamins supplemented with vitamin C (1g/day) and E (400IU/day) during the training period. FM and FFM were assessed by DEXA. Multiple 3 x 2 (group x time) mixed-factor ANOVA with Tukey adjustment was performed to examine differences in the dependent variables. The significance level was set at P ≤ .05. Only placebo increased total FFM (34.9 ± 4.9 vs 36.3 ± 4.8 kg, P<0.05) and decreased total FM (21.8 ± 7.8 vs 21.0 ± 8.3 kg, P<0.05) after training for 10 weeks. Moreover, only placebo presented a significantly greater FFM percent change from pre to post-intervention compared to control (4.0 ± 3.4 vs −0.7 ± 3.1%, respectively, P < 0.05). These results suggest that chronic AS can mitigate ST related improvements of body composition in young women.
Keywords: Fat mass, Fat-free mass, Hypertrophy, Vitamin C, Vitamin E
INTRODUCTION
Oxidative stress (OS) is a well-known phenomenon that may cause oxidative damage to different types of cells, including muscle cells. Oxidative damage is associated with the action of reactive metabolites generally named reactive oxygen species (ROS) (18, 21). It has been demonstrated that acute strength exercise can trigger the generation of ROS in skeletal muscle (4, 21). Of note, it is argued that ROS produced during exercise may increase muscle fatigue and, as a consequence, diminish performance (27). To counteract this, supplementation with different kinds of antioxidants have become popular among athletes and non-athletes in the last decades (29). Particularly, vitamins C and E have been commonly used among physically active people, including strength training (ST) participants (35).
However, it has been suggested that OS caused by a ST session may play a positive role in chronic adaptations to training. For instance, previous studies showed that ROS generated by exercise are signaling molecules for protein synthesis and improvement of the endogenous antioxidant system (2, 16, 23). Moreover, chronic ST is known to promote adaptations that result in a plethora of physiological and functional benefits to men and women without taking supplementation of any kind (15, 24). Body composition improvements is within those benefits. In general, the positive adaptations induced by ST in body composition are body fat mass (FM) reduction and increased fat-free mass (FFM) (e.g. hypertrophy) (7, 8, 14).
Muscle hypertrophy occurs when protein synthesis is greater than protein breakdown (25). Interestingly, there is some evidence that antioxidant supplementation may blunt protein synthesis pathways probably by reducing ROS activity in muscle cells (20,23,32). The consequences of that may be the mitigation of ST adaptations, as recently shown by Dutra et al (10), who reported that supplementation with vitamin C (1000 mg) and E (400 IU) attenuate neuromuscular and hypertrophy gains (e.g. rectus femoris thickness) of young women after 10 weeks of ST. However, it cannot be assumed that the thickness of a single muscle is representative of whole body FFM. In fact, very few studies have investigated the chronic effect of ST combined with antioxidant supplementation on whole body FFM and FM, and they present important methodological differences (5–7, 23).
For instance, Bobeuf and colleagues (7) reported that supplementation with vitamin C (1g) and E (600 mg) for six months boosted FFM gain (+1.5 kg) associated with ST in elderly men and women. Contrarily, Bjørnsen et al. (5) found that after 12 weeks of ST, legs FFM of elderly men increased more (5% vs 2% gain) in the placebo than in the supplemented group (1g vitamin C and 235 mg vitamin E). Noteworthy, as elderly people are more prone to OS because of the aging process (9), to combine antioxidant supplementation with ST in the elderly could be part of a comprehensive intervention to counteract physiological decrements of that process (30). However, very little attention has been given to antioxidant supplementation in healthy young women who participate in ST, even though consumption is common among this population (33, 34).
According to our knowledge, only one study analyzed body composition alterations (e.g. FFM and FM) after ST combined with antioxidant supplementation in recreationally strength-trained men and women (23). The authors found that 10 weeks of ST combined with vitamin C (1000 mg) and E (235 mg) supplementation evoked similar FFM gain when compared to a placebo group (1.8 ± 1.6% vs 1.7 ± 1.6%, respectively), while FM was unchanged in both groups of men and women. However, it is known that estrogen may work as an antioxidant (11), so gender could be a source of bias to this study. Thus, in spite of the results reported by Dutra et al. (10) regarding muscle thickness, and by Paulsen et al. regarding FFM and FM (23), it is still uncertain if antioxidant supplementation could mitigate FFM and FM alterations induced by ST in young healthy adults. Therefore, the aim of this study was to investigate the chronic effect of ST combined with vitamin C and E supplementation on FFM and FM of young women.
METHODS
Participants
This is a double-blinded randomized study. Tests were conducted before and after 10 weeks of ST. Two sessions of familiarization to the exercises were performed prior to baseline measurements. At those sessions, participants performed two sets of four exercises (chest press, seated row, deadlifts and dumbbell lunges) with light loads and were instructed about the proper technique of exercise execution. Rest interval between sets was 90 seconds. After that, subjects were allocated into three groups: 1) vitamins, 2) placebo or 3) control. An external researcher performed the randomization of vitamins and placebo groups by sortition. Participants from vitamins and placebo performed ST for 10 weeks. As women commonly perform less exercises for the upper body muscles (12), the ST protocol included exercises for this region of the body, but focused on lower body muscle groups. The vitamins group supplemented with vitamin C and E during the ST period. A power analysis conducted a priori with G*POWER 3.1.9.2 (Universitat Kiel, Germany) determined that 42 participants were needed in the present study for a power of 0.80, with an effect size f of 0.25 and an α = 0.05. Fifty-three college students volunteered to participate. The following inclusion criteria were adopted: non-smoking women aged 18 to 30 years who were previously untrained in ST for at least six months. All participants answered a face-to-face questionnaire addressing medical and exercise history, as well as medication and supplement usage. Participants who reported musculoskeletal and/or cardiometabolic disorders, and supplementation with any antioxidant compound were excluded. At this point, three were excluded and the others were assigned to vitamins, placebo or control groups. To be included in the analysis, compliance to training and supplementation had to be at least 85% (13). Seven volunteers dropped out the intervention due to pregnancy (n = 1), started another ST program (n = 1), started nutritional treatment not related to the study (n = 1), injury (n = 2) and lack of interest to finish the intervention (n = 2). Ten participants were not included in the analysis due to low adherence to training (n = 6) or supplementation (n = 4). So, this report present data from the 33 subjects who completed the experiment. Sample size in each group was 12, 11 and 10 for vitamins, placebo and control, respectively. A new power analysis was conducted after the end of the intervention to determine the statistical power of the tests. A sensitivity power analysis showed an effect size f = 0.28. Then, a post hoc analysis showed a power of 0.78. Enrollment was voluntary, and written consent was obtained from each participant. The procedures were conducted according to the Helsinki Declaration and the experimental protocol was approved by the Institutional Review Board of the University Center of Brasília, process number 1.515.933/2016.
Protocol
Vitamins and placebo groups performed two exercises for upper body (chest press and seated row) and two for lower body region (deadlifts and dumbbell lunges) throughout the training period. Sessions were performed twice per week with a minimum of 48 hours interval between them. Exercises for the upper body muscles were performed as follows: two sets of 10–12 repetitions (reps) for both exercises throughout the training period. Loads for the lower body exercises were increased as follows: weeks 1–3, two sets of 12–15 reps for both exercises; weeks 4–6, three sets of 10–12 reps for deadlift and two sets of 10–12 reps for lunges; weeks 7–8, three sets of 10–12 reps for both exercises; weeks 9–10, four sets of 8–10 reps for deadlift and three sets of 8–10 reps for lunges. This protocol and progression scheme is in line with the American College of Sports Medicine recommendations to maximize strength and hypertrophy (1). Subjects were instructed to perform all sets until momentary failure, according to the definition of Steele and colleagues (31). If necessary, loads were adjusted from set to set to maintain the designated number of repetitions. Also, concentric and eccentric actions were performed in approximately two seconds each, without pause between them. Rest intervals between sets ranged from 1.5 to 2.0 minutes. All sessions were supervised by experienced exercise scientists. At the end of the intervention, estimates of strength gain were derived using the “Training Load Chart” of the National Strength and Conditioning Association (3). These data are reported as change from pre to post intervention (in kg).
Body composition: Body weight was measured to the nearest 0.05 kg using a calibrated electronic scale with volunteers dressed in light clothes. Height was determined without shoes to the nearest 0.1 cm using a wall stadiometer. Body mass index (BMI) was derived as body weight divided by height squared (kg/m2). Body composition measurements were performed with Dual energy X-ray absorptiometry (DEXA; Lunar model 8743, GE Medical Systems, Madison, Wisconsin, USA) to assess FFM and FM according to procedures specified elsewhere (19). Participants were oriented to maintain dietary habits (except for avoiding calcium ingestion) and to refrain from physical activity 24h prior to the test. Also, they were instructed to come to the test dressed in comfortable clothing without metal on it (zippers, buttons etc.). All measurements were carried out by the same trained technician, and the equipment was calibrated daily according to the manufacturer’s specifications. Intraclass correlation coefficient (ICC) for this technician is 0.97 and 0.99 for FFM and FM, respectively.
Supplementation
All pills (vitamins and placebo) were produced by a local manufacturing pharmacy. Each vitamin pill contained 333.3 mg of ascorbic acid and 133.3 IU of α-tocopherol. The placebo pills had the same shape and appearance as the vitamin pills and contained magnesium stearate, colloidal silicon dioxide, pharmaceutical talc and cornflour. Participants were oriented to ingest three pills per day for 67 days, from the beginning till the end of ST. Hence, daily dosage was 1000 mg of vitamin C and 400 IU of vitamin E. This is a commonly available dosage in commercial supplements, and was previously used in recent studies (5, 6, 9). Adherence to supplementation was checked after the intervention by counting the remaining pills in the pack of pills of each participant. All subjects filled out a 3-day food recall diary to assess macronutrients and vitamins intake before the start of the ST. This was not repeated after the end of the study. Moreover, they were instructed to avoid the following beverages during the study: coffee, tea, alcoholic beverages and juices rich in antioxidants (such as grape and orange juice).
Statistical Analysis
Homogeneity of variance was tested using Levene’s test. Multiple 3 x 2 (group x time) mixed-factor ANOVA with Tukey adjustment was performed to examine differences in the dependent variables (FFM and FM). One-Way ANOVA was performed to compare percent changes between groups after the intervention. Strength gains of vitamins and placebo groups were analyzed using multiple 2 x 2 (group x time) mixed-factor ANOVA. Independent samples T-test was applied to compare adherence to training sessions and to supplementation between vitamins and placebo groups. The significance level was set at P ≤ .05. All analyses were performed using the Statistical Package for Social Sciences (IBM SPSS 20.0, Armonk, New York, USA) for Windows. Values are given as mean ± standard deviation (± SD).
RESULTS
Descriptive characteristics of participants at baseline and adherence to the intervention are shown in Table 1. No significant differences between groups was found for any variable (P > 0.05).
Table 1.
Sample Characteristics at baseline and adherence to the intervention (Mean ± SD).
| Group | |||
|---|---|---|---|
| Variable | Vitamins (n=12) | Placebo (n=11) | Control (n=10) |
| Age (years) | 22.6 ± 2.3 | 22.6 ± 2.1 | 23.7 ± 3.2 |
| Body mass (kg) | 59.3 ± 6.9 | 58.7 ± 9.9 | 54.9 ± 8.5 |
| Height (m) | 1.64 ± 0.07 | 1.66 ± 0.11 | 1.66 ± 0.07 |
| BMI (kg/m2) | 21.9 ± 2.9 | 21.8 ± 3.1 | 20.1 ± 3.1 |
| Total energy intake (cal) | 1600.0 ± 416.1 | 1418.0 ± 280.8 | 1819.4 ± 441.7 |
| Carbohydrates (%) | 52.8 ± 6.3 | 51.0 ± 7.4 | 52.2 ± 7.4 |
| Lipids (%) | 32.3 ± 5.7 | 30.6 ± 7.0 | 30.3 ± 6.1 |
| Proteins (%) | 14.7 ± 2.1 | 17.3 ± 4.6 | 17.4 ± 2.6 |
| Vitamin C (mg) | 249.7 ± 388.0 | 302.3 ± 792.2 | 262.5 ± 491.3 |
| Vitamin E (mg) | 3.3 ± 2.5 | 4.0 ± 2.8 | 4.4 ± 1.9 |
| Training Adherence (%) | 90.4 ± 2.6 | 91.4 ± 2.3 | NA |
| Supplements Adherence (%) | 97.0 ± 3.3 | 97.3 ± 4.0 | NA |
Note: All P > .05. NA: not applicable. BMI: body mass index.
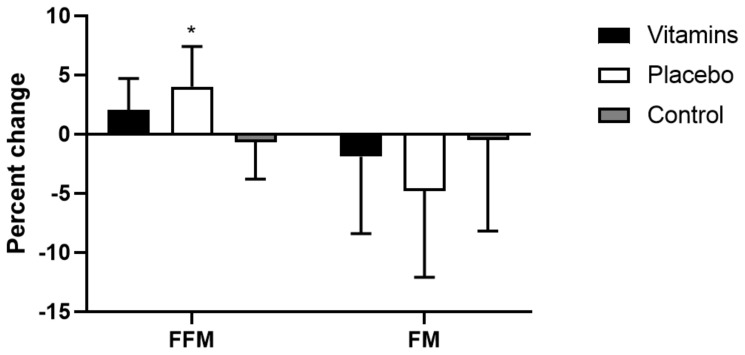
Regarding FFM and FM, there was no significant difference between groups before and after the ST period for both variables. However, the placebo group showed a significant increase in FFM (34.9 ± 4.9 to 36.3 ± 4.8 kg; P < .05) after the intervention, while vitamins (34.8 ± 2.4 to 35.5 ± 2.2 kg; P > .05) and control (35.3 ± 4.0 to 35.0 ± 3.5; P > 0.05) did not. In addition, the placebo group showed a significant decrease in FM (21.8 ± 7.8 to 21.0 8.3 kg; P < 0.05) after the intervention, while vitamins (22.4 ± 6.2 to 21.7 ± 4.9 kg; P > 0.05) and control (17.7 ± 5.4 to 17.8 ± 6.0; P > 0.05) did not. Only placebo presented a significantly greater FFM percent change from pre to post-intervention compared to control (P < 0.05, Figure 1), with a larger effect size compared to vitamins and control (Partial η2 = 0.38, 0.14, and 0.03 for placebo, vitamins and control, respectively).
Figure 1.
Percent change from pre to post-training (mean ± SD). FFM: Fat-free mass. FM: Fat mass. * P < 0.05 vs control.
Both placebo (deadlift: + 29.6 ± 10.7 kg; dumbbell lunges: + 38.2 ± 9.4 kg) and vitamins (deadlift: + 35.0 ± 7.7 kg; dumbbell lunges: + 39.5 ± 9.0 kg) increased strength after the training period when compared to baseline values in both exercises (P < 0.05). However, no differences between groups were observed before and after the training period.
DISCUSSION
The purpose of this study was to analyze chronic effects of ST combined with vitamin C and E supplementation on body composition of college women. The main finding was that only placebo showed FFM adaptations of greater magnitude (P < .05) when compared to control (Figure 1). Also, placebo was the only group that presented a significant decrease in FM after 10 weeks of ST compared to baseline values.
Previous studies that analyzed the effect of antioxidant supplementation on body composition adaptations induced by ST are scarce and diverge from the present study with regard to sample characteristics (e.g. elderly vs young), dosage of supplementation and ST protocol. Bobeuf and colleagues (7) reported beneficial effects of antioxidant supplementation (1000 mg vitamin C and 400 IU vitamin E) on FFM (1.5 kg gain) in an elderly sample of men and women (65.6 ± 3.8 years) after six months of ST (3 sets, 8 reps, 80% 1RM). Interestingly, none of the three other groups (ST alone, placebo pills alone and vitamin supplementation alone) analyzed by those authors increased FFM. They discuss that vitamin C and E supplementation likely reduced damage and/or increased protein synthesis induced by muscle contraction associated to ST. However, they did not measure protein oxidation or synthesis, and there is no support in the literature to that assumption, especially regarding protein synthesis. Worthy of note, this result was not repeated in a second study by the same authors (6) adopting the same supplementation scheme and ST protocol.
Nevertheless, as OS is believed to increase with advancing age (9), it would not be contradictory that older people benefit from antioxidant supplementation, particularly if they present vitamins deficiency (28, 30). However, the study of Bjørnsen et al. (5) found that after 12 weeks of ST, legs FFM of elderly men (60–81 years) increased more (5% vs 2% gain) in the placebo than in the supplemented group (1g vitamin C and 235 mg vitamin E). Moreover, they showed that rectus femoris thickness increased more (16.2 vs 10.9%, P < .05) in the placebo than in an antioxidant group at the 12th week of the intervention. Similarly, the recent study by Dutra et al. reported that rectus femoris thickness, as well as neuromuscular gains (e.g. peak torque and total work) are attenuated in young women after 10 weeks of ST combined with vitamin C (1g/day) and E (400 IU/day) supplementation compared to a placebo group. Although it cannot be assumed that the thickness of a single muscle is representative of whole body FFM, the result of the present study corroborates the aforementioned studies with the novel finding that supplementation with vitamin C and E attenuate FFM and FM improvements in young women.
In this sense, there is a growing body of evidence showing that ROS produced during exercise are important regulators of muscle growth (5, 20, 26). So, it appears that the use of high-dose antioxidant supplementation could suppress physiologically important ROS mediated actions, such as the activation of hypertrophy pathways (5). Indeed, animal models and acute human studies support this explanation. For instance, Makanae et al. (20) demonstrated that oral administration of vitamin C attenuated hypertrophy induced by mechanical overload in rats. By the same token, Paulsen and colleagues (23) reported that vitamin C and E interfered with exercise-induced signaling in muscle cells after a session of ST performed by young men and women. It seems like excess vitamin C and E may reduce the phosphorylation of important hypertrophy pathways mediated by ROS, such as p38, ERK1/2 and p70S6K. Results from the present investigation also support this explanation, once participants taking supplementation had their FFM adaptations attenuated in relation to placebo.
It is worthy to note that effect size values in the present work were all higher for placebo compared to vitamins and control groups. Moreover, only participants from the placebo group presented a significant reduction in FM when compared to baseline values. This is likely related to the more pronounced FFM gains in this group, once muscle growth is related to an elevated fat oxidation in the liver (17). In line with this, a previous report showed that increases in muscle mass after resistance training for 9 months are inversely correlated with fat mass gain (r= −0.63, P < .05) in women (22). Based on this data, it is plausible to infer that the additional antioxidants delivered from the supplementation blunted favorable redox signaling pathways induced by ST and negatively interfered with body composition adaptation.
This work has both strengths and limitations. The main limitation is that protein synthesis and oxidative stress markers were not analyzed. These assessments could have helped to identify mechanistic pathways influencing the results. Secondly, tests may have been performed in different phases of the menstrual cycle. In addition, contraceptive drug usage was not controlled during the study. It has been suggested that estrogen may work as an antioxidant (11). However, it is unlikely the results were influenced in this way once participants who reported contraceptive usage were randomly spread across groups. Thirdly, data about vitamins and other antioxidants intake during and after the intervention was not collected, and this can be a source of bias to the study. However, participants received orientations from a nutritionist about food and drink consumption during the intervention. Finally, no strength assessment was performed, only estimates were done. However, strength assessment was not the focus of the present investigation. Strengths of the study include a robust experimental design (double-blinded, placebo-controlled, randomized study), use of a gold standard method of body composition assessment and the high adherence of participants to both ST and supplementation.
More research is needed to identify the situations that may warrant antioxidant supplementation in the context of ST (hypovitaminotic cases, oxidative stress). Future studies should address the effect of this kind of intervention on the oxidant/antioxidant status and its implications for strength and hypertrophy in non-healthy people, such as sarcopenic obese and cancer survival patients. In addition, different daily dosages of antioxidants, anabolic hormonal status, as well as longer periods of training should also be examined in future research.
In summary, the present results show that vitamin C and E supplementation attenuate body composition adaptations related to ST, as only participants that received placebo pills presented alterations of greater magnitude when compared to control. Effect sizes support this assumption by demonstrating higher values for placebo. Considering these results, vitamin C and E supplementation should be avoided by healthy young women who want to increase FFM and reduce FM.
REFERENCES
- 1.American College of Sports Medicine. Progression models in resistance training for healthy adults. [Internet] 2009. American College of Sports Medicine position stand. [DOI] [PubMed] [Google Scholar]
- 2.Azizbeigi K, Azarbayjani Ma, Peeri M, Agha-Alinejad H, Stannard S. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int J Sport Nutr Exerc Metab. 2013;23(3):230–238. doi: 10.1123/ijsnem.23.3.230. [DOI] [PubMed] [Google Scholar]
- 3.Baechle TR, Earle RW. Essentials of Strength Training and Conditioning 3a. Human Kinetics Publishers; 2008. [Google Scholar]
- 4.Bailey DM, Lawrenson L, McEneny J, Young IS, James PE, Jackson SK. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic Res. 2007 Feb;41:182–190. doi: 10.1080/10715760601028867. [DOI] [PubMed] [Google Scholar]
- 5.Bjørnsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. 2016;26:755–763. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 6.Bobeuf F, Labonte M, Dionne IJ, Khalil A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging. 2011;15(10):883–889. doi: 10.1007/s12603-011-0097-2. [DOI] [PubMed] [Google Scholar]
- 7.Bobeuf F, Labonté M, Khalil A, Dionne IJ. Effects of resistance training combined with antioxidant supplementation on fat-free mass and insulin sensitivity in healthy elderly subjects. Diabetes Res Clin Pract. 2010;87(1):2009–2011. doi: 10.1016/j.diabres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Bottaro M, Veloso J, Wagner D, Gentil P. Resistance training for strength and muscle thickness: Effect of number of sets and muscle group trained. Sci Sport. 2011;26(5):259–264. [Google Scholar]
- 9.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 10.Dutra MT, Alex S, Mota MR, Sales NB, Brown LE, Bottaro M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl Physiol Nutr Metab. 2018;43(8):775–781. doi: 10.1139/apnm-2017-0866. [DOI] [PubMed] [Google Scholar]
- 11.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle sex matters. Sport Med. 2010;40(1):41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Gentil P. The effects of resistance training on lower and upper body strength gains in young women. Int J Kinesiol Sport Sci. 2015;3(3) [Google Scholar]
- 13.Gentil P, Bottaro M. Effects of training attendance on muscle strength of young men after 11 weeks of resistance training. Asian J Sports Med. 2013;4(2):101–106. doi: 10.5812/asjsm.34489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentil P, Soares S, Bottaro M. Single vs. multi-joint resistance exercises: Effects on muscle strength and hypertrophy. Asian J Sports Med. 2015;6(2):e24057. doi: 10.5812/asjsm.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentil P, Steele J, Pereira MC, Castanheira RPM, Paoli A, Bottaro M. Comparison of upper body strength gains between men and women after 10 weeks of resistance training. PeerJ. 2016;4:e1627. doi: 10.7717/peerj.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handayaningsih A-E, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152(3):912–921. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- 17.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7(2):159–72. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson MJ, Vasilaki A, McArdle A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic Biol Med. 2016;98:13–17. doi: 10.1016/j.freeradbiomed.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Lima RM, Bezerra LMa, Rabelo HT, Silva MaF, Silva AJR, Bottaro M. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitom. 2009;12(1):35–41. doi: 10.1016/j.jocd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Makanae Y, Kawada S, Sasaki K, Nakazato K, Ishii N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiol. 2013;208(1):57–65. doi: 10.1111/apha.12042. [DOI] [PubMed] [Google Scholar]
- 21.Mason SA, Morrison D, McConell GK, Wadley GD. Muscle redox signalling pathways in exercise. Role of antioxidants. Free Radic Biol Med. 2016;98:29–45. doi: 10.1016/j.freeradbiomed.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Orsatti FL, Nahas EAP, Orsatti CL, Oliveira EP, Nahas-Neto J, Mota GR. Muscle mass gain after resistance training is inversely correlated with trunk adiposity gain in postmenopausal women. J Strength Cond Res. 2012;26(8):2130–2139. doi: 10.1519/JSC.0b013e318239f837. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen G, Hamarsland H, Cumming KT, Johansen RE, Hulmi JJ, Børsheim E. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol. 2014;592(Pt 24):5391–5408. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips BE, Williams JP, Greenhaff PL, Smith K, Atherton PJ. Physiological adaptations to resistance exercise as a function of age. JCI Insight. 2017;2(17):1–16. doi: 10.1172/jci.insight.95581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers SK. Can Antioxidants Protect Against Disuse Muscle Atrophy? Sport Med. 2014;44(S2):155–165. doi: 10.1007/s40279-014-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95(1):1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid MB. Reactive oxygen species as agents of fatigue. Med Sci Sports Exerc. 2016;48(11):2239–2246. doi: 10.1249/MSS.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt H, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A. Antioxidants in translational medicine. Antioxid Redox Signal. 2015;00(00):1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senchina DS, Burke LM, Stear SJ, Castell LM. A - Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 39. Br J Sports Med. 2012;46(14):1145–1146. doi: 10.1136/bjsports-2011-090836. [DOI] [PubMed] [Google Scholar]
- 30.Stea TH, Stølevik SB, Berntsen S, Ezzathkah Bastani N, Paulsen G, Lohne Seiler H. Effect of Omega-3 and Vitamins E + C supplements on the concentration of serum b-vitamins and plasma redox aminothiol antioxidant status in elderly men after strength training for three months. Ann Nutr Metab. 2016;68(2):145–155. doi: 10.1159/000443847. [DOI] [PubMed] [Google Scholar]
- 31.Steele J, Fisher J, Giessing J, Gentil P. Clarity in reporting terminology and definitions of set endpoints in resistance training. Muscle Nerve. 2017;56:368–374. doi: 10.1002/mus.25557. [DOI] [PubMed] [Google Scholar]
- 32.Vincent HK, Bourguignon CM, Vincent KR, Weltman AL, Bryant M, Taylor AG. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity (Silver Spring) 2006;14(12):2224–2235. doi: 10.1038/oby.2006.261. [DOI] [PubMed] [Google Scholar]
- 33.Williams MH. Dietary supplements and sports performance: Introduction and vitamins. J Int Soc Sports Nutr. 2004;1(2):1–6. doi: 10.1186/1550-2783-1-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010;42(7):1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 35.Yfanti C, Tsiokanos A, Fatouros IG, Theodorou AA, Deli CK, Koutedakis Y. Chronic eccentric exercise and antioxidant supplementation : effects on lipid profile and insulin sensitivity. J Sport Sci Med. 2017;16:375–382. [PMC free article] [PubMed] [Google Scholar]