Abstract
Fine particles (PM2.5) are known to increase risks of cardiovascular diseases, but it is unclear how they affect plasma lipid levels. In this study, we examined the associations between PM2.5 exposure and lipid/lipoprotein levels from 2,289 midlife women enrolled in the longitudinal Study of Women’s Health Across the Nation. The average exposure to PM2.5 and gaseous co-pollutants during the prior one year, six months, 30 days, and one day were estimated for each woman based on US Environmental Protection Agency ambient monitoring data. Blood samples were collected annually from 1999 to 2005 and analyzed for lipids/lipoproteins. Mixed-effect models were used to account for repeated measures for each woman, adjusted for demographic, health and behavior covariates. PM2.5 exposures, especially the long-term exposure, were negatively associated with protective lipoproteins, and positively associated with atherogenic lipoproteins. For example, each 3 μg/m3 increase of one-year PM2.5 exposure was associated with decreases of −0.7% (−1.4%, − 0.1%) in high-density lipoprotein cholesterols and −0.6% (−1.1%, −0.1%) in apolipoprotein A1 (ApoA1), as well as increases of 3.8% (1.0%, 6.6%) in lipoprotein(a) and 1.4% (0.5%, 2.3%) in the ratio of apolipoprotein B (ApoB) / ApoA1. In stratified analysis, increased atherogenic lipoproteins were mainly observed in women without dyslipidemia, and both increased atherogenic lipoproteins and reduced protective lipoproteins were observed among women in perimenopause. In summary, PM2.5 exposure was associated with adverse lipid level changes, and thus, may increase cardiovascular risks in midlife women.
Keywords: PM2.5, long-term exposure, lipoprotein, dyslipidemia, menopause
Introduction
Particulate matter smaller than 2.5 micrometers (PM2.5 or fine particles) has been associated with a variety of adverse health effects. The strongest associations have been with morbidity and mortality from cardiovascular diseases (CVDs) (Dockery et al. 1993; Miller et al. 2007; Pope et al. 2004). Having reviewed relevant studies, the U.S. Environmental Protection Agency (EPA) concluded that a causal association exists between both short- and long-term PM2.5 exposure and cardiovascular morbidity and mortality (U.S. EPA 2009). The American Heart Association also confirmed this consistent causal relationship (Brook et al. 2010).
Given their established associations with cornary heart disease, several components of serum cholesterols have been used to predict cardiac risks (Blaton 2003; Navab et al. 2011). Elevated circulating lipoproteins that contain apolipoprotein B (ApoB), for example, low-density lipoprotein (LDL) and lipoprotein(a) (Lp(a)), could penetrate the endothelial surface of arterial walls to form fatty streaks in the subendothelial space and lead to development of atherosclerotic plaques (Carmena et al. 2004). High levels of triglycerides (TG) have also been identified as a risk factor for CVD (Miller et al. 2011). In contrast, high-density lipoproteins (HDL) protect against CVD by transporting excess cholesterol back to the liver for disposal as bile salts, reversing endothelial cell dysfunction, inhibiting endothelial cell apoptosis, decreasing platelet aggregation, and inhibiting LDL oxidation (Nofer et al. 2002; Toth 2003). Apolipoprotein A1 (ApoA1) is the main initiator and driver of reverse cholesterol transport (Lu et al. 2011).
A few epidemiologic studies have shown some modest adverse associations between PM and plasma lipid levels, and in general, the trends were that elevated exposures to PM2.5 were positively associated with “bad” cholesterols, including LDL, TG, total cholesterol (TC), and ApoB, as well as negatively associated with “good” cholesterol, namely, HDL, in various exposure windows (Bell et al. 2017; Cai et al. 2017; Chen et al. 2016; Chuang et al. 2010; Chuang et al. 2011; Shanley et al. 2016; Yitshak Sade et al. 2016). However, some studies reported no statistically significant association with HDL-c with either long-term, intermediate-term or episodic PM2.5 exposure (Bell et al. 2017; Chen et al. 2016; O’Toole et al. 2010). Thus, the evidence has been inconsistent. Further, differences in study design, population characteristics, geography, and statistical methods do not permit a direct comparison, but very few studies have examined associations in different exposure intervals in the same cohort.
Moreover, such associations between PM2.5 exposure and serum lipid levels may be modified by other factors. Midlife women, for example, experience accelerated adverse changes in some lipids during the menopausal transition (Derby et al. 2009; Matthews et al. 2009). Disorders of lipids and lipoprotein metabolism (also called dyslipidemia), characterized by elevated levels of LDL and TG or low levels of HDL, have pervaded the adult U.S. population, elevating the risk for CVD (Burnett 2004; Navab et al. 2011; Toth et al. 2012). People experiencing these health conditions may be susceptible to additional burdens to their lipid metabolism.
Therefore, in this study, we aimed to evaluate the longitudinal association between exposure to ambient PM2.5 and lipids/lipoproteins in multiple exposure windows and to understand whether dyslipidemia and menopause transition status influenced those associations.
Methods
Study population
Data for this study are derived from the longitudinal data collected in the Study of Women’s Health Across the Nation (SWAN), which has been following a large midlife cohort of women through the menopausal transition in multiple sites in the U.S. (Sowers et al. 2000). Details of the SWAN participant recruitment and study design have been described in detail elsewhere (Sowers et al. 2000). Briefly, starting in 1995, SWAN recruited females 42–52 years of age who had an intact uterus and at least one ovary, were not using exogenous sex hormones, were not pregnant or lactating, and had at least one menstrual period in the past three months. Women were recruited from six sites for the present study, with each site targeting enrollment to white women and one other racial/ethnic group: Hispanics in Newark, NJ; Chinese in Oakland, CA; Japanese in Los Angeles, CA; African Americans in Detroit, MI, Chicago, IL, and Pittsburgh, PA. Approximately 450 women recruited at each site who participated in annual clinical assessments and completed questionnaires and interviews. The Institutional Review Boards at all participating sites approved SWAN protocols, and all participants provided written informed consent. The analyses presented in this paper were based on serum lipid/lipoprotein data for visits 3 through 7 (1999–2005), as PM2.5 only became available from 1999.
Data collection
Air pollutant data
Ambient air pollutant data were obtained from the U.S. EPA’s Air Data website (https://www.epa.gov/airquality/airdata, accessed October 2016). PM2.5 data were typically collected every three days, sometimes daily or every six days, and reported as 24-hour average concentrations. Ambient gases, such as ozone (O3), carbon monoxide (CO), nitrogen dioxide (NO2) and sulfur dioxide (SO2), were examined for potential confounding. They were monitored on an hourly basis, downloaded as eight-hour average concentrations for O3 as well as one-hour average concentrations for CO, NO2 and SO2.
We obtained the residential history for each participant, which allowed us to geocode each residence, with the coordinates randomly moved within a block (up to 400 feet) to enhance confidentiality. A circular buffer area was created around each location using ArcGIS v10.0 (Environmental Systems Research Institute 1995–2016). After considering the potential for exposure misclassification, sample size, and population characteristics, we chose a 20 km radius as the buffer area distance, in which PM2.5 level is considered homogeneous (Bell et al. 2011; Ebisu et al. 2014). An algorithm was developed to choose a representative monitor for each residence based on distance and measurement availability (Green et al. 2016). If a participant moved (13% of all women over this study’s time period), exposure data from multiple addresses during the year prior to her visit were weighted based on the time of move or evenly weighted if the relocation date was not available. We examined multiple exposure windows, including short-term (prior one day), intermediate term (prior 30 days), and long-term (prior six-month and one-year) exposures, and calculated average exposure levels for these windows prior to each blood draw. More details about exposure classification can be found in our previous publications (Green et al. 2016; Wu et al. 2017).
Blood measurement and analysis
Blood was drawn at each SWAN clinic visit on an approximately annual basis and analyzed for many CVD markers as described previously (Matthews et al. 2017). Lipid/lipoproteins associated with CVD risk were measured, including HDL-c, LDL-c, TG, TC, Lp(a), lipoprotein A1 (LpA1), ApoA1, and ApoB (Burnett 2004; Kaptoge et al. 2012; Lowe et al. 2004; Smith et al. 2005). The ratio of ApoB/ApoA1, a better marker of cardiovascular risks than the traditional lipid profile, was also calculated (Carmena et al. 2004).
Most markers were measured during each visit, except LpA1, which was measured every other visit (visits 3 and 5 in our study) for all women and in a subset of women at visit 7.
Covariates
Demographic and health information were collected at baseline and during annual clinic visits by in-person interviews and self-administered questionnaires. Race/ethnicity was classified as African American, Asian (Chinese or Japanese), Caucasian, and Hispanic. Educational attainment was grouped into high school or less, some college, or college graduate. Participants were asked to report current smoking status and history, alcohol use, physical activity level (Visits 3, 5 and 6 only), medication use, hormone use, major surgeries, and chronic health conditions (e.g., whether they had ever been diagnosed with diabetes). Weight and height were measured at each visit to calculate body mass index (BMI) as weight in kg/[height in m]2.
Menopausal status was determined at each visit based on the self–reported menstrual patterns in the past year, and was classified as: premenopause (menses in the past 3 months with no change in regularity); early perimenopause (menses within the past 3 months but becoming less predictable); late perimenopause (3 to 11 months of amenorrhea); natural postmenopause (12 or more months of amenorrhea); postmenopause due to bilateral salpingo-oophorectomy (BSO); and unknown status due to hormone use or hysterectomy (Derby et al. 2009; Gold et al. 2006).
Statistical analysis
The distribution of lipids/lipoproteins and ambient PM2.5 exposure were examined. Correlations between PM2.5 and other air pollutants, including O3, CO, NO2 and SO2, were also calculated. To study the association between PM2.5 and lipid/lipoprotein markers and account for repeated measurements in each woman, we applied linear mixed-effects regression models so that each woman had her own random intercept. First-order ante-dependence structure was specified for the covariance structure because longitudinal visits from each participant were not necessarily equidistant in time (Zimmerman and Núñez-Antón 1997). The endpoints, lipid/lipoprotein levels and the ratio of ApoB/ApoA1, were log-transformed to meet the normality assumption more closely and were included as continuous dependent variables. PM2.5 concentration of each exposure window was included in a single-pollutant model.
The regression model was adjusted for race/ethnicity, education, and time-varying variables, including visit number, season of the visit, participant’s age at the visit, menopause status, BMI, smoking status, diabetic status, alcohol consumption in the 24 hours before the blood draw, hormone use since last visit, and anti-lipidemic medication use. These covariates were selected based on their associations to the outcomes reported in the literature (Bind et al. 2016; Chuang et al. 2010), and the Akaike Information Criterion (AIC) values of the model were evaluated. These covariates remained in further stratified and sensitivity analyses.
Given the potential susceptibility of midlife women to adverse lipid changes, we also examined the associations between PM2.5 and lipid/lipoprotein level in stratified analyses by dyslipidemia and menopausal status. Dyslipidemia was present if any of the following criteria were met: fasting TG level ≥150 mg/dL (1.70 mmol/L) or non-fasting TG level ≥200 mg/dL (2.26 mmol/L); LDL-c ≥190 mg/dL (4.92 mmol/L); HDL-c <50 mg/dL (1.29 mmol/L); or use of any prescription lipid-lowering medications (e.g., statins) (Miller et al. 2011; Stone et al. 2014). A woman was considered dyslipidemic if she presented dyslipidemia in any completed visit(s), while those who were free of dyslipidemia in all visits were considered non-dyslipidemic. Given the small sample sizes, we combined early perimenopause and late perimenopause into perimenopause, in contrast to natural postmenopause. Those who were considered postmenopause due to surgery were not included in this stratified analysis.
We conducted a number of sensitivity tests. Because our previous analyses revealed associations between ambient gases and lipid level flutuations (Wu et al. 2017), we examined potential confounding by ambient gases, including O3, CO, NO2 and SO2, using two-pollutant models incorporating each gaseous pollutant and PM2.5 concentrations in separate models. In sensitivity analyses, we also excluded visits in which a participant reported having used hormones or took anti-lipidemic medications since the prior visit, women who had a hysterectomy, and women who had diabetes. As non-work physical activity scores were only available at Visits 3, 5 and 6, we assigned the values to Visits 4 and 7 with either the value from the previous visit or the mean of the visits before and after, and included it in the model.
Visits conducted after any major cardiovascular events, including myocardial infarction, congestive heart failure, stroke, percutaneous coronary intervention and coronary artery bypass graft, were excluded. Only a few participants from New Jersey had data for visits 6 and 7; so these data were censored. The association between PM2.5 exposure for each lipid/lipoprotein in each exposure window was considered individually. No adjustment was made for multiple comparisons to allow fair comparisons with other studies (Rothman 1990). All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Results were based on two-sided t-tests and 95% confidence intervals (CI).
Results
Distributions of Biomarkers and Exposures
The final study population included 8,698 serum samples collected from 2,289 women, with an average age of 49±3 years at visit 3 when air quality data became available. Study participants completed a mean of four annual visits, with a total of 62% of participants identified as having dyslipidemia, with higher rates among Hispanic (71%) and African American (68%) women. Seventy-four percent of women had entered perimenopause by visit 3. This proportion rose to 91% at visit 7, in which 56% of active participants were postmenopausal. Women with dyslipidemia and higher BMI had less healthy lipid profile, higher atherogenic lipoproteins and lower protective lipoproteins, and women in late peri- and natural postmenopause also showed higher levels of atherogenic lipoproteins (Table 1).
Table 1.
Median (interquartile range) lipid/lipoprotein concentrations (mg/dl) for the SWAN cohort, stratified by dyslipidemia status, BMI, and menopausal status, 1999–2005a
| Na | HDL | LDL | TG | TC | Lp(a) | LpA1 | ApoA1 | ApoB | ||
|---|---|---|---|---|---|---|---|---|---|---|
| All participants baseline | 1838 | 57 (21) | 111 (39) | 102 (76) | 195 (46) | 15 (34) | 54 (21) | 163 (37) | 110 (37) | |
| Dyslipidemia | Yes | 5,437 | 50 (14) | 120 (45) | 134 (87) | 201 (52) | 16 (40) | 47 (17) | 157 (35) | 116 (39) |
| No | 3,259 | 68 (17) | 107 (37) | 79 (34) | 194 (41) | 16 (36) | 62 (19) | 182 (33) | 95 (28) | |
| BMI | <25 | 3,221 | 65 (21) | 110 (40) | 89 (50) | 197 (45) | 12 (26) | 59 (23) | 177 (38) | 101 (34) |
| 25+ | 5,262 | 53 (17) | 118 (42) | 119 (86) | 200 (49) | 19 (46) | 50 (19) | 161 (36) | 112 (39) | |
| Menopausal status | Pre | 494 | 53 (21) | 111 (38) | 98 (68) | 189 (38) | 12 (32) | 51 (21) | 161 (37) | 105 (35) |
| Early peri | 3,342 | 56 (19) | 111 (38) | 98 (67) | 191 (44) | 16 (38) | 53 (20) | 163 (35) | 104 (35) | |
| Late peri | 883 | 57 (21) | 122 (45) | 109 (85) | 206 (51) | 19 (47) | 52 (20) | 167 (38) | 112 (39) | |
| Natural post | 2,617 | 58 (21) | 122 (46) | 111 (76) | 207 (50) | 17 (37) | 53 (20) | 171 (41) | 112 (38) | |
N is the number of observations, not women; each participant has data for multiple visits, and could be in different categories for subsequent visits. Sample size varied by biomarkers. Visits without any blood data (N=69) or any matching exposure data (N=5147) were excluded. Visits 6 and 7 in New Jersey were censored due to small sample size (N=55). Data from visits after any CVD events (N=11 from 4 participants) were excluded. Marker values out of reasonable ranges (0.02–8.2% varied by markers) were excluded.
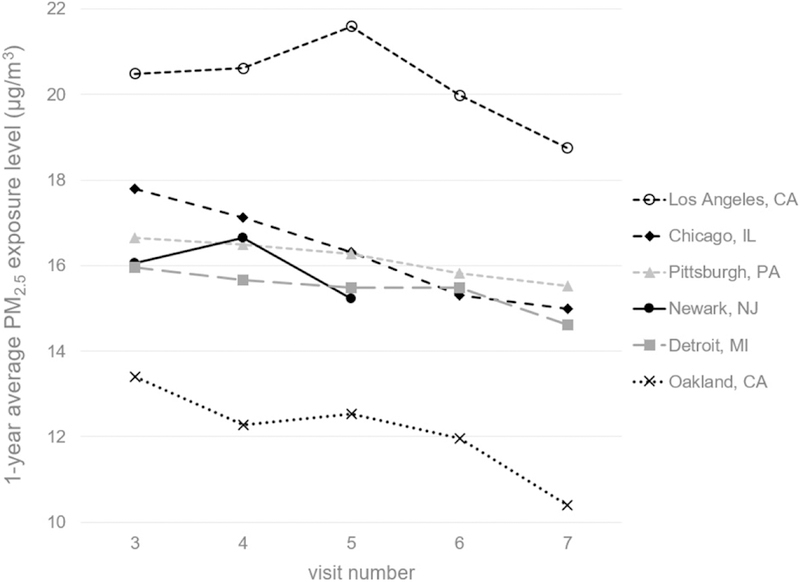
Among the six participating sites, PM2.5 exposure levels were highest in Los Angeles, CA and lowest in Oakland, CA for both short- and long-term (Table S1). PM2.5 exposure levels declined for each site over time during the study period (Fig 1), which was likely attributable to the nationalwide standards to reduce particle emissions. Based on the data from visit 3, PM2.5 concentrations were moderately correlated with NO2 concentrations, with Spearman correlation coefficients (r) ranging from 0.42 to 0.63, depending on exposure windows. One-year average PM2.5 concentrations were also moderately correlated with O3 concentrations (r = 0.36); otherwise, PM2.5 was barely or weakly correlated with ambient O3, CO or SO2 (r < 0.30).
Figure 1.
Average one-year PM2.5 exposure levels of SWAN participants at six sites across visits 3 to 7 (1999–2005).
Associations in total cohort
Based on the interquartile values of different exposure windows (Table S1), we calculated the percent changes in lipid/lipoprotein levels per 10, 5 and 3 μg/m3 increase of PM2.5 concentrations for short-, intermediate- and long-term exposure, respectively. In the total cohort, long-term PM2.5 exposures, especially one-year average exposure, were positively associated with Lp(a) and ApoB and negatively associated with HDL and ApoA1 (Table 2). The effect of long-term PM2.5 exposure on the ratio of ApoB/ApoA1 was prominent, with 1.4% (95% CIs: 0.5%, 2.3%) and 0.8% (0.3%, 1.3%) increases per 3 μg/m3 increase in the one-year and six-month average PM2.5 concentrations, respectively. The average prior 30-day PM2.5 exposure was marginally associated with increased TC. No association was observed with short-term PM2.5 exposure.
Table 2.
Percent of change in lipid/lipoprotein levels per an interquartile increase in PM2.5 exposure for SWAN cohort, 1999–2005a.
| Marker | Exposure window | N | 95%CI |
|---|---|---|---|
| HDL-c | 1-day | 5,115 | −0.01 (−0.3, 0.3) |
| 30-day | 6999 | 0.2 (−0.1, 0.6) | |
| 6-month | 6830 | −0.1 (−0.5, 0.3) | |
| 1-year | 6359 | −0.7 (−1.4, −0.1)** | |
| LDL-c | 1-day | 5054 | 0.03 (−0.4, 0.5) |
| 30-day | 6918 | 0.4 (−0.1, 0.8) | |
| 6-month | 6751 | −0.04 (−0.6, 0.5) | |
| 1-year | 6284 | 0.4 (−0.5, 1.3) | |
| TG | 1-day | 5120 | −0.3 (−1.1, 0.5) |
| 30-day | 7002 | 0.1 (−0.7, 0.9) | |
| 6-month | 6833 | 0.04 (−0.9, 1.0) | |
| 1-year | 6362 | −0.2 (−1.6, 1.4) | |
| TC | 1-day | 5113 | −0.1 (−0.4, 0.2) |
| 30-day | 6997 | 0.3 (−0.01, 0.6)* | |
| 6-month | 6828 | −0.01 (−0.4, 0.3) | |
| 1-year | 6357 | −0.02 (−0.6, 0.5) | |
| Lp(a) | 1-day | 4812 | −0.3 (−1.5, 0.9) |
| 30-day | 6581 | 0.3 (−1.0, 1.5) | |
| 6-month | 6414 | 0.5 (−1.0, 2.1) | |
| 1-year | 5957 | 3.8 (1.0, 6.6)*** | |
| LpA1 | 1-day | 2476 | −0.05 (−0.8, 0.7) |
| 30-day | 3460 | 0.4 (−0.3, 1.1) | |
| 6-month | 3301 | 0.05 (−0.7, 0.8) | |
| 1-year | 2920 | −0.4 (−1.4, 0.6) | |
| ApoA1 | 1-day | 5028 | −0.1 (−0.4, 0.2) |
| 30-day | 6876 | −0.03 (−0.3, 0.3) | |
| 6-month | 6711 | −0.2 (−0.6, 0.1) | |
| 1-year | 6244 | −0.6 (−1.1, −0.1)** | |
| ApoB | 1-day | 4992 | 0.2 (−0.2, 0.6) |
| 30-day | 6833 | 0.2 (−0.2, 0.5) | |
| 6-month | 6668 | 0.5 (0.04, 1.0)** | |
| 1-year | 6207 | 0.5 (−0.3, 1.2) | |
| ApoB/ApoA1 | 1-day | 4924 | 0.3 (−0.1, 0.7) |
| 30-day | 6740 | 0.2 (−0.2, 0.7) | |
| 6-month | 6579 | 0.8 (0.3, 1.3)*** | |
| 1-year | 6122 | 1.4 (0.5, 2.3)*** |
Analyses were based on log-transformed biomarker levels, adjusted for race/ethnicity, education, visit number, season of the visit, age at visit, menopausal status, BMI, smoking status, diabetic status, alcohol consumption in the last 24 hours, hormone use, and anti-lipidemic medication use. The unit changes of PM2.5 exposure used in this calculation were 10 μg/m3 for short-term exposure (1-day window), 5 μg/m3 for intermediate-term exposure (30-day window), and 3 μg/m3 for long-term exposure (6-month and 1-year windows).
The associations mentioned above remained robust in the sensitivity analyses, excluding women who had a previous hysterectomy, visits in which a participant reported having used hormones or took anti-lipidemic medications since the prior visit, and excluding women who had reported having diabetes mellitus. Including non-work physical activity scores in the model did not change the statistical significance of these associations.
Most of the associations mentioned above remained after adjusting for ambient gases with few exceptions (Fig S1). Briefly, the associations between ApoB and 6-month PM2.5 exposure were slightly weaker when adjusting for ambient gases, especially SO2; and the associations of HDL and ApoA1 with one-year PM2.5 exposure were reduced by NO2, possibly due to the correlation between one-year average PM2.5 and NO2 concentrations.
Stratification by dyslipidemia status
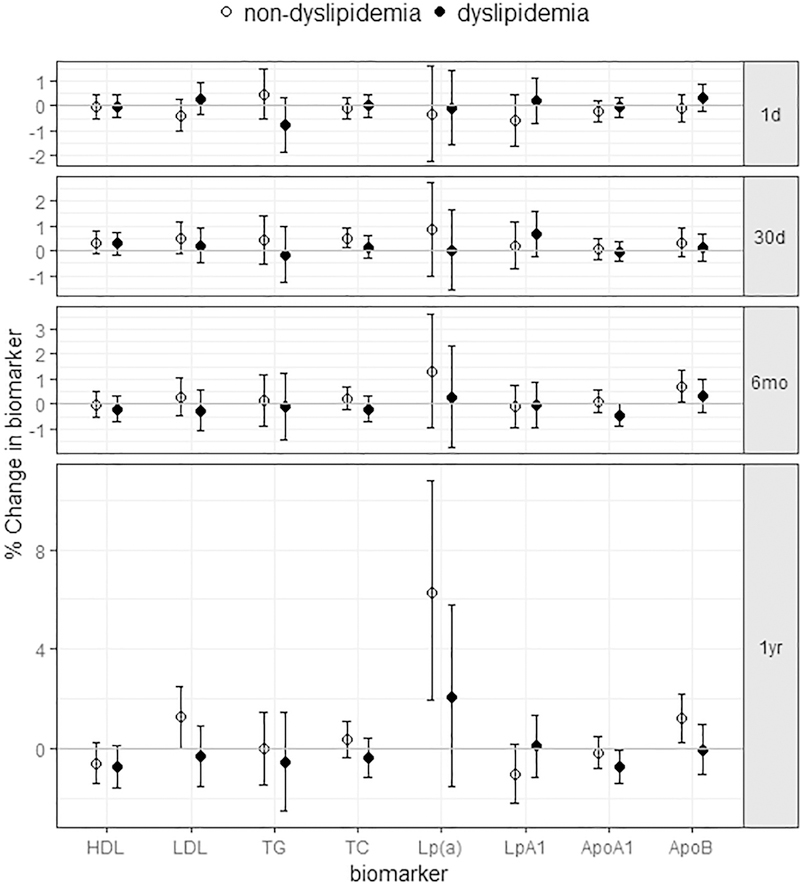
The stratification by dyslipidemia status revealed different patterns with the associations between PM2.5 exposure and lipids/lipoproteins among women with differing health profiles (Fig. 2, Table S2). Again, most associations were observed in the long-term exposure windows. Positive associations were observed between one-year or six-month PM2.5 exposure and atherogenic lipoproteins, including LDL-c, Lp(a) and ApoB, as well as between 30-day PM2.5 exposure and TC, only among women without dyslipidemia. Specifically, a 1.3% (0.03%, 2.5%) increase in LDL, 6.3% (1.9%, 10.8%) increase in Lp(a), and a 1.2% (0.3%, 2.2%) increase in ApoB were associated per 3 μg/m3 increase of the one-year average PM2.5 concentration. In contrast, negative associations for protective lipoproteins, i.e., HDL-c and ApoA1, only appeared among women with dyslipidemia. The ApoB/ApoA1 ratios increased with long-term PM2.5 exposure in both women with and without dyslipidemia (not shown).
Figure 2.
The estimated associations between PM2.5 exposure in different time windows and lipid/lipoproteins, stratified by dyslipidemia status. (Data shown are the mean estimates and error bars represent 95% confidence intervals.)
Stratification by menopausal status
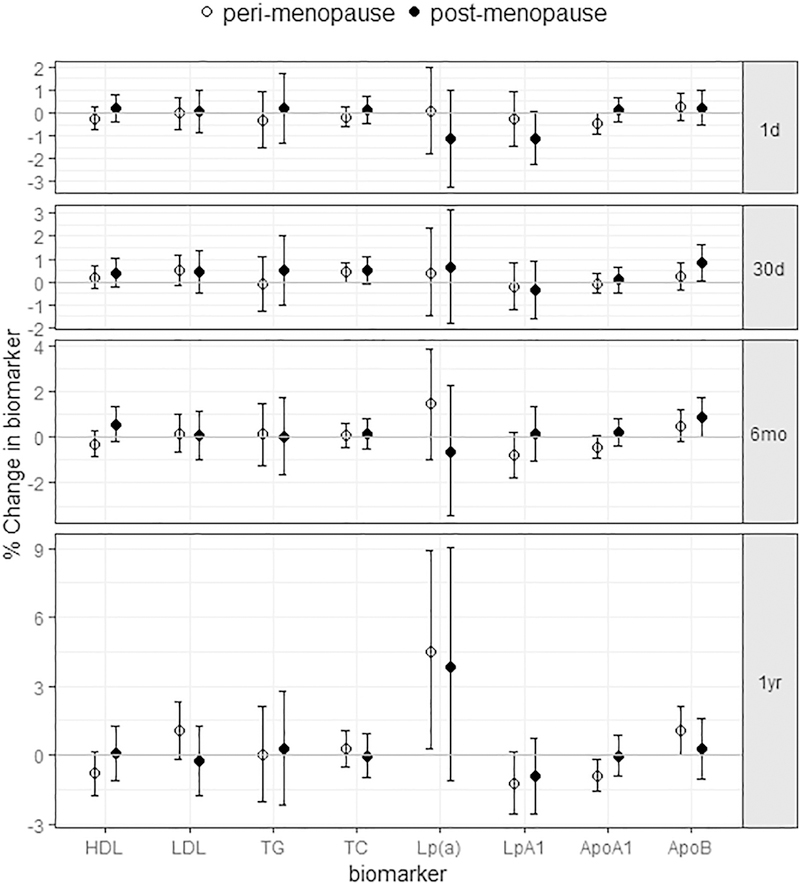
Both the positive associations with atherogenic lipoproteins and the negative associations with protective lipoproteins with PM2.5 exposure were observed among perimenopausal women; specifically, each 3 μg/m3 increase of one-year PM2.5 concentrations was associated with increases of 1.1% (−0.2%, 2.3%) in LDL-c, 4.5% (0.3%, 8.9%) in Lp(a), and 1.1% (0.02%, 2.1%) in ApoB, as well as decreases of −0.8% (−1.8%, 0.1%) in HDL-c, −1.2% (−2.6%, 0.1%) in LpA1, and −0.9% (−1.6%, −0.2%) in ApoA1, which appeared stronger than those in the total cohort (Fig 3, Table S3). With the same increment in exposure, ApoB/ApoA1 increased 2.4% (1.2%, 3.7%) during perimenopause. In addition, we also observed an increase in TC associated with 30-day PM2.5 exposure and decreased ApoA1 associated with one-day PM2.5 exposure.
Figure 3.
The estimated associations between PM2.5 exposure in different time windows and lipid/lipoproteins for perimenopause and postmenopause. (Data shown are the mean estimates and error bars represent 95% confidence intervals.)
Associations with one-year PM2.5 exposure became attenuated among postmenopausal women. For them, changes in lipids/lipoproteins tended to be associated with more recent exposure, instead of long-term exposure. Every 5 μg/m3 increase of 30-day PM2.5 concentrations was associated with 0.5% (−0.1%, 1.1%) increase in TC, 0.8% (0.04, 1.6%) increase in ApoB, and 0.9% (−0.03%, 1.7%) increase in ApoB/ApoA1 ratio. LpA1 dropped −1.1% (−2.3%, 0.04%) per 10 μg/m3 increase of one-day PM2.5 concentrations.
Discussion
Using longitudinal serum data from the SWAN cohort, we examined the relationship between PM2.5 exposure and lipids/lipoprotein levels among midlife women. We observed elevated levels of atherogenic lipoproteins, such as, LDL-c, ApoB, and Lp(a), as well as reduced protective lipoproteins, namely, HDL-c, ApoA1 and LpA1, associated with PM2.5 exposure, particularly with long-term exposure. Stratification by dyslipidemia and menopausal status revealed different patterns of lipid changes among women with differing health profiles, but in all subgroups, increasing annual PM2.5 exposure was associated with increased ApoB/ApoA1 ratios. These results suggested that PM2.5 exposure, especially long-term exposure, exhibit adverse associations with lipids/lipoproteins, and thus, increase the chance of developing CVD.
The associations we observed were consistent with a few earlier studies. Long-term exposures to PM2.5 and/or PM10 exposure have been associated with elevated LDL-c, TC, ApoB, and reduced HDL (Cai et al. 2017; Chen et al. 2016; Chuang et al. 2011; Shanley et al. 2016). Three-month exposures to PM2.5 were found to increase LDL-c, TG, TC, as well as decreases in HDL-c (Yitshak Sade et al. 2016) or HDL particle number (Bell et al. 2017). However, we did not observe a negative association between 30-day exposure with HDL-c or ApoA1. Regarding short-term exposure, a controlled exposure assessment study suggested decreased HDL anti-oxidant/anti-inflammatory capacity shortly after exposure to a high level of PM2.5 (150 μg/m3) (Ramanathan et al. 2016). Most other studies reported no associations with short-term exposure (up to a 7-day average) to ambient PM2.5 at moderate levels (Bell et al. 2017; Yeatts et al. 2007; Yitshak Sade et al. 2016), also consistent with our findings.
Adjustment for co-pollutants suggested confounding by ambient gases for some associations we observed. Our earlier study reported NO2 and SO2 to be associated with changes in lipid/lipoprotein levels in this cohort (Wu et al. 2017). In that study, a residual model was employed to account for the correlation between ambient PM and NO2 concentrations, which revealed that both one-year exposures to PM2.5 and NO2 were associated with decreased HDL-c and ApoA1. SO2 reduced the association between one-year PM2.5 exposure and ApoB, due to its strong positive association with ApoB. Ozone and CO also slightly reduced the associations between one-year PM2.5 exposure and ApoB, though they were not statistically associated with ApoB.
The different change patterns of lipids/lipoproteins by women’s dyslipidemia status indicated that the lipid metabolism in women with and without pre-existing health conditions may respond to PM2.5 exposure differently. Upon exposure to PM2.5, the atherogenic lipoproteins Lp(a) and ApoB increased among women without dyslipidemia, which was a relatively healthier subgroup in the overall cohort. For women with dyslipidemia, whose atherogenic lipoproteins may have already been at unfavorable levels, protective lipids/lipoproteins further decreased with PM2.5 exposure. Both pathways could increase CVD risks.
This study results brings attention to the susceptibility of women during the menopausal transition. The menopausal transition is marked by rising LDL and ApoB levels around the final menstrual period, as previous studies and our study have observed, which has been associated with greater risk of CVD (de Aloysio et al. 1999; Derby et al. 2009; Matthews et al. 2009, 2017). We also observed reduced protective lipids/lipoproteins associated with PM2.5 among women during perimenopausal visits, highlighting the potential susceptibility of perimenopausal women to environmental influences on these lipid measures. Among women who transitioned to postmenopause, strong associations were not observed. Given that the sample size of postmenopausal visits included in this analysis was 40% less than that of perimenopausal visits, the power to detect associations in this subgroup was limited compared to perimenopausal subgroup. The physiological mechanism behind the different associations by menopausal status were still unclear, since previous menopausal studies usually treated perimenopausal and postmenopausal periods together. Future studies with additional follow-up in the postmenopausal period are needed to address fully the impact of PM2.5 on lipids/lipoproteins in postmenopausal women.
The biological mechanisms involved in PM-induced cardiovascular associations have been studied extensively, and new evidence continues to emerge (Brook et al. 2010; Libby et al. 2002). Besides causing systemic oxidative stress and inflammation, recent toxicologic evidence suggests that PM2.5 caused aggregation of ApoA1, accelerated oxidation of LDL, and degradation of ApoB, and thus, disrupts their normal functions (Kim et al. 2015; Sun et al. 2009). Aggregation of ApoA1 could reduce the number of exposed epitopes, so that the amount of functioning ApoA1 would be reduced. Meanwhile, as the ligand for the LDL receptor, degradation of ApoB makes it harder to clear LDL from circulation. Further evidence suggests that ultrafine component (diameters < 1 microm) is primarily responsible for the changes in lipids, inducing lipid peroxidation and impairing HDL anti-oxidant and anti-inflammatory function (Araujo et al. 2008; Huang et al. 2003; Li et al. 2013; Yin et al. 2013).
We note that the toxicological evidence was mostly observed under short- or intermediate-term exposures to high concentrations of PM2.5. HDL and LDL turn over quickly within hours after episodic oxidative stress (Bowry et al. 1992). Therefore, the changes in lipid levels due to short- or intermediate-term PM2.5 exposures are expected to be smaller and may not be detectable. However, consistent oxidative stress could significantly reduce HDL levels, inhibiting its protective function, which has been observed among hemodialysis patients and patients with rheumatoid arthritis (Morena et al. 2000; Rho et al. 2010). Long-term PM2.5 exposure may similarly exert consistent oxidative stress, resulting in reduced HDL-related lipoprotein levels.
The present study has three advantages. First, it was based on a relatively large cohort from multiple sites across the U.S., with valid longitudinal documentation of exposures and outcome measures as well as potential confounding variables. Secondly, we examined the associations between short-, intermediate-, and long-term ambient PM2.5 exposure and lipid/lipoprotein levels in the same cohort, providing an overview of the impact and relative importance of PM2.5 exposures in different time windows on lipid mechanism. Lastly, our analyses explored these associations in particular subgroups, those with dyslipidemia and perimenopausal women, and revealed different pathways of adverse changes in lipids/lipoproteins varying by health condition. Learning these impacts may help medical practitioners make care or treatment plan for individuals in the sensitive subgroups.
Meanwhile, results should be interpreted considering the following limitations. First, we assigned air pollution exposure using the regulatory monitoring networks around a buffer area, which may have introduced exposure misclassification. Regulatory monitoring of PM2.5 started in 1999 or 2000. Satellite or inventory data at that time were very limited and did not necessarily cover all of our study areas. Therefore, we had to rely on central-site ambient monitoring data as many similar studies conducted in those early years did. We observed fair variations of PM2.5 levels from multiple monitoring stations within each site (Table S1), similar to those reported by another large multi-site study, the Multi-Ethnic Study of Atherosclerosis (Bell et al. 2017). With the understanding of these limitations, we based exposure on participants’ residential history and optimized the method used to select each monitor. Secondly, some known CVD risk factors and potential confounding factors, such as noise and dietary intake that may have resulted in residual confounding, were not considered in this study. Future studies with consideration of these factors are warranted. Lastly, as mentioned above, to allow fair comparison with similar studies, we did not make adjustment for multiple comparisons. It is up to readers if they would like to interpret results with adjustment.
Conclusions
In summary, our findings indicate PM2.5 exposure, especially the long-term exposure, harms lipid metabolism among midlife women. Midlife women are potentially subject to greater risks of CVD due to the physiological changes during the menopausal transition and the high prevalence of dyslipidemia in this population. The findings of this study raise concern about the impact of long-term exposure to fine particles on lipids/lipoproteins, which merit further toxicological studies on air pollution induced lipid/lipoprotein metabolism.
Supplementary Material
Highlights.
We examined the associations of ambient PM2.5 exposure with lipids/lipoproteins.
PM2.5 exposures could increase atherogenic and reduce protective lipoproteins.
Most of the adverse impact was observed in long-term exposure windows.
PM2.5 influenced different lipoproteins depending on one’s health status.
Long-term exposure to ambient PM2.5 may increase CVD risks in midlife women.
Acknowledgement / Source of Financial Support
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, NIH, the State of California, or the California Environmental Protection Agency.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016 - present; Winifred Rossi 2012 – 2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chai
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflicts of Interest: The authors declare they have no actual or potential competing financial interests.
References
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. 2008. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Wu X, Malig BJ, Broadwin R, Gold EB, Qi L, et al. 2016. Estimating the associations of apparent temperature and inflammatory, hemostatic, and lipid markers in a cohort of midlife women. Environ Res 152:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G, Mora S, Greenland P, Tsai M, Gill E, Kaufman JD. 2017. Association of air pollution exposures with high-density lipoprotein cholesterol and particle number: The multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 37:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD. 2011. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. J Expo Sci Environ Epidemiol 21:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaton V 2003. The role of lipids in the development of atherosclerosis and coronary heart disease: Guidelines for diagnosis and treatment. In: electronic Journal of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), Vol. 14. [PMC free article] [PubMed] [Google Scholar]
- Bowry VW, Stanley KK, Stocker R. 1992. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A 89:10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- Burnett JR. 2004. Lipids, lipoproteins, atherosclerosis and cardiovascular disease. Clin Biochem Rev 25:2. [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Hansell AL, Blangiardo M, Burton PR, BioShaRe, de Hoogh K, et al. 2017. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the hunt and lifelines cohorts. Eur Heart J 38:2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena R, Duriez P, Fruchart JC. 2004. Atherogenic lipoprotein particles in atherosclerosis. Circulation 109:III2–7. [DOI] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, et al. 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in mexican americans. Diabetes Care 39:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Cheng TJ. 2010. Effect of air pollution on blood pressure, blood lipids, and blood sugar: A population-based approach. J Occup Environ Med 52:258–262. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in taiwan. Occup Environ Med 68:64–68. [DOI] [PubMed] [Google Scholar]
- de Aloysio D, Gambacciani M, Meschia M, Pansini F, Bacchi Modena A, Bolis PF, et al. 1999. The effect of menopause on blood lipid and lipoprotein levels. The icarus study group. Atherosclerosis 147:147–153. [DOI] [PubMed] [Google Scholar]
- Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. 2009. Lipid changes during the menopause transition in relation to age and weight: The study of women’s health across the nation. Am J Epidemiol 169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. 1993. An association between air pollution and mortality in six u.S. Cities. N Engl J Med 329:1753–1759. [DOI] [PubMed] [Google Scholar]
- Ebisu K, Belanger K, Bell ML. 2014. The association between airborne pm2.5 chemical constituents and birth weight-implication of buffer exposure assignment. Environ Res Lett 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. 2006. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health 96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, et al. 2016. Long- and short-term exposure to air pollution and inflammatory/hemostatic markers in midlife women. Epidemiology 27:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SL, Hsu MK, Chan CC. 2003. Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environ Health Perspect 111:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. 2012. Emerging risk factors collaboration: C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 367:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lee EY, Choi I, Kim J, Cho KH. 2015. Effects of the particulate matter 2.5 (pm2.5) on lipoprotein metabolism, uptake and degradation, and embryo toxicity. Mol Cells 38:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, et al. 2013. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLr-null mice. J Lipid Res 54:1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. 2002. Inflammation and atherosclerosis. Circulation 105:1135–1143. [DOI] [PubMed] [Google Scholar]
- Lowe GD, Danesh J, Lewington S, Walker M, Lennon L, Thomson A, et al. 2004. Tissue plasminogen activator antigen and coronary heart disease. Prospective study and meta-analysis. Eur Heart J 25:252–259. [DOI] [PubMed] [Google Scholar]
- Lu M, Lu Q, Zhang Y, Tian G. 2011. Apob/apoa1 is an effective predictor of coronary heart disease risk in overweight and obesity. Journal of Biomedical Research 25:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. 2009. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol 54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas-Mitchell EJ, et al. 2017. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke 48:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. 2007. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356:447–458. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. 2011. Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation 123:2292–2333. [DOI] [PubMed] [Google Scholar]
- Morena M, Cristol JP, Dantoine T, Carbonneau MA, Descomps B, Canaud B. 2000. Protective effects of high-density lipoprotein against oxidative stress are impaired in haemodialysis patients. Nephrol Dial Transplant 15:389–395. [DOI] [PubMed] [Google Scholar]
- Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. 2011. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 8:222–232. [DOI] [PubMed] [Google Scholar]
- Nofer J-R, Kehrel B, Fobker M, Levkau B, Assmann G, Eckardstein Av 2002. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 161:1–16. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Hellmann J, Wheat L, Haberzettl P, Lee J, Conklin DJ, et al. 2010. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ Res 107:200–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. 2004. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 109:71–77. [DOI] [PubMed] [Google Scholar]
- Ramanathan G, Yin F, Speck M, Tseng CH, Brook JR, Silverman F, et al. 2016. Effects of urban fine particulate matter and ozone on HDL functionality. Part Fibre Toxicol 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, et al. 2010. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res (Hoboken) 62:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. 1990. No adjustments are needed for multiple comparisons. Epidemiology 1:43–46. [PubMed] [Google Scholar]
- Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J. 2016. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 27:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Patterson C, Yarnell J, Rumley A, Ben-Shlomo Y, Lowe G. 2005. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? The caerphilly study. Circulation 112:3080–3087. [DOI] [PubMed] [Google Scholar]
- Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, et al. 2000. Swan: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Menopause: Biology and pathobiology, (RA Lobo JK, Marcus R, ed). San Diego:Academic Press, 175–188. [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2014. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation 129:S1–45. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PP. 2003. Reverse cholesterol transport: High-density lipoprotein’s magnificent mile. Current Atherosclerosis Reports 5:386–393. [DOI] [PubMed] [Google Scholar]
- Toth PP, Potter D, Ming EE. 2012. Prevalence of lipid abnormalities in the united states: The national health and nutrition examination survey 2003–2006. J Clin Lipidol 6:325–330. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. 2009. Final report: Integrated science assessment for particulate matter EPA/600/R-08/139F. Washington, DC. [Google Scholar]
- Wu X, Basu R, Malig B, Broadwin R, Ebisu K, Gold EB, et al. 2017. Association between gaseous air pollutants and inflammatory, hemostatic and lipid markers in a cohort of midlife women. Environ Int 107:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, et al. 2007. Coarse particulate matter (pm2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect 115:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Lawal A, Ricks J, Fox JR, Larson T, Navab M, et al. 2013. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 33:1153–1161. [DOI] [PubMed] [Google Scholar]
- Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. 2016. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab:jc20161378. [DOI] [PubMed]
- Zimmerman DL, Núñez-Antón V. 1997. Structured antedependence models for longitudinal data. In: Modelling longitudinal and spatially correlated data:Springer; New York, 63–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.