Abstract
Bicuspid aortic valve disease is the most common congenital cardiac disorder, being present in 1% to 2% of the general population. Associated aortopathy is a common finding in patients with bicuspid aortic valve disease, with thoracic aortic dilation noted in approximately 40% of patients in referral centers. Several previous consensus statements and guidelines have addressed the management of bicuspid aortic valve–associated aortopathy, but none focused entirely on this disease process. The current guidelines cover all major aspects of bicuspid aortic valve aortopathy, including natural history, phenotypic expression, histology and molecular pathomechanisms, imaging, indications for surgery, surveillance, and follow-up, and recommendations for future research. It is intended to provide clinicians with a current and comprehensive review of bicuspid aortic valve aortopathy and to guide the daily management of these complex patients.
Central Message
The current document is the full online-only version of “The American Association for Thoracic Surgery Consensus Guidelines on Bicuspid Aortic Valve–Related Aortopathy.”
Perspective
BAV-related aortopathy is a common clinical entity. An increasing amount of literature has recently shown that BAV aortopathy is less dangerous than previously described. The current document is a comprehensive review of BAV-related aortopathy and its management.
Graphical Abstract
Typical patient with BAV and associated aortopathy.
1. INTRODUCTION
Bicuspid aortic valve (BAV) disease is the most common congenital cardiac disorder, being present in 1% to 2% of the general population.1 Associated aortopathy is a common finding in patients with BAV disease, with thoracic aortic dilation noted in approximately 40% of patients in referral centers.2 The risk of acute aortic emergencies, most commonly aortic dissection, is higher in patients with BAV disease than in the general population.3 Optimal timing of surgical intervention, to avoid aortic emergencies, is defined as that time point when the risk of conservative management exceeds the risk of surgery. However, precise determination of this time point is difficult and depends on several factors, including patient age, risk factors, comorbidities, family history, and presence or absence of significant aortic valvular disease.
Historically, aortopathy observed in patients with BAV disease was thought to be no different than that associated with tricuspid aortic valve (TAV) disease. That is, aortic dilation was thought to be due to turbulent blood flow downstream from a stenotic aortic valve. In the 1990s and 2000s, however, several observations and studies led investigators to think that a strong genetic role contributed to BAV-associated aortopathy and that the risk of acute aortic complications was substantially increased in this patient population.4 Such hypotheses led to recommendations for a more aggressive surgical approach to this disease, with some suggesting that BAV aortopathy was roughly equivalent to Marfan syndrome.5 Subsequent studies and observations have led to a middle ground, however, suggesting that hemodynamic and genetic components play varying roles in different subgroups of patients with BAV6 and that the risk of aortic emergencies is not as high as previously thought in this patient population.7,8 Determination of the cause of BAV aortopathy is important because of the therapeutic implications for patients with isolated aneurysmal dilation of the aorta and in those undergoing aortic valve surgery for BAV disease.
Several previous documents have addressed the management of BAV-associated aortopathy, with the first being a set of multisocietal guidelines published in 2010.5 This document made aggressive recommendations for the management of BAV aortopathy, grouping such patients in with Marfan and other connective tissue disorders. However, multiple studies reported since that time have provided new insights into the pathophysiology and mechanistic aspects of BAV aortopathy. As such, a more conservative set of recommendations was made in the more recently published valvular heart disease guidelines by the American Heart Association (AHA) and the American College of Cardiology (ACC).9 The marked difference in the positions of these 2 sets of guidelines resulted in the recent publication of a clarification statement.10 The European Society of Cardiology (ESC) also published guidelines on the management of valvular heart disease in 201211 and aortic disease specifically in 2014.12 Both of these documents contained more conservative recommendations for BAV aortopathy, in line with the 2014 ACC/AHA guidelines.
Of these publications, none focused entirely on patients with BAV aortopathy. Therefore, the current consensus statement differs in that it covers all major aspects of BAV aortopathy, including its natural history, phenotypic expression, histology and molecular pathomechanisms, imaging, indications for surgery, surveillance and follow-up, and recommendations for future research. Such research will hopefully lead to new insights into this common disease and the need for an update to the current consensus statement in a few years.
BAV aortopathy is a markedly heterogeneous entity. Dilation may occur in the aortic root, the tubular ascending aorta, the proximal aortic arch, or any contiguous combination of these 3.13 The current document uses the term “aortic root” to refer to the proximal aorta extending from the nadir of the aortic annulus to the sinotubular junction (STJ) including the coronary ostia. For purposes of consistency, the term “tubular aorta” (also known as the “supracoronary aorta”) is used to describe the area between the STJ and the takeoff of the brachiocephalic artery. The “aortic arch” refers to the area extending from the brachiocephalic to the left subclavian artery. In addition, the Sievers’ classification14 is used to describe BAV morphology (Figure 1). Although several different classification systems have been used to describe BAV morphology, the Sievers’ system is the one that is used most commonly within the cardiac surgery literature. However, it is clear that a more comprehensive classification system that takes into consideration BAV morphology, BAV pathology (ie, stenosis, insufficiency, or mixed), and location and extent of associated BAV aortopathy is required.
FIGURE 1.
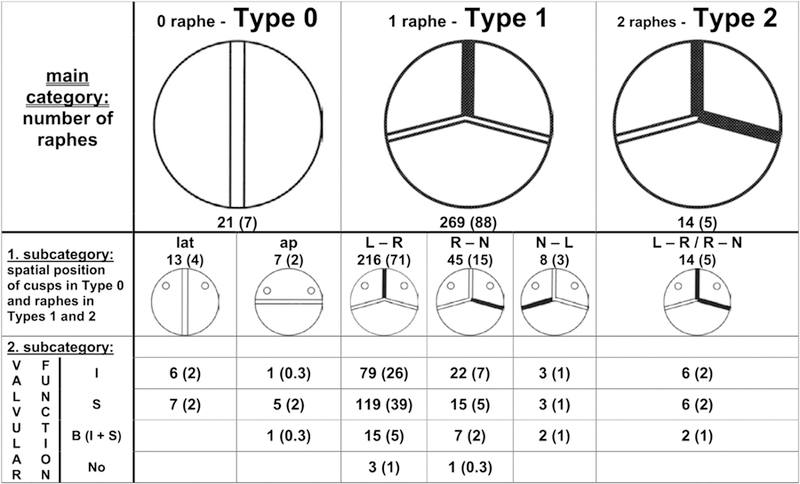
Sievers’ classification system for BAVas viewed from the surgeon’s side with the left coronary artery at left. The number of specimens is given, and the percentage is shown in parentheses. The blackened lines represent raphe. The main category is based on the number of raphes, the first subcategory is based on spatial position, and the second subcategory reflects valve function. Ap, Anterior-posterior; B, balanced valvular lesion; I, insufficiency; L, left coronary sinus; lat, lateral; N, noncoronary sinus; No, normal function; R, right coronary sinus; S, stenosis. Used with permission from Sievers and Schmidtke.14
Because BAV aortopathy is a relatively common disorder, decisions regarding its therapeutic management must be made by cardiovascular clinicians on a regular basis. Despite this, there is significant confusion within the cardiovascular community regarding appropriate decision-making in this patient population.15 The confusion is not surprising, however, given the described differences in recommendations made by various societies and the shifting discussion on the cause and pathophysiology of BAV aortopathy. The purpose of this consensus statement is to provide clinicians with a current and comprehensive review of all major aspects of BAV aortopathy and to serve as a guide in the daily management of these complex patients.
2. EPIDEMIOLOGY AND NATURAL HISTORY
A. Epidemiology
Aortic enlargement and aneurysm formation, especially in the ascending aorta, are part and parcel of BAV disease—the so-called bicuspid aortopathy.
I. Prevalence
The prevalence of BAV in the general population is known to be 1% to 2%.1,16–20 Hoffman and Kaplan1 report a prevalence in this range based on “unselected consecutive necropsies,” which they consider the “standard” for detection. This makes BAV the most common congenital anomaly affecting the human heart (if one excludes tiny muscular ventricular septal defects that close spontaneously by 1 year of age). It is said that BAV accounts for more morbidity and mortality than all other congenital heart lesions combined.18,21 This mortality may be incurred via multiple disease mechanisms: aortic stenosis (AS), aortic insufficiency (AI), or ascending aortic aneurysm and dissection. Male patients are thought to predominate, by a margin of approximately 2 to 1.22 It has been shown in a single-center experience that 50% of all aortic valve operations performed on patients aged more than 50 years are done for BAV disease.23 Likewise, 50% of all valve operations performed in patients with coarctation of the aorta can be attributed to BAV disease.24
Although these statistics are staggering, the true burden of BAV disease may be grossly underestimated, because it may remain asymptomatic in childhood and even into adulthood, so that no imaging studies are indicated or performed.25
II. Likelihood of aneurysm development in patients with bicuspid aortic valve disease
Multiple studies have quantified the risk over time of development of dilatation of the ascending aorta (to a size of 4.0–4.5 cm) in patients with BAV. These studies indicate that 20% to 30% of patients with BAV develop aneurysmal enlargement during a follow-up of 9 to 25 years.26–28 A recent review article suggests that up to 84% of patients with BAV may ultimately develop an aneurysm (based on 8 individual studies). The risk of aneurysm development was found to be 80-fold higher than for the general population.28
III. Aneurysm location
Heterogeneity is the rule in terms of the segment of the ascending aorta involved by bicuspid aneurysm.26 The tubular ascending aorta is most commonly involved (60%−70% of bicuspid aneurysms), although all segments, including the aortic root and the aortic arch, can be involved (Figure 2). There is evidence that the “root phenotype” of BAV aortopathy, in which the predominant dilatation is at the level of the sinuses of Valsalva, represents a more malignant and rapidly progressive aortopathy (Section 3.C).29–32
FIGURE 2.
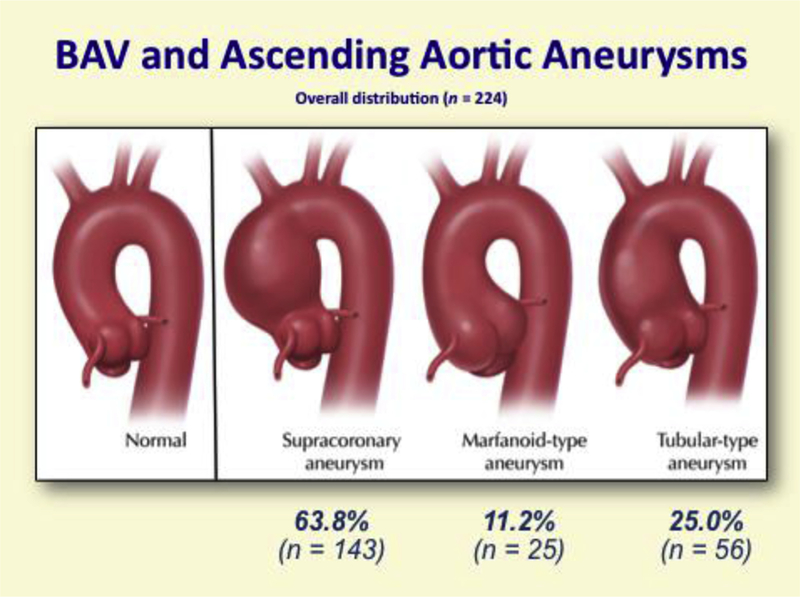
Anatomic distribution of aortic aneurysms in patients with BAV. BAV, Bicuspid aortic valve. (Aortic Institute at Yale-New Haven, unpublished data, June 30, 2017.) These data are similar to those reported by Michelena and colleagues.26
The marked heterogeneity of BAV-associated aneurysm location is distinctly different from other common types of ascending aortic aneurysms. Degenerative aneurysms tend to start in the mid-ascending aorta and then progress distally and proximally, whereas those associated with connective tissue disease are usually confined to the aortic root. BAV morphology and pathology seem to play a role in determining where the BAV-associated aneurysm is located (Section 3).
IV. Major role in causation of aortic dissection
An important point to note is the large number of aortic dissections associated with BAV aortopathy. Although only 5% or less of patients with BAV will have aortic dissection over a lifetime,21 BAV disease affects 1 in every 50 to 100 human beings. Thus, not only the better appreciated Marfan syndrome but also BAV is an important cause of aortic dissection.3 However, recent studies have found substantially lower rates of aortic dissection in patients with BAV than previously determined, especially in younger patients.27,33 For instance, in a recent study by Itagaki and colleagues,7 the rate of aortic dissection in patients with BAV 15 years after aortic valve replacement (AVR) was 0.55% and not significantly different from patients with TAV (0.41%). However, patients with Marfan syndrome had a substantially higher rate of aortic dissection (5.5%) that was significantly greater than the rate in patients with BAV or TAV (P <.001).
V. Genetics
Although there is a definite genetic component to BAV disease, the precise patterns of inheritance have been elusive. Approximately 9% to 15% of first-order family members also have BAV disease, with men and women equally affected within those families.34–36 These percentages are higher than in the general population (1%−2%), demonstrating the influence of genetics in this disease. Missense mutations in the NOTCH1 gene have been implicated in some patients with BAV disease.37–39 The vital NOTCH signaling pathway, involved in differentiation of multiple organs (including skeletal muscle, central nervous system, pancreas, and blood vessels), is highly evolutionarily conserved (ie, in humans, mice, and zebrafish).40 High evolutionary conservation indicates critical pathways whose aberration is likely to lead to significant or life-threatening disease. NOTCH genes play an important role in familial bicuspid valve disease, but they are found in only 4% of spontaneous cases.41 The variety and complexity of inheritance of BAVare under intense investigation but remain to be fully clarified.
VI. Associated lesions
Many other lesions and syndromes are strongly associated with BAV (Table 1). Associated anatomic lesions include aortic coarctation and patent ductus arteriosus. Coarctation-mediated hypertension greatly increases the risk of aortic dissection.42 In the presurgical era, death from aortic dissection occurred in 19% of patients with BAV, but in 50% of patients with concomitant BAV disease and coarctation.43,44Aortic coarctation accompanies BAV more commonly in men (4:1) than in women.42 Syndromes associated with BAV disease include Turner’s syndrome (monosomy X, characterized by short stature, lymphedema of the hands and feet, and amenorrhea) and William’s syndrome (abnormal facial appearance, low nasal bridge, unusually cheerful demeanor). In addition to those lesions described in Table 1, patients with BAV are also known to have an increased prevalence of anterior mitral valve leaflet elongation and prolapse.45,46
TABLE 1.
Cardiovascular conditions associated with bicuspid aortic valve disease
| Condition | Incidence of BAV |
|---|---|
| Coarctation of the aorta | 50% |
| Turner syndrome | 30% |
| Supravalvular AS | 30% |
| Sinus of Valsalva aneurysm | 15%−20% |
| Ventricular septal defect | 30% |
| Shone complex | 60%−85% |
| Ascending aortic aneurysm | Common |
| Loeys–Dietz syndrome | 2.5%−17% |
| ACTA2 mutation familial thoracic aneurysm syndrome | 3% |
| Anterior mitral leaflet prolongation/prolapse | Common45,46 |
BAV, Bicuspid aortic valve; AS, aortic stenosis. Modification of Braverman A. BAV and associated conditions. Graphic 83657, version 1.0. In: Up-to-Date. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2016. Available at: http://www.uptodate.com. Accessed June 30, 2017.
B. Natural History
BAV disease can cause morbidity and mortality through the valve disease (stenosis or insufficiency) or by ascending aortic aneurysm (leading to aortic dissection or, in rare cases, rupture). However, recent studies have demonstrated, in the modern era of diagnosis and care, an overall survival for patients with BAV identical to that of the normal population.26 As long as patients are followed regularly and surgery is offered in a timely fashion, then the risk of catastrophic aortic events is low. However, some important observations should be kept in mind when following patients with BAV.
I. Aortic valve dysfunction is not needed for aortic dissection to occur
It is important to recognize that neither AS nor AI needs to be present for aortic dissection to occur.47 In BAV disease, valvular complications—AS or regurgitation—progress at their own independent rates, different from the rate of progression of the bicuspid aneurysm. Thus, the lack of AS does not preclude aortic dissection from occurring. However, patients with BAVand valvular disease (stenosis or insufficiency) are at increased risk of rupture and dissection of the aorta.48 Evidence increasingly demonstrates regurgitant BAVs having a more malignant phenotype than stenotic BAVs, with a higher risk of aortic dissection.49 This topic will be discussed in more detail in Sections 3 and 6.
II. More malignant behavior of bicuspid aorta?
Many have thought of BAV aortopathy as “Marfan syndrome light”—that is, more severe than ordinary aortic aneurysm disease, but not quite as virulent as the Marfanoid aorta. Despite the clinical impression that BAV aortopathy is a malignant actor, supportive concrete evidence has been elusive.
A study by Davies and colleagues50 looked for disparities in behavior between patients with ascending aortic aneurysm with and without BAV disease. Patients with BAV disease presented at a smaller aortic diameter (4.6 vs 4.9 cm for patients without BAV). Also, their aortas grew more rapidly than those of patients with TAV: 1.9 mm/year compared with 1.3 mm/year.50 A higher proportion of patients with BAV required operative treatment of their aortas (72.8% vs 44.8%) at a significantly younger age (48.9 vs 63.1 years).50 However, among unoperated patients, there was no detriment in survival for the bicuspid group, who actually did better than the TAV group (8.6% vs 25.7% rate of rupture, dissection, or death at 5 years of follow-up).50 This likely reflects the substantially younger age at presentation of the bicuspid group (49 vs 64 years).50 However, in the BAV group, those patients with AS in addition to the aneurysm had an increased risk of aortic rupture, dissection, or death before operative repair when compared with patients with a normally functioning bicuspid valve.50 One possible limitation of these data is the fact that they are derived from a thoracic aortic referral center, and therefore may not accurately reflect the natural history of BAV aneurysms in the general population.
Other studies have also reported “mild” behavior with near normal long-term survival and low overall growth rates for the bicuspid aorta, on the order of 0.4 to 0.6 mm/year, with no differences noted according to specific pattern of leaflet fusion.29,51 These studies did not find a relationship between growth rate and original aortic size. The discrepancies between these studies and the study by Davies and colleagues50 have several possible explanations. First, these were studies derived from echocardiographic databases and not from a thoracic aortic referral center, which may have implications on patient selection bias. Second, the investigators may not have adequately imaged the uppermost portion of the ascending aorta in some patients, because this is a known limitation of echocardiography. Finally, the patients from the echocardiographic-based studies initially presented with smaller aortic diameters (4.1 and 3.8 cm) than those in the study by Davies and colleagues50 (4.6 cm).
III. Medical therapy for bicuspid aortopathy?
It is unclear whether any medical therapy is effective in preventing adverse events in aortic aneurysms of any kind, even in the most thoroughly studied Marfan population. Although beta-blockers and angiotensin receptor blocking drugs are commonly applied to “protect” the BAV, supportive evidence is lacking.52–54 However, such agents should be given to patients with documented hypertension. Statins have been shown to be ineffective, whereas other studies show a possible protective effect.55
IV. Contemporary clinical outcomes
Contemporary clinical outcomes for patients with BAV have been summarized in a comprehensive table by Michelena and colleagues28 from the International BAV Consortium (Table 2). Age at presentation, survival, and likelihood of heart failure, aortic valve surgery, endocarditis, aneurysm formation, aneurysm surgery, and aortic dissection are described for 8 contemporary clinical studies. Table 2 demonstrates excellent overall survival of patients with BAV in community, population-based studies, whereas outcomes are poorer in referral center patients who have required AVR. Heart failure is particularly uncommon in patients with BAV, and AS is a more common indication for surgery than AI. Aneurysm formation (aortic diameter >45 mm) occurs in 25% to 45% of patients over prolonged periods of follow-up, but aortic dissection is a rare event (~1%) outside of tertiary referral center populations, where it is more common (~10%).28
TABLE 2.
Contemporary clinical outcomes in patients with bicuspid aortic valve disease
| Contemporary clinical
outcomes of BAV studies* |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study features, clinical outcomes | Michelena and colleagues26 | Tzemos and colleagues27 | Michelena and colleagues33 | Davies and colleagues50,† | Russo and colleagues56 | Borger and colleagues57,‡ | McKellar and colleagues58 | Girdauskas and colleagues30,§ |
| Publication year | 2008 | 2008 | 2011 | 2007 | 2002 | 2004 | 2010 | 2012 |
| Clinical setting | Community, population-based | Tertiary referral center | Community, population-based | Tertiary referral center | ‘Tertiary referral center | Tertiary referral center | Tertiary referral center | Tertiary referral center |
| Inclusion characteristics | Minimal BAV dysfunction | Any BAV dysfunction | Any BAV dysfunction | Any BAV dysfunction with aortic aneurysm (mean baseline diameter 4.6 mm) | Status post-AVR | Status post-AVR | Status post-AVR | Status postisolated AVR with aortic aneurysm (mean baseline diameter 4.6 mm) |
| N | 212 | 642 | 416 | 70 | 50 | 201 | 1286 | 153 |
| Baseline age, y, mean T SD | 32 ± 20 | 35 ± 16 | 35 ± 21 | 49 | 51 ± 12 | 56 ± 15 | 58 ± 14 | 54 ± 11 |
| Follow-up y, mean T SD | 15 ± 6 | 9 ± 5 | 16 ± 7 | 5 | 20 ± 2 | 10 ± 4 | 12 ± 7 | 12 ± 3 |
| Survival | 90% at 20 y | 96% at 10 y | 80% at 25 y | 91% at 5 y | ≈40% at 15 y | 67% at 15 y | 52% at 15 y | 78% at 15 y |
| Heart failure | 7% at 20 y | 2% | – | – | – | – | – | – |
| Aortic valve surgery | 24% at 20 y | 21% | 53% at 25 y | 68% | – | – | – | – |
| Reason for aortic valve surgery | AS 67% AR 15% | AS 61% AR 27% | AS 61% AR 29% | – | – | – | – | – |
| Endocarditis | 2% | 2% | 2% | – | 4% | 2% | – | – |
| Aneurysm formation (definition, mm) | 39% (>40 mm) | 45% (>35 mm) | 26% at 25 y (≥45 mm) | – | – | 9% (≥50 mm) | 10% (≥50 mm) | 3% (≤50 mm) |
| Aortic surgery (for aneurysm) | 5% at 20 y | 7% | 9% | 73% | 6% | 9% | 1% | 3% |
| Aortic dissection | 0% at 20 y | 1% | 0.5% at 25 y | 9% | 10% at 20 y | 0.5% | 1% at 15 y | 0% |
BAV, Bicuspid aortic valve; AVR, aortic valve replacement; SD, standard deviation; AS, aortic stenosis; AR, aortic regurgitation.
Outcomes reported as percentage only were not reported within Kaplan–Meier survival analyses. Survival in the first 3 studies26,27,33 was not different than that of the general population. Survival in the study by McKellar and colleagues58 was inferior to that of the general population, and the rest of the studies were not compared with the general population.
This study compared patients with BAV with aneurysms versus patients with TAV with aneurysms. The incidence of aortic dissection was the same for both groups with superior survival in patients with BAVand both groups with dissection at similar aortic diameters.
This study suggested that patients with aortic dimension 45 mm or greater at the time of AVR should have the aorta concomitantly repaired, the basis of the current recommendations.
This study included consecutive patients with isolated AVR performed for AS only. However, 21 patients with predominant dilatation of the root (mean diameter, 44 mm) and severe aortic regurgitation who underwent AVR were followed in parallel for a mean of 10 years, and 2 acute dissections occurred. Reproduced with permission from Michelena and colleagues.28
3. PATIENT PHENOTYPES
A. Introduction
Evidence of phenotypic heterogeneity of BAV aortopathy has emerged in the last decade from several observational studies and stimulated a critical reappraisal of literature and treatment recommendations. The hypothesis has been proposed that different types of BAV aortopathy (ie, so-called aortic phenotypes) may be caused by distinct pathogenetic mechanisms and therefore require individualized surgical approaches.59,60 In particular, the 2 long-debated theories on BAV aortopathy pathogenesis, namely, the genetic and the hemodynamic theories, could both be plausible inasmuch as different phenotypic forms might be subtended by different contributions of both causative factors. Phenotypic heterogeneity of BAV aortopathy also may explain to some extent the inconsistencies in published natural history and follow-up studies, especially regarding the risk of aortic events in BAV disease. Previous data from mixed BAV cohorts resulted in a broad spectrum of surgical treatment methods being suggested, ranging from very conservative approaches to very aggressive recommendations, usually extrapolated from guidelines for management of patients with connective tissue disorders (eg, Marfan syndrome).5
Although the evidence of BAV aortopathy heterogeneity has gained increasing recognition in the last decade, data on individual aortic phenotypes are still scarce. The majority of published natural history/follow-up studies contain mixed BAV cohorts and include different stages of BAV disease. A better understanding of the interaction among morphologic features, functional characteristics of the aortic root, and transvalvular hemodynamics is required.
Scientific efforts to address the phenotypic heterogeneity in BAV aortopathy were mainly based on valve-related factors, including BAV morphology (ie, number and location of fused cusps), functional lesion (ie, stenosis, insufficiency or mixed lesions), and shape/configuration of the proximal aorta, which are addressed in detail next.
B. Bicuspid Aortic Valve Morphology
As mentioned in the Introduction, BAV morphology is described throughout this article according to the classification of Sievers and Schmidtke14 (Figure 1). BAV morphology with fusion of the right-left coronary cusps (ie, Sievers type I, R/L) and right noncoronary cusp (Sievers type I, R/N) represents the 2 most common BAV morphologies, accounting for approximately 75% and 20% of clinical cases, respectively.14 Sievers type I L/N and patients without a raphe (ie, Sievers type 0) are uncommon. The low prevalence of these specific fusion morphologies resulted in exclusion of these patients in most case series.
An embryogenetic study by Fernandez and colleagues61 demonstrated that the 2 most common BAV morphologies (ie, R/L and R/N) develop at different embryonic stages through distinct mechanisms and therefore should be interpreted as separate etiologic entities. The authors further suggested that the etiological factors giving rise to the specific BAV morphologies might be involved in the occurrence of distinct forms of BAV aortopathy.61 However, it is known that both morphologies can frequently appear within the same pedigree.62
Recent 4-dimensional (4D) flow magnetic resonance imaging (MRI) studies demonstrated that distinct aortic cusp fusion patterns result in specific orientations of eccentric flow jets (Figure 3),63–65 which in turn may lead to differential distributions of aortic wall shear stress (WSS)63,65 and subsequent focal flow-induced vascular remodeling.64 Eccentric transvalvular flow results in elevated regional WSS at the right-anterior wall of the proximal aorta for R/L BAV and right-posterior walls for R/N BAV.65 Bissell and colleagues63 demonstrated more severe flow abnormalities (complex flow, higher in-plane WSS) and larger aortas in the R/N BAV versus R/L BAV. However, propagation patterns of transvalvular flow are still not uniform in patients with BAV with the same cusp fusion morphology,63–66 and thus the impact of additional functional parameters (eg, subvalvular components, geometric orientation of residual aortic valve orifice) has been postulated.66
FIGURE 3.
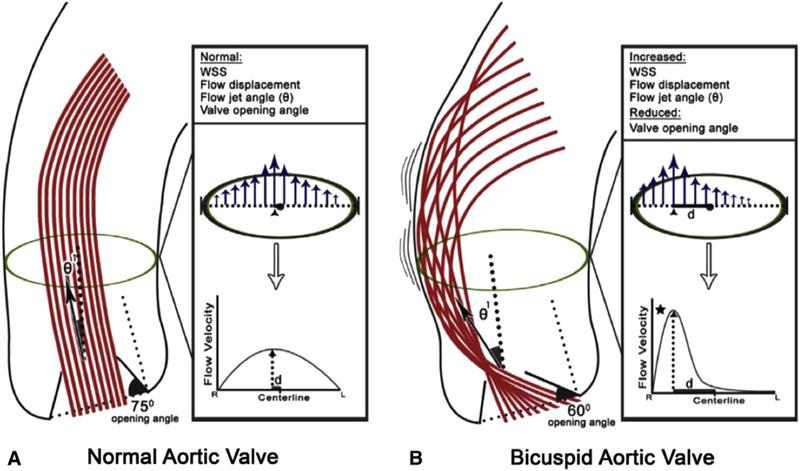
A, Normal aortic valve anatomy with an opening angle of 75 degrees, flow jet angle (θ1, which measures displacement of peak systolic flow [arrow] from vessel midline). B, BAV showing restricted valve opening (opening angle of 60 degrees), displaced flow jet with associated θ1, displaced high-flow velocities near the vessel wall leading to asymmetrically increased WSS at the aortic convexity. WSS, Wall shear stress. Reproduced with permission from Burris and Hope.97
The R/L morphology has been associated with younger patient age and absence of significant AS or regurgitation,67 whereas a greater prevalence of female patients is observed in those with R/N.68,69 Regarding the associated aortopathy, BAV R/L fusion morphology has been linked with increased diameters of the sinuses of Valsalva.51,68–71 In contrast, R/N fusion morphology is associated with a smaller dimension of the aortic root and larger aortic arch diameters.68–71 However, some authors found no significant correlation between aortic dimensions and BAV morphology.72,73 In addition, the published data on progression of BAV aortopathy in R/L versus R/N fusion morphologies are inconsistent. Some authors found that patients with BAV with R/L fusion are at increased risk of rapid aortic dilatation,71,74 whereas others reported the same findings in patients with R/N fusion.75 Different statistical approaches and different age ranges of the study populations may explain the differences between studies, because the 2 types of BAV morphology seem to progress in an age-dependent manner.69,76 Other studies have found no correlation between ascending aortic dilatation rates and BAV morphology.29,51
The data on proximal aortopathy in unicuspid aortic valve (UAV) disease are limited. Sievers and colleagues77 reported on more extensive aortopathy, including aortic root and ascending aorta, at a very young age in patients with UAV disease. Moreover, patients with UAV disease are more likely to require ascending aortic repair during their valvular procedure.78 Furthermore, significantly higher expression of GATA5 and endothelial nitric oxide synthase in the ascending aortas of patients with UAV disease versus BAV and TAV disease has been recently reported.79 Another recent study80 demonstrated decreased long-term survival in patients with UAV undergoing isolated AVR when compared with patients with UAV who underwent simultaneous aortic surgery.
C. Valve Function
The most common clinical presentation of BAV disease is calcific AS (BAV-AS), usually presenting between the fifth and seventh decades of life in both male and female patients. In contrast, pure/predominant BAV AI (BAV-AI) tends to occur in younger male patients and accounts for only 10% to 15% cases of BAV lesions in autopsy series.81 Distinct patterns of associated aortopathy have been observed in BAV-AS versus BAV-AI.47,67,82 Moreover, differences in histologic and extracellular matrix (ECM) protein changes,83–85 consistent with the clinical evidence from post-AVR follow-up studies,86 have also led investigators to suggest different pathobiological mechanisms of BAV aortopathy for these 2 groups of patients. BAV-AS is strongly associated with an asymmetric dilatation of the tubular ascending aorta, which represents the most common aortic phenotype.47,78,82,87 In contrast, BAV-AI is mainly accompanied by aortic root dilatation (ie, so-called root phenotype).78,82,86 However, these associations are not absolute, and heterogeneity within subgroups of valve function has been observed.48,82 As stated previously, BAV aortopathy is a heterogeneous disease.
Aortic dilation observed in patients with normally functioning BAVs has been previously used as an argument for a genetic origin of BAV aortopathy.48 However, recent experimental in vitro models88 and in vivo 4D flow MRI studies89 demonstrate that even clinically normally functioning BAVs (ie, without transvalvular pressure gradient or significant insufficiency) are associated with eccentric transvalvular flow and asymmetrically increased WSS in the proximal aorta. Aortic dilatation in normally functioning BAVs occurs most frequently in the tubular ascending aorta90 and has a natural history that is comparable to their TAV counterparts in terms of rate of dilatation and occurrence of aortic events.90
D. Shape of the Proximal Aorta
Della Corte and co-authors82 were the first to introduce a phenotypic classification of the proximal aorta based on the aortic segment involved and suggested the terms “root phenotype” and “ascending phenotype” (Figure 4). This classification system separated patients with BAV with a possible greater expression of genetically triggered aortopathy (ie, root phenotype) from those with a presumed hemo-dynamic cause of aortopathy (ie, ascending phenotype). Other classification systems for the pattern of dimensions of the different aortic segments have been proposed: Each of them has merits and flaws, and none can completely cover the entire spectrum of forms of dilatation.
FIGURE 4.
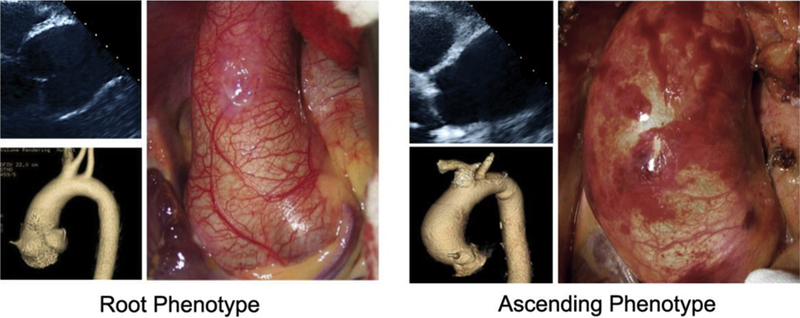
Root phenotype versus ascending phenotype of BAV aortopathy. Echocardiographic imaging is shown in the upper left corner, 3-dimensional reconstruction is shown in lower left corner, and intraoperative findings are shown at right. In the root phenotype, the diameter of the aorta at the level of the sinuses of Valsalva is greater than that of the tubular ascending aorta, whereas in the ascending phenotype the diameter of the tubular ascending aorta is greater than that of the sinuses.
Four distinct patterns of aortic dilatation in patients with BAV have been suggested by Fazel and coauthors13 from Stanford using a model of hierarchical clustering and integrating nonechocardiographic imaging (ie, computed tomography [CT] scan and MRI). They identified 4 “clusters”: aortic root dilatation alone (cluster I), tubular ascending aorta dilatation alone (cluster II), simultaneous involvement of the tubular portion and aortic arch (cluster III), and more diffuse dilatation involving the aortic root, tubular portion, and aortic arch (cluster IV).13 Cluster IV was the most frequent pattern of aortopathy; however, no risk factor analysis or longitudinal data were included. A slightly modified CT scan–based classification system of proximal aortic shape has been used by Kang and co-authors,91 confirming the previously reported association of the atypical morphologies (mainly R/N) with arch involvement.68
From an echocardiographic analysis, Schaefer and colleagues68 defined 3 shapes of the proximal aorta based on the relative dimensions of sinuses of Valsalva, STJ, and tubular segment: type N, with dilation of the sinuses with preservation of the STJ; type A, dilation of the tubular aorta with preserved STJ; and type E, dilation of the STJ with preserved sinus of Valsalva, regardless of the diameter of tubular aorta.
Finally, Park and associates92 proposed a classification scheme of BAV aortic phenotypes in a surgical study based on the segment involved (root vs tubular aorta) in the aneurysmal disease. These authors classified “type I” dilatation as that involving the tubular ascending aorta only, “type II” involving both the tubular ascending aorta and the root, and “type III” confined to the aortic root.92
In a recent longitudinal study that sought to validate the 3 different echocardiography-based BAV phenotypic classifications of the proximal aorta,68,82,92 only the classification system distinguishing between ascending phenotype and root phenotype showed a potential prognostic value in predicting growth rate of the aorta over time.93 Consistent with these findings, a prior longitudinal study demonstrated that the root phenotype is associated with a significantly greater risk of aortic events after isolated AVR.30 This classification system also has practical value, because the root phenotype frequently requires a Bentall or valve-sparing procedure, whereas replacement of the tubular aorta is usually sufficient to treat the ascending phenotype (Figure 5). Of note, 2 aortic dissection series revealed that patients with BAV received a Bentall operation significantly more often (85%−94%) than patients with TAV (20%−30%) because of the presence of a preexisting root aneurysm or the involvement of the sinuses by the dissecting process.31,32 It should be stressed that although valve morphology is a congenital feature of a patient with BAV, the aortic phenotype can change during life. That is, a proportion of patients with root phenotype can progress to an ascending phenotype over time.29 Further research in this area will undoubtedly lead to further insights into BAV-aortopathy patterns. Hopefully, a single classification system will emerge that encompasses BAV morphology, BAV lesion, and location and extent of associated aortopathy in a clinically meaningful manner.
FIGURE 5.
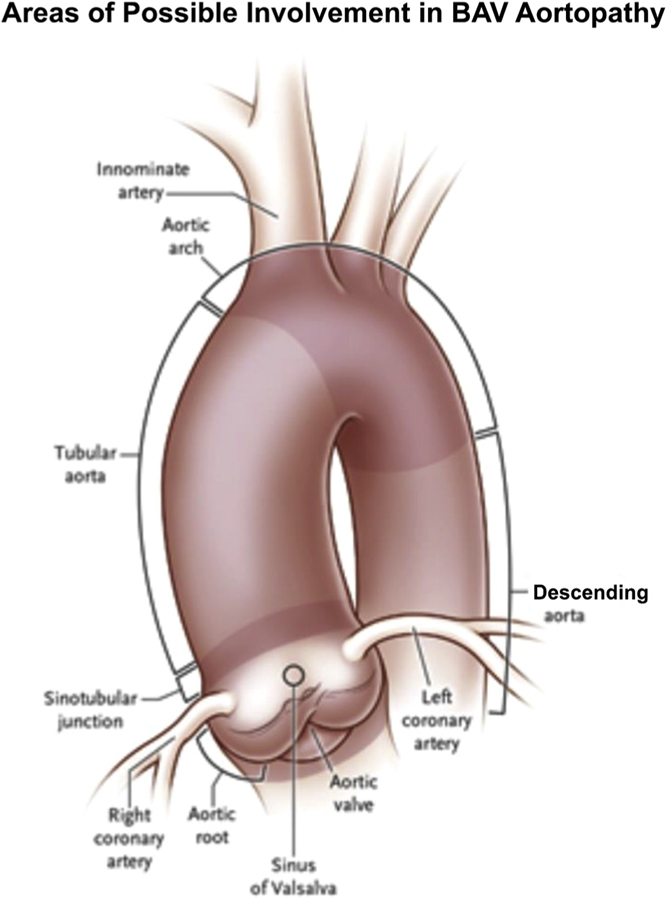
Subdivision of proximal aortic involvement in BAV aortopathy. Reproduced with permission from Verma and Siu.18
E. Symmetry Versus Asymmetry of Aortic Dilatation
Symmetry versus asymmetry of aortic dilatation also may provide an important clue regarding the predominant pathogenesis of BAV aortopathy inasmuch as asymmetrical aortic involvement might be an indicator of rheological factors involved, whereas symmetrical involvement might be more predictive of genetically triggered vessel wall weakness. By “asymmetric dilatation,” predominant enlargement of the greater, outer curvature (usually referred to by the misnomer “convexity” as opposite to the lesser, inner curvature or “concavity”) of the tubular aorta is meant. Pre-operative imaging methods can be used to identify asymmetric aneurysms.94,95 Several studies found a significant correlation between functional BAV lesion (ie, BAV-AS) and asymmetric dilatation of the tubular aorta.95,96 Asymmetric patterns of histologic lesions and ECM protein expression have been demonstrated between the concavity (where less severe changes are observed) and the convexity (more severe structural changes) in such patients.66,85 A recent study looked at the expression of transforming growth factor (TGF) β−1 and matrix metalloproteinases (MMPs) in BAV aortopathy and found that wall areas that had been mapped as regions of increased WSS by preoperative 4D-flow MRI exhibited greater expression of these markers of vascular remodeling.64 No similar study has thus far been performed in patients with BAV-AI.
4. HISTOPATHOLOGIC AND BIOMECHANICAL FINDINGS OF BICUSPID AORTIC VALVE AORTOPATHY
A. Histopathologic Studies
BAV aortopathy consists of premature cystic medial degeneration in approximately one half of surgically excised BAVs.98 Histopathologic changes in the media have been well documented and specifically delineated for the BAV-associated aneurysms.85,99–102 It is also well established that the aortic ECM plays an important role in maintaining the aorta through both the binding/storing secreted proteins and maintaining the structural integrity of the vascular wall.100 The presence of thin, fragmented elastin fibers, reduced fibrillin-1 content,99 and decreased types I and III collagen have suggested elevated proteolytic activity.85,100 The degradation of ECM is under the balanced control of MMPs and their specific tissue inhibitors (tissue inhibitors of metalloproteinases [TIMPs]), which are secreted by vascular smooth muscle cells (SMCs), fibroblasts, and endothelial cells.103 Various studies have shown a disturbance in the ECM of surgically resected BAVs with increased activity of MMPs, with MMP-1, MMP-2, MMP-9, MMP-12, and MMP-14 (MT1-MMP) being most often implicated.104–108 The critical role of MMP-2 as a key molecular mediator was supported by a recent meta-analysis.109 Wang and colleagues110 further showed MMP-2 as a circulating biomarker of aortic dilatation in patients with BAV.
Like MMPs, the expression of TIMPs is controlled during tissue remodeling and physiologic conditions to maintain a balance in the metabolism of the ECM. Studies have demonstrated increased TIMP-1, TIMP-2, and TIMP-4 levels associated with BAV aortopathy.107 Altered MMP/TIMP stoichiometry leads to apoptosis and degeneration of the aortic wall (ie, loss of elastic tissue and SMCs) and the eventual progression of aneurysms. Of note, histologic grading has revealed more extensive degradation of the ascending aortic wall in patient with Sievers 1 R/L fusion.101 This has further been supported with data showing elevated proteolytic indices (ie, MMP abundance corrected for TIMP abundance) for MMP-1, MMP-2, MMP-9, and MMP-12.111 Consistent with findings in patients with Marfan syndrome,112 early data point to the involvement of TGF-β signaling contributing to the progression of BAV aortopathy, although this remains some-what controversial.113–115
Phillippi and co-workers116 further characterized the medial matrix remodeling of the BAVand found unique patterns compared with TAV.116 Grewal and co-workers117 compared the histopathology of BAV, TAV, and Marfan aortic tissue and found both similarities and differences among all 3 groups with respect to parameters of matrix remodeling and vascular smooth muscle markers. The complexity of the histopathologic findings is substantial, and it is not clear what molecular pathways are unique to the BAV. The complexity is further confounded by the findings of Heng and colleagues.118 In this recent study, tissue pathology was compared between TAV and patients with BAV at matched aortic diameters. At odds with conventional wisdom, more severe histologic abnormalities were found in TAV compared with BAVs, especially when stratified by diameter.
In addition to ECM degradation, SMC loss is a prominent feature of BAV aortopathy. SMC phenotype, oxidative stress patterns, and responsiveness to oxidative stress appear altered in the BAV wall.115,119–124 Metallothionein, a free radical scavenger, expression is dysregulated, and consequent SMC cell viability is reduced in response to oxidative stress in the BAV. Regional differences in apoptotic activity also appear to be present comparing the concave and convex portions of the bicuspid aorta.125 Given the mounting evidence of regional differences in both the biology and the biomechanics of BAVaortopathy, it is likely that they correlate with one another. Aims to leverage these collective insights toward better diagnostics and risk adjudication are approaching.
B. Biomechanical Studies
Biomechanical functional testing of aortic tissues may provide further insights into BAV aortopathy. Ascending aortic wall properties are biomechanically anisotropic. Despite total collagen and elastin content and histopathologic findings that are similar, microarchitectural and biomechanical differences are apparent when comparing aortic wall characteristics in BAV versus TAV. The tensile strength, particularly in the circumferential and longitudinal directions, is higher in surgically resected ascending aortas in BAV compared with TAV,126,127 whereas the delamination strength of the aortic wall of the BAV is lower than the TAV.128 Aortic wall remodeling characteristics in patients with BAV also appear distinct with more highly aligned collagen fibers, more undulating and lessaligned elastin fibers, thinner elastic lamellae, and greater distances between elastic lamellae.100,116,127,129,130 Aortic stiffness is associated with progressive aortic dilatation and aneurysm formation, which is characteristic of BAV aortopathy.131 A recent study of abdominal aortic aneurysms found that segmental stiffening of the aorta preceded aneurysm growth and introduced the concept that stiffening may act as an early mechanism triggering elastin break-down and aneurysm growth.132 Nonetheless, the evidence regarding cellular and molecular mechanisms for BAV aortopathy remains complex and contradictory, with a need for larger cohort, well-controlled studies.
Although stimuli for BAV aortopathy are likely multifactorial, results from recent studies provide strong evidence that a hemodynamic (ie, rheologic) stimulus, the WSS, may change local matrix homeostasis and, in turn, ascending aortic structure and associated functional properties.64,133–137 WSS is known to affect MMP2 levels138 and has been implicated in the development of aortopathy.89,139 Aortic WSS calculations demonstrate differences in regional and radial wall stresses,140,141 but how these differences correspond to aortic wall remodeling, biology, and clinical outcome is not yet known. Proof-of-concept data were recently obtained using a novel ex vivo tissue model. Atkins and co-workers142 modeled regional WSS from a TAV compared with a BAV in an ex vivo porcine tissue model, and the impact of BAV-mediated WSS was determined on aortic wall remodeling. The investigators found cellular, molecular (ie, increased MMP-2 activity), and structural changes that are characteristic of human BAV aortopathy. As highlighted by the investigators, the study indicates that altered WSS resulting from a BAV can focally mediate aortic medial degradation. These unique experimental findings provide compelling support for an important role of hemodynamics in mediating BAV aortopathy.
Recent advances in MRI have permitted unobstructed in vivo assessment of time-resolved 3-dimensional (3D) blood velocity, using a volumetric technique referred to as “4D flow MRI.” 4D flow MRI provides the unique ability to quantify complex 3D blood flow patterns in vivo and has facilitated new insights and discovery with respect to complex cardiovascular hemodynamics.65,143–147 Multidimensional 4D flow MRI data (3 spatial dimensions describing 3D velocity over time) enables aortic blood flow visualization, quantification of regional flow and velocity,144,148–150 and WSS quantification.134–137,151,152 Recent MRI studies provide strong evidence that valve-mediated local flow dynamics143 and regional differences in WSS65 are associated with changes in regional aortic wall histology and proteolytic events,64 which are known to drive adverse aortic remodeling. Early studies used less-sophisticated MRI techniques (2-dimensional [2D] phase-contrast MRI) to demonstrate BAV-mediated changes in flow and WSS152 and their association with aortic enlargement.153 Subsequent 4D flow MRI studies have conclusively documented that aortic WSS is increased in subjects with BAV independent of stenosis severity when compared with age- and aortic size–matched controls.143 Moreover, regional variation of WSS within the aorta is dependent on aortic valve fusion phenotype65,143,154 and is associated with aortic diameter.63 A recent study with 30 patients with BAV and 30 age-appropriate TAV controls provided evidence that altered aortic hemodynamics may be a pathophysiologic mechanism by which R/L or R/N BAV fusion patterns influence the expression of aortopathy.65
Similar to the findings of Atkins and Sucosky in the porcine model, aortic hemodynamic alterations were found to be related to medial wall degeneration.142 In a recent study that included both in vivo 4D flow MRI and aortic tissue resection in 20 patients with BAV, elastin content and structure were severely disrupted in regions of high WSS with a shift in the expression of specific MMPs and TGF-b. Girdauskas and colleagues66 found a similar correlation between systolic transvalvular flow patterns and proximal aortic wall changes in the setting of BAV-AS. With more extensive investigation, it is conceivable that quantitative metrics of valve-mediated hemodynamics could be used to guide more precise and individualized surgical resection strategies beyond contemporary empirical size thresholds.
5. DIAGNOSTIC MODALITIES
A. General Vascular Imaging Concepts
Transthoracic echocardiography (TTE) is the recommended imaging modality for the initial assessment of the aortic valve and thoracic aorta, including the assessment of hemodynamic valve function (Table 3; Figures 6 and 7).155 If any part of the examination is not possible by TTE, CTor MRI is recommended to assess the presence and extent of aortopathy (Figures 8 and 9). Hemodynamic valve assessment also can be performed by MRI,156 although TTE remains the gold standard. TTE assessment of aortic valve function is usually sufficient, but transesophageal echocardiography (TEE) should be performed in patients with AI that is difficult to quantify with TTE. BAV-AI may result in an eccentric jet that can be better visualized with TEE, particularly if patients have less-than-severe AI by TTE and unexplained left ventricular dilation or dysfunction. In addition, TEE may best determine the mechanism of AI when aortic valve repair is being considered.
TABLE 3.
Recommendations for initial imaging of the aorta in patients with bicuspid aortic valve
| Recommendation | Class/LOE |
|---|---|
| TTE is the initial imaging modality of choice for assessment of the aortic valve and thoracic aorta in patients with BAV. | I/C5,159 |
| The entire thoracic aorta should be measured by TTE, reporting each aortic segment separately in millimeters: root (sinuses of Valsalva), STJ, tubular ascending aorta (proximal, mid, and distal), arch, and descending thoracic aorta (Figure 11). Maximum diameter, regardless of location, should be reported. Aortic coarctation should be ruled out with Doppler evaluation of the descending thoracic aorta and abdominal aorta. | I/C5,159,180 |
| If TTE cannot visualize any aortic segment, any segment measures ≥45 mm, or aortic coarctation cannot be ruled out, recommend assessment of the entire thoracic aorta with ECG-gated cardiac MRA or CTA. | I/C33,57 |
| If a patient is undergoing cardiac surgery and root or tubular ascending aorta measure 40–44 mm by TTE, recommend assessment of the thoracic aorta with MRA or CTA before surgery. | I/C33,57,166 |
| If aortic coarctation is present, screening for cerebral aneurysms is recommended. | I/B180 |
LOE, Level of evidence; TTE, transthoracic echocardiography; BAV, bicuspid aortic valve; STJ, sinotubular junction; ECG, electrocardiogram; MRA, magnetic resonance angiography; CTA, computed tomography angiography.
FIGURE 6.
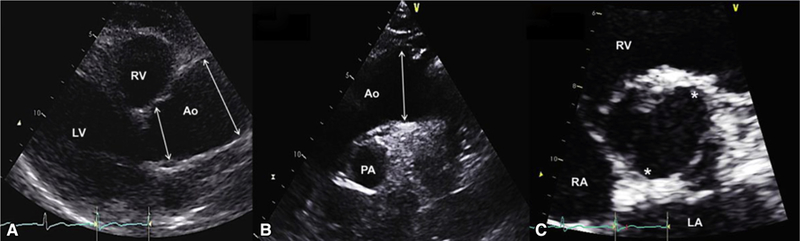
Typical echocardiographic findings in a patient with BAV with tubular ascending aorta dilatation phenotype. A, Echocardiogram of a 60-year-old woman with R/N BAV, no aortic valve regurgitation, and a fusiform ascending tubular aortic aneurysm. Left parasternal long-axis view in diastole shows root measurement of 36 mm (first arrow from left) and midtubular ascending aorta measurement of 47 mm (second arrow from left). B, Suprasternal diastolic view shows the mildly dilated proximal arch (36 mm, arrow) and normal upper descending aorta. C, Parasternal short-axis en face view of the aortic valve in systole shows 2 commissures (asterisks) at 1 and 7 o’clock with right nonfusion. LV, Left ventricle; RV, right ventricle; Ao, aorta; PA, pulmonary artery; RA, right atrium; LA, left atrium.
FIGURE 7.
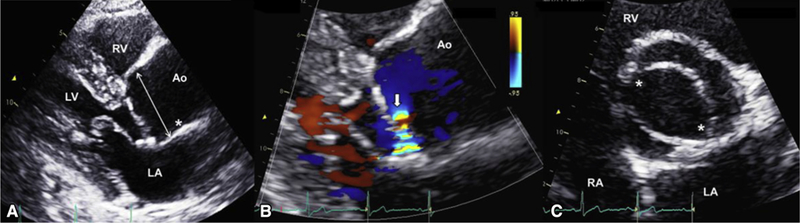
Patient with BAV with root phenotype aortic dilation. A, Echocardiogram of a 53-year-old man with R/L BAV, severe aortic valve regurgitation, and root-proximal ascending aortic aneurysm. Left parasternal long-axis view in diastole shows root measurement of 46 mm (arrow), STJ effacement (asterisk), and proximal tubular ascending aorta dilatation. B, Left parasternal long-axis zoomed color-Doppler view in diastole shows the flow convergence (arrow) of a posteriorly directed jet that quantified to 78 mL per beat of regurgitant volume. C, Parasternal short-axis en face view of the aortic valve in systole shows 2 commissures (asterisks) at 4 and 10 o’clock with right-left fusion. LV, Left ventricle; RV, right ventricle; LA, left atrium; Ao, aorta; RA, right atrium.
FIGURE 8.
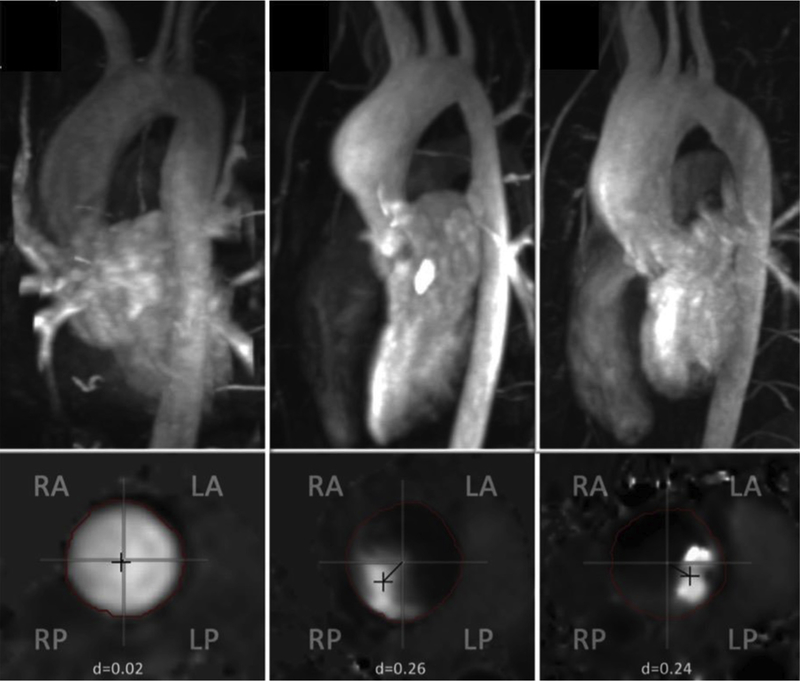
MRI assessment of patients with BAV showing normal aortas (left) and different types of BAV aortopathy (middle and right). Each of the 3 upper panels shows maximum intensity projection of magnetic resonance angiography with the corresponding inferior panel demonstrating the planar analysis of systolic flow. The left panel demonstrates imaging from a normal patient. The middle panel demonstrates aneurysmal dilation at the level of the sinuses with flow directed rightward and posteriorly in a patient with a left-right cusp fusion. The right panel shows more diffuse aneurysmal dilation in a patient with right-noncoronary cusp fusion and flow directed leftward and posteriorly. RA, Right anterior; RP, right posterior; LA, left anterior; LP, left posterior. Adapted from Burris and Hope.181
FIGURE 9.
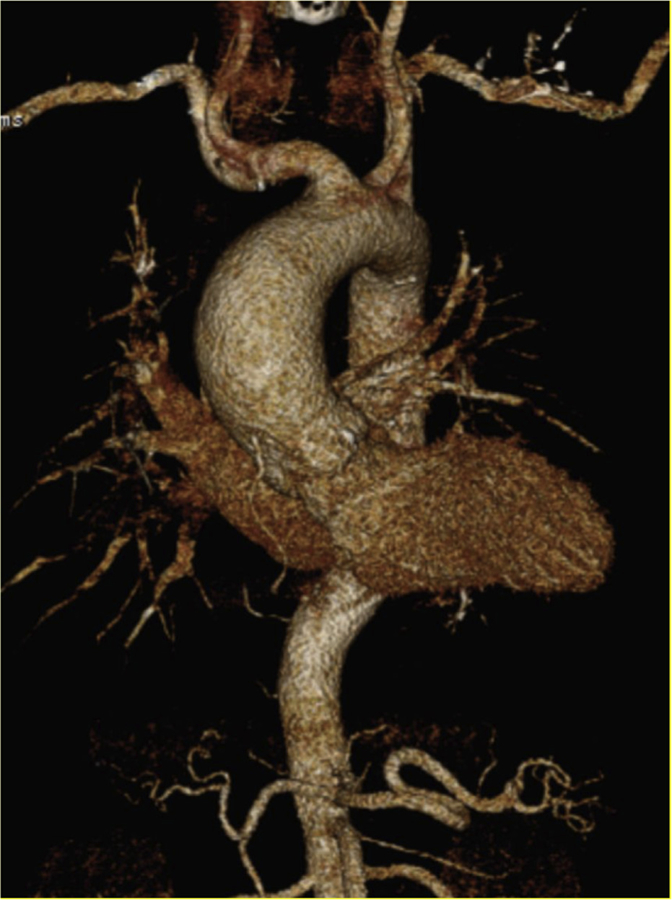
CT imaging with 3 dimensional reconstruction of a patient with BAV with associated aortopathy.
When evaluating the BAV with echocardiography, the entire thoracic aorta should be assessed: aortic root (aortic annulus, sinuses of Valsalva, and STJ), tubular ascending (proximal, mid and distal), aortic arch, and descending thoracic aorta, including diameter measurement and Doppler assessment for the presence of coarctation (Table 3 and Figure 10). It is important to recognize that the term “aortic root” has been loosely used in the past to include the ascending aorta, and it is critical that both the components of the aortic root and the tubular ascending aorta are measured separately and reported as such. The abdominal and pelvic aorta need not be assessed in isolated BAV disease, unless a family history of abdominal or iliac aneurysms is present or suspicion of coarctation exists. In addition, although intracranial arterial aneurysms have been found in 10% of patients with BAV versus 1% of control patients,157 these are small (ie, <10 mm in diameter), and no increased prevalence of BAV has been found in patients with intracranial aneurysm–related subarachnoid hemorrhages.158 Therefore, routine brain angiography is not recommended in patients with BAV unless coarctation of the aorta is present (Table 3).42 Intracranial hemorrhage is a complication of coarctation of the aorta, independent of the presence of BAV.
FIGURE 10.
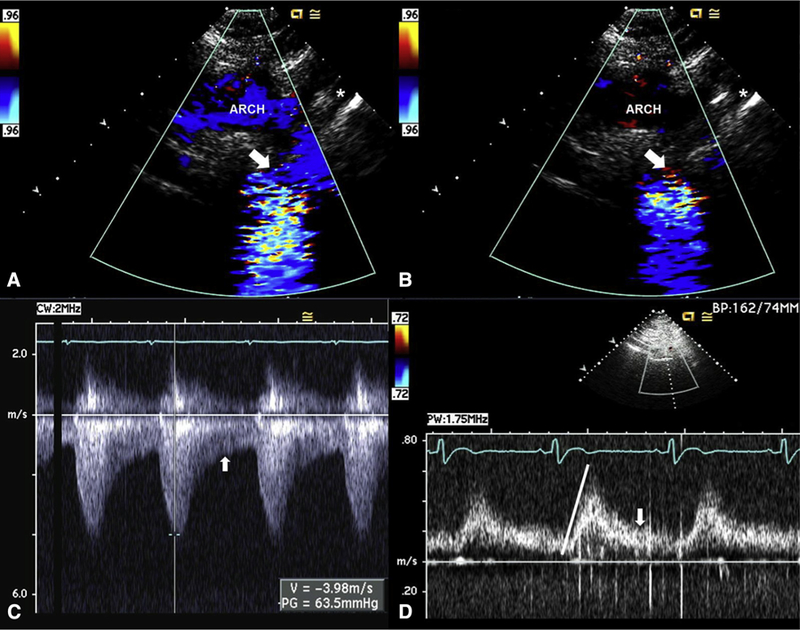
Transthoracic assessment for aortic coarctation. A, Echocardiogram of a 31-year-old woman with BAV and severe aortic coarctation. Suprasternal systolic still frame shows laminar Doppler flow through the proximal portion of the arch (“ARCH”) before becoming turbulent flow across a tight coarctation (arrow) just distal to the left subclavian (asterisk). B, Suprasternal diastolic still frame shows no Doppler flow through the proximal portion of the arch but persistent diastolic turbulent flow across the coarctation (arrow) just distal to the left subclavian (asterisk). C, Continuous-wave Doppler signal across the coarctation shows a systolic (measurement) peak gradient of 64 mm Hg through the coarctation, with persistent flow in diastole (arrow). D, Pulsed-wave Doppler signal of the abdominal aorta shows a delayed peaking of the systolic signal (line) with prominent persistent flow in diastole (arrow), pathognomonic of coarctation.
B. Image Acquisition and Analysis: Echocardiography, Magnetic Resonance Imaging, and Computed Tomography
There is no consensus regarding a standard method to measure and compare aortic measurements across echocardiography, MRI, and CT,159 and different methods are frequently used within the same institution. Although it is clear that end-diastolic leading-edge to leading-edge is the method of choice for TTE in adults,159 no such consensus exists for CT/MRI. Some advocate end-diastolic outer wall-to-outer wall measurements,5 and others advocate inner wall-to-inner wall dimensions.159,160 Recent data suggest no systematic measurement bias when comparing the current echocardiographic method with the CT/MRI inner wall-to-inner wall method for measuring the ascending aorta in the absence of root asymmetry.160
Care must be taken when interpreting results across modalities. The maximum diameter observed in the aorta, regardless of the position in which it is measured, should be reported in addition to the measurements obtained at pre-defined anatomic locations (Table 3). In this article, we recommend the best practices for each modality.
Measurements of the adult thoracic aorta by TTE and TEE should be obtained in diastole (ie, at the QRS complex) with the leading-edge to leading-edge technique (Figure 11).159 For TTE, measurements of root and tubular ascending aorta are made in the left parasternal long-axis view (Figures 6 and 7), but other views such as left “high” parasternal and right parasternal are complementary and recommended. For TEE, the midesophageal long-axis view and high-esophageal mid-ascending aorta view are used (Figure 12). Both TTE and TEE modalities have the disadvantage of potentially measuring obliquely and not perpendicular to the long axis of the tubular ascending aorta, which could render inaccurate aortic diameter measurements.
FIGURE 11.
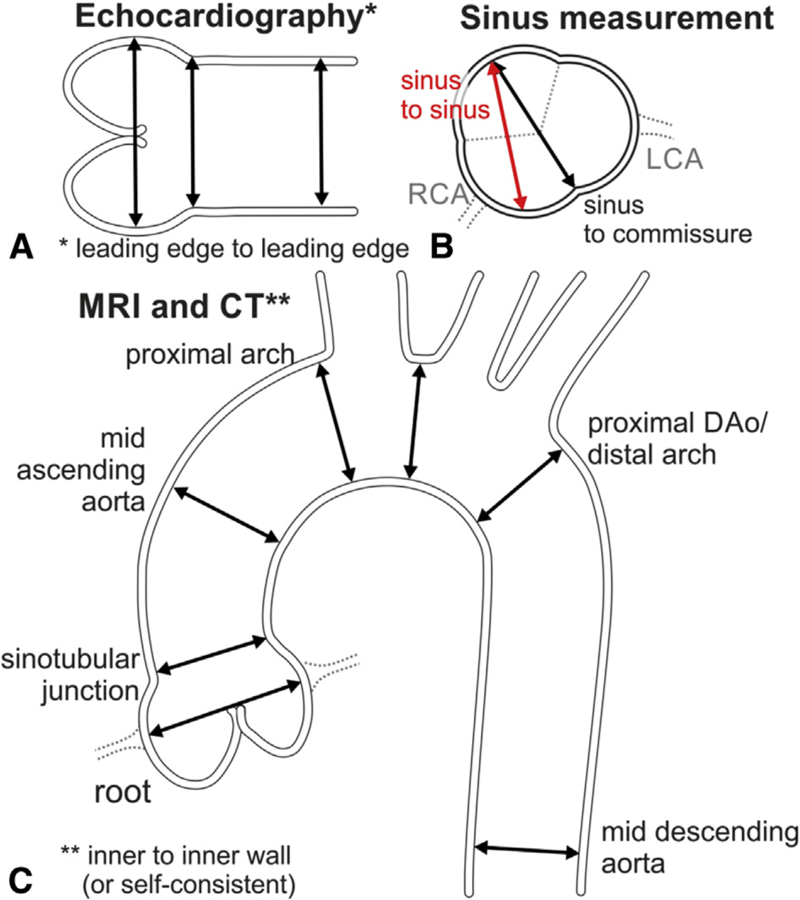
A, Schematic shows the leading-edge to leading-edge measurement technique used in echocardiography, from left to right: measurement of the sinuses of Valsalva, STJ, and proximal tubular ascending aorta. B, Inner-to-inner measurements used in MRI and CT. In addition, a consistent approach to measuring all 3 sinuses with MRI and CT is necessary. The sinus-to-commissure and sinus-to-sinus measurements can both be used, but consistency is necessary for interval surveillance. C, Standard measurement locations for MRI and CTwith the inner-wall to inner-wall technique. RCA, Right coronary artery; LCA, left coronary artery; MRI, magnetic resonance imaging; CT, computed tomography; DAo, descending aorta.
FIGURE 12.
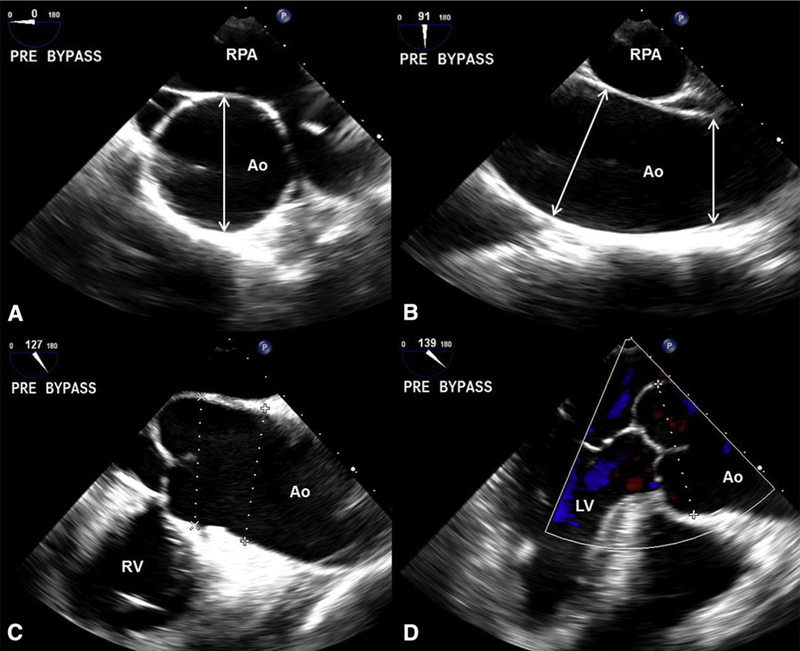
TEE aorta assessment. A, Prebypass echocardiogram of a 79-year-old man with typical BAV (right-left cusp fusion), mild AS, and severe generalized aorta dilatation. High-esophageal mid-ascending aorta short-axis measurement (arrow) at 0°. B, Same imaging position as A, now at 91°, reveals the mid-ascending aorta at 52 mm (long arrow) and the distal aorta (short arrow) at 49 mm. C, Mid-esophageal long axis at 127° allows measurements of the proximal ascending aorta and root (dotted lines). D, Mid-esophageal long axis at 139° allows improved visualization of the root, which measured 49 mm (dotted line). The patient underwent a Bentall procedure. Ao, Aorta; RPA, right pulmonary artery; RV, right ventricle; LV, left ventricle.
MRI and CT acquire 3D fields of view, and thus the aorta diameter should be measured with multiplanar reconstruction to obtain double-oblique cross-sectional views of the vessel (perpendicular to the longitudinal axis of the aorta). The double oblique view corrects for measurement errors caused by projecting the 3D aorta on a 2D screen. For the same reason, it is recommended that ascending aortic diameters 45 mm or greater obtained by echocardiography be further investigated by electrocardiogram (ECG)-gated MRI or CT angiography during diastole. CT or MRI may be performed in patients with aortic dilation (ie, 40–44 mm) and poor-quality echocardiographic images. Recommended CTand MRI measurement locations are displayed in Figure 11. If measurements are comparable and reproducible between techniques, then future interval measurements can be obtained by TTE alone, with repeat CT or MRI examination every 3 years to re-verify reproducibility and agreement. If initial measurements are discrepant, then CT or MRI should be the technique of choice for interval aortic diameter measurements.
Regarding the aortic root, TTE measurements are consistently lower than those measured by ECG-gated CT angiography.160,161 This is particularly true for the asymmetric dilated BAV root whose dimensions are frequently underestimated by single 2D parasternal long-axis and standard short-axis TTE views. When root dilatation is visually suspected by TTE or the root is significantly asymmetric, we recommend measuring diastolic leading-edge to leading-edge sinus-to-sinus diameters in parasternal short-axis TTE view or alternatively go straight to CT/MRI ECG-gated root assessment.
Because of the highly reproducible nature of ECG-gated CT and MRI, these techniques should be used for accurate assessment of aortic root measurements. However, it is important to note that clinical cutoffs for intervention largely have been derived from echocardiography,28 a difficult conundrum to reconcile.155 Nevertheless, akin to the tubular aorta, echocardiography-derived aortic root diameters 45 mm or greater should be verified by ECG-gated CT or MRI (Table 3). Given that the sinuses can dilate asymmetrically, all 3 sinus-to-commissure (or sinus-to-sinus) dimensions should be measured.162 The CT measurements that correlate best with echocardiographic-derived values are inner wall-to-inner wall dimensions, which require the administration of contrast medium (Figure 11).163
The choice between CT and MRI is dependent on their availability, institutional expertise, and age of the patient. Younger patients (ie, <50 years) would benefit from MRI to avoid CT-associated radiation exposure, but ECG-gated MRI is not commonly performed in most institutions. Ideally, interval measurements should be performed with the same imaging modality and technique (ie, ECG-gated), and compared side-by-side by an experienced reader.155
It should be noted that radiologists occasionally recognize signs of BAV aortopathy in a patient with no previous diagnosis of BAV. Suggestive signs are aortic leaflet calcification at a young age (ie, <60 years) and an asymmetric shape to the ascending aorta, with bulging of the outer curvature.164 Such patients should undergo TTE to confirm the diagnosis.
C. Aortic Imaging Surveillance
After the first echocardiographic evaluation, the thoracic aorta should be reassessed entirely on a yearly basis if greater than 45 mm (Table 4). A first interval repeat measurement could be considered at 6 months before proceeding to yearly assessments, especially if other risk factors are present, such as aortic coarctation or family history of dissection. As opposed to Marfan syndrome in which the aortic root is predominantly involved,51 the most common segment involved in patients with BAV is the tubular ascending aorta (ie, 60%−70% of BAV dilated aortas).28 It is vitally important that images of any type not be compared with the last prior image, but rather with the oldest prior image, which can be harder to access. Otherwise, gradual growth can go undetected.165
TABLE 4.
Recommendations for interval monitoring imaging of the aorta in patients with bicuspid aortic valve
| Recommendation | Class/LOE |
|---|---|
| Interval imaging should be performed with the same imaging technique and measurement method, and compared side-by-side with previous study by an expert in that imaging technique. | I/C12,155,159 |
| Interval aorta imaging recommendations apply to patients with native BAV and those who have undergone AVR, given that aorta complications may occur in patients with BAV postsurgery. | I/B58,166 |
| In patients with normal initial aortic diameters by TTE, the thoracic aorta should be reimaged every 3 to 5 y. | I/C51,155 |
| In patients with initial aortic dilatation (root or tubular ascending aorta measure 40–49 mm), the thoracic aorta should be reimaged at 12 mo. If stability is confirmed, then reimaging can be performed every 2 or 3 y. | I/C12,29,51,155 |
| In patients with more advanced initial aortic dilatation (root or tubular ascending aorta measure 50–54 mm), the thoracic aorta should be reimaged at least every 12 mo (yearly). | I/C12,51,155 |
| If thoracic aortic dilation (≥45 mm) noted by TEE is not reproducible with CTA or MRA (ie, >2-mm difference between modalities), then interval imaging follow-up should be performed with MRA or CTA. | I/C155,159 |
LOE, Level of evidence; BAV, bicuspid aortic valve; AVR, aortic valve replacement; TTE, transthoracic echocardiography; CTA, computed tomography angiography; MRA, magnetic resonance angiography.
Aortic growth rates for the tubular ascending segment in adults with BAV have recently been reported to range from 0.4 to 0.6 mm/year,29,51 whereas earlier studies demonstrated maximal dilatation rates of 1 to 2 mm/year. Few patients are observed to have dilation rates of more than 2 mm/year.29,51 Although these represent “artificially annualized” rates, it remains unlikely that patients with BAV will have dilation of 3 mm or more per year. It is also important to note that an interval diameter change of 1 or 2 mm by current imaging modalities is within the realm of error. Therefore, an interval dilatation of 3 mm or more should be considered clinically significant.12 Absolute echocardiographic aortic diameters at baseline are not reliable predictors of the rate of dilatation during follow-up29,51; thus, regular interval imaging (Table 3) is recommended regardless of baseline diameter. Previous AVR is more common in patients with BAV presenting with aortic dissection compared with patients with TAV with dissection.166 Patients with BAV with previous AVR have the highest reported risk of aortic dissection after 15 years of follow-up (ie, 1%),58 particularly if the original operation was performed for BAV with aortic regurgitation.49 Therefore, continued interval monitoring of the unrepaired aorta post-AVR is suggested.
D. Abnormal Aortic Diameter Values and Indexing
The sinuses of Valsalva are normally larger than the STJ and tubular ascending aorta, and the latter is larger than the arch and descending thoracic aorta. Normal values in adults by age, body size, and gender have been reported for the aortic root159,167 and tubular ascending aorta.159,168,169 An aneurysm is defined as a permanent focal dilatation of an artery having at least 50% increase in diameter compared with expected.5,170 In clinical practice, however, it is generally considered that a tubular ascending aorta greater than 37 mm or aortic root greater than 40 mm represents aortic dilation (but not aneurysm formation) in adult patients.171
It is important to recognize that the aforementioned cut-offs are not absolute, such that 50 mm could represent moderate dilatation in a large man but may be severe dilatation in a small woman. Thus, correction for body size parameters has been proposed (Table 3). Surgical repair has been suggested for patients with Turner syndrome who have an indexed aortic diameter of 2.75 cm/m2 or greater.172 The ratio of aortic cross-sectional area divided by height [ratio r2 π(cm2)/height (m)] has also been proposed as a method to correct for dissimilarities in body size.173 A ratio greater than 10 cm2/m has been recommended as the cut-off for elective aorta repair in both Marfan syndrome and BAV disease,5 and a recent study involving 380 patients with BAV with dilated aortas found that a cutoff of 13 cm2/m for the tubular ascending aorta and 10 cm2/m for the root exhibited superior predictive accuracy for the occurrence of dissection than absolute cutoffs.174 Another indexed measure of the aorta, the “aortic size index,” in which the maximum aortic size in centimeters divided by the body surface area has been validated in a large database of patients with aneurysm and was found to be more predictive of adverse events than maximum aortic dimension alone.175 Likewise, a large database of patients with TAV with aortic aneurysms found that indexed aortic size improved the ability to predict long-term events.176 However, more research needs to be done to confirm these findings.
E. Emerging Imaging Technology and Imaging Biomarkers
Imaging research for risk factors associated with BAV aortopathy has primarily focused on degree of coexisting aortic valve stenosis or regurgitation. These functional metrics alone do not reflect the rheologic burden on the aortic wall due to BAV. With this in mind, a number of techniques have shown promising initial results in the search for imaging biomarkers predictive of rheology-associated aortopathy development. For example, Della Corte and colleagues153 investigated the valve opening angle obtained via 2D balanced steady-state free precession cine images to compute a proxy measurement for understanding the impact of flow eccentricity on aortic growth. In this 36- subject cohort, they found the fused leaflet opening angle predicted ascending aorta diameters and growth rates. By using a similar hypothesis, Burris and colleagues177 computed the barycenter of the velocity field from 2D phase-contrast MRI to obtain “flow displacement,” a parameter representative of the eccentricity of vessel cross-section velocity field. The baseline displacement measurement was found to be predictive of ascending aorta growth in a small cohort of subjects.
With the use of insight from 2D phase-contrast MRI studies,152 a number of investigators have assessed the rheologic forces at the aorta wall using 4D flow MRI and the computation of WSS.89,178,179 These studies have directly measured the impact of eccentric flow and their forces on the aortic wall (Figures 13 and 14) and found correlations to the aorta phenotype65 and regional tissue aortopathy.64,179 Although further study is needed, these preliminary findings indicate that rheologically mediated aorta remodeling is an important factor to consider in the design of future studies.
FIGURE 13.
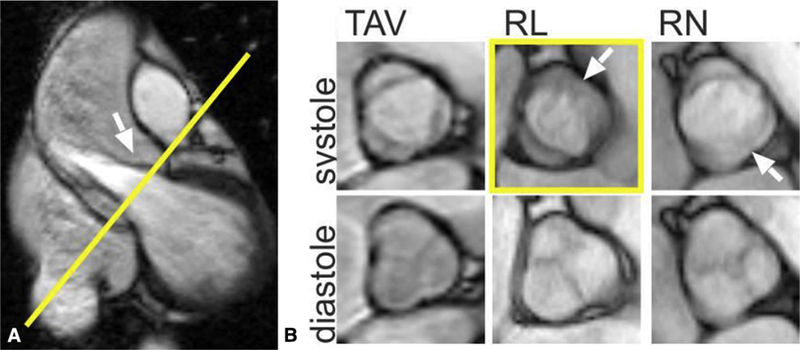
A, 2D balanced steady-state free precession cine MRI showing the left ventricular outflow tract and the position of the aortic valve imaging plane shown in yellow. B, TAV and the 2 most common BAV phenotypes: R/L and R/N cusp fusion. Arrows show the location of the raphe (if present) between the conjoined cusps. The conjoined R/L cusp (yellow box, arrow) is also seen to be doming in the corresponding left ventricular outflow tract view (A, arrow). Bicuspidality of the aortic valve should be assessed in systole rather than diastole, because the valves often appear tricuspid when closed. TAV, Tricuspid aortic valve; RL, right-left; RN, right noncoronary. Adapted from Entezari and colleagues.182
FIGURE 14.
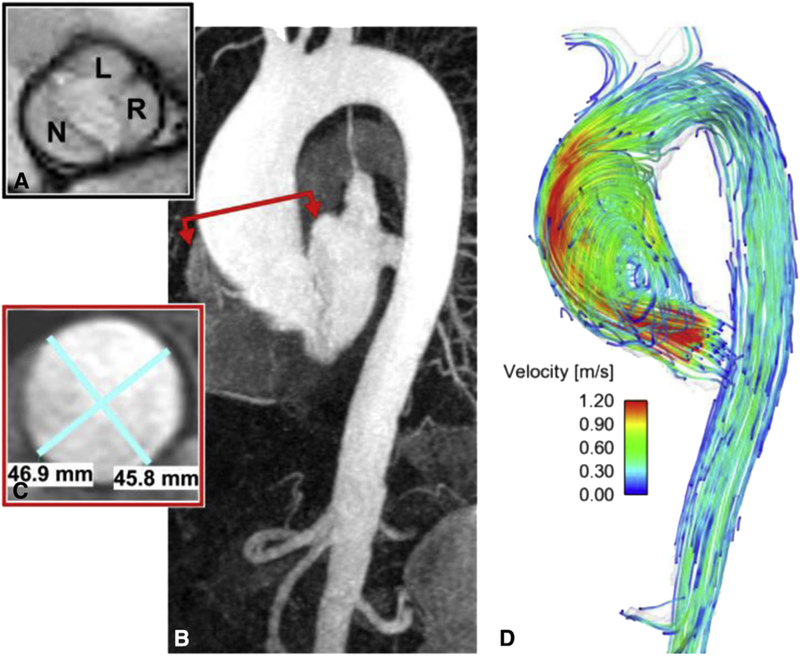
MRI of a 73-year-old man shows the (A) balanced steady-state free precession valve cines of a patient with BAV with R/L fusion and no stenosis. B, Contrast-enhanced magnetic resonance angiography shows mild dilation of the sinus of Valsalva with a maximal dimension of 40 mm and (C) a 47-mm dilation of the mid-ascending aorta. D, An eccentric jet is observed downstream from the nonstenotic bicuspid valve that impacts along the anterior portion of the tubular aorta. L, Left coronary cusp; N, noncoronary cusp; R, coronary cusp.
6. INDICATIONS FOR SURGERY
The most important clinical decision for patients with BAV-associated aortopathy is the appropriate timing of surgical intervention. Optimally, surgery should be recommended as soon as the risk of watchful waiting exceeds the risk of surgical intervention. Unfortunately, the precise time point when this occurs is patient-and surgeon-/center-specific and therefore oftentimes difficult to identify. Prophylactic aortic repair is recommended to prevent catastrophic aortic complications, particularly aortic dissection and rupture. When examining data obtained from retro-spective and natural history studies, it is important to include patients experiencing sudden, unexplained cardiac death as presumed (or at least possible) aortic complications.
Factors that need to be considered when recommending aortic repair include maximum aortic diameter, presence of aortic risk factors (ie, rate of aortic growth, BAV phenotype, systemic hypertension, family history of aortic complications, or other aortic conditions such as coarctation or connective tissue disorders), presence of surgical risk factors (eg, advanced age, decreased left ventricular function, redo surgery), concomitant indications for cardiac surgery (most commonly aortic valvular stenosis or insufficiency), and surgeon/team experience and level of expertise. Although many different factors need to be considered when making this clinical decision, it is worth-while noting that operative risk usually plays a lesser role for experienced aortic surgeons because the majority of patients with BAV are relatively young with few surgical risk factors.
Despite the multitude of factors that need to be simulta-neously assessed, we describe our general recommendations for surgical repair in patients with BAV with aortopathy. For the purposes of clarity, indications have been divided into patients with and without concomitant indications for AV surgery. In addition, recommendations for management of the aortic arch are listed at the end of this section.
A. Risk–Benefit Assessment of Additional Aortic Repair: General Considerations
As in all surgical decision-making, the decision to repair the aortic root or ascending aorta must be based on the risk–benefit for a given patient in a given institution or surgeon’s hands. In this clinical scenario, the risk of any complications related to aortic surgery must be weighed against the potential benefit from preventing aneurysm-related complications. According to recent Society of Thoracic Surgeons data, isolated ascending aorta replacement surgery is associated with a 3.4% risk of mortality and 3.2% risk of stroke,183 whereas aortic arch surgery is associated with a 5.1% risk of in-hospital mortality and 5.3% risk of stroke.183 In contrast, the corresponding risks for isolated AVR are 2.5% and 1.5%.184 Although the addition of an aortic procedure to AVR is associated with no demonstrable increase in morbidity or mortality at some large-volume centers,185–187 this is not the case for most cardiac surgery institutions. Center- and surgeon-specific volumes have consistently been shown to have an influence on outcomes in a wide variety of technically complex operations, and aortic surgery is no exception. For instance, Hughes and colleagues188 examined patients undergoing aortic root or AVR plus ascending aortic replacement surgery and found that operative mortality was 58% lower in high-volume centers compared with low-volume centers.
B. Indications for Aortic Repair in Patients With Bicuspid Aortic Valve With Significant Aortic Valve Dysfunction
For those patients with BAV with valve dysfunction significant enough to meet indications for AV surgery, the recommended cutoff for concomitant ascending aortic replacement is 4.5 cm (Class IIa, level of evidence C in AHA/ACC 2014 guidelines,9 ESC 2014 aortic guidelines,12 ESC valvular guidelines11) (Table 5). This recommendation is primarily based on a retrospective study by Borger and colleagues57 that showed a higher incidence of subsequent aortic events in patients with BAV undergoing AVR with an aortic diameter of 4.5 cm or more. It should be noted that the majority of follow-up events were simple replacement of the ascending aorta during elective reoperative AVR surgery. Another study supporting this cutoff showed that the majority of patients with BAV status post-AVR who developed aortic dissection had aortic dilation of 4.5 cm or greater,166 and a second study demonstrated an increased risk of dissection among patients with BAV with aortic dilation of 4.5 cm or more.33 The incidence of aortic dissection and other aortic catastrophes post-AVR is low, particularly in patients with BAV with AS.49,57
TABLE 5.
Recommendations for aortic repair in patients with bicuspid aortic valve aortopathy
| Recommendation | Class/LOE |
|---|---|
| Repair of the ascending aorta/root is recommended when the aortic diameter is ≥55 mm in patients without risk factors | I/B26,27,33,155,226 |
| Repair of the ascending aorta/root should be performed when the aortic diameter is ≥50 mm in patients with risk factors (ie, root phenotype or predominant AI, uncontrolled hypertension, family history of aortic dissection/sudden death, coarctation, aortic growth >3 mm/y) | IIa/B26,27,33,155,226 |
| Repair of the ascending aorta/root may be performed in patients with an aortic diameter of ≥50 mm when the patients are at low surgical risk and operated on by an experienced aortic team in a center with established surgical results. | IIb/C2,174 |
| Concomitant repair of the ascending aorta/ root should be performed when the aortic diameter is ≥45 mm in patients undergoing cardiac surgery. | IIa/B26,33,57,155,166,191 |
| Repair of the aortic arch is recommended in patients with an aortic arch diameter of ≥55 mm. | I/B221,227 |
| Concomitant repair of the aortic arch should be performed in patients undergoing cardiac surgery with an aortic arch diameter of ≥50 mm. | IIa/C228 |
| Concomitant repair of the aortic arch may be performed in patients undergoing cardiac surgery with an aortic arch diameter of ≥45 mm, provided the patients are at low surgical risk and operated on by an experienced aortic team with established surgical results. | IIb/C220 |
| It is recommended that patients undergoing elective aortic arch repair be referred to an experienced aortic team with established surgical results. | I/B224,225 |
LOE, Level of evidence; AI, aortic insufficiency.
One argument supporting concomitant replacement of the aorta during AV surgery, regardless of future risk of aortic complications, is the fact that the aortic wall tends to be quite thin when the diameter exceeds 4.5 cm. Therefore, surgeons may elect to replace the aorta in such patients, rather than risk experiencing catastrophic tears in the suture line of an effaced aorta at the end of the procedure. In contrast, avoidance of prophylactic aortic repair in patients with moderate aortic dilation (ie, 4.5–5.0 cm) is prudent when extension of the myocardial ischemic time should be avoided (eg, patients with poor left ventricular function).
C. Specific Surgical Considerations for Patients With Bicuspid Aortic Valve Undergoing Aortic Valve Surgery
Most patients with BAV undergoing AVR do not require aortic root replacement surgery. Indeed, the incidence of significant aortic root dilation post-AVR in patients with BAV is low, similar to patients with TAV disease.92,189,190 However, root replacement is recommended in patients with BAV with an aortic root diameter exceeding 4.5 cm.26,57,155,191 Root replacement is oftentimes required in patients with BAV presenting with acute aortic dissection, because the proximal root is frequently involved in the dissection process.32 However, performing ascending aortic replacement alone and leaving a modestly dilated root if the valve is intact may be prudent in patients in extremis in whom an expedient operation may decrease operative mortality.187 Leaving the root “for another day” should not be considered a failure in surgery for acute aortic dissection.
Patients with BAV may present with aneurysmal dilation and effacement of the ascending aorta that extends preferentially into the noncoronary sinus. Such patients may be effectively treated with a modified remodeling operation with a tongue of graft extending into the noncoronary sinus. Such an approach spares the patient from the added complexity and increased risk associated with a complete root replacement operation, and is associated with a low rate of subsequent aortic events. Studies have shown excellent midterm results using this technique.192
Type of implanted valve at the time of AVR may influence the extent of aortic repair in patients with BAV. In patients with moderate aortic root dilation (ie, 4.5–5.0 cm) who have opted for a mechanical valve, complete root replacement is reasonable. Isolated AVR is preferable, however, in young patients who have opted for a biological valve because of the low risk of subsequent aortic root rupture/dissection92,190 and the increased technical difficulty associated with repeat aortic root replacement surgery.193
AVR surgery in patients with BAV may be complicated by coronary anomalies, which are far more common than in patients with TAV.194 The most common BAV-associated coronary anomaly is a nondominant right coronary artery, which can have implications for myocardial protection during AV surgery. The position of the coronary ostia is also more variable in patients with BAV, with ostia frequently positioned directly adjacent to an aortic valve commissure. Such anomalies are important to note preoperatively and may cause the surgeon to take a less aggressive approach to aortic root repair in such patients.
D. Role of Valve-Sparing Aortic Root Replacement and Aortic Valve Repair
Patients with bicuspid aortopathy and relatively normal aortic cusps with good mobility can be considered for valve-sparing aortic root replacement surgery (ie, David operation) in select centers. However, the indications for aortic repair should be the same as for patients undergoing more conventional forms of surgery (Table 5). With careful patient selection, studies from high-volume centers have shown that valve-sparing aortic root replacement can be performed in patients with BAV without increasing the risk of reoperation or recurrent aortic regurgitation when compared with patients with TAV.195–197 However, long-term results remain pending, and some have expressed concerns of increased long-term risk of AS or recurrent insufficiency.198–200 Further research insights into the different BAV phenotypes will possibly shed more light on the actual risk of these complications after repair/sparing procedures. Given that valve-sparing aortic root replacement is more technically challenging in patients with BAV, such operations should be performed in referral centers by surgeons with substantial clinical experience with the David operation.
Isolated AV repair has also been applied in select patients with BAV. On the basis of the pathophysiologic classification of aortic regurgitation developed by El Khoury and colleagues,201 various approaches to AV repair have been developed.202,203 Although good midterm results in patients with BAV have been demonstrated in expert hands,204,205 debate continues regarding the optimal method and even necessity of annular stabilization in such patients.206–209 Another topic of debate is the optimal commissural geometry for AV repair in patients with BAV.210–212 The lack of consensus regarding these issues, the lack of long-term follow-up data, and the increased technical complexity of AV repair in patients with BAV have resulted in a lack of widespread adoption of these techniques by the general cardiac surgery community.
E. Indications for Aortic Repair in Patients With Bicuspid Aortic Valve Without Significant Aortic Valve Dysfunction
Current guidelines recommend intervention on the aorta in patients with BAV without significant aortic valvular dysfunction (ie, valvular dysfunction does not meet criteria for surgical valve repair/replacement) if the maximal aortic diameter exceeds 5.5 cm and patients are lacking any high-risk characteristics (Table 5) (Class I, level B in AHA/ACC guidelines9,10 and Class I, level C in ESC 2014 guidelines12). Such recommendations are based on the observation that 6.0 cm represents a definite inflection point in the risk of aortic complications in all patients regardless of AV morphology213 and that natural history studies demonstrating a definitively increased risk of such complications in those with BAV (vs TAV) are debatable. Although Michelena and colleagues33 demonstrated that patients with BAV have a higher risk of aortic dissection than the general population, it is unknown at what aortic diameter these dissections tend to occur. In addition, the observation that many patients with aortic dissection present with an aortic diameter of less than 5.5 cm (ie, “aortic size paradox”)214,215 is difficult to interpret given that the denominator size for this group of patients is large.213 Indeed, some studies of patients presenting with acute aortic dissection have demonstrated larger ascending aortic diameters in patients with BAV,32,166 refuting the notion that the BAV is less stable than the TAV. The larger aortic diameters in patients with BAV may be a result of longer periods of exposure to increased aortic shear stress in patients born with a congenital anomaly, as opposed to acquired disorders such as hypertension or atherosclerosis. Such an explanation would be consistent with the increasing amount of data supporting the hemodynamic theory of BAV aortopathy, as opposed to the genetic theory (Section 3.A). It would also underscore the importance of ongoing surveillance of the aorta in patients with BAV, with surgical intervention being recommended only when appropriate thresholds have been reached. These observations and the definite, albeit low, risk of surgical intervention argue against routine replacement of the aorta in patients with BAV with smaller aortic diameters at the current time.
Certain factors may increase the risk of aortic complications in patients with BAV and therefore lead to earlier intervention. Current guidelines recommend surgical intervention at an aortic diameter of 5.0 cm in patients with any of the following risk factors: aortic coarctation, systemic hypertension, a family history of aortic dissection, or rapid aortic growth (>3–5 mm/year) in experienced hands. In the AHA/ACC guidelines, this is a Class IIa, level of evidence C recommendation,9,10 and in the ESC 2014 aortic guidelines, this is a Class I, level C recommendation.12 Intervention at lower dimensions can be considered in patients with small body surface area or stature, particularly if they have Turner syndrome. Surgical repair is reasonable in patients with Turner syndrome with an indexed aortic diameter of 2.75 cm/m2 or greater.172 A similar indexed aortic diameter cutoff175 or an aortic cross-sectional area to height ratio of greater than 10 cm2/m174 may be used to guide earlier surgical intervention in patients with small stature. A lower threshold for aortic repair (ie, diameter of 5.0 cm) may be considered in women planning pregnancy because of an increased risk of aortic complications in such patients.216 Finally, earlier intervention may be occasionally justified in patients with a strong preference for early surgery, particularly if their condition causes undue emotional stress.
A statement of clarification on management of aortopathy in BAV, released by the AHA/ACC in 2016, also recommended surgery if the aortic diameter is 5.0 cm or greater, the patient is at low operative risk, and the operation is performed by an experienced aortic surgical team in a center with established expertise (class IIa, level of evidence B).10 Masri and colleagues2 found that surgical intervention in patients with BAV restored them to a normal population survival curve and that patients with an aortic diameter greater than 5.0 cm who did not undergo operative repair had a modestly increased risk of death or aortic dissection during follow-up. However, the perioperative mortality in this study was only 0.4%. Wojnarski and colleagues,174 from the same center, also demonstrated a modestly increased risk of aortic dissection starting at 5.0 cm in patients with BAV, with a more pronounced increase in risk starting at 5.5 cm. On the basis of these findings, the current document makes a class IIb, level of evidence C recommendation for surgical repair of low-risk patients in experienced aortic centers if the aortic diameter is greater than 5.0 cm (Table 5).
These recommendations reflect a general change toward a more conservative approach for BAV-associated aortopathy when compared with previous guidelines, which stated that such patients should be managed as aggressively as those with connective tissue disorders.5 Studies published subsequent to these earlier guidelines have demonstrated that patients with BAV have a markedly lower risk of aortic complications and aortic dilation than those with Marfan syndrome.7,33,51 A recent joint statement of clarification was published to address these issues.
Recent evidence suggests a marked difference in the natural history of patients with BAV with aortic regurgitation compared with stenosis. Girdauskas and colleagues86 found that the 10-year freedom from adverse aortic events (dissection/rupture, death, and need for proximal aortic surgery) was 78% in BAV with aortic regurgitation versus 93% in patients with BAV stenosis patients. Wang and colleagues217 recently confirmed these results in a retrospective study. Other studies have demonstrated more rapid progression of BAV aortopathy in patients with aortic root phenotype (ie, dilation with greater diameter at the sinuses of Valsalva than the tubular ascending aorta, typically associated with aortic regurgitation; Section 3.C).29,51,82 In addition, a meta-analysis found patients with BAV with aortic regurgitation to be 10 times more likely to experience aortic dissection than those with AS.218 Therefore, it is reasonable to consider aortic repair in patients with BAV with aortic regurgitation and root phenotype of aortic dilation at an aortic diameter of 5.0 cm. Such patients may particularly benefit from a valve-sparing aortic root replacement (David) operation, if done in an experienced center with known outcomes.
F. Management of Aortic Arch
In contrast to the ascending aortopathy, the natural history of the aortic arch in patients with BAV is not well established. Although there is correlation between BAV morphology (eg, Sievers type 1, R/L) and proximal aortic aneurysm, the association with regard to aortic arch pathology is less clear.219 Although some investigators have found a correlation among Sievers type 1, R/N, and aortic arch dilation,13,68 this association is not consistent. The relative lack of natural history studies is further confounded with the denominator neglect phenomenon, whereas a few studies report the complications of aortic arch aneurysm (eg, numerator).13,219,220 Data are lacking on the risk of development of aortic arch pathologies with BAV (eg, denominator). Furthermore, the gap in knowledge also applies to nonsize criteria for intervention, such as risk factors, genetic clusters, aortic wall thickness, and strain measurements for patients with BAV.
BAV aortopathy has been briefly addressed in multiple guidelines and consensus statements, but most do not address the aortic arch specifically. Neither the 2010 ACC/AHA guidelines5 nor the ESC guidelines12 discuss indications for aortic arch repair in patients with BAV. The 2014 Canadian Cardiovascular Society Position statement was the first to recommend a threshold of 5.5 cm for replacement of aortic arch aneurysm associated with BAV.221
I. Aortic arch dissection
According to the International Registry of Acute Aortic Dissection registry, the risk that the aortic arch is involved during acute type A aortic dissection is lower in those with BAV than TAV and Marfan syndrome.222 Furthermore, a Mayo clinic study reported that among patients with known aortic dilatation before dissection, the mean diameter was lower for those with TAV compared with BAV.166 One can conclude from these observations that patients presenting with acute aortic syndromes (acute dissection, intramural hematoma, or penetrating atherosclerotic ulcer) of the aortic arch should be treated following the recommended guidelines for these pathologies, regardless of the aortic valve morphology.
II. Aortic arch aneurysm
There is controversy regarding the indication for surgical therapy in aneurysmal aortic arch disease in patients with BAV. Although experts agree that symptomatic aneurysmal aortic arch disease should be treated regardless of size, there is disagreement in regard to the asymptomatic patient and the extent of the distal aortic repair.
Park and co-authors220 reported on a series of 422 patients with BAV undergoing replacement of the ascending aorta without intervention on the aortic arch. These patients were followed up for a median of 4 years with no reoperations for arch dilatation. They concluded that subsequent enlargement of the aortic arch after ascending aortic replacement is rare.220 They recommended tailoring the extent of distal aortic operation in patients with BAV and avoiding arch repair if the transverse arch is not significantly enlarged (ie, ≥4.5 cm).220
A contrarian view has been expressed from investigators at Stanford University.13,219 With a study based partly on embryological studies showing migration of cells of neural crest origin into the aortic arch, Fazel and co-authors13 performed hierarchical cluster analysis and found 5 distinct patterns of aortic involvement in 127 patients with BAV. In clusters III and IV, they found more frequent involvement of the aortic arch and therefore recommended aggressive hemiarch or total arch replacement in experienced centers. However, this study did not provide longitudinal information about the fate of the various clusters. Furthermore, the amount of arch dilation found in such patients (median of 3.5 cm in cluster III) was well below the recommended thresholds for aortic arch repair.
Malaisrie and colleagues223 recently advocated extending resection into the arch when the distal ascending or proximal arch was larger than 4.0 cm.223 They compared 177 patients who underwent hemiarch replacement with 207 patients who received isolated ascending aortic replacement. The mortality rate increased from 1.5% to 3.0% in the hemiarch group, although the difference did not reach statistical significance in this small series. However, there was a statistically significant 54% increase in cardiopulmonary bypass times and 35% increase in crossclamp times in the hemiarch group.
This information suggests that indications for repair of the aortic arch should be no different in the setting of BAV compared with TAV. If a patient with BAV presents for AVR and has an ascending aortic aneurysm with a normal aortic diameter below the takeoff of the innominate artery, ascending aortic repair without arch intervention is recommended. If the aortic arch has a diameter of greater than 4.5 cm at the innominate artery takeoff, hemiarch replacement is reasonable in experienced centers, with the understanding that operative mortality and risk of stroke may be mildly increased. A total arch replacement is reasonable in patients with BAV undergoing AVR with a mid-aortic arch diameter of 4.5 cm or greater at the level of left carotid artery as measured by 3D aortic centerline reconstructions. However, such pathology is rare and usually found in patients with BAV with other causes of aortic arch dilation (eg, previous aortic coarctation repair, concomitant connective tissue disorder or Turner syndrome, chronic aortic dissection). Given the complexity of the latter operations, a referral to experienced aortic centers is recommended. In emergency situations (eg, aortic dissection), even experienced aortic surgeons may wisely opt to avoid compete resection of the diseased aorta and deal only with the most critical aspects of the procedure. Complete resection of all affected aorta, if necessary, can then be considered electively at a later time.
III. Operative volume and outcome
Situations in which an operation should be extended to a more aggressive approach should take into consideration the expertise and comfort level of the surgeon and the experience of the center. One set of previously published guide-lines attempted to define an experienced aortic center, but the recommended benchmark mortality rates (ie, <1% for elective repair of ascending aorta and aortic root aneurysm repair) is far below most reported series.221
A Japanese cooperative study examined 2875 patients undergoing thoracic aortic surgery in 36 centers between 2003 and 2005224 and found an important impact of hospital and surgeon volume on operative mortality. They found in young patients (<65 years of age), outcomes improved with increased hospital volume.224 Risk-adjusted mortality was 10% for centers performing less than 20 thoracic aortic operations during a 3-year period compared with 4% for centers performing more than 20 operations.224 In addition, observed outcomes in high-risk patients (ie, Japan Adult Cardiovascular Surgery Database predicted risk of mortality ≥6%) improved with increased hospital volume.224 Risk-adjusted mortality in these high-risk patients was 20% for centers performing less than 20 thoracic aortic operations, compared with 12% for centers performing more than 20 operations.224 Gazoni and colleagues225 also compared low-volume centers (<40 cases in 3 years) with high-volume centers (>80 cases in 3 years) in the Virginia Cardiac Surgery Quality Initiative. They found no difference in mortality for ascending aneurysm with valve procedure (3.4% high vs 5.2% low, P = .40), but increased mortality in low-volume centers for isolated ascending aneurysms (17% vs 3%, P = .01) and arch aneurysms (25% vs 5%, P = .01).225 Thus, ascending aortic replacement with or without aortic arch repair may be associated with a higher complication rate than previously identified in centers with limited experience. By taking into account these findings, patients requiring more extensive aortic repair involving the aortic arch should be referred to a center of expertise for nonemergency surgery.
7. SURGICAL FOLLOW-UP, MEDICAL MANAGEMENT/WATCHFUL WAITING, FAMILY SCREENING
The current section provides information regarding management and radiologic follow-up of patients with BAV undergoing watchful waiting, as well as patients with BAV who have undergone aortic repair surgery. Recently published guidelines and review articles on the subject may also be instructive.18,221
A. Imaging Postsurgery
Imaging of the aorta soon after initial surgical repair is aimed at detecting anastomotic leaks and pseudoaneurysms, as well as establishing a baseline for future comparisons. For this purpose, an ECG-gated cardiac CT is preferred to TTE because echocardiography is often limited by the presence of prosthetic aortic valves and provides incomplete aortic imaging. In younger individuals (<50 years of age), however, MRI may be preferable to repeat CT examinations to avoid the risk of radiation-induced malignancy. Furthermore, in the setting of acute aortic syndromes, MRI is particularly helpful in distinguishing mural thrombus from intramural blood.229,230
The interval at which repeat imaging is performed after aortic surgery is often dictated by the extent of the initial operation and whether areas of aortic dilatation were not addressed during the initial surgery. For example, if a supracoronary graft replacement was performed in the setting of a moderate root dilatation that was not addressed, then the imaging surveillance interval may be shorter. A similar situation may arise if a moderately dilated aortic arch was left untreated during replacement of the ascending aorta or if limited resection was performed in the setting of a type A dissection. In patients who underwent complex hybrid reconstructions of the aorta (ie, debranching combined with endovascular repair), more frequent imaging is also advisable. In the absence of residual aortic dilation/pathology, it is reasonable to suggest that the entire aorta be imaged by CT or MRI once every 3 to 5 years after aortic repair (Table 6). When possible, these studies should be performed at the same institution using similar imaging techniques and protocols to minimize variation.
TABLE 6.
Recommendations for postsurgical repair, medical management, and watchful waiting
| Recommendation | Class/LOE |
|---|---|
| Radiologic imaging (with CTA or MRA) may be performed after aortic surgery to establish a postrepair baseline. | IIb/C |
| Ongoing postoperative surveillance intervals should be individualized on the basis of the clinical, anatomic, and surgical features. In the presence of residual aortic dilation/ pathology, it is reasonable to image the entire aorta every 3–5 y by CT or MRI after repair. | IIa/B12,29,51,58,155,166 |
| MRI should be considered for repeat examinations in an adolescent or in the adult population aged <50 y. | IIa/B159 |
| Treatment of hypertension is recommended according to country- and region-specific guidelines. | I/C52–54,155,250,251 |
| Beta-blockers and inhibitors of the renin-angiotensin system should be considered for blood pressure control based on evidence extrapolated from populations with connective tissue disease. Nonpharmacologic approaches (salt reduction, weight reduction) should be advocated as part of blood pressure control strategies. | IIa/C52–54,155,250,251 |
| Patients with aortic aneurysms that are at or near surgical thresholds for correction should avoid strenuous lifting, pushing, or straining that would require a Valsalva maneuver. | IIa/C5,221,241,242,252 |
| It is recommended to avoid heavy weight lifting or competitive athletics involving isometric exercise when the ascending aortic diameter is >45 mm. | I/B5,221,241,242,252 |
| Patients with BAV and dilated aorta should be precluded from private driving if the ascending aorta diameter is >6.0 and restricted from commercial driving if the ascending thoracic aorta diameter is >5.5 cm. | IIa/C221,240 |
| It is recommended that prepregnancy evaluation and postpregnancy management of women with BAV with or without associated aortopathy be performed by practitioners with expertise in the management of pregnant women with heart disease. | I/C253 |
| First-degree relatives of patients with BAV should undergo screening echocardiography. | IIa/B249 |
LOE, Level of evidence; CTA, computed tomography angiography; MRA, magnetic resonance angiography; CT, computed tomography; MRI, magnetic resonance imaging; BAV, bicuspid aortic valve.
B. Medical Management and Watchful Waiting
The medical management of patients with BAV aortopathy who are subject to ongoing watchful waiting is usually focused on blood pressure control and overall cardiovascular risk reduction via pharmacologic and nonpharmacologic measures. The rationale for antihypertensive therapy is based on mechanistic and animal studies,231,232 as well as observational reports linking aortic dissection to hypertension.233,234 In patients with BAV aortopathy, there are no randomized trials or observational studies to help guide decision-making. Treating hypertension with beta-blockers or inhibitors of the renin-angiotensin system has been suggested, based largely on extrapolation of data from Marfan cases.18,221 At the present time, there are no data to support lower blood pressure thresholds for patients with dilated aortas in the setting of BAV; therefore, country-and region-specific guidelines for treatment of hypertension should be followed. Target blood pressure thresholds in individuals without diabetes, aged more than 60 years, and with multiple risk factors are likely going to change after the results of the SPRINT trial, which demonstrated a reduction in overall cardiovascular mortality with an intensive blood pressure target of 120/80 mm Hg in such individuals.235 Although clinical data are lacking, it may be reasonable to achieve these targets in subjects who do not meet the SPRINT criteria who have specific factors, including patient or family history of acute aortic syndrome or sudden death, or aortic aneurysm growth despite medical therapy.
In patients with chronic aortic dissection, observational reports suggest lower risk for operative repair with beta-blocker therapy.236 In patients with type A and type B aortic dissections, beta-blockers are associated with improved survival.237 Use of calcium-channel blockers has also been associated with improved survival in type B aortic dissections,237 as well as decreased rate of aortic expansion.238 One study identified an association between angiotensin-converting enzyme inhibitor and better survival in patients with type B aortic dissection,239 although this was not confirmed in a more recent study.237
General counseling on nonpharmacologic approaches to risk reduction should be part of watchful waiting in those with BAV aortopathy. Such recommendations include limiting salt intake (to reduce hypertension), a diet low in saturated fats, exercise (with caveats, discussed later), and smoking cessation. Management of dyslipidemia should follow regional or national guidelines based on primary or secondary prevention thresholds and targets, where applicable.
There are no specific recommendations regarding automobile driving within the 2010 ACC/AHA guidelines.5 However, the Canadian Medical Association has recommended that patients with abdominal aortic aneurysm be precluded from driving when the rupture risk exceeds 10% per year. On the basis of the best observational data available, these thresholds of risk occur for thoracic aortic aneurysms greater than 6.0 cm in the ascending aorta or arch, and greater than 6.5 cm in the descending aorta.175 A lower threshold for rupture risk is reasonable for commercial driving.221,240
Exercise prescription or restrictions should be individualized in patients with aortic aneurysms. Patients with previously repaired aortic dissection should avoid strenuous lifting, pushing, or straining that would require a Valsalva maneuver.5,221,241,242 Strenuous strength training may be dangerous for patients with BAV aortopathy, because aortic dissection has been linked to weight lifting.242 The proposed mechanism is transiently elevated blood pressure associated with isometric exercise or Valsalva maneuver.241 Heavy weight lifting or competitive athletics involving isometric exercise may trigger aortic dissection; therefore, such activities should be avoided in patients with moderately dilated aortas (ie, >4.5 cm) or when there has been a significant interval increase in aortic size. However, individuals with bicuspid aortopathy can and should undergo aerobic or endurance exercise, because these exercises are beneficial for blood pressure lowering.243 If patients want to engage in vigorous aerobic exercise, such as running or basketball, one might consider performing a symptom-limited stress test to ensure that the patient does not have a hypertensive response to exercise. In patients with a normal bicuspid valve and no associated dilated aorta, no restrictions of activity are required.
The management of pregnancy in the setting of BAV is not well studied, and this area has been recently summarized in a review.18 In general, women with BAV should undergo imaging of the entire aorta before pregnancy,244 and prepregnancy evaluation in women with known BAV aortopathy should be performed by practitioners with expertise in the management of pregnant women with heart disease. The exact threshold to recommend against pregnancy is not known, but it would reasonable to suggest that if the ascending aortic diameter is close to the threshold of surgical intervention, then the risks and benefits should be weighed and individualized decisions be made. Some studies have suggested that women with ascending aorta or root dimension greater than 4.5 cm should be advised against pregnancy, although this is controversial.180,221 A thorough interdisciplinary, team-based approach is recommended to discuss case-by-case scenarios.
Pregnant women with a dilated aorta, including BAV aortopathy, should have strict blood pressure control and repeated echocardiographic imaging every 4 to 12 weeks during pregnancy.244 MRI (without gadolinium) is recommended if there is an indication for imaging of the distal ascending aorta, aortic arch, or descending aorta during pregnancy. TEE is an alternative to MRI for imaging of aorta during pregnancy.
Beta adrenergic blockers, to reduce shear stress on the aorta, may be considered during pregnancy in women with a dilated aorta. Women with bicuspid aortopathy or history of aortic dissection should deliver in a center where cardiothoracic surgery is available.244 The type of delivery (eg, Caesarean section) and peripartum anesthetic requirements should be determined in advance by the obstetrics and anesthesia teams.
C. Family Screening
Most cases of BAV disease are sporadic, but familial clustering has been a long-recognized phenomenon.245 Genetic studies have suggested an autosomal dominant pattern with incomplete penetrance and variable expressivity as the likely mode of inheritance.246 However, the preponderance of male patients with BAV and the association with Turner syndrome have suggested an X-linked pattern.247 Several different gene mutations have been linked to BAV disease, including NOTCH1, TGF-ß2, ACTA2, FNB1, KCNJ2, GATA5, Nkx2–5, and SMAD6.248 The degree of genetic heterogeneity is not surprising, given the marked heterogeneity of clinical findings in patients with BAV.
The heritability of BAV disease is more than 80%, and approximately 9% to 15% of first-degree relatives have the disorder.249 Therefore, it is recommended that first-degree relatives of patients with BAV are screened with echocardiography.
8. KNOWLEDGE GAPS AND FUTURE RESEARCH
Key questions remain unanswered with respect to BAV-associated aortopathy. First, specific genetic and developmental causes of the congenital bicuspid valve malformation itself remain unclear. A thorough genetic understanding has been especially elusive, likely reflecting multifactorial genetic mechanisms. This critical gap in knowledge influences the understanding of the wider spectrum of manifestations of BAV-related diseases, including the heterogeneous expression of different phenotypes of BAV aortopathy. It is reasonable to assume that the underlying pathogenesis of the bicuspid valve itself may play a role in the propensity, development, and progression of BAV aortopathy. Further investigations of these root causes are necessary for a more complete understanding of bicuspid aortopathy. Second, the causes of valve dysfunction in the majority of patients with BAV over time are unclear. Like-wise, the causes of BAV aortopathy in many, but a smaller proportion of, patients with BAV remain elusive. Third, the marked clinical heterogeneity of BAV disease and the specific risk factors that predispose individual patients with BAV to valve dysfunction or to aortic dilatation/dissection remain mysterious. Further research to clarify the pathophysiology of BAV disease progression and to more precisely identify risk profiles for individual patients with BAV is needed. Addressing these knowledge gaps could dramatically change our clinical and surgical approach to BAV aortopathy.254
Advances in knowledge may have been hampered thus far by a circuitous debate about the pathogenesis of BAV aortopathy as “genetic” versus “hemodynamic.” In light of the increasing recognition of the heterogeneity of BAV aortopathy, this dichotomy has begun to be questioned.82,255 In a recent study, aortic dilatation progressed at a yearly rate that varied within a wide range in a cohort of patients with BAV followed for 3 years, and did not progress at all in 43% of them.51 This marked heterogeneity suggests a more complex pathogenesis than just “genetically determined” or “hemodynamically driven.” Both genetic variants and rheological abnormalities may coexist, resulting in diverse clinical phenotypes with distinct natural histories.
Most studies on BAV aortopathy generally disregarded the described heterogeneous nature of BAV disease in terms of clinical features (age of onset, velocity of progression, risk of acute events), valve morphology, and phenotypes. Future studies on BAV aortopathy should be adequately designed to differentiate among distinct forms of the disease.
Another common limitation of previous research in this field is the observational nature of the majority of previous studies. Such retrospective studies report the association of clinical factors, flow features, histopathology, or molecular findings with aortic dilatation in patients with BAV and consequently infer their role in the underlying pathogenesis. However, it is unclear whether the associated findings are a consequence of the dilatation itself rather than a determinant or risk factor.256 Future research must advance beyond associative studies toward more informative mechanistic investigations, functional studies, and experimental validations. Clinical randomized trials, particularly of pharmacologic treatments to slow the progression of aortic dilatation or prevent acute events, are particularly warranted. However, conducting these studies will be challenging because of the slow progression of BAV disease and the need for large cohorts to account for the different phenotypes. Nevertheless, multisite longitudinal studies that link clinical, genetic, and hemodynamic risk factors to patient outcomes are urgently needed.
Clinical studies may be confounded by patient referral patterns or selection criteria for enrollment. These issues may explain contradictory studies, which are common in the field of BAV research. Clinical features of BAV aortopathy can vary according to whether they are analyzed in population studies or with specific hospital referral patterns, as well as between surgical and nonsurgical studies. Failure to account for these confounding factors and sources of bias can lead to misleading conclusions.
Another limitation of past research on BAV aortopathy has been the use of inconsistent or ambiguous terminology, making it difficult to compare results between studies.93,257 In addition, many clinical series were obtained from single-center experiences with a limited number of patients observed. Future collaborative multi-center efforts with clearly defined terminology are warranted. Such studies may be spearheaded by international organizations, such as the recently established Bicuspid Aortic Valve Consortium research group.28 Previous studies, especially those on molecular and cellular aspects, tended to address novel pathways and pathogenetic hypotheses rather than verify and expand previously acquired knowledge. Thus, various findings from different research efforts cannot converge into the establishment of a definite pathogenetic sequence, that is, all the subsequent mechanistic steps from the first cause to the ultimate effect. It may be necessary to merge large amounts of patient data and different investigators’ expertise to ensure adequately powered study populations and correct study designs.
At the present time, the greatest unmet clinical research need is the identification of optimal criteria for risk stratification. Prognostic stratification of BAV aortopathy suffers from 2 important issues: the gaps in knowledge on the pathogenesis of bicuspid aortopathy and the unknown mechanisms of acute aortic complications, namely, aortic dissection. It has been demonstrated that dissection occurs in the majority of cases at aortic sizes well below the threshold recommending prophylactic aortic resection,258 although this observation can be explained by the “size paradox.”213 Some investigators have advocated nondimensional criteria for risk stratification.259 These should be derived from the validation of novel methods to detect aortopathy in its early stages and predict aortic disease development via preclinical aortic wall dysfunction or aortic tissue disarray. Circulating biomarkers that are associated with aortopathy may be particularly helpful in this regard, although preliminary studies in this area have not been fruitful. A greater prognostic armamentarium would support a patient-specific approach to BAV aortopathy,59,69,254 especially in terms of criteria informing surveillance, surgical indications, and follow-up. Future advances may be best achieved using emerging diagnostic imaging modalities, such as 4D-flow MRI and computation fluid dynamics,143 possibly combined with novel molecular biomarkers.259
9. CONCLUSIONS
Research on BAVaortopathy is challenging for many reasons. There remain significant gaps in our current knowledge that limit best practices and adherence to clinical guidelines. Future investigations should account for epidemiologic and phenotypic heterogeneity of the disease. Multicenter and multidisciplinary teams should be lever-aged to perform robust hypothesisdriven analyses.28 Basic and translational approaches may help inform clinical studies.
Footnotes
Conflict of Interest Statement
Dr Borger received speakers’ honoraria and/or consulting fees from Edwards Lifesciences, Medtronic, St Jude Medical, and CryoLife. Dr Moon received speakers’ honoraria and/or consulting fees from Edwards Lifesciences and Medtronic. All other authors have nothing to disclose with regard to commercial support.
References
- 1.Hoffman J, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–900. [DOI] [PubMed] [Google Scholar]
- 2.Masri A, Kalahasti V, Alkharabsheh S, Svensson LG, Sabik JF, Roselli EE, et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2016;151:1650–1659.e1. [DOI] [PubMed] [Google Scholar]
- 3.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol 2004;43:665–9. [DOI] [PubMed] [Google Scholar]
- 4.Fedak P, David T, Borger M, Verma S, Butany J, Weisel R. Bicuspid aortic valve disease: recent insights in pathophysiology and treatment. Expert Rev Cardiovasc Ther 2005;3:295–308. [DOI] [PubMed] [Google Scholar]
- 5.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 121:e266–369. [DOI] [PubMed] [Google Scholar]
- 6.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg 2011;39:809–14. [DOI] [PubMed] [Google Scholar]
- 7.Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus Marfan syndrome. J Am Coll Cardiol 2015;65:2363–9. [DOI] [PubMed] [Google Scholar]
- 8.Sherrah AG, Andvik S, van der Linde D, Davies L, Bannon PG, Padang R, et al. Nonsyndromic thoracic aortic aneurysm and dissection: outcomes with Marfan syndrome versus bicuspid aortic valve aneurysm. J Am Coll Cardiol 2016;67: 618–26. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Thorac Cardiovasc Surg 2014; 148:e1–132. [DOI] [PubMed] [Google Scholar]
- 10.2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease Representative Members, Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG, 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease Representative Members, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016;151: 959–66. [DOI] [PubMed] [Google Scholar]
- 11.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012; 42:S1–44. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35: 2873–926. [DOI] [PubMed] [Google Scholar]
- 13.Fazel SS, Mallidi HR, Lee RS, Sheehan MP, Liang D, Fleischman D, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg 2008;135:901–7. 907.e1-e2. [DOI] [PubMed] [Google Scholar]
- 14.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–33. [DOI] [PubMed] [Google Scholar]
- 15.Verma S, Yanagawa B, Kalra S, Ruel M, Peterson MD, Yamashita MH, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg 2013; 146:1033–1040.e4. [DOI] [PubMed] [Google Scholar]
- 16.Friedman T, Mani A, Elefteriades JA. Bicuspid aortic valve: clinical approach and scientific review of a common clinical entity. Expert Rev Cardiovasc Ther 2008;6:235–48. [DOI] [PubMed] [Google Scholar]
- 17.Mills P, Leech G, Davies M, Leathan A. The natural history of a non-stenotic bicuspid aortic valve. Br Heart J 1978;40:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014;370:1920–9. [DOI] [PubMed] [Google Scholar]
- 19.Sagie A, Vaturi M, Shapira Y. [Update in aortic valve diseases]. Harefuah 2007; 146:867–71. 909. [PubMed] [Google Scholar]
- 20.Williams DS. Bicuspid aortic valve. J Insur Med 2006;38:72–4. [PubMed] [Google Scholar]
- 21.Ward C Clinical significance of the bicuspid aortic valve. Heart 2000;83:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Movahed MR, Hepner AD, Ahmadi-Kashani M. Echocardiographic prevalence of bicuspid aortic valve in the population. Heart Lung Circ 2006;15:297–9. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920–5. [DOI] [PubMed] [Google Scholar]
- 24.Roos-Hesselink JW, Scholzel BE, Heijdra RJ, Spitaels SE, Meijboom FJ, Boersma E, et al. Aortic valve and aortic arch pathology after coarctation repair. Heart 2003;89:1074–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandenburg RO Jr, Tajik AJ, Edwards WD, Reeder GS, Shub C, Seward JB. Accuracy of 2-dimensional echocardiographic diagnosis of congenitally bicuspid aortic valve: echocardiographic-anatomic correlation in 115 patients. Am J Cardiol 1983;51:1469–73. [DOI] [PubMed] [Google Scholar]
- 26.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25. [DOI] [PubMed] [Google Scholar]
- 28.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014;129:2691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Corte A, Bancone C, Buonocore M, Dialetto G, Covino FE, Manduca S, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging 2013;6: 1301–10. [DOI] [PubMed] [Google Scholar]
- 30.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012;42:832–8. [DOI] [PubMed] [Google Scholar]
- 31.Rylski B, Desai ND, Bavaria JE, Vallabhajosyula P, Moser W, Pochettino A, et al. Aortic valve morphology determines the presentation and surgical approach to acute type A aortic dissection. Ann Thorac Surg 2014;97: 1991–7. [DOI] [PubMed] [Google Scholar]
- 32.Etz CD, von Aspern K, Hoyer A, Girrbach FF, Leontyev S, Bakhtiary F, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve. Eur J Cardiothorac Surg 2015;48: 142–50. [DOI] [PubMed] [Google Scholar]
- 33.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104–12. [DOI] [PubMed] [Google Scholar]
- 34.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol 1997;30:1809–12. [DOI] [PubMed] [Google Scholar]
- 35.Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet 1996;62: 336–8. [DOI] [PubMed] [Google Scholar]
- 36.Emanuel R, Withers R, O’Brien K, Ross P, Feizi O. Congenitally bicuspid aortic valves. Clinicogenetic study of 41 families. Br Heart J 1978;40:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM III. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2007;134: 290–6. [DOI] [PubMed] [Google Scholar]
- 38.Prakash SK, Bosse Y, Muehlschlegel JD, Michelena HI, Limongelli G, Della Corte A, et al. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the International BAVCon (Bicuspid Aortic Valve Consortium). J Am Coll Cardiol 2014;64:832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270–4. [DOI] [PubMed] [Google Scholar]
- 40.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol 2003;23:543–53. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, Wrobel S, et al. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem Biophys Res Commun 2006;345: 1460–5. [DOI] [PubMed] [Google Scholar]
- 42.Warnes CA. Bicuspid aortic valve and coarctation: two villains part of a diffuse problem. Heart 2003;89:965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbott M Coarctation of the aorta of adult-type. Am Heart J 1928;3:574–628. [Google Scholar]
- 44.Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol 2005;30:470–522. [DOI] [PubMed] [Google Scholar]
- 45.Lad V, David TE, Vegas A. Mitral regurgitation due to myxomatous degeneration combined with bicuspid aortic valve disease is often due to prolapse of the anterior leaflet of the mitral valve. Ann Thorac Surg 2009;87:79–82. [DOI] [PubMed] [Google Scholar]
- 46.Charitos EI, Hanke T, Karluss A, Hilker L, Stierle U, Sievers HH. New insights into bicuspid aortic valve disease: the elongated anterior mitral leaflet. Eur J Cardiothorac Surg 2013;43:367–70. [DOI] [PubMed] [Google Scholar]
- 47.Hahn RT, Roman MJ, Mogtader AH, Devereux RB. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol 1992;19:283–8. [DOI] [PubMed] [Google Scholar]
- 48.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000;102:III35–9. [DOI] [PubMed] [Google Scholar]
- 49.Girdauskas E, Rouman M, Disha K, Espinoza A, Misfeld M, Borger MA, et al. Aortic dissection after previous aortic valve replacement for bicuspid aortic valve disease. J Am Coll Cardiol 2015;66:1409–11. [DOI] [PubMed] [Google Scholar]
- 50.Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM Jr, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338–44. [DOI] [PubMed] [Google Scholar]
- 51.Detaint D, Michelena HI, Nkomo VT, Vahanian A, Jondeau G, Sarano ME. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014;100:126–34. [DOI] [PubMed] [Google Scholar]
- 52.Chun AS, Elefteriades JA, Mukherjee SK. Medical treatment for thoracic aortic aneurysm - much more work to be done. Prog Cardiovasc Dis 2013;56:103–8. [DOI] [PubMed] [Google Scholar]
- 53.Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation 2011;124:1469–76. [DOI] [PubMed] [Google Scholar]
- 54.Ziganshin BA, Mukherjee SK, Elefteriades JA. Atenolol versus Losartan in Marfan’s Syndrome. N Engl J Med 2015;372:977–8. [DOI] [PubMed] [Google Scholar]
- 55.Regeer MV, van Rosendael PJ, Kamperidis V, Schalij MJ, Bax JJ, Marsan NA, et al. Effect of statins on aortic root growth rate in patients with bicuspid aortic valve anatomy. Int J Cardiovasc Imaging 2015;31:1583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo CF, Mazzetti S, Garatti A, Ribera E, Milazzo A, Bruschi G, et al. Aortic complications after bicuspid aortic valve replacement: long-term results. Ann Thorac Surg 2002;74:S1773–6; discussion S1792–9. [DOI] [PubMed] [Google Scholar]
- 57.Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg 2004;128:677–83. [DOI] [PubMed] [Google Scholar]
- 58.McKellar SH, Michelena HI, Li Z, Schaff HV, Sundt TM III. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol 2010;106:1626–33. [DOI] [PubMed] [Google Scholar]
- 59.Fedak PW, Verma S. Bicuspid aortopathy and the development of individualized resection strategies. J Thorac Cardiovasc Surg 2014;148:2080–1. [DOI] [PubMed] [Google Scholar]
- 60.Della Corte A Phenotypic heterogeneity of bicuspid aortopathy: a potential key to decode the prognosis? Heart 2014;100:96–7. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez B, Duran AC, Fernandez-Gallego T, Fernandez MC, Such M, Arque JM, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol 2009;54:2312–8. [DOI] [PubMed] [Google Scholar]
- 62.Robledo-Carmona J, Rodriguez-Bailon I, Carrasco-Chinchilla F, Fernandez B, Jimenez-Navarro M, Porras-Martin C, et al. Hereditary patterns of bicuspid aortic valve in a hundred families. Int J Cardiol 2013;168:3443–9. [DOI] [PubMed] [Google Scholar]
- 63.Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging 2013;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, et al. Valve-related hemodynamics mediate human bicuspid aortopathy: insights from wall shear stress mapping. J Am Coll Cardiol 2015;66:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 2014;129: 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girdauskas E, Rouman M, Disha K, Scholle T, Fey B, Theis B, et al. Correlation between systolic transvalvular flow and proximal aortic wall changes in bicuspid aortic valve stenosis. Eur J Cardiothorac Surg 2014;46:234–9. [DOI] [PubMed] [Google Scholar]
- 67.Sievers HH, Stierle U, Hachmann RM, Charitos EI. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg 2016;49:439–46. [DOI] [PubMed] [Google Scholar]
- 68.Schaefer B, Lewin M, Stout K, Gill E, Prueitt A, Byers P, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008;94:1634–8. [DOI] [PubMed] [Google Scholar]
- 69.Della Corte A, Bancone C, Dialetto G, Covino FE, Manduca S, D’Oria V, et al. Towards an individualized approach to bicuspid aortopathy: different valve types have unique determinants of aortic dilatation. Eur J Cardiothorac Surg 2014;45:e118–24. [DOI] [PubMed] [Google Scholar]
- 70.Merritt BA, Turin A, Markl M, Malaisrie SC, McCarthy PM, Carr JC. Association between leaflet fusion pattern and thoracic aorta morphology in patients with bicuspid aortic valve. J Magn Reson Imaging 2014;40:294–300. [DOI] [PubMed] [Google Scholar]
- 71.Page M, Mongeon FP, Stevens LM, Souliere V, Khairy P, El-Hamamsy I. Aortic dilation rates in patients with bicuspid aortic valve: correlations with cusp fusion phenotype. J Heart Valve Dis 2014;23:450–7. [PubMed] [Google Scholar]
- 72.Buchner S, Hulsmann M, Poschenrieder F, Hamer OW, Fellner C, Kobuch R, et al. Variable phenotypes of bicuspid aortic valve disease: classification by cardiovascular magnetic resonance. Heart 2010;96:1233–40. [DOI] [PubMed] [Google Scholar]
- 73.Cecconi M, Manfrin M, Moraca A, Zanoli R, Colonna PL, Bettuzzi MG, et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol 2005;95:292–4. [DOI] [PubMed] [Google Scholar]
- 74.Thanassoulis G, Yip JW, Filion K, Jamorski M, Webb G, Siu SC, et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med 2008;5:821–8. [DOI] [PubMed] [Google Scholar]
- 75.Holmes KW, Lehmann CU, Dalal D, Nasir K, Dietz HC, Ravekes WJ, et al. Progressive dilation of the ascending aorta in children with isolated bicuspid aortic valve. Am J Cardiol 2007;99:978–83. [DOI] [PubMed] [Google Scholar]
- 76.Fernandes SM, Khairy P, Sanders SP, Colan SD. Bicuspid aortic valve morphology and interventions in the young. J Am Coll Cardiol 2007;49: 2211–4. [DOI] [PubMed] [Google Scholar]
- 77.Sievers HH, Stierle U, Mohamed SA, Hanke T, Richardt D, Schmidtke C, et al. Toward individualized management of the ascending aorta in bicuspid aortic valve surgery: the role of valve phenotype in 1362 patients. J Thorac Cardiovasc Surg 2014;148:2072–80. [DOI] [PubMed] [Google Scholar]
- 78.Sievers HH, Stierle U, Hachmann RM, Charitos EI. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg 2016;49:439–46. [DOI] [PubMed] [Google Scholar]
- 79.Henn D, Perttunen H, Gauer S, Schmied W, Porras C, Such M, et al. GATA5 and endothelial nitric oxide synthase expression in the ascending aorta is related to aortic size and valve morphology. Ann Thorac Surg 2014;97:2019–25. [DOI] [PubMed] [Google Scholar]
- 80.Zhu Y, Roselli EE, Idrees JJ, Wojnarski CM, Griffin B, Kalahasti V, et al. Out-comes after operations for unicuspid aortic valve with or without ascending repair in adults. Ann Thorac Surg 2016;101:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a liter-ature review of 2,715 additional cases. Mayo Clin Proc 1999;74:14–26. [DOI] [PubMed] [Google Scholar]
- 82.Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007;31:397–405. [DOI] [PubMed] [Google Scholar]
- 83.Roberts WC, Vowels TJ, Ko JM, Filardo G, Hebeler RF Jr, Henry AC, et al. Comparison of the structure of the aortic valve and ascending aorta in adults having aortic valve replacement for aortic stenosis versus for pure aortic regurgitation and resection of the ascending aorta for aneurysm. Circulation 2011; 123:896–903. [DOI] [PubMed] [Google Scholar]
- 84.Girdauskas E, Rouman M, Borger MA, Kuntze T. Comparison of aortic media changes in patients with bicuspid aortic valve stenosis versus bicuspid valve insufficiency and proximal aortic aneurysm. Interact Cardiovasc Thorac Surg 2013;17:931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, et al. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg 2005;130:504–11. [DOI] [PubMed] [Google Scholar]
- 86.Girdauskas E, Disha K, Secknus M, Borger M, Kuntze T. Increased risk of late aortic events after isolated aortic valve replacement in patients with bicuspid aortic valve insufficiency versus stenosis. J Cardiovasc Surg 2013;54:653–9. [PubMed] [Google Scholar]
- 87.Girdauskas E, Rouman M, Disha K, Espinoza A, Dubslaff G, Fey B, et al. Aortopathy in patients with bicuspid aortic valve stenosis: role of aortic root functional parameters. Eur J Cardiothorac Surg 2016;49:635–44. [DOI] [PubMed] [Google Scholar]
- 88.Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg 2004; 77:177–85. [DOI] [PubMed] [Google Scholar]
- 89.Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010;255:53–61. [DOI] [PubMed] [Google Scholar]
- 90.La Canna G, Ficarra E, Tsagalau E, Nardi M, Morandini A, Chieffo A, et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol 2006;98:249–53. [DOI] [PubMed] [Google Scholar]
- 91.Kang JW, Song HG, Yang DH, Baek S, Kim DH, Song JM, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging 2013;6:150–61. [DOI] [PubMed] [Google Scholar]
- 92.Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM III. Fate of nonreplaced sinuses of Valsalva in bicuspid aortic valve disease. J Thorac Cardiovasc Surg 2011;142:278–84. [DOI] [PubMed] [Google Scholar]
- 93.Della Corte A, Bancone C, Dialetto G, Covino FE, Manduca S, Montibello MV, et al. The ascending aorta with bicuspid aortic valve: a phenotypic classification with potential prognostic significance. Eur J Cardiothorac Surg 2014;46: 240–7. [DOI] [PubMed] [Google Scholar]
- 94.Lu MT, Thadani SR, Hope MD. Quantitative assessment of asymmetric aortic dilation with valve-related aortic disease. Acad Radiol 2013;20:10–5. [DOI] [PubMed] [Google Scholar]
- 95.Bauer M, Gliech V, Siniawski H, Hetzer R. Configuration of the ascending aorta in patients with bicuspid and tricuspid aortic valve disease undergoing aortic valve replacement with or without reduction aortoplasty. J Heart Valve Dis 2006;15:594–600. [PubMed] [Google Scholar]
- 96.Cotrufo M, Della Corte A. The association of bicuspid aortic valve disease with asymmetric dilatation of the tubular ascending aorta: identification of a definite syndrome. J Cardiovasc Med 2009;10:291–7. [DOI] [PubMed] [Google Scholar]
- 97.Burris NS, Hope MD. 4D flow MRI applications for aortic disease. Magn Reson Imaging Clin N Am 2015;23:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niwa K, Perloff J, Bhuta S, Laks H, Drinkwater D, Child J, et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation 2001;103:393–400. [DOI] [PubMed] [Google Scholar]
- 99.Fedak PW, de Sa MP, Verma S, Nili N, Kazemian P, Butany J, et al. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg 2003;126:797–806. [DOI] [PubMed] [Google Scholar]
- 100.Bauer M, Pasic M, Meyer R, Goetze N, Bauer U, Siniawski H, et al. Morphometric analysis of aortic media in patients with bicuspid and tricuspid aortic valve. Ann Thorac Surg 2002;74:58–62. [DOI] [PubMed] [Google Scholar]
- 101.Russo CF, Cannata A, Lanfranconi M, Vitali E, Garatti A, Bonacina E. Is aortic wall degeneration related to bicuspid aortic valve anatomy in patients with valvular disease? J Thorac Cardiovasc Surg 2008;136:937–42. [DOI] [PubMed] [Google Scholar]
- 102.Della Corte A, De Santo LS, Montagnani S, Quarto C, Romano G, Amarelli C, et al. Spatial patterns of matrix protein expression in dilated ascending aorta with aortic regurgitation: congenital bicuspid valve versus Marfan’s syndrome. J Heart Valve Dis 2006;15:20–7. [PubMed] [Google Scholar]
- 103.Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics 2010;9:2048–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kutz WE, Wang LW, Bader HL, Majors AK, Iwata K, Traboulsi EI, et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J Biol Chem 2011;286:17156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg 2007;133:1028–36. [DOI] [PubMed] [Google Scholar]
- 106.Tzemos N, Lyseggen E, Silversides C, Jamorski M, Tong JH, Harvey P, et al. Endothelial function, carotid-femoral stiffness, and plasma matrix metalloproteinase-2 in men with bicuspid aortic valve and dilated aorta. J Am Coll Cardiol 2010;55:660–8. [DOI] [PubMed] [Google Scholar]
- 107.Wilton E, Bland M, Thompson M, Jahangiri M. Matrix metalloproteinase expression in the ascending aorta and aortic valve. Interact Cardiovasc Thorac Surg 2008;7:37–40. [DOI] [PubMed] [Google Scholar]
- 108.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res 2005;123:40–8. [DOI] [PubMed] [Google Scholar]
- 109.Rabkin SW. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm - comparison with and without bicuspid aortic valve: a meta-analysis. Vasa 2014;43:433–42. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Wu B, Dong L, Wang C, Wang X, Shu X. Circulating matrix metallo-proteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart Vessels 2016;31:189–97. [DOI] [PubMed] [Google Scholar]
- 111.Ikonomidis JS, Ruddy JM, Benton SM Jr, Arroyo J, Brinsa TA, Stroud RE, et al. Aortic dilatation with bicuspid aortic valves: cusp fusion correlates to matrix metalloproteinases and inhibitors. Ann Thorac Surg 2012;93:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011;332:358–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Girdauskas E, Schulz S, Borger MA, Mierzwa M, Kuntze T. Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann Thorac Surg 2011;91: e70–1. [DOI] [PubMed] [Google Scholar]
- 114.Paloschi V, Gadin JR, Khan S, Bjorck HM, Du L, Maleki S, et al. Aneurysm development in patients with a bicuspid aortic valve is not associated with transforming growth factor-beta activation. Arterioscler Thromb Vasc Biol 2015;35: 973–80. [DOI] [PubMed] [Google Scholar]
- 115.Forte A, Della Corte A, Grossi M, Bancone C, Provenzano R, Finicelli M, et al. Early cell changes and TGFbeta pathway alterations in the aortopathy associated with bicuspid aortic valve stenosis. Clin Sci (Lond) 2013;124:97–108. [DOI] [PubMed] [Google Scholar]
- 116.Phillippi JA, Green BR, Eskay MA, Kotlarczyk MP, Hill MR, Robertson AM, et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg 2014;147:1056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grewal N, Franken R, Mulder BJ, Goumans MJ, Lindeman JH, Jongbloed MR, et al. Histopathology of aortic complications in bicuspid aortic valve versus Marfan syndrome: relevance for therapy? Heart Vessels 2016;31:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heng E, Stone JR, Kim JB, Lee H, MacGillivray TE, Sundt TM. Comparative histology of aortic dilatation associated with bileaflet versus trileaflet aortic valves. Ann Thorac Surg 2015;100:2095–101. [DOI] [PubMed] [Google Scholar]
- 119.Grewal N, Gittenberger-de Groot AC, Poelmann RE, Klautz RJ, Lindeman JH, Goumans MJ, et al. Ascending aorta dilation in association with bicuspid aortic valve: a maturation defect of the aortic wall. J Thorac Cardiovasc Surg 2014; 148:1583–90. [DOI] [PubMed] [Google Scholar]
- 120.Phillippi JA, Klyachko EA, Kenny JP IV, Eskay MA, Gorman RC, Gleason TG. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation 2009;119:2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohamed SA, Radtke A, Saraei R, Bullerdiek J, Sorani H, Nimzyk R, et al. Locally different endothelial nitric oxide synthase protein levels in ascending aortic aneurysms of bicuspid and tricuspid aortic valve. Cardiol Res Pract 2012;2012:165957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arcucci A, Ruocco MR, Albano F, Granato G, Romano V, Corso G, et al. Analysis of extracellular superoxide dismutase and Akt in ascending aortic aneurysm with tricuspid or bicuspid aortic valve. Eur J Histochem 2014;58:2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phillippi JA, Eskay MA, Kubala AA, Pitt BR, Gleason TG. Altered oxidative stress responses and increased type I collagen expression in bicuspid aortic valve patients. Ann Thorac Surg 2010;90:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blunder S, Messner B, Aschacher T, Zeller I, Turkcan A, Wiedemann D, et al. Characteristics of TAV-and BAV-associated thoracic aortic aneurysms–smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis 2012;220:355–61. [DOI] [PubMed] [Google Scholar]
- 125.Mohamed SA, Misfeld M, Hanke T, Charitos EI, Bullerdiek J, Belge G, et al. Inhibition of caspase-3 differentially affects vascular smooth muscle cell apoptosis in the concave versus convex aortic sites in ascending aneurysms with a bicuspid aortic valve. Ann Anat 2010;192:145–50. [DOI] [PubMed] [Google Scholar]
- 126.Pichamuthu JE, Phillippi JA, Cleary DA, Chew DW, Hempel J, Vorp DA, et al. Differential tensile strength and collagen composition in ascending aortic aneurysms by aortic valve phenotype. Ann Thorac Surg 2013;96:2147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Forsell C, Bjorck HM, Eriksson P, Franco-Cereceda A, Gasser TC. Biomechanical properties of the thoracic aneurysmal wall: differences between bicuspid aortic valve and tricuspid aortic valve patients. Ann Thorac Surg 2014;98: 65–71. [DOI] [PubMed] [Google Scholar]
- 128.Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg 2012;143:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wagsater D, Paloschi V, Hanemaaijer R, Hultenby K, Bank RA, Franco-Cereceda A, et al. Impaired collagen biosynthesis and cross-linking in aorta of patients with bicuspid aortic valve. J Am Heart Assoc 2013;2: e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsamis A, Phillippi JA, Koch RG, Pasta S, D’Amore A, Watkins SC, et al. Fiber micro-architecture in the longitudinal-radial and circumferential-radial planes of ascending thoracic aortic aneurysm media. J Biomech 2013;46:2787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prakash A, Adlakha H, Rabideau N, Hass CJ, Morris SA, Geva T, et al. Segmental aortic stiffness in children and young adults with connective tissue disorders: relationships with age, aortic size, rate of dilation, and surgical root replacement. Circulation 2015;132:595–602. [DOI] [PubMed] [Google Scholar]
- 132.Raaz U, Zollner AM, Schellinger IN, Toh R, Nakagami F, Brandt M, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015;131:1783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harloff A, Wallis W, Strecker C, Brendecke S, Simon J, Weiller C, et al. Global pulse wave velocity in 87 patients with acute ischemic stroke and aortic atherosclerosis. Proc Intl Soc Mag Reson Med 2010;18:4434. [Google Scholar]
- 134.Bock J, Frydrychowicz A, Lorenz R, Hirtler D, Barker AJ, Johnson KM, et al. In vivo noninvasive 4D pressure difference mapping in the human aorta: phantom comparison and application in healthy volunteers and patients. Magn Reson Med 2011;66:1079–88. [DOI] [PubMed] [Google Scholar]
- 135.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med 2008;60:1218–31. [DOI] [PubMed] [Google Scholar]
- 136.Harloff A, Nussbaumer A, Bauer S, Stalder AF, Frydrychowicz A, Weiller C, et al. In vivo assessment of wall shear stress in the atherosclerotic aorta using flow-sensitive 4D MRI. Magn Reson Med 2010;63:1529–36. [DOI] [PubMed] [Google Scholar]
- 137.van Ooij P, Potters WV, Nederveen AJ, Allen BD, Collins J, Carr J, et al. A methodology to detect abnormal relative wall shear stress on the full surface of the thoracic aorta using four-dimensional flow MRI. Magn Reson Med 2015;73:1216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.den Reijer PM, Sallee D III, van der Velden P, Zaaijer ER, Parks WJ, Ramamurthy S, et al. Hemodynamic predictors of aortic dilatation in bicuspid aortic valve by velocity-encoded cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, et al. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging 2011;4:781–7. [DOI] [PubMed] [Google Scholar]
- 140.Nathan DP, Xu C, Plappert T, Desjardins B, Gorman JH III, Bavaria JE, et al. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg 2011;92:1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pasta S, Rinaudo A, Luca A, Pilato M, Scardulla C, Gleason TG, et al. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J Biomech 2013;46:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Atkins SK, Cao K, Rajamannan NM, Sucosky P. Bicuspid aortic valve hemodynamics induces abnormal medial remodeling in the convexity of porcine ascending aortas. Biomech Model Mechanobiol 2014;13:1209–25. [DOI] [PubMed] [Google Scholar]
- 143.Barker AJ, Markl M, Burk J, Lorenz R, Bock J, Bauer S, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012;5:457–66. [DOI] [PubMed] [Google Scholar]
- 144.Markl M, Geiger J, Arnold R, Stroh A, Damjanovic D, Foll D, et al. Comprehensive 4-dimensional magnetic resonance flow analysis after successful heart transplantation resolves controversial intraoperative findings and reveals complex hemodynamic alterations. Circulation 2011;123:e381–3. [DOI] [PubMed] [Google Scholar]
- 145.Markl M, Wegent F, Zech T, Bauer S, Strecker C, Schumacher M, et al. In vivo wall shear stress distribution in the carotid artery: effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ Cardiovasc Imaging 2010;3:647–55. [DOI] [PubMed] [Google Scholar]
- 146.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, et al. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke 2010;41:1145–50. [DOI] [PubMed] [Google Scholar]
- 147.Harloff A, Strecker C, Dudler P, Nussbaumer A, Frydrychowicz A, Olschewski M, et al. Retrograde embolism from the descending aorta: visualization by multidirectional 3D velocity mapping in cryptogenic stroke. Stroke 2009;40:1505–8. [DOI] [PubMed] [Google Scholar]
- 148.Bock J, Frydrychowicz A, Stalder AF, Bley TA, Burkhardt H, Hennig J, et al. 4D phase contrast MRI at 3 T: effect of standard and blood-pool contrast agents on SNR, PC-MRA, and blood flow visualization. Magn Reson Med 2010;63: 330–8. [DOI] [PubMed] [Google Scholar]
- 149.Frydrychowicz A, Arnold R, Harloff A, Schlensak C, Hennig J, Langer M, et al. Images in cardiovascular medicine. In vivo 3-dimensional flow connectivity mapping after extracardiac total cavopulmonary connection. Circulation 2008;118:e16–7. [DOI] [PubMed] [Google Scholar]
- 150.Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 2007;25: 824–31. [DOI] [PubMed] [Google Scholar]
- 151.Garcia J, Markl M, Schnell S, Allen B, Entezari P, Mahadevia R, et al. Evaluation of aortic stenosis severity using 4D flow jet shear layer detection for the measurement of valve effective orifice area. Magn Reson Imaging 2014;32: 891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barker AJ, Lanning C, Shandas R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng 2010;38:788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Della Corte A, Bancone C, Conti CA, Votta E, Redaelli A, Del Viscovo L, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: a new risk marker for aortopathy. J Thorac Cardiovasc Surg 2012;144: 360–9. 369.e1. [DOI] [PubMed] [Google Scholar]
- 154.Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, et al. Bicuspid aortic cusp fusion morphology alters aortic 3D outflow patterns, wall shear stress and expression of aortopathy. Circulation 2014;129: 673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Michelena HI, Della Corte A, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: incidence, etiology, and clinical significance. Int J Cardiol 2015;201:400–7. [DOI] [PubMed] [Google Scholar]
- 156.Malaisrie SC, Carr J, Mikati I, Rigolin V, Yip BK, Lapin B, et al. Cardiac magnetic resonance imaging is more diagnostic than 2-dimensional echocardiography in determining the presence of bicuspid aortic valve. J Thorac Cardiovasc Surg 2012;144:370–6. [DOI] [PubMed] [Google Scholar]
- 157.Schievink WI, Raissi SS, Maya MM, Velebir A. Screening for intracranial aneurysms in patients with bicuspid aortic valve. Neurology 2010;74:1430–3. [DOI] [PubMed] [Google Scholar]
- 158.Shaulov A, Leibowitz D, Rott D. Prevalence of bicuspid aortic valve in patients presenting with subarachnoid hemorrhage related to an intracerebral aneurysm. Int J Cardiol 2012;157:142–3. [DOI] [PubMed] [Google Scholar]
- 159.Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015;28:119–82. [DOI] [PubMed] [Google Scholar]
- 160.Rodriguez-Palomares JF, Teixido-Tura G, Galuppo V, Cuellar H, Laynez A, Gutierrez L, et al. Multimodality assessment of ascending aortic diameters: comparison of different measurement methods. J Am Soc Echocardiogr 2016;29:819–826.e4. [DOI] [PubMed] [Google Scholar]
- 161.Ocak I, Lacomis JM, Deible CR, Pealer K, Parag Y, Knollmann F. The aortic root: comparison of measurements from ECG-gated CTangiography with transthoracic echocardiography. J Thorac Imaging 2009;24:223–6. [DOI] [PubMed] [Google Scholar]
- 162.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park JY, Foley TA, Bonnichsen CR, Maurer MJ, Goergen KM, Nkomo VT, et al. Transthoracic echocardiography versus computed tomography for ascending aortic measurements in patients with bicuspid aortic valve. J Am Soc Echocardiogr 2017;30:625–35. [DOI] [PubMed] [Google Scholar]
- 164.Hope MD, Urbania TH, Yu JP, Chitsaz S, Tseng E. Incidental aortic valve calcification on CT scans: significance for bicuspid and tricuspid valve disease. Acad Radiol 2012;19:542–7. [DOI] [PubMed] [Google Scholar]
- 165.Elefteriades JA, Rizzo JA, Coady MA. Thoracic aorta. Radiology 1999;211: 889. [DOI] [PubMed] [Google Scholar]
- 166.Eleid MF, Forde I, Edwards WD, Maleszewski JJ, Suri RM, Schaff HV, et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart 2013;99: 1668–74. [DOI] [PubMed] [Google Scholar]
- 167.Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989;64:507–12. [DOI] [PubMed] [Google Scholar]
- 168.Biaggi P, Matthews F, Braun J, Rousson V, Kaufmann PA, Jenni R. Gender, age, and body surface area are the major determinants of ascending aorta dimensions in subjects with apparently normal echocardiograms. J Am Soc Echocardiogr 2009;22:720–5. [DOI] [PubMed] [Google Scholar]
- 169.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, et al. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr 2008;2:298–308. [DOI] [PubMed] [Google Scholar]
- 170.Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991;13:452–8. [DOI] [PubMed] [Google Scholar]
- 171.Wolak A, Gransar H, Thomson LE, Friedman JD, Hachamovitch R, Gutstein A, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008;1:200–9. [DOI] [PubMed] [Google Scholar]
- 172.Gravholt CH, Landin-Wilhelmsen K, Stochholm K, Hjerrild BE, Ledet T, Djurhuus CB, et al. Clinical and epidemiological description of aortic dissection in Turner’s syndrome. Cardiol Young 2006;16:430–6. [DOI] [PubMed] [Google Scholar]
- 173.Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892–3. [DOI] [PubMed] [Google Scholar]
- 174.Wojnarski CM, Svensson LG, Roselli EE, Idrees JJ, Lowry AM, Ehrlinger J, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg 2015;100:1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Davies RR, Gallo A, Coady MA, Tellides G, Botta DM, Burke B, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg 2006;81:169–77. [DOI] [PubMed] [Google Scholar]
- 176.Masri A, Kalahasti V, Svensson LG, Roselli EE, Johnston D, Hammer D, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016;134:1724–37. [DOI] [PubMed] [Google Scholar]
- 177.Burris NS, Sigovan M, Knauer HA, Tseng EE, Saloner D, Hope MD. Systolic flow displacement correlates with future ascending aortic growth in patients with bicuspid aortic valves undergoing magnetic resonance surveillance. Invest Radiol 2014;49:635–9. [DOI] [PubMed] [Google Scholar]
- 178.Hope MD, Meadows AK, Hope TA, Ordovas KG, Reddy GP, Alley MT, et al. Images in cardiovascular medicine. Evaluation of bicuspid aortic valve and aortic coarctation with 4D flow magnetic resonance imaging. Circulation 2008;117:2818–9. [DOI] [PubMed] [Google Scholar]
- 179.Girdauskas E, Rouman M, Disha K, Fey B, Dubslaff G, Theis B, et al. Functional aortic root parameters and expression of aortopathy in bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2016;67:1786–96. [DOI] [PubMed] [Google Scholar]
- 180.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714–833. [DOI] [PubMed] [Google Scholar]
- 181.Burris NS, Hope MD. Bicuspid valve-related aortic disease: flow assessment with conventional phase-contrast MRI. Acad Radiol 2015;22:690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Entezari P, Schnell S, Mahadevia R, Malaisrie C, McCarthy P, Mendelson M, et al. From unicuspid to quadricuspid: influence of aortic valve morphology on aortic three-dimensional hemodynamics. J Magn Reson Imaging 2014;40: 1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Williams JB, Peterson ED, Zhao Y, O’Brien SM, Andersen ND, Miller DC, et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol 2012;60:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thourani VH, Suri RM, Gunter RL, Sheng S, O’Brien SM, Ailawadi G, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015;99:55–61. [DOI] [PubMed] [Google Scholar]
- 185.Rinewalt D, McCarthy PM, Malaisrie SC, Fedak PW, Andrei AC, Puthumana JJ, et al. Effect of aortic aneurysm replacement on outcomes after bicuspid aortic valve surgery: Validation of contemporary guidelines. J Thorac Cardiovasc Surg 2014;148:2060–9. [DOI] [PubMed] [Google Scholar]
- 186.Opotowsky AR, Perlstein T, Landzberg MJ, Colan SD, O’Gara PT, Body SC, et al. A shifting approach to management of the thoracic aorta in bicuspid aortic valve. J Thorac Cardiovasc Surg 2013;146:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peterss S, Charilaou P, Dumfarth J, Li Y, Bhandari R, Tranquilli M, et al. Aortic valve disease with ascending aortic aneurysm: Impact of concomitant root-sparing (supracoronary) aortic replacement in nonsyndromic patients. J Thorac Cardiovasc Surg 2016;152:791–798.e1. [DOI] [PubMed] [Google Scholar]
- 188.Hughes GC, Zhao Y, Rankin JS, Scarborough JE, O’Brien S, Bavaria JE, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg 2013;145:166–70. [DOI] [PubMed] [Google Scholar]
- 189.Charitos EI, Stierle U, Petersen M, Mohamed SA, Hanke T, Schmidtke C, et al. The fate of the bicuspid valve aortopathy after aortic valve replacement. Eur J Cardiothorac Surg 2014;45:e128–35. [DOI] [PubMed] [Google Scholar]
- 190.Regeer MV, Versteegh MI, Klautz RJ, Schalij MJ, Bax JJ, Marsan NA, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016;102:1981–7. [DOI] [PubMed] [Google Scholar]
- 191.Svensson LG, Kim KH, Blackstone EH, Rajeswaran J, Gillinov AM, Mihaljevic T, et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011;142:622–629.e1–3. [DOI] [PubMed] [Google Scholar]
- 192.Ugur M, Schaff HV, Suri RM, Dearani JA, Joyce LD, Greason KL, et al. Late outcome of noncoronary sinus replacement in patients with bicuspid aortic valves and aortopathy. Ann Thorac Surg 2014;97:1242–6. [DOI] [PubMed] [Google Scholar]
- 193.Garrido-Olivares L, Maganti M, Armstrong S, David TE. Clinical outcomes of aortic root replacement after previous aortic root replacement. J Thorac Cardiovasc Surg 2013;146:611–5. [DOI] [PubMed] [Google Scholar]
- 194.Lerer PK, Edwards WD. Coronary arterial anatomy in bicuspid aortic valve. Necropsy study of 100 hearts. Br Heart J 1981;45:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH, et al. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg 2012;145:117–26. 127.e1–5; discussion 126–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kunihara T, Aicher D, Rodionycheva S, Groesdonk HV, Langer F, Sata F, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg 2012;143:1389–95. [DOI] [PubMed] [Google Scholar]
- 197.David TE, Feindel CM, David CM, Manlhiot C. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg 2014; 148:872–80. [DOI] [PubMed] [Google Scholar]
- 198.Miller DC. Rationale and results of the Stanford modification of the David V reimplantation technique for valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2015;149:112–4. [DOI] [PubMed] [Google Scholar]
- 199.Svensson LG, Pillai ST, Rajeswaran J, Desai MY, Griffin B, Grimm R, et al. Long-term survival, valve durability, and reoperation for 4 aortic root procedures combined with ascending aorta replacement. J Thorac Cardiovasc Surg 2016;151:764–771.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kari FA, Doll KN, Hemmer W, Liebrich M, Sievers HH, Richardt D, et al. Residual and progressive aortic regurgitation after valve-sparing root replacement: a propensity-matched multi-institutional analysis in 764 patients. Ann Thorac Surg 2016;101:1500–6. [DOI] [PubMed] [Google Scholar]
- 201.El Khoury G, Glineur D, Rubay J, Verhelst R, d’Acoz Y, Poncelet A, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115–21. [DOI] [PubMed] [Google Scholar]
- 202.Aicher D, Schafers HJ. Aortic valve repair–current status, indications, and outcomes. Semin Thorac Cardiovasc Surg 2012;24:195–201. [DOI] [PubMed] [Google Scholar]
- 203.Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Noirhomme P, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg 2010;140:276–284.e1. [DOI] [PubMed] [Google Scholar]
- 204.Vohra HA, Whistance RN, De Kerchove L, Punjabi P, El Khoury G. Valve-preserving surgery on the bicuspid aortic valve. Eur J Cardiothorac Surg 2013;43: 888–98. [DOI] [PubMed] [Google Scholar]
- 205.Svensson LG, Al Kindi AH, Vivacqua A, Pettersson GB, Gillinov AM, Mihaljevic T, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg 2014;97:1539–48. [DOI] [PubMed] [Google Scholar]
- 206.Vallabhajosyula P, Komlo C, Szeto WY, Wallen TJ, Desai N, Bavaria JE. Root stabilization of the repaired bicuspid aortic valve: subcommissural annuloplasty versus root reimplantation. Ann Thorac Surg 2014;97:1227–34. [DOI] [PubMed] [Google Scholar]
- 207.de Kerchove L, Boodhwani M, Glineur D, Vandyck M, Vanoverschelde JL, Noirhomme P, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430–8. [DOI] [PubMed] [Google Scholar]
- 208.Schafers HJ, Raddatz A, Schmied W, Takahashi H, Miura Y, Kunihara T, et al. Reexamining remodeling. J Thorac Cardiovasc Surg 2015;149:S30–6. [DOI] [PubMed] [Google Scholar]
- 209.Aicher D, Schneider U, Schmied W, Kunihara T, Tochii M, Schafers HJ. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg 2013;145:S30–4. [DOI] [PubMed] [Google Scholar]
- 210.Kari FA, Kvitting JP, Stephens EH, Liang DH, Merk DR, Fischbein MP, et al. Tirone David procedure for bicuspid aortic valve disease: impact of root geometry and valve type on mid-term outcomes. Interact Cardiovasc Thorac Surg 2014;19:375–81. [DOI] [PubMed] [Google Scholar]
- 211.Vallabhajosyula P, Szeto WY, Komlo CM, Ryan LP, Wallen TJ, Gorman RC, et al. Geometric orientation of the aortic neoroot in patients with raphed bicuspid aortic valve disease undergoing primary cusp repair and a root reimplantation procedure. Eur J Cardiothorac Surg 2014;45:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aicher D, Kunihara T, Abou Issa O, Brittner B, Graber S, Schafers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178–85. [DOI] [PubMed] [Google Scholar]
- 213.Paruchuri V, Salhab KF, Kuzmik G, Gubernikoff G, Fang H, Rizzo JA, et al. Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology 2015;131:265–72. [DOI] [PubMed] [Google Scholar]
- 214.Parish LM, Gorman JH III, Kahn S, Plappert T, St John-Sutton MG, Bavaria JE, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg 2009;35:941–6. [DOI] [PubMed] [Google Scholar]
- 215.Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’Gara PT, Evangelista A, et al. Aortic diameter>or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120–7. [DOI] [PubMed] [Google Scholar]
- 216.Lansman SL, Goldberg JB, Kai M, Tang GH, Malekan R, Spielvogel D. Aortic surgery in pregnancy. J Thorac Cardiovasc Surg 2017;153(suppl):S44–88. [DOI] [PubMed] [Google Scholar]
- 217.Wang Y, Wu B, Li J, Dong L, Wang C, Shu X. Impact of aortic insufficiency on ascending aortic dilatation and adverse aortic events after isolated aortic valve replacement in patients with a bicuspid aortic valve. Ann Thorac Surg 2016; 101:1707–14. [DOI] [PubMed] [Google Scholar]
- 218.Girdauskas E, Rouman M, Disha K, Espinoza K, Misfeld M, Borger MA, et al. Meta-analysis of aortic dissection after previous aortic valve replacement for bicuspid aortic valve disease. J Am Coll Cardiol 2015;66:1409–11. [DOI] [PubMed] [Google Scholar]
- 219.Kari FA, Fazel SS, Mitchell RS, Fischbein MP, Miller DC. Bicuspid aortic valve configuration and aortopathy pattern might represent different pathophysiologic substrates. J Thorac Cardiovasc Surg 2012;144:516–7. [DOI] [PubMed] [Google Scholar]
- 220.Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM III. Should the proximal arch be routinely replaced in patients with bicuspid aortic valve disease and ascending aortic aneurysm? J Thorac Cardiovasc Surg 2011; 142:602–7. [DOI] [PubMed] [Google Scholar]
- 221.Boodhwani M, Andelfinger G, Leipsic J, Lindsay T, McMurtry MS, Therrien J, et al. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol 2014;30:577–89. [DOI] [PubMed] [Google Scholar]
- 222.Siddiqi H, Isselbacher E, Suzuki T, Montgomery D, Pape L, Fattori R, et al. Is size a good predictor of dissection risk in patients with Marfan syndrome or bicuspid aortic valves? Insights from the International Registry of Acute Aortic Dissection (IRAD). J Am Coll Cardiol 2012;59:E1883. [Google Scholar]
- 223.Malaisrie SC, Duncan BF, Mehta CK, Badiwala MV, Rinewalt D, Kruse J, et al. The addition of hemiarch replacement to aortic root surgery does not affect safety. J Thorac Cardiovasc Surg 2015;150:118–124.e2. [DOI] [PubMed] [Google Scholar]
- 224.Miyata H, Motomura N, Ueda Y, Tsukihara H, Tabayashi K, Takamoto S. Toward quality improvement of thoracic aortic surgery: estimating volume-outcome effect from nationwide survey. Eur J Cardiothorac Surg 2009;36: 517–21. [DOI] [PubMed] [Google Scholar]
- 225.Gazoni LM, Speir AM, Kron IL, Fonner E, Crosby IK. Elective thoracic aortic aneurysm surgery: better outcomes from high-volume centers. J Am Coll Surg 2010;210:855–60. [DOI] [PubMed] [Google Scholar]
- 226.Etz CD, Zoli S, Brenner R, Roder F, Bischoff M, Bodian CA, et al. When to operate on the bicuspid valve patient with a modestly dilated ascending aorta. Ann Thorac Surg 2010;90:1884–92. [DOI] [PubMed] [Google Scholar]
- 227.Coady MA, Stockwell PH, Robich MP, Poppas A, Sellke FW. Should aortas in patients with bicuspid aortic valve really be resected at an earlier stage than tricuspid? CON. Cardiol Clin 2010;28:299–314. [DOI] [PubMed] [Google Scholar]
- 228.Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O’Gara PT, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg 2013;95:S1–66. [DOI] [PubMed] [Google Scholar]
- 229.Scheske JA, O’Brien JM, Earls JP, Min JK, LaBounty TM, Cury RC, et al. Coronary artery imaging with single-source rapid kilovolt peak-switching dual-energy CT. Radiology 2013;268:702–9. [DOI] [PubMed] [Google Scholar]
- 230.So A, Lee TY, Imai Y, Narayanan S, Hsieh J, Kramer J, et al. Quantitative myocardial perfusion imaging using rapid kVp switch dual-energy CT: preliminary experience. J Cardiovasc Comput Tomogr 2011;5:430–42. [DOI] [PubMed] [Google Scholar]
- 231.Elefteriades JA. Editorial comment: does medical therapy for thoracic aortic aneurysms really work? Are beta-blockers truly indicated? Cardiol Clin 2010;28: 271–2. [DOI] [PubMed] [Google Scholar]
- 232.Boucek RJ, Gunja-Smith Z, Noble NL, Simpson CF. Modulation by propranolol of the lysyl cross-links in aortic elastin and collagen of the aneurysm-prone turkey. Biochem Pharmacol 1983;32:275–80. [DOI] [PubMed] [Google Scholar]
- 233.Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108:II312–7. [DOI] [PubMed] [Google Scholar]
- 234.Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, et al. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol 2002;40:685–92. [DOI] [PubMed] [Google Scholar]
- 235.Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Genoni M, Paul M, Jenni R, Graves K, Seifert B, Turina M. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg 2001;19:606–10. [DOI] [PubMed] [Google Scholar]
- 237.Suzuki T, Isselbacher EM, Nienaber CA, Pyeritz RE, Eagle KA, Tsai TT, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol 2012;109:122–7. [DOI] [PubMed] [Google Scholar]
- 238.Jonker FH, Trimarchi S, Rampoldi V, Patel HJ, O’Gara P, Peterson MD, et al. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg 2012; 94:1223–9. [DOI] [PubMed] [Google Scholar]
- 239.Takeshita S, Sakamoto S, Kitada S, Akutsu K, Hashimoto H. Angiotensin-converting enzyme inhibitors reduce long-term aortic events in patients with acute type B aortic dissection. Circ J 2008;72:1758–61. [DOI] [PubMed] [Google Scholar]
- 240.Canadian Medical Association. CMA Driver’s Guide: Determining Medical Fitness to Operate Motor Vehicles Toronto, Canada: Canadian Medical Association; 2012. [Google Scholar]
- 241.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2007;116:572–84. [DOI] [PubMed] [Google Scholar]
- 242.Hatzaras I, Tranquilli M, Coady M, Barrett PM, Bible J, Elefteriades JA. Weight lifting and aortic dissection: more evidence for a connection. Cardiology 2007; 107:103–6. [DOI] [PubMed] [Google Scholar]
- 243.Cornelissen VA, Buys R, Smart NA. Endurance exercise beneficially affects ambulatory blood pressure: a systematic review and meta-analysis. J Hypertens 2013;31:639–48. [DOI] [PubMed] [Google Scholar]
- 244.European Society of Gynecology, Association for European Paediatric Cardiology, German Society for Gender Medicine, Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–97. [DOI] [PubMed] [Google Scholar]
- 245.Gale AN, McKusick VA, Hutchins GM, Gott VL. Familial congenital bicuspid aortic valve: secondary calcific aortic stenosis and aortic aneurysm. Chest 1977;72:668–70. [DOI] [PubMed] [Google Scholar]
- 246.Laforest B, Nemer M. Genetic insights into bicuspid aortic valve formation. Cardiol Res Pract 2012;2012:180297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Prakash SK, Bondy CA, Maslen CL, Silberbach M, Lin AE, Perrone L, et al. Autosomal and X chromosome structural variants are associated with congenital heart defects in Turner syndrome: The NHLBI GenTAC registry. Am J Med Genet A 2016;170:3157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Andreassi MG, Della Corte A. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol 2016;31:585–92. [DOI] [PubMed] [Google Scholar]
- 249.Hales AR, Mahle WT. Echocardiography screening of siblings of children with bicuspid aortic valve. Pediatrics 2014;133:e1212–7. [DOI] [PubMed] [Google Scholar]
- 250.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47: 296–308. [DOI] [PubMed] [Google Scholar]
- 251.Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014;63:878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Braverman AC, Harris KM, Kovacs RJ, Maron BJ, American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 7: aortic diseases, including Marfan syndrome: a scientific statement from the American Heart Association and American College of Cardiology. Circulation 2015;132: e303–9. [DOI] [PubMed] [Google Scholar]
- 253.McKellar SH, MacDonald RJ, Michelena HI, Connolly HM, Sundt TM III. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol 2011;107:96–9. [DOI] [PubMed] [Google Scholar]
- 254.Della Corte A, Body SC, Booher AM, Schaefers HJ, Milewski RK, Michelena HI, et al. Surgical treatment of bicuspid aortic valve disease: knowledge gaps and research perspectives. J Thorac Cardiovasc Surg 2014;147: 1749–1757.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fedak PW. Bicuspid aortic valve syndrome: heterogeneous but predictable? Eur Heart J 2008;29:432–3. [DOI] [PubMed] [Google Scholar]
- 256.Della Corte A Trying to overcome the ‘chicken or egg’ impasse in bicuspid aortopathy research. Eur J Cardiothorac Surg 2016;49:644–5. [DOI] [PubMed] [Google Scholar]
- 257.Sievers HH, Sievers HL. Aortopathy in bicuspid aortic valve disease - genes or hemodynamics? or Scylla and Charybdis? Eur J Cardiothorac Surg 2011;39: 803–4. [DOI] [PubMed] [Google Scholar]
- 258.Rylski B, Branchetti E, Bavaria JE, Vallabhajosyula P, Szeto WY, Milewski RK, et al. Modeling of predissection aortic size in acute type A dissection: more than 90% fail to meet the guidelines for elective ascending replacement. J Thorac Cardiovasc Surg 2014;148: 944–948.e1. [DOI] [PubMed] [Google Scholar]
- 259.Fedak PW, Verma S. The molecular fingerprint of bicuspid aortopathy. J Thorac Cardiovasc Surg 2013;145:1334. [DOI] [PubMed] [Google Scholar]


