Abstract
Maternal exposure to di(2-ethylhexyl) phthalate (DEHP) decreased the plasma triglyceride in prepartum mice. To identify the fatty acid (FA) species involved and to understand the underlying mechanisms, pregnant Sv/129 wild-type (mPPARα), peroxisome proliferator-activated receptor α-null (Pparα-null) and humanized PPARα (hPPARα) mice were treated with diets containing 0%, 0.01%, 0.05% or 0.1% DEHP. Dams were dissected on gestational day 18 together with fetuses, and on postnatal day 2 together with newborns. n-3/n-6 polyunsaturated, saturated, and monounsaturated FAs in maternal plasma and in liver of wild-type offspring, and representative enzymes for FA desaturation and elongation in maternal liver, were measured. The plasma levels of linoleic acid, α-linolenic acid, palmitic acid and oleic acid were higher in the pregnant control mPPARa mice than in Ppara-null and hPPARα mice. DEHP exposure significantly decreased the levels of these four FAs only in pregnant mPPARα mice. Plasma levels of many FAs were higher in pregnant mice than in postpartum ones in a genotype-independent manner, while it was lower in the livers of fetuses than pups. DEHP exposure slightly increased hepatic arachidonic acid, α-linolenic acid, palmitoleic acid and oleic acid in fetuses, but not in pups. However, DEHP exposure did not clearly influence FA desaturase 1 and 2 nor elongase 2 and 5 expressions in the liver of all maternal mice. Taken together, the levels of plasma four FAs with shorter carbon chains were higher in pregnant mPPARα mice than in other genotypes, and DEHP exposure decreased these specific FA concentrations only in mPPARα mice, similarly to triglyceride levels.
Keywords: Di(2-ethylhexyl) phthalate, Fatty acids, Postpartum, Pregnancy, Pups
1. Introduction
Plasticizers are used to improve the plasticity and elasticity of the polyvinylchloride (PVC) materials. Phthalate ester is the most commonly used plasticizer in the world, the output of which was about 320,000 tons in 2005 in Japan. Although its output is decreasing year by year and stood at about 200,000 tons in 2011, phthalate ester still accounts for about 75% of all plasticizer production in Japan (Japan Plasticizer Industry Association, 2013; Ministry of Economy, Trade and Industry, 2012). Among all the phthalate esters in Japan, di(2-ethylhexyl) phthalate (DEHP) accounts for more than 60% of PVC products, such as construction materials, wire covering, and vinyl sheets used for agriculture and daily-life products like food wrappers (Japan Plasticizer Industry Association, 2010). DEHP easily elutes from those materials because it does not chemically bind to PVC. As a result, humans are mainly exposed to DEHP through food (Koch et al., 2006).
DEHP exerts adverse effects on liver, kidney, and reproductive organs, and in some organs causes carcinogenicity (Lamb et al., 1987; Rusyn et al., 2006; Christiansen et al., 2009). Hayashi et al. (2011) reported that maternal exposure to DEHP decreased the number of alive fetuses and pups, while it increased those of resorption in wild-type (mouse peroxisome proliferator-activated receptor α: mPPARα) and humanized PPARα (hPPARα) mice. Interestingly, these adverse effects were not observed in Pparα-null mice. A decrease in the concentration of maternal plasma triglyceride (TG) was observed only in mPPARα mice, and this was suspected to be a pathogenesis for the adverse effects of DEHP. Therefore, DEHP exposure may also influence the concentration of several fatty acid (FA) components which constitute a major part of TG. Indeed, FAs are essential for early human development (Hornstra, 2001), and exposure to DEHP and/or mono (2-ethylhexyl) phthalate (MEHP) changed serum lipid profiling including TG and n-3/n-6 in families of rats (Oishi, 1984) and in a rat placental cell line (Xu et al., 2006), which may have resulted in abnormal fetal development.
DEHP absorbed in the body is metabolized into MEHP by lipase (Gunnarsson et al., 2008), a ligand for PPARα and γ (Maloney and Waxman, 1999). After PPARα is activated, β- or ω-oxidizing enzymes of the FAs are up-regulated, and the metabolism is accelerated. FAs that humans and other animals cannot synthesize de novo and must be ingested from food for optimum development and health, are called essential fatty acids (EFA) (Hornstra, 2001). Linoleic acid (LA, a n-6 FA, carbon chain length (C) 18) and α-linolenic acid (ALA, a n-3 FA, C18) are two EFAs and serve as precursors for the synthesis of the respective n-6 and n-3 longer-chain, polyunsaturated fatty acids (LC-PUFA) via sequential desaturation, elongation and partial degradation steps. Arachidonic acid (AA, C20) is the most important member of n-6 LC-PUFA, and eicosapentaenoic acid (EPA, C20) and docosahexaenoic acid (DHA, C22) are the most important members of n-3 LC-PUFA (Hornstra, 2001; Guillou et al., 2010). In the conversion of linoleic acid and α-linolenic acid to their respective LC-PUFA groups, fatty acid desaturase (Fads) 2 catalyzes the initial and rate-limiting desaturation of linoleic acid and α-linolenic acid. Fads1 promotes desaturation to yield arachidonic acid and EPA, and elongation of very-long-chain FAs by elongase (Elovl) 2 and 5 are more selective for PUFA elongation (Guillou et al., 2010). In contrast to PUFA, saturated FAs such as palmitic acid (C16) and stearic acid (C18), and monounsaturated FAs such as palmitoleic acid (C16) and oleic acid (C18), can be synthesized de novo and have undesirable effects on health, although they are essential barrier components of the plasma membrane (Guillou et al., 2010).
Considering the above, in the present study, we investigated the effects of DEHP exposure on the levels of EFA and n-3/n-6 LC-PUFA, as well as saturated and monounsaturated FAs in the plasma of pregnant and postpartum mice, and on the livers of their offspring. We then discussed the relationship between the decrease in TG and individual FA components induced by DEHP exposure.
2. Materials and methods
2.1. Laboratory animal and diet
Three genotyped male and female mice, i.e. (1) mPPARα, (2) Pparα-null (Lee et al., 1995) and (3) hPPARα,Tet-Off express human PPARα only in the liver of Pparα-null mice (Cheung et al., 2004) with a Sv/129 genetic background, were obtained from the US National Cancer Institute, and housed in a constant environment (23–25°C room temperature, 57–60% humidity, and 12 h light/dark cycles). They were fed a solid diet (CLEA Rodent Diet CE-2; Japan Clea Co., Tokyo, Japan) and tap water ad libitum. The mice were then bred. At the age of 12 weeks, the genotyped mice were divided into 4 groups, and given diets containing 0, 0.01, 0.05, and 0.1% DEHP, respectively, ad libitum. Their dietary intakes were as follows: 10–12, 55–64 and 119–145 mg per kg body weight per day, respectively, throughout the observed periods.
Four weeks after the commencement of DEHP exposure, the genotyped mice with the same exposure level were mated. The day on which the plug was identified was designated as gestational day (GD) 0. All the pregnant mice were divided into 2 groups: one was dissected on GD18 together with fetuses and the other on postnatal day (PND) 2 together with newborn pups. Under these conditions, DEHP exposures at dosages of 0.05% and/or 0.1% decreased the number of live fetuses and pups, and increased resorption in mPPARα and hPPARα. In addition, the plasma TG level in pregnant mice was decreased at a 0.1% dose only in mPPARα mice (Hayashi et al., 2011). The blood and livers were collected. Blood was processed with heparin and the removed livers were snap-frozen in liquid nitrogen. These samples were stored at −80°C until use.
All the experiments were conducted according to the Guidelines for Animal Experiments of the Nagoya University Animal Center.
2.2. Measurement of maternal plasma FAs concentration
FA levels in maternal plasma of mice were measured according to the method of Masood et al. (2005): 25 μl of plasma was mixed with 1.2 ml of methanol, 75 μl of acetyl chloride, and 75 μl of 10 μg/100 μl tricosanoic acid ethyl ester/methanol (internal standard) in screw-capped glass test tubes. Air in the test tubes was then replaced by nitrogen and heated at 100°C for 60 min in a heating block. After the tubes were allowed to cool to room temperature, n-hexane (500 μl) was added and shaken for 30 s by vortex. Then the tubes were centrifuged at 3000 rpm for 2 min, and the upper organic layer was collected and moved into another vial followed by replacing the air of the vial with nitrogen. After the n-hexane extraction was repeated once more, the concentration of FA methyl ester contained in the n-hexane layer was measured by gas chromatography–mass spectrometry (GC–MS). Nine FA components targeted for measurement included palmitic acid and stearic acid of saturated FAs, palmitoleic acid and oleic acid of monounsaturated FAs, linoleic acid and arachidonic acid of the n-6 family, and α-linolenic acid, EPA and DHA of the n-3 family.
Details of the conditions for the GC–MS (Agilent Technologies, GC: 6890N, MS: 5975, Injector: 7683B Series, auto sampler 7683 series) were as follows: capillary column, DB-FFAP (15 m × 0.10 mm × 0.10 μm, Agilent Technologies); carrier gas, helium with velocity of 0.2 ml/min with a constant head pressure of 279.6 kPa. Initial column and injection port temperature, 150°C with 280°C in interface temperature. The temperature program was as follows: initial temperature of 150°C for 0.25 min, raised to 200°C in the next 1 min, held at 200°C for 4.5 min, raised to 225°C in the next 2 min, held at 225°C for 8.5 min, raised to 245°C in the next 0.25 min, and then held at 245°C for 1.5 min. Analysis mode, EI; positive ion, 70 eV; SIM parameters: tricosanoic acid (internal standard), palmitic acid, palmitoleic acid, stearic acid, oleic acid 74 m/z, linoleic acid 67 m/z, α-linolenic acid, arachidonic acid, EPA, DHA 79 m/z. Split ratio was 200:1. Under these conditions, the detection limits were 2.4 μg/ml for palmitic acid, 1.3 μg/ml for stearic acid, 0.69 μg/ml for palmitoleic acid, 3.6 g/ml for oleic acid, and 2.0 μg/ml for EFA and n-3/n-6 PUFA.
2.3. Measurement of liver FAs in offspring
Because we could not collect enough blood samples from offspring for the measurement of FA, liver concentrations in fetuses and pups were measured. Livers from fetuses and newborn pups were homogenized with 50 mM K2HPO4/KH2PO4 buffer with a ratio of 1:3. The lipids were extracted from the homogenate according to the method of Folch et al. (1957). The chloroform layer obtained was evaporated and dried. Then the sediment was resolved by 1.2 ml of methanol, and the 9 FA components were measured by the same method mentioned above.
2.4. Western blot analysis
Fads 1, 2 and Elovl 2, 5 are localized in the microsomes; therefore, maternal liver microsomal fraction was prepared. Samples containing the same quantity of protein were subjected to SDS-PAGE as described elsewhere (Aoyama et al., 1998; Jia et al., 2012; Nakajima et al., 2000; Nakamura et al., 2009). Briefly, the samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the membranes were incubated with the following antibodies: Fads 1 (V-21), Fads 2 (M-50), and Elovl2 (S-16) (Santa Cruz Biotechnology, Santa Cruz, CA), and Elovl5 (ab81326) (Abcam, Cambridge, UK). Actin (H-196) (Santa Cruz Biotechnology, Santa Cruz, CA) antibody was performed for loading control. 1-step™ NBT/BCIP (Pierce Biotechnology, Rockford, IL, USA) was used for the detection of specific proteins. Each band was quantified by densitometry using the Lane and Spot Analyzer version 5.0 (ATTO Corporation, Tokyo, Japan).
2.5. Real-time quantitative PCR
Total RNA was extracted from whole livers using RNeasy Mini Kit (Qiagen, Tokyo, Japan). Quantitative real-time PCR analysis was performed using 1× SYBR Green PCR master Mix (Applied Biosystems) as described elsewhere (Ito et al., 2007; Ramdhan et al., 2009; Nakamura et al., 2009). We normalized all of the mRNA levels to glyceraldehyde-3-phosphate dehydrogenase. The sequences for the forward and reverse primers are shown in Supplemental Table 1.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tox.2013.04.010.
2.6. Statistical analysis
All the data are presented as mean ± standard deviation (SD). Statistical analyses were performed by a Tukey–Kramer HSD test while comparing the genotype effects. Dunnett’s test was performed to compare the DEHP exposure in each genotyped mouse, and t-test was used to compare the control groups of fetuses and pups, and pregnant and postpartum dams. Data were converted into logarithm form when it was not a normal distribution. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Maternal plasma FA concentrations
3.1.1. n-6 family PUFA
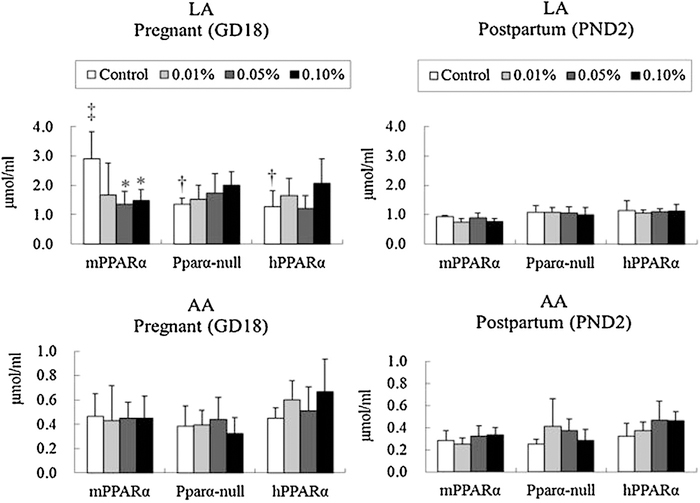
Comparing each genotype of pregnant control mice, the levels of linoleic acid were significantly higher in mPPARα mice than in their counterpart Pparα-null and hPPARα mice (Fig. 1), although such a difference was not observed in the level of postpartum control mice. Comparing pregnant mice with postpartum control mice in each genotype, linoleic acid was significantly higher in pregnant mPPARα mice than in postpartum ones. DEHP exposure significantly decreased linoleic acid levels at 0.05 and 0.1% dosages in pregnant mPPARα mice, but not in pregnant Pparα-null and hPPARα mice nor in postpartum mice of any genotype. Arachidonic acid concentrations were not influenced by pregnancy, genotypes, or DEHP exposure.
Fig. 1.
Maternal plasma fatty acid concentrations of n-6 family. Data represent mean ± SD. LA, linoleic acid; AA, arachidonic acid. *p < 0.05 compared with control in each genotyped mouse. †p < 0.05 compared with control of mPPARα. ‡p < 0.05 compared with postpartum control in each genotyped mouse.
3.1.2. n-3 family PUFA
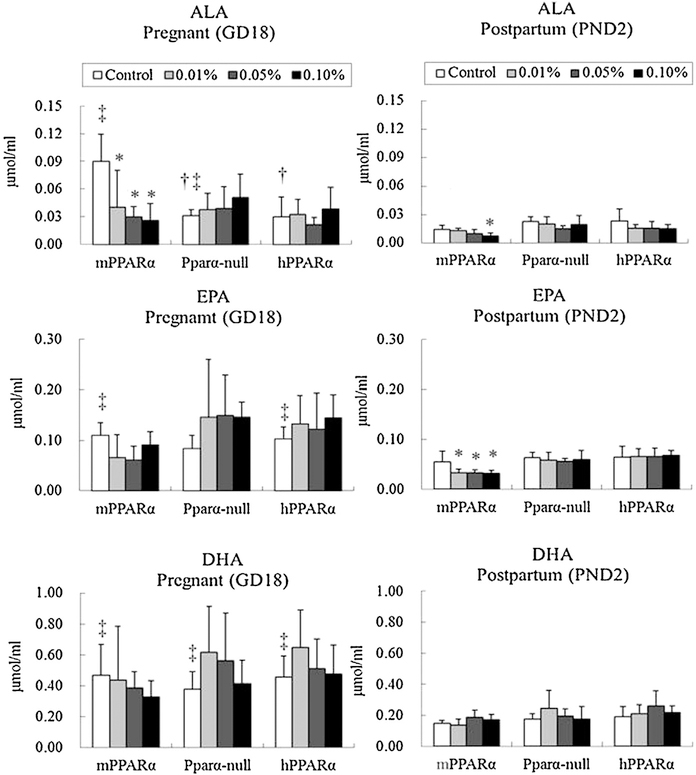
As shown in Fig. 2, similar to linoleic acid, the levels of linolenic acid were significantly higher in pregnant control mPPARα mice than Pparα-null and hPPARα mice in their counterparts, but no differences were noted in the levels of EPA and DHA. In postpartum mice, no significant difference was observed between the control groups of different genotypes regarding the levels of plasma α-linolenic acid, EPA, and DHA. While comparing the control groups of pregnant and postpartum mice, levels of α-linolenic acid in mPPARα and Pparα-null mice were found to be significantly higher in the pregnant mice than in the postpartum ones, as well as EPA in mPPARα and hPPARα mice, and DHA in all three genotypes.
Fig. 2.
Maternal plasma fatty acid concentrations of n-3 family. Data represent mean ± SD. ALA, alpha-linorenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. *p < 0.05 compared with control in each genotyped mouse. †p < 0.05 compared with control of mPPARα. ‡p < 0.05 compared with postpartum control in each genotyped mouse.
DEHP exposure significantly decreased the concentrations of plasma α-linolenic acid in pregnant mPPARα mice at all dosages. The concentrations of plasma EPA and DHA in any genotyped pregnant mice were not influenced by any dosage of DEHP exposure. Among the postpartum mice, DEHP exposure significantly decreased the levels of plasma α-linolenic acid at the 0.1% dosage and the levels of EPA at all three dosages in mPPARα mice.
3.1.3. Saturated and monounsaturated FAs
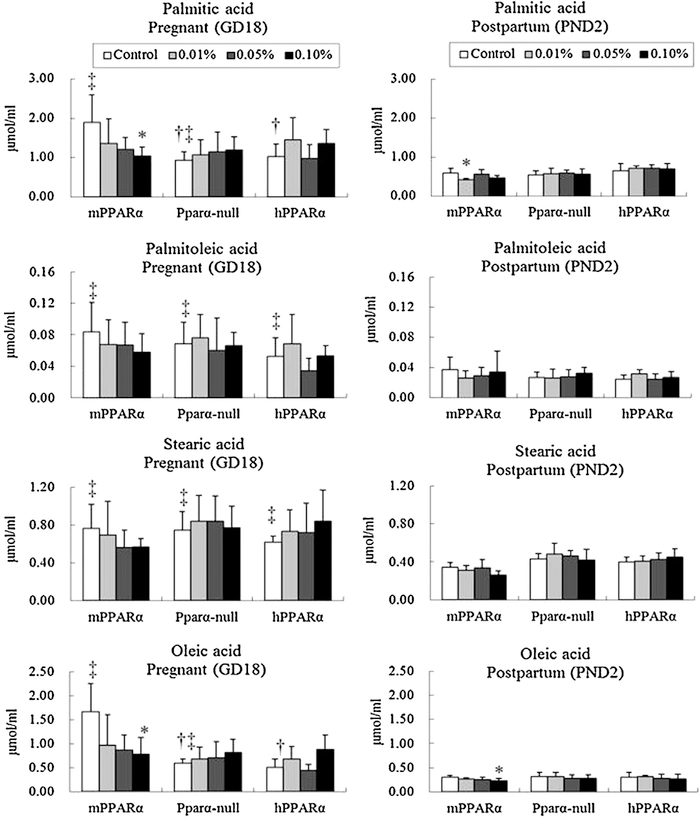
Comparison among the controls of different by genotyped pregnant mice indicated that levels of plasma palmitic acid and oleic acid in mPPARα mice were significantly higher than those in Pparα-null and hPPARα mice (Fig. 3). No significant difference was noted for the levels of palmitoleic acid and stearic acid in any genotype.
Fig. 3.
Maternal plasma levels of n-9 family and saturated fatty acids. Data represent mean ± SD. *p < 0.05 compared with control in each genotyped mouse. †p < 0.05 compared with control of mPPARα. ‡p < 0.05 compared with postpartum control in each genotyped mouse.
While comparing the control groups between pregnant and postpartum mice in their respective genotypes, the levels of palmitoleic acid and stearic acid in all genotypes were found to be significantly higher in the pregnant mice. For palmitic acid and oleic acid, the levels were significantly higher in mPPARα and Pparα-null pregnant mice than in the respective postpartum mice.
DEHP exposure at a 0.1% dosage significantly decreased the levels of palmitic and oleic acid in mPPARα pregnant mice. In contrast, exposure to 0.01% and 0.1% slightly decreased the levels of palmitic acid and oleic acid, respectively, in postpartum mPPARα mice. The plasma levels of these FA components in Pparα-null and hPPARα mice were not influenced by any dosage of DEHP exposure during pregnancy or postpartum.
3.2. Liver FA concentrations in fetuses and pups
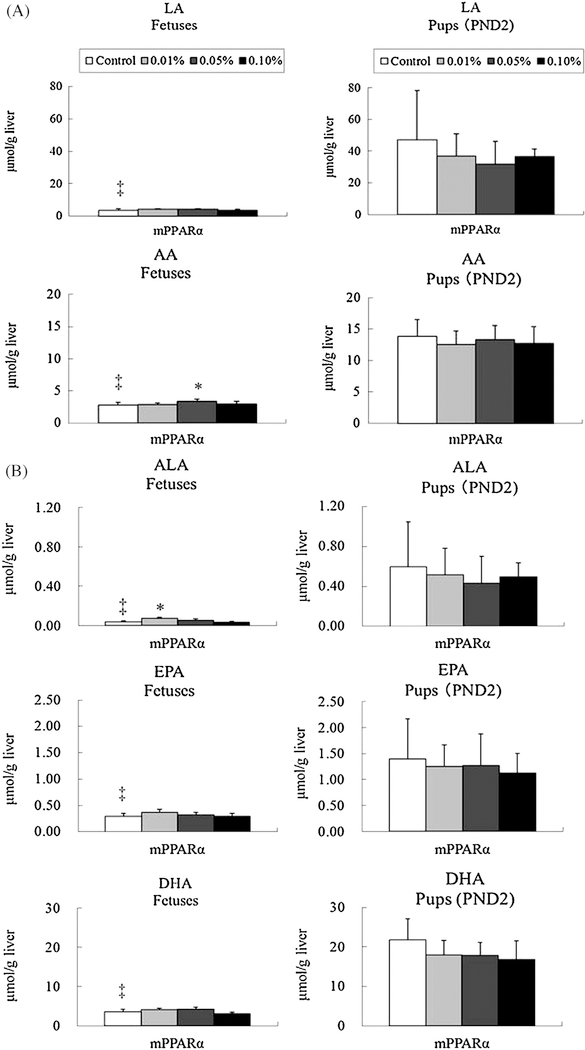
Since DEHP decreased maternal plasma concentrations of various FAs in pregnant mPPARα mice, we also measured corresponding FA concentrations in livers of fetuses (GD18) and pups (PND2) in the mPPARα genotype.
3.2.1. n-6 family PUFA
Levels of linoleic acid and arachidonic acid were both significantly lower in fetuses than pups (Fig. 4A). DEHP exposure at the 0.05% dosage significantly increased the level of arachidonic acid in fetus, but no influence was noted in the concentrations of linoleic acid in fetus and those of both FAs in pups at any dosage.
Fig. 4.
Hepatic levels of n-6 family (A) and n-3 family (B) fatty acids in fetuses and pups of mPPARα mice. Data represent mean ± SD. LA, linoleic acid; AA, arachidonic acid; ALA, alpha-linorenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. *p < 0.05 compared with control. ‡p < 0.05 compared with pups.
3.2.2. n-3 family PUFA
Levels of α-linolenic acid, EPA and DHA were also strikingly lower in fetuses than pups (Fig. 4B). In fetus, DEHP exposure slightly increased α-linolenic acid levels at the 0.01% dosage, but not at 0.05 and 0.1% dosages. However, DEHP exposure did not influence the levels of EPA and DHA in fetus, nor in any n-3 PUFA in pups.
3.2.3. Saturated and monounsaturated FAs
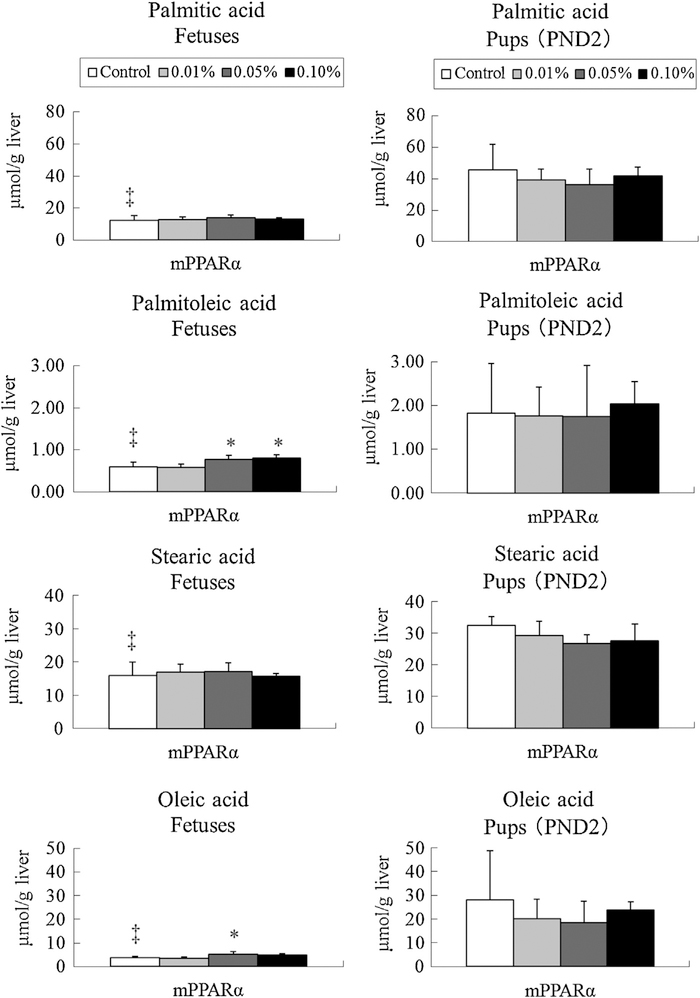
Levels of palmitic, palmitoleic, stearic, and oleic acids were found to be significantly lower in fetuses than pups (Fig. 5). DEHP exposure of 0.05 and 0.1% dosages significantly increased the level of palmitoleic acid in fetuses. The exposure slightly increased oleic acid levels only at the 0.05% dosage. No influence from DEHP exposure was responsible for the concentrations of these FA in pups.
Fig. 5.
Levels of n-9 family and saturated fatty acids in fetuses and pups of mPPARα mice. Data represent mean ± SD. *p < 0.05 compared with control. ‡p < 0.05 compared with pups.
3.3. Proportion of each FA component in wild-type control mice and their offspring
The sum of nine FAs was designated as total FAs in wild-type control mice and their offspring, and the proportion of each component was calculated (Table 1). It was obvious that total FAs were 3.1-fold higher in plasma of pregnant mice than in postpartum ones, whereas the level was 4.5-fold higher in the liver of newborn pups than in fetuses. The most abundant FA was linoleic acid, accounting for 34% of total FAs in both pregnant and postpartum mice. Palmitic acid ranked second in both groups, followed by oleic acid and stearic acid. The profile of FAs in the liver of newborn pups was very similar to that of the maternal plasma, while the profile of liver of fetuses was quite different from that of the maternal plasma. In the liver of fetuses, stearic acid was the most abundant FA, followed by palmitic acid.
Table 1.
Proportion of each FA component in wild-type control mice and their offspring.
| Pregnant (μmol/ml plasma) | Postpartum (μmol/ml plasma) | Fetus (μmol/g liver) | New-born pups (μmol/g liver) | |
|---|---|---|---|---|
| Linoleic acid | 2.91 ± 0.92 (34.4) | 0.93 ± 0.05 (34.5) | 3.69 ± 0.73 (8.6) | 3.69 ± 0.73 (8.6) |
| Arachidonic acid | 0.46 ± 0.19 (5.5) | 0.28 ± 0.09 (10.5) | 2.76 ± 0.44 (6.4) | 13.86 ± 2.67 (7.2) |
| α-Linorenic acid | 0.09 ± 0.03 (1.1) | 0.01 ± 0.00 (0.5) | 0.04 ± 0.01 (0.1) | 0.60 ± 0.45 (0.3) |
| EPA | 0.11 ± 0.03 (1.3) | 0.06 ± 0.02 (2.0) | 0.29 ± 0.06 (0.7) | 1.40 ± 0.77 (0.7) |
| DHA | 0.47 ± 0.20 (5.5) | 0.15 ± 0.02 (5.4) | 3.58 ± 0.68 (8.3) | 21.76 ± 5.31 (11.3) |
| Palmitic acid | 1.90 ± 0.71 (22.4) | 0.59 ± 0.12 (21.7) | 12.32 ± 2.93 (28.7) | 45.66 ± 16.17 (23.7) |
| Palmitoleic acid | 0.08 ± 0.04 (1.0) | 0.04 ± 0.02 (1.4) | 0.60 ± 0.10 (1.4) | 1.83 ± 1.13 (0.9) |
| Stearic acid | 0.76 ± 0.25 (9.0) | 0.34 ± 0.05 (12.6) | 15.85 ± 4.08 (36.9) | 32.57 ± 2.78 (16.9) |
| Oleic acid | 1.67 ± 0.59 (19.7) | 0.31 ± 0.04 (11.3) | 3.81 ± 0.67 (8.9) | 27.92 ± 20.78 (14.5) |
| Total FAs | 8.46 ± 2.83 | 2.71 ± 0.15 | 42.95 ± 8.90 | 192.80 ± 78.65 |
Total FAs represents sum of nine FAs.
Figures in parentheses represent percent of each FA for total FAs.
3.4. Measurements of representative enzymes involved inLC-PUFA synthesis
To examine the relationship between DEHP exposure and EFA conversion into LC-PUFA, representative enzymes including Fads 1, Fads 2, Elovl 2 and Elovl 5 were measured in maternal liver tissues by Western blot and quantitative real-time PCR.
While comparing the control groups among three genotyped pregnant mice, the level of Fads2 in mPPARα mice was significantly higher than in Pparα-null mice (Supplemental Fig. 1). In contrast, the level of Elovl5 was significantly lower in mPPARα mice than Pparα-null mice. There was no difference in the levels of Fads1 and Elovl2 in any genotyped pregnant control mice. As for the control group postpartum mice, the level of Fads1 was significantly higher in mPPARα than Pparα-null mice, and Elovl2 was higher in hPPARα mice than in Pparα-null mice. No difference was observed in the levels of Fads2 and Elovl5 among control groups of each genotype.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tox.2013.04.010.
Comparisons of control groups from each genotype between pregnant and postpartum mice showed that the level of Fads1 in hPPARα mice was significantly higher during pregnancy. However, the levels of Fads2 in Pparα-null mice and Elovl2 in mPPARα mice were significantly lower during pregnancy. No significant difference was observed for Elovl5 in any of the genotyped mice.
DEHP exposure did not influence the protein levels of these enzymes in any genotyped pregnant mice, nor in mPPARα postpartum mice. Although DEHP exposure of 0.01 and 0.1% dosages in Pparα-null mice did not induce changes, the level of Fads2 was significantly decreased by the 0.05% dosage. For hPPARα mice, DEHP exposure increased the levels of Fads1 and Fads2 at 0.05 and 0.1%, and 0.1% dosages, respectively. DEHP did not influence the levels of Elovl2 and Elovl5 in postpartum mice.
While comparing the control groups of three genotyped pregnant or postpartum mice, no difference in the mRNA level of any enzyme was observed in pregnant mice (data not shown). The level of Fads2 in postpartum mPPARα mice was significantly lower than in their hPPARα mice counterparts, but there was no difference in other enzymes between strains. Comparisons of control groups from each genotype between pregnant and postpartum mice showed that levels of Fads1 and Fads2 were significantly higher in pregnant mPPARα mice than in their postpartum mice counterparts. No difference was detected in the levels of Elovl2 and Elovl5.
The influence of DEHP exposure was not observed on the mRNA levels of each enzyme in any genotyped pregnant mice (data not shown). DEHP exposure significantly increased Fads1 mRNA in postpartum Pparα-null mice at 0.05% dosage alone, but did not influence it in postpartum mPPARα and hPPARα mice. DEHP exposure also did not influence the levels of Fads2, Elovl2 and Elovl5 in any genotyped postpartum mice.
4. Discussion
In the present study, DEHP significantly decreased maternal plasma concentrations of linoleic acid, α-linolenic acid, palmitic acid, and oleic acid, but only in the pregnant mPPARα mice. Interestingly, these four specific FAs levels were significantly higher in pregnant control mPPARα mice than in Pparα-null and hPPARα mice. These findings were quite similar to the effects of DEHP exposure on maternal plasma TG levels (Hayashi et al., 2011). Since the major components of FAs are linoleic acid, palmitic acid, and oleic acid, accounting for 76.5% of total plasma FAs in the pregnant mice, it is not doubtful that there is a similar relationship between FAs and TG. Additionally, the plasma concentrations of many FAs were generally higher in pregnant mice than in postpartum ones regardless of the genotypes, though unchanged components were different among the three genotypes. Thus, FA concentrations in plasma of mice can be influenced not only by DEHP exposure, but also by mouse PPARα gene and pregnancy condition. However, DEHP exposure did not obviously decrease plasma FAs of postpartum mice except for EPA, α-linolenic acid and oleic acid in mPPARα mice, which were not major components of FAs.
The first point under discussion in this research is why maternal exposure to DEHP decreased the major concentrations of plasma FAs, linoleic acid, α-linolenic acid, palmitic acid and oleic acid, only in mPPARα mice. DEHP exposure activated maternal PPARα target genes that promote FA β-oxidation in mPPARα mice; however, these enzymes were also induced in hPPARα mice (Hayashi et al., 2011). Additionally, DEHP did not influence Fads 1, 2 and Elovl 2, 5 involved in the FA desaturation or elongation process, respectively. Therefore, these enzymes may not be involved in the DEHP-induced decrease of plasma FAs in pregnant mice. Since the administration of fibrate, a pharmacological ligand for PPARα of mouse, caused a significant increment in the mRNA expression of Fads 1 and 2 (Matsuzaka et al., 2002), there may be differences in the metabolic impact on FAs between fibrates and DEHP. This means that the action on lipid degradation via PPARα by DEHP is different from that of other ligands such as fibrates. Though the plasma EPA concentration of pregnant wild-type mice was not influenced by DEHP exposure, the levels were significantly decreased by DEHP exposure during the postpartum period. After DEHP exposure, mRNA expression of mitochondrial β-oxidation enzymes in pregnant mice was unaffected, but significantly increased in postpartum mice (Hayashi et al., 2011). Therefore, mitochondrial β-oxidation of EPA in postpartum mice may be induced, which may result in the decrease of EPA content. As a whole, short chain FAs with fewer than 20 carbon atoms were more susceptible to DEHP exposure in pregnant or postpartum mice. The length of the carbon chain may correlate with the specificity of FA to DEHP toxicity. Since DEHP downregulated MTP-mRNA levels only in mPPARα mice (Hayashi et al., 2011), this may be a potential factor in the decrease of four specific FAs.
Although a decrease in the concentrations of PUFA by DEHP exposure at 10–145 mg/kg/day was observed in plasma of mPPARα maternal mice, no such influence was observed on liver FA concentrations in fetuses and pups, similar to TG effects (unpublished observation). Of these, DEHP effects on α-linolenic acid may be more sensitive than those on TG, because the effect on α-linolenic acid was seen from the lowest exposure level, whereas at the highest dose (~145 mg/kg/day) for TG. Exposure to DEHP at 1500 mg/kg/day in pregnant rats decreased arachidonic acid concentrations in plasma, brain, liver, heart and intestine, as well as DHA in plasma and brain of fetus (Xu et al., 2008). Therefore, it is possible that the effects of DEHP exposure on FA concentrations in fetuses or pups may occur at a higher exposure level than what we used in this study. In our earlier research (Hayashi et al., 2011), the number of alive fetuses and newborns decreased by DEHP exposure. Therefore, we did not measure FAs in the dead fetuses and pups, which may have been the reason why no difference in FA levels was observed in the offspring. It may also be possible that the lower DEHP dosages adopted in our study do not influence the FA levels of offspring.
Next, it is particularly worth noting that the four FAs mentioned above showed significantly higher levels during pregnancy in mPPARα control mice compared to their Pparα-null and hPPARα mice counterparts. Different from palmitic acid and oleic acid, linoleic acid and α-linolenic acid are EFAs, which cannot be synthesized in the body, and must be given by food. However, there was no difference in food consumption among the three genotypes (Hayashi et al., 2011), which may not contribute to genotype differences in EFAs. In the liver, FAs are synthesized to TG by diacylglycerol acyltransferase (Dgat) 1 and 2, then TG is transported from liver to blood by microsomal triglyceride transfer protein (MTP) (Tietge et al., 1999; Chandler et al., 2003; Yen et al., 2008), and can be hydrolyzed into FAs by lipoprotein lipase (Rodrigues et al., 2010). No difference was noted in the expression of Dgat 1 and 2 in the liver among three genotypes. In contrast, hepatic MTP mRNA levels were significantly higher in mPPARα control mice than in their Pparα-null and hPPARα mice counterparts (Hayashi et al., 2011). On the other hand, FAs are metabolized in the liver by β-oxidizing enzymes, and this process is promoted by activating PPARα (Yoon, 2009; van Raalte et al., 2004). Although the expressions of these enzymes were considerably higher in mPPARα control mice than Pparα-null control mice, no obvious difference was noted in the levels between mPPARα control and hPPARα control mice. Two EFAs are precursors to the synthesis of LC-PUFA via sequential desaturation by Fads 1 and 2 and elongation by Elovl 2 and 5 (Guillou et al., 2010). However, no differences were noted in the hepatic expressions of these enzymes among control mice of three genotypes. Taken together, regarding the reason for the four FAs with significant genotype differences in pregnant mice, the higher expressions of hepatic MTP mRNA levels in mPPARα than Pparα-null and hPPARα control mice may be the most plausible explanation, similar to the different plasma TG levels (Hayashi et al., 2011): the special FAs observed among three mouse genotypes were thought to be dependent of the different types of TG transportation by MTP (Herrera, 2002).
Several FAs showed higher concentrations during pregnancy compared to postpartum periods in each control group: all FAs except arachidonic acid for mPPARα mice; all FAs except n-6 PUFA and EPA in Pparα-null mice; and, EPA, DHA, palmitoleic acid and stearic acid in hPPARα mice. These results also correspond to the elevated plasma TG during pregnancy in these strains in our earlier report (Hayashi et al., 2011). Human studies revealed that plasma TG and LC-PUFA had a physiologic antepartum rise and a return to baseline postpartum (Montes et al., 1984; Al et al., 2000). Animal studies also reported higher TG concentrations in serum during late pregnancy in mice (Sweeney et al., 2006). Hayashi et al. (2011) reported remarkably higher expression of MTP mRNA in the liver of pregnant dams than in those of postpartum dams. The differences in the concentrations of FA between pregnant and postpartum mice might depend on different TG transportation systems, as many of the above-mentioned FA can exist in the form of TG (Herrera, 2002). Additionally, McMullin et al. (2008) reported that TG in milk was 100-fold higher than in serum of rats during lactation. Here, total FAs in the liver of newborns were 71-fold higher than in maternal plasma. Therefore, the transfer of TG including FAs from mother to newborn through maternal milk during postpartum is a possible explanation for decreased plasma FAs in postpartum mice in our present study. It also should be noted that there was no difference in the FA profile between newborns and their mothers, while whereas profile was quite different between fetuses and their mothers: in fetuses, stearic acid was the most abundant FA. Further measurement of FAs in placenta may be warranted.
Finally, hepatic concentrations of all FAs measured were remarkably higher in pups than in fetuses in mPPARα mice, as shown in another study (McMullin et al., 2008) in which the serum lipid level progressively increased throughout the postnatal period. FAs are supplied to fetuses and pups through placenta and milk, respectively. The high fat content of milk, the sole source nutrition in pups, coupled with the high rate of lipolysis and increased liver lipoprotein lipase activity in neonates, likely contribute to the increase in serum lipids (McMullin et al., 2008). This situation explained our observations. Additionally, the abundant liver FAs in pups are critical for postnatal development.
In summary, plasma FA concentration can be influenced by DEHP exposure, as well as by mouse PPARα gene and gestational status. Various changes in FA concentrations, especially the decrease in the four FAs concentrations mentioned above by DEHP exposure during the pregnancy of mPPARα mice, may have been related to the decrease in TG and the reduction of the number of fetuses and newborns observed in our previous study (Hayashi et al., 2011).
Supplementary Material
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B20390171) from the Japan Society for the Promotion of Science from Food Safety Commission, Japan (1002) and Health and Labour Sciences Research Grants from Research on Food Safety of the Ministry of Health, Labour and Welfare (200939055A).
Footnotes
Conflict of interest statement
None.
References
- Al MD, van Houwelingen AC, Hornstra G, 2000. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am. J. Clin. Nutr 71, 285S–291S. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ, 1998. Altered constitutive expression of fatty acid metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem 273, 5678–5684. [DOI] [PubMed] [Google Scholar]
- Chandler CE, Wilder DE, Pettini JL, Savory YE, Petras SF, Chang G, Vincent J, Harwood HJ Jr., 2003. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J. Lipid Res. 44, 1887–1901. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ, 2004. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res. 64, 3849–3854. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, Hass U, 2009. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect 117, 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH, 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 226, 497–509. [PubMed] [Google Scholar]
- Guillou H, Zadravec D, Martin PG, Jacobsson A, 2010. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res 49, 186–199. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D, Leffler P, Ekwurtzel E, Martinsson G, Liu K, Selstam G, 2008. Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction 135, 693–703. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ito Y, Yamagishi N, Yanagiba Y, Tamada H, Wang D, Ramdhan DH, Naito H, Harada Y, Kamijima M, Gonzales FJ, Nakajima T, 2011. Hepatic peroxisome proliferator-activated receptor α may have an important role in the toxic effects of di(2-ethylhexyl)phthalate on offspring of mice. Toxicology 289 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- Herrera E, 2002. Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development—a review. Placenta 23 (Suppl. A), S9–S19. [DOI] [PubMed] [Google Scholar]
- Hornstra G, 2001. Importance of polyunstarated fatty acids of the n-6 and n-3 families for early human development. Eur. J. Lipid Sci. Technol 103, 379–389. [Google Scholar]
- Ito Y, Yamanoshita O, Kurata Y, Kamijima M, Aoyama T, Nakajima T, 2007. Induction of peroxisome proliferator-activated receptor alpha (PPARα)-related enzymes by di(2-ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch. Toxicol 81, 219–226. [DOI] [PubMed] [Google Scholar]
- Japan Plasticizer Industry Association, 2010. Kasozai Information No. 23. http://www.kasozai.gr.jp/kasoinfo/pdf/no23.pdf
- Japan Plasticizer Industry Association, 2013. http://www.kasozai.gr.jp/data/toukei-pdf/2013-02seisan.pdf
- Jia X, Naito H, Yetti H, Tamada H, Kitamori K, Hayashi Y, Yamagishi N, Wang D, Yanagiba Y, Ito Y, Wang J, Tanaka N, Ikeda K, Yamori Y, Nakajima T, 2012. The modulation of hepatic adenosine triphosphate and inflammation by eicosapentaenoic acid during severe fibrotic progression in the SHRSP5/Dmcr rat model. Life Sci. 90, 934–943. [DOI] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J, 2006. Di(2-ethylhexyl) phthalate (DEHP):human metabolism and internal exposure – an update and latest results. Int. J. Androl 29, 155–165. [DOI] [PubMed] [Google Scholar]
- Lamb JC IV, Capin RE, Teague J, Lawton AD, Reel JR, 1987. Reproductive effects of four phthalic acid esters in the mouse. Toxicol. Appl. Pharmacol 88, 255–269. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Krotez DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ, 1995. Targeted disruption of the alpha isoform of the peroxsome proliferators-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ, 1999. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol 161, 209–218. [DOI] [PubMed] [Google Scholar]
- Masood A, Stark KD, Salem N Jr., 2005. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res 46, 2299–2305. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasty AH, Tamura Y, Osuga J, Okazaki H, Iizuka Y, Takahashi A, Sone H, Gotoda T, Ishibashi S, Yamada N, 2002. Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J. Lipid Res 43, 107–114. [PubMed] [Google Scholar]
- McMullin TS, Lowe ER, Bartels MJ, Marty MS, 2008. Dynamic changes in lipids and proteins of maternal, fetal, and pup blood and milk during perinatal development in CD and Wistar rats. Toxicol. Sci 105, 260–274. [DOI] [PubMed] [Google Scholar]
- Ministry of Economy, Trade and Industry, 2012. 2011 Yearbook of chemical industry statistics. http://www.meti.go.jp/statistics/tyo/seidou/result/ichiran/02kagaku.html
- Montes A, Walden CE, Knopp RH, Cheung M, Chapman MB, Alberts JJ, 1984. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of prelipemia. Arteriosclerosis 4, 407–417. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kamijo Y, Usuda N, Liang Y, Fukushima Y, Kametani K, Gonzalez FJ, Aoyama T, 2000. Sex-dependent regulation of hepatic peroxisome proliferation in mice by trichloroethylene via peroxisome proliferator-activated receptor alpha (PPARalpha). Carcinogenesis 21, 677–682. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ito Y, Yanagiba Y, Ramdhan DH, Kono Y, Naito H, Hayashi Y, Li Y, Aoyama T, Gonzalez FJ, Nakajima T, 2009. Microgram-order ammonium perfluorooctanoate may activate mouse peroxisome proliferator-activated receptor alpha, but not human PPARalpha. Toxicology 265, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S, 1984. Effects of di-2-ethylhexyl phthalate on lipid composition of serum and testis in rats. Toxicol. Lett 23, 67–72. [DOI] [PubMed] [Google Scholar]
- Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, Kamijima M, Nakajima T, 2009. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol. Lett 191, 103–108. [DOI] [PubMed] [Google Scholar]
- Rodrigues CE, Bonfa E, Carvalho JF, 2010. Review on anti-lipoprotein lipase antibodies. Clin. Chim. Acta 411, 1603–1605. [DOI] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML, 2006. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol 36, 459–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney TR, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR, 2006. Decreased nuclear hormone receptor expression in the livers of mice in late pregnancy. Am. J. Physiol. Endocrinol. Metab 290, E1313–E1320. [DOI] [PubMed] [Google Scholar]
- Tietge UJ, Bakillah A, Maugeais C, Tsukamoto K, Hussain M, Rader DJ, 1999. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res 40, 2134–2139. [PubMed] [Google Scholar]
- van Raalte DH, Li M, Pritchard PH, Wasan KM, 2004. Peroxisome proliferator-activated receptor (PPAR) alpha: a pharmacological target with a promising future. Pharm. Res 21, 1531–1538. [DOI] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT, 2008. Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta 29, 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Knipp GT, Cook TJ, 2006. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch. Toxicol 80, 293–298. [DOI] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr., 2008. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res 49, 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, 2009. The role of PPARα in lipid metabolism and obesity: focusing on the effect of estrogen on PPARα actions. Pharmacol. Res 60, 151–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.