Abstract
Background
Strongyloides stercoralis infection, a neglected tropical disease, is widely distributed. Autochthonous cases have been described in Spain, probably infected long time ago. In recent years the number of diagnosed cases has increased due to the growing number of immigrants, travelers and refugees, but endemically acquired cases in Spain remains undetermined.
Methodology
We systematically searched the literature for references on endemic strongyloidiasis cases in Spain. The articles were required to describe Strongyloides stercoralis infection in at least one Spanish-born person without a history of travel to endemic areas and be published before 31st May 2018. Epidemiological data from patients was collected and described individually as well as risk factors to acquisition of the infection, diagnostic technique that lead to the diagnosis, presence of eosinophilia and clinical symptoms at diagnosis.
Findings
Thirty-six studies were included, describing a total of 1083 patients with an average age of 68.3 years diagnosed with endemic strongyloidiasis in Spain. The vast majority of the cases were described in the province of Valencia (n = 1049). Two hundred and eight of the 251 (82.9%) patients in whom gender was reported were male, and most of them had current or past dedication to agriculture. Seventy percent had some kind of comorbidity. A decreasing trend in the diagnosed cases per year is observed from the end of last decade. However, there are still nefigw diagnoses of autochthonous cases of strongyloidiasis in Spain every year.
Conclusions
With the data provided by this review it is likely that in Spain strongyloidiasis might have been underestimated. It is highly probable that the infection remains undiagnosed in many cases due to low clinical suspicion among Spanish population without recent travel history in which the contagion probably took place decades ago.
Author summary
S. stercoralis is a soil transmitted helminth that is common in many subtropical and tropical countries, but is also found in other regions of the world. In this study we reviewed all published material on endemic infection of S. stercoralis acquired in Spain issued before 31st May 2018. We collected data from these articles and reported clinical and epidemiological characteristics of patients. Our systematic review of the articles showed a clear geographical pattern; nearly 97% of the cases described had been acquired in the Valencia province. Most of them (82.9%) were male, and most had current or past dedication to agriculture. Our results showed that 70.3% had at least one condition or treatment that could have made them more vulnerable to suffer a severe form of this helminthic disease. Our data suggests that S. stercoralis infection probably remains underdiagnosed in Spanish population. Due to the scarce information available about endemic strongyloidiasis in Spain until now, we believe that the present work will be relevant and the conclusions derived from it might raise awareness about underdiagnosis. Transmission risk factors described in the people diagnosed may be key for prevention and control strategies implementation.
Introduction
Strongyloidiasis is a disease caused by soil-transmitted helminths, mainly by the species Strongyloides stercoralis. This intestinal nematode infects an estimated 300 million people worldwide, although this is probably underestimated. It is one of the most neglected of the neglected tropical diseases (NTD) and is widely distributed [1–2]. Although it generally occurs in subtropical and tropical countries, transmission is also possible in countries with temperate climates. Autochthonous cases have been described in Spain, possibly infected long time ago. It remains uncertain whether S. stercoralis is currently endemic in Spain. Still, some authors consider this country and some other southern European countries as endemic [3].
The life cycle of S. stercoralis is complex and follows multiple routes, including a complete life cycle outside the human host. The most frequent mechanism of infection is percutaneous entry of the filariform larvae. In healthy people, most of the cases are asymptomatic, although it can cause intermittent symptoms that mainly affect the intestine, the lungs or the skin [4].
About criterion used to establish the diagnosis of strongyloidiasis is not homogenized among the centers. The diagnostic laboratory criterion of strongyloidiasis is the observation of larval stages. However, in chronic infection, larvae excretion may be low and fluctuating, and microscopic observation is not sensitive enough and multiple stool specimens should be analyzed to increase the sensitivity of the method. The clinical criterion is a patient with epidemiological antecedents and any of the associated clinical manifestations, especially if it is an immunosuppressed patient.
These methods are laborious, time consuming, and in the case of fecal culture, requires well trained technicians in order to differentiate S. stercoralis. Several immunological tests have also been described (ELISA, IFAT and Western blot) with variable sensitivity and specificity depending on the population tested among other factors [1].
Alternative diagnostic methods, such as molecular biology techniques (mostly polymerase chain reaction, PCR) have been implemented. However, PCR might not be suitable for screening purpose, whereas it might have a role as a confirmatory test, since it still misses a relevant proportion of infected people [5].
Due to the subtle symptoms, low sensitivity of diagnostic techniques and the complex lifecycle that can cause asymptomatic autoinfection for decades, the prevalence of S. stercoralis is thought to be severely underestimated.
Typically risk factors for severe infection include immunosuppression, certain malignancies, human T-cell lymphotropic virus type 1 infection, and alcoholism. Likewise, S. stercoralis has been associated with agricultural or mining activities. In Germany, it was recognized as a parasitic professional disease in miners [6]. S. stercoralis infection has also been linked to low socioeconomic factors and infrastructure, indicating that it as a disease of disadvantage [7–8]. In recent years the number of diagnosed cases has been increasing in high income countries due to the growing number of immigrants, travelers and refugees [9–10].
To provide information on this topic, a systematic review of the cases of endemic strongyloidiasis in Spain was carried out, as well as the description of the epidemiological characteristics of these patients.
Methods
Aiming to assemble all scientific articles based on endemic strongyloidiasis diagnosed in Spain, a systematic review was carried out. Relevant articles were retrieved from PubMed, EMBASE, Scielo, ISI Web of Knowledge, and Cochrane Library databases using combinations of the search terms adapted to each database. Additionally, Gray Literature in the form of communications presented at national congresses was performed, as well as OpenGrey. As a secondary source, Google Scholar and free internet search was used for non-indexed articles. The keywords were “Strongyloides stercoralis”, “soil-transmitted helminthiasis”, “endemic”, and “Spain”. The following combinations of MeSH were used in PubMed: (Strongy* [MeSH] AND Spain), ("Strongyloidiasis" [MeSH] AND Spain NOT "imported" NOT "immigrant"), and ("Strongyloidiasis" [MeSH] AND "endemic" AND "Spain").
The selection criteria were articles published in any language until May 31st 2018 that contained the description of at least one human case of infection with S. stercoralis acquired in Spain without a history of travel to endemic areas. No restrictions were applied based on the study design or data collection. Human filter was applied. A manual search of the bibliographical references cited in the relevant articles was carried out.
All potential articles were analyzed by two researchers to assess compliance with the selection criteria. In situations of missed information, the corresponding author of the paper was contacted to gather the information. If the author answered the required information to fulfill the inclusion criteria, those articles were considered. If not, they were excluded because they could not ensure the endemic acquisition.
The exclusion criteria included: animal studies, cases in which endemic infection could not be assured, cases of foreigners from an endemic country for S. stercoralis, native people with trips to endemic or probably endemic countries in the past (e.g. Italy, France or Portugal), transplanted people in which this contagion route could not be excluded, and duplicated cases.
Based on these criteria the articles were reviewed in two stages. In the first stage, articles were selected by titles and abstracts according to selection criteria. In the second stage, the full text of the articles was analyzed. Finally, the articles that met the selection criteria were included in the study.
From each study the following data was extracted: the study period, year of publication and number of endemic cases described. The following epidemiological data from patients described in the studies was collected: age, gender, geographical origin, medical comorbidities and concomitant treatments, occupation (or hobbies if relevant), other risk factors, year of diagnosis, diagnostic technique used for diagnosis, presence of eosinophilia and clinical symptoms.
Results
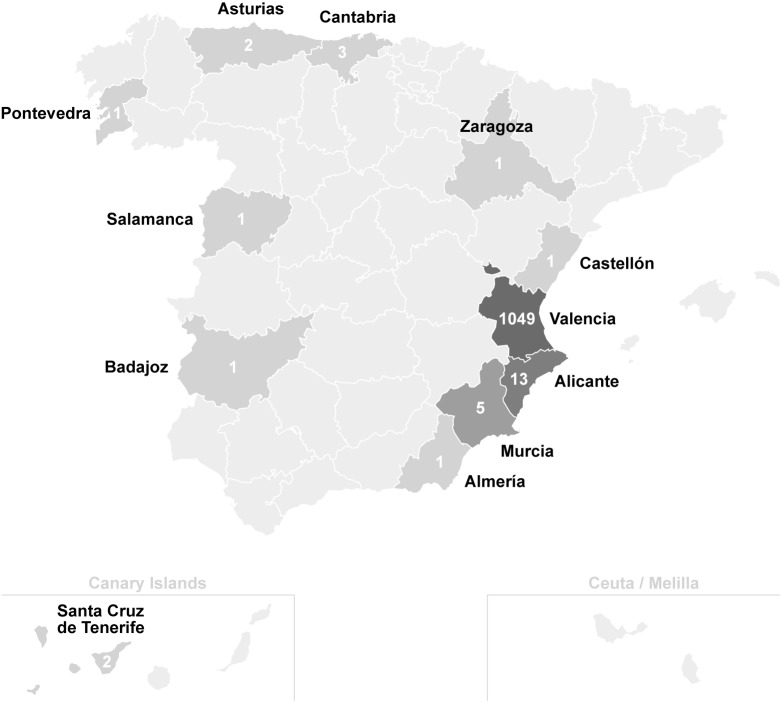
Thirty-six studies were included describing a total of 1083 patients with endemic strongyloidiasis in Spain (see Tables 1 and 2) [11–46]. The average age of the described cases was 68.35 years, ranging from 17 to 100 years old. Two hundred and eight of the 251 (82.9%) patients in whom gender was reported were male, and most of them had current or past dedication to agriculture. The province in whom most cases were described was Valencia, with 1049 people diagnosed. Alicante had 13 and Murcia 5, eventually describing cases in provinces of coastal oceanic climate with abundant rainfall most of the year and temperatures below 22°C (Cantabria, Asturias, and Pontevedra). See Fig 1.
Table 1. Main characteristics of included articles.
| Reference | Study period | Number of cases | Year of publication |
|---|---|---|---|
| Duvignaud et al [11] | 1 | 2016 | |
| Valerio et al [12] | 01/2003–12/2012 | 2 | 2013 |
| Fernández Rodríguez et al [13] | 1 | 2012 | |
| Mayayo et al [14] | 1 | 2005 | |
| Oltra Alcaraz et al [15] | 261 | 2004 | |
| Martinez-Vazquez et al [16] | 1 | 2003 | |
| Román Sanchez et al [17] | 31 | 2003 | |
| Román Sanchez et al [18] | 152 | 2001 | |
| Rodríguez Calabuig* et al [19] | 10/1997–10/1999 | 15 | 2001 |
| Cremades Romero et al [20] | 1 | 1996 | |
| Rodríguez Calabuig* et al [21] | 01/1994–06/1997 | 15 | 1998 |
| Llagunes et al [22] | 1 | 2010 | |
| Esteve-Martínez et al [23] | 1 | 2013 | |
| Olmos et al [24] | 1997 | 1 | 2004 |
| López Gaona et al [25] | 1 | 2009 | |
| Escudero-Sanchez et al [26] | 1 | 2017 | |
| Pacheco-Tenza et al [27] | 01/1999–03/2016 | 4 | 2016 |
| Esteban Ronda et al [28] | 1 | 2016 | |
| Martinez-Perez et al [29] | 2000–2015 | 1 | 2018 |
| Igual Adell et al [30] | 2007 | ||
| Pretel Serrano et al [31] | 1994–1999 | 3 | 2001 |
| Ortiz Romero et al [32] | 1 | 2007 | |
| Batista et al [33] | 1 | 1992 | |
| Llenas García et al [34] | 01/1999–12/2017 | 9 | 2018 |
| Tornero et al [35] | 01/2002–12/2017 | 423 | 2018 |
| Corbacho Loarte et al [36] | 01/2008–12/2015 | 1 | 2017 |
| García García et al [37] | 2009–2010 | 2 | 2011 |
| Igual et al [38] | 2004–2005 | 112 | 2006 |
| Lozano Polo et al [39] | 1 | 2005 | |
| Cremades Romero et al [40] | 04/1994–10/1995 | 32 | 1997 |
| Sanchis-Bayarri et al [41] | 1 | 1981 | |
| Tirado et a [42] | 1 | 2002 | |
| Nevado et al [43] | 1 | 1996 | |
| Sampedro et al [44] | 1 | 1988 | |
| Lopez Gallardo et al [45] | 1 | 1997 | |
| Toldos et al [46] | 1 | 1995 |
* Data obtained through clarification of the main author
Table 2. Clinical and epidemiological characteristics of patients with autochtonous strongyloidiasis in Spain.
| Gender and age (in years) | City and province of residence | Occupation | Comorbidity or concomitant treatment | Other risk factors | Year of diagnosis | Diagnostic techniques | Presence of eosinophilia. Eosinophils count per mm3. Percentage of eosinophils in white blood cell /percentage of patients with eosinophilia if relevant |
Clinical symptoms | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| W 17 | La Safor, Valencia | No data | No data | Walking barefoot | 2005 | Fresh stool examination and/or fecal culture | Yes, 16,900 |
Abdominal pain, diarrhea and weight loss | [11] |
| W 61 | No data | No data | Asthma and chronic eosinophilic pneumonia. | No data | 2011 | Serology | Yes 1,480 20% |
Chronic urticaria and abdominal pain | [13] |
| M 79 | Zaragoza, Aragón | No data | Mild malnutrition | No data | 2004 | Bronchial aspirate examination | No | Abdominal pain | [14] |
| 261 M+W patients, 21–100 years old | La Safor, Valencia | 124 agriculture activities, 18 construction activities | No data | 33 irrigation ditches cleaners, 6 ditch baths |
1995–1999 | Fresh stool examination, Baermann test and fecal culture | No data | No data | [15] |
| M 25 | Fornelos de Montes, Pontevedra | Logging company | No data | Occasional agricultural work and bathing in river | 2002 | Fresh stool examination, and examination after concentration (Ritchie) | Yes 22,000 |
Nonspecific skin lesions and abdominal pain | [16] |
| 31 M patients, mean age 68.6 ± 8.0 | Gandía, Valencia | Farmers | No data | Work barefoot | 2003 | Fresh stool examination and/or fecal culture | No data | No data | [17] |
| 120 M and 32 W, mean age 67 | Gandía, Valencia | Farmers and farmer´s wives | 44 COPD(no further details), 38 heart disease, 7 solid neoplasia, and 1 HIV infection (no further details |
99 work barefoot or were spouses of farmers, 13 drink non-potable water | 1990–1997 | Fresh stool examination and fecal culture | 82% (n = 125) had eosinophilia median count: 1,357+-811 severe cases median count 694 +-309 |
41.65% (n = 63) were asymptomatic, 13% (n = 20) had hyperinfection syndrome or disseminated strongyloidiasis |
[18] |
| 15 patients, mean age 66 ± 10 | Oliva, Valencia | 68% farmers (rice and citric) | No data | No data | 1997–1999 | Fresh stool examination and fecal culture | 100% (n = 15) patients had eosinophilia (>500 eosinophils) | 49% cough, 47% pruritus, and 38% dyspepsia | [19] |
| M 70 | Ribera Baixa, Valencia | Farmer | COPD (FEV1 48%) + corticosteroids | No data | 1995 | Fresh stool examination and/or fecal culture | Yes 12,600 34% |
Nausea, vomits, weight loss and abdominal pain | [20] |
| 15 M+W, mean age 65 (SD 11.5) | Oliva, Valencia | Farmers | No data | 66.6% had some risk factor (work barefoot, drink non-potable water) | 1994–1997 | Fresh stool examination and/or fecal culture | No data | 56.6% symptoms (12% cough) | [21] |
| M 76 | Valencia Province | No data | Crohn´s disease + corticosteroids | No data | 2009 | Bronchoalveolar lavage | No | Fever, arthralgia, dyspnea | [22- 23] |
| M 57 | Santander, Cantabria | No data | HIV infection CD4 42/mm3 Rheumatoid arthritis Corticosteroids+ Immunosuppression |
No data | 2003 | Postmortem histopathological examination (trachea, lungs, ileum, cecum and pericolonic lymph nodes) | No | Anorexia, dysphagia, odynophagia, night sweats, weight loss, diarrhea, and acute respiratory distress | [24] |
| W 82 | Restiello-Grado, Asturias |
No data | No data | Gardening hobby | 2008 | Fresh stool examination and/or fecal culture | Yes 6,120 38% |
Abdominal pain and diarrhea | [25] |
| M 85 | Born in Extremadura, living in Madrid | Farmer | No data | No data | 2017 | Serology | No data | No data | [26] |
| M 69 | Orihuela, Alicante | Farmer | Lung carcinoma, chemotherapy, inhaled corticosteroids | No data | 2007 | Sputum examination | Yes 600 |
Hemoptysis, hyperinfection syndrome | [27] |
| W 73 | Redovan / Vega Baja del Segura. Alicante | Farmer | Diverticular disease | No data | 2015 | Serology | Yes 2,700 |
Abdominal pain and diarrhea | [27] |
| M 80 | Orihuela, Alicante | Farmer | Bladder tumor | No data | 2015 | Fresh stool examination and/or fecal culture | Yes 700 |
Abdominal pain, pruritus | [27] |
| M 72 | Orihuela, Alicante | Carrier | COPD (no further details)+ corticosteroids | Farmer's work in his free time | 2015 | Fecal culture, serology, histopathological examination (colon)biopsy) | Yes 2,400 |
Hyperinfection syndrome | [27] |
| M 84 | Chella, Valencia* | Farmer | Gastrectomy, asplenia, malnutrition and treatment with corticosteroids | Walk barefoot | 2016 | Bronchial aspirate examination | No | Asthenia, dysphagia, low-grade fever, anorexia, and weight loss | [28] |
| M 40 | Sta Cruz de Tenerife, Canarias | Construction worker | Immunosuppression | Walk barefoot in mud | 2006 | Fresh stool examination | Yes No further data |
Hyperinfection syndrome | [29] |
| M 81 | Vega del Segura, Murcia | Farmer | COPD (FEV1 42%) | Walk barefoot | 1998 | Fresh stool examination | Yes 1500 21% |
Abdominal pain and pruritus | [31] |
| M 77 | Vega del Segura, Murcia | Farmer | COPD(FEV1 30%)+ corticosteroids | Walk barefoot | 1999 | Fresh stool examination | Yes 1,100 11% |
Bronchospasm, hemoptysis | [31] |
| M 82 | Vega del Segura. Murcia | Farmer | COPD (no further details) + corticosteroids | Walk barefoot | 1999 | Fresh stool examination | Yes 3,900 29% |
Dyspnea, wheezing, abdominal pain, meteorism, and pruritus | [31] |
| M 85 | Murcia | No data | COPD (FEV1 50%) Corticosteroids for the last month |
No data | 2008 | Bronchial aspirate examination | Yes 3,100 40.8% |
Cough and dyspnea | [32] |
| M 35 | Sta Cruz de Tenerife, Canarias | No data | HIV infection CD4 36 |
No data | 1992 | Fresh stool examination and/or fecal culture | No | Cough, fever, vomits, and diarrhea | [33] |
| 8 M and 1 W, mean age 79 | Orihuela, Alicante | 6 farmers | 2 neoplasia, 3 COPD (no further details) + corticosteroids | No data | 1999–2017 | Fresh stool examination and/or fecal culture, and serology | 88.9% (n = 8) patients had eosinophilia median count 700 |
[34] | |
| 54 patients, mean age 72.6 (SD 9) | La Safor. Valencia | 60% former rice farmers | No data | In 4 of them bathing in marshy waters for recreational reasons was assumed, or parents had worked on rice fields | 2009:17 cases 2010: 12 cases 2011: 10 cases 2012: 5 cases 2013: 1 case 2014: 4 cases 2015:2 cases 2016: 1 case 2017: 2 cases |
Fresh stool examination and/or fecal culture | No data | No data | [35] |
| No data | No data | No data | No data | No data | 2008–2015 | Harada Mori, Baerman and/or fecal culture | No data | No data | [36] |
| 2 patients | Valencia. Valencia | No data | No data | No data | 2009–2010 | Fecal culture and Ritchie concentration method | No data | No data | [37] |
| 112 patients | Gandía. Valencia | No data | No data | No data | 2004–2005 | Fresh stool examination and/or fecal culture | No data | No data | [38] |
| W 64 | Santander, Cantabria | No data | Asthma + corticosteroids | No data | 2005 | Fresh stool examination and/or fecal culture | Yes 700 14% |
Bilateral pleural effusion and respiratory failure | [39] |
| 28 M + 4 W, mean age 68 (SD 7) | La Safor, Valencia | Farmers | 62% of patients with comorbidities: COPD, asthma, alcoholism, diabetes mellitus, and neoplasia | Walk barefoot | 1994–1995 | Fresh stool examination and/or fecal culture, and bronchoalveolar lavage | 100% (n = 32) had eosinophilia (>600) | 65% had respiratory, digestive and/or cutaneous symptoms | [40] |
| M 71 | Oliva. Valencia | Farmer | No data | Walk barefoot | 1981 | Cytological examination of an abdominal puncture, fresh stool examination and/or fecal culture | Yes 6,840 (38%) |
Vomits and abdominal pain | [41] |
| M 71 | Vinaroz, Castellón | Farmer | COPD (no further details) + corticosteroids | Walk barefoot | 2002 | Bronchial aspirate examination | No | Acute respiratory and renal failure, rash, and hemoptysis | [42] |
| W 63 | Cantabria | No data | Asthma + corticosteroids | No data | 1996 | Fresh stool examination | Yes 19% |
Dyspnea, cough, expectoration, diarrhea, vomits and pruritus | [43] |
| M 77 | Oviedo, Asturias | Coal Miner | COPD (FEV1 30–50%) + inhaled corticosteroids and alcohol | No data | 1988 | Histopathological examination (duodenal biopsy) | No | Weight loss and gastrointestinal symptoms | [44] |
| M 71 | Almería, Almería | No data | Ulcerative colitis + corticoids | No data | 1997 | Bronchial aspirate, stool and sputum examination, histopathological examination (colon biopsy) |
No | Abdominal pain, diarrhea, fever, dyspnea, cough, and expectoration | [45] |
| M 58 | Vega Media del Segura, Murcia | Farmer | COPD (no further details) + corticosteroids | No data | 1994 | Histopathological examination (duodenal biopsy), fecal culture | Yes 12,496 44% |
Abdominal pain, diarrhea, vomits, and weight loss | [46] |
* Data obtained through clarification of the main author.
Fig 1. Geographical distribution of autochthonous Strongyloides stercoralis infection in Spain.
The map was obtained from the open access website http://mapsvg.com/maps.
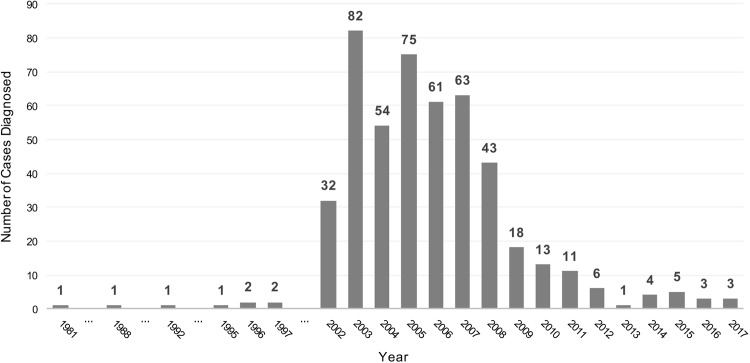
Regarding the number of diagnosed cases per year, a decreasing trend is observed since the beginning of this decade. The year with higher number of diagnosed cases was 2003, with 82 patients. Since 2011, no more than 10 cases have been reported annually (Fig 2).
Fig 2. Number of patients diagnosed with autochthonous Strongyloides stercoralis infection in Spain per year.
Only patients from articles that clearly specified the year of diagnosis of each case were included.
The technique that led to the diagnosis of strongyloidiasis was described in 743 patients from twenty-six different articles. In some cases, different techniques were used for the same diagnosis. In 692 patients (93.1%), the technique used for the definitive diagnosis of strongyloidiasis was the fresh stool examination, specific fecal culture, the Baermann test, the Ritchie technique or the Harada Mori technique. In 39 patients (5.2%) the diagnosis was made by the sputum or bronchoalveolar lavage examination. In 6 cases (0.8%) the diagnosis was made by serological techniques and in another 6 cases (0.8%) the diagnosis was made by histopathological analysis.
In 26 of the 37 patients individually described, comorbidities were reported. Out of those, most frequent were diseases that associate the use of corticosteroids such as: chronic obstructive pulmonary disease (COPD), asthma, and inflammatory bowel diseases or immunosuppressive conditions due to advanced HIV infection (AIDS stage) or malignancies. In all patients diagnosed with COPD, severity of airflow limitation (FEV1) was according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria at least moderate GOLD 2 (50% ≤ FEV1 < 80% predicted) if not severe: GOLD 3 (30% ≤ FEV1 < 50% predicted).
Overall, 70.3% of these patients had at least one comorbidity. In patients in whom blood test results were reported, 41 out of the 50 (82%) exhibited eosinophilia. The median eosinophil count in patients with eosinophilia was 4,057 eosinophil/mm3; considering 24 individual reported counts.
Discussion
Strongyloidiasis prevalence may be underestimated in many countries. With the data provided by this review it is likely that underestimation could have been a reality for the last five decades in Spain. The main cause would be the lack of clinical suspicion. But also the subtle symptoms, the decades-long persistence of infection in untreated hosts and the absence of a diagnostic test of choice with high sensitivity and specificity would ultimately contribute.
An important finding of our work is that almost 97% of all published infections occurred in the province of Valencia. The fact that most cases diagnosed and published are in the province of Valencia, can respond to various reasons. Firstly, the area had the perfect combination of temperature and humidity, population exposed to S. stercoralis for occupational reasons such as rice farmers or irrigation ditch cleaners (activities that were characteristically carried out barefoot) and hygiene factors of rural areas during the 1960s (lack of drinking water and toilets in some homes). It is noteworthy that no cases of strongyloidiasis have been reported in other areas with similar climatology and population equally dedicated to the cultivation of rice fields, such as the Delta del Ebro in Tarragona province. We consider highly probable that there has been transmission in other areas outside those described. Secondly, health care professionals in the area of Valencia probably had a greater awareness of the infection, with a higher suspicion and therefore a higher number of diagnoses.
Although we concur that the estimated prevalence of S. stercoralis by one highly cited article is not representative of the entire country, we disagree that Spain should not be considered an endemic country [17]. However, autochthonous cases have been anecdotal in the last decade, as indicated by Martinez-Perez [47].
The results of the individuals diagnosed showed an average age close to 70 years old.
Given the known characteristics of the disease the contagion probably took place decades before the diagnosis, coinciding with the postwar period where hygienic conditions and infrastructure were affected. On one hand, factors of unavoidable mention that directly affect the transmission of this helminth are the improvement in hygienic conditions and the mechanization of agricultural work. On the other hand, the increase of awareness by health care workers, especially from the most affected communities, may have led to the diagnosis of new cases in recent years.
An overall higher incidence rate in male gender is described, which is consistent with previous studies [15, 17, 21, 27]. This might be explained due to a gender biased; since some articles focus on screening high risk population (farmers or smokers with COPD), traditionally associated with gender roles.
Regarding the diagnostic techniques used, there is great heterogeneity among the different studies. The sensitivity of techniques based on microscopy is not good enough, particularly in chronic infections. Serology is a useful tool but could overestimate the prevalence of the disease due to cross-reactivity with other nematode infections and its difficulty distinguishing recent and past (and cured) infections. However, current serological tests are specific enough and negativization or a decrease in the titers could be observed 6–12 month after treatment, making this tool very useful [48].
There are some limitations that have to be mentioned. Inevitably there are cases of strongyloidiasis that have not been written for publication. In addition, ten articles had to be excluded due to lack of information about travel history or did not comply with the minimum information required. Therefore, it is highly probable that there were more than 1083 cases. Lastly, given the characteristics of this review, it is possible that there are some duplicate cases in multiple description articles and described individually by another researcher.
In summary, there are still new diagnoses of autochthonous cases of strongyloidiasis in Spain every year, especially as occupational hazard in a specific Spanish region. Although the number of diagnoses is much lower than in the past decade, it is highly probable that the infection remains undiagnosed due to low clinical suspicion among Spanish population without recent travel history. Epidemiological studies in at risk areas based on serological techniques could give more information about the real situation of autochthonous cases of strongyloidiasis in Spain.
Supporting information
(DOC)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. World Health Organisation. Intestinal Worms. Strongyloidiasis. Epidemiology. Available from: http://www.who.int/intestinal_worms/epidemiology/strongyloidiasis/en/. Cited 5 August 2018.
- 2.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967–72. 10.1016/j.trstmh.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Toledo R, Muñoz-Antoli C, Esteban JG. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol. 2015;88:165–241. 10.1016/bs.apar.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 4.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391(10117):252–65. 10.1016/S0140-6736(17)31930-X [DOI] [PubMed] [Google Scholar]
- 5.Buonfrate D, Requena-Mendez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, et al. (2018) Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—A systematic review and meta-analysis. PLoS Negl Trop Dis 12(2): e0006229 10.1371/journal.pntd.0006229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7(7):e2288 10.1371/journal.pntd.0002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beknazarova M, Whiley H, Ross K. Strongyloidiasis: A Disease of Socioeconomic Disadvantage. Int J Environ Res Public Health. 2016;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durrheim DN. Simply wearing footwear could interrupt transmission of Strongyloides stercoralis. BMJ. 2013;347:f5219 10.1136/bmj.f5219 [DOI] [PubMed] [Google Scholar]
- 9.Barrett J, Warrell CE, Macpherson L, Watson J, Lowe P, Armstrong M, et al. The changing aetiology of eosinophilia in migrants and returning travellers in the Hospital for Tropical Diseases, London 2002–2015: An observational study. J Infect. 2017;75(4):301–8. 10.1016/j.jinf.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Belhassen-Garcia M, Alonso-Sardon M, Martinez-Perez A, Soler C, Carranza-Rodriguez C, Perez-Arellano JL, et al. Surveillance of strongyloidiasis in Spanish in-patients (1998–2014). PLoS One. 2017;12(12):e0189449 10.1371/journal.pone.0189449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvignaud A, Pistone T, Malvy D. Strongyloidiasis in a young French woman raises concern about possible ongoing autochthonous transmission in Spain. Int J Infect Dis. 2016; 42:43–4. 10.1016/j.ijid.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 12.Valerio L, Roure S, Fernández-Rivas G, Basile L, Martinez-Cuevas O, Ballesteros AL, et al. Strongyloides stercoralis, the hidden worm. Epidemiological and clinical characteristics of 70 cases diagnosed in the North Metropolitan Area of Barcelona, Spain, 2003–2012. Trans R Soc Trop Med Hyg. 2013;107(8):465–70. 10.1093/trstmh/trt053 [DOI] [PubMed] [Google Scholar]
- 13.Fernández Rodríguez C, Enríquez-Matas A, Sanchéz Millán ML, Mielgo Ballesteros R, Jukic Beteta KD, Valdez Tejeda M et al. Strongyloides stercoralis Infection: A Series of Cases Diagnosed in an Allergy Department in Spain. J Investig Allergol Clin Immunol. 2012; Vol. 22(6): 437–459. [PubMed] [Google Scholar]
- 14.Mayayo E, Gomez-Aracil V, Azua-Blanco J, Azua-Romeo J, Capilla J, Mayayo R. Strongyloides stercolaris infection mimicking a malignant tumour in a non-immunocompromised patient. Diagnosis by bronchoalveolar cytology. J Clin Pathol. 2005;58(4):420–2. 10.1136/jcp.2004.017756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oltra Alcaraz C, Igual Adell R, Sánchez Sánchez P, Viñals Blasco MJ, Andreu Sanchez O, Sarrión Aunon A, et al. Characteristics and geographical profile of strongyloidiasis in healthcare area 11 of the Valencian community (Spain). J Infect. 2004;49(2):152–8. 10.1016/j.jinf.2004.01.016 [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Vázquez C, González Mediero G, Núñez M, Pérez S, García-Fernández JM, Gimena B. Strongyloides stercoralis en el sur de Galicia. An. Med. Interna (Madrid) 2003. September;20(9):477–9. [PubMed] [Google Scholar]
- 17.Román-Sánchez P, Pastor-Guzman A, Moreno-Guillen S, Igual-Adell R, Suner-Generoso S, Tornero-Estébanez C et al. High prevalence of Strongyloides stercoralis among farm workers on the Mediterranean coast of Spain: analysis of the predictive factors of infection in developed countries. Am J Trop Med Hyg. 2003;69 (3):336–40. [PubMed] [Google Scholar]
- 18.Román Sánchez P, Pastor Guzman A, Moreno Guillen S, Igual Adell R, Martín Estruch A, Navarro Gonzalo I, et al. Endemic strongyloidiasis on the Spanish Mediterranean coast. QJM 2001;94: 357–63. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez Calabuig D, Igual Adell R, Oltra Alcaraz C, Sánchez Sánchez P, Bustamante Balen M, Parra Godoy F et al. Actividad laboral agrícola y estrongiloidiasis. Estudio caso-control. Rev Clin Esp. 2001; 201, 81–84. [DOI] [PubMed] [Google Scholar]
- 20.Cremades Romero MJ, Martínez García MA, Menéndez Villanueva R, Cremades Romero ML, Pemán García J. Infección por Strongyloides stercoralis en un paciente con obstrucción crónica al flujo aéreo corticodependiente. Arch Bronconeumol. 1996;32(8):430–1. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez Calabuig D, Oltra Alcaraz C, Igual Adell R, Parra Godoy F, Martínez Sánchez J, Ángel Rodenas C, et al. Treinta casos de estrongiloidiasis en un centro de atención primaria: características y posibles complicaciones. Aten Primaria. 1998; 31:271–274 [PubMed] [Google Scholar]
- 22.Llagunes J, Mateo E, Peña JJ, Carmona P, de Andrés J. Hiperinfección por Strongyloides stercoralis. Med Intensiva. 2010;34(5):353–6. [DOI] [PubMed] [Google Scholar]
- 23.Esteve-Martinez A, Sanchez-Carazo JL, Alegre-de-Miquel V. [Respiratory distress and purpura in an immunocompromised patient: hyperinfestation with dissemination of Strongyloides stercoralis]. Med Clin (Barc). 2013;141(11):512. [DOI] [PubMed] [Google Scholar]
- 24.Olmos JM, Gracia S, Villoria F, Sales R, J Gonzalez-Macıa J. Disseminated strongyloidiasis in a patient with acquired immunodeficiency syndrome. Eur J Intern Med. 2004;15(8):529–30. 10.1016/j.ejim.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Lopez Gaona V, Miñana Climent JC, Delgado Parada E, Gutierrez Vara S, Vazquez Valdes F, Solano Jaurrieta JJ. Strongyloides stercoralis infestation in an immunocompetent 82-year-old woman. Rev Esp Geriatr Gerontol. 2009;44(3):155–8. 10.1016/j.regg.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 26.Escudero Sanchez R, Comeche Fernandez B. Strongyloides, a dangerous unknown. Med Clin (Barc). 2017;149(7):310–1. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco-Tenza MI, Ruiz-Macia JA, Navarro-Cots M, Gregori-Colome J, Cepeda-Rodrigo JM, Llenas-Garcia J. Strongyloides stercoralis infection in a Spanish regional hospital: Not just an imported disease. Enferm Infecc Microbiol Clin. 2018;36(1):24–8. 10.1016/j.eimc.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 28.Esteban Ronda V, Franco Serrano J, Briones Urtiaga ML. Pulmonary Strongyloides stercoralis infection. Arch Bronconeumol. 2016;52(8):442–3. 10.1016/j.arbres.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Perez A, Díez S, Roure Belhassen-Garcia M, Torrús-Tendero D, Perez-Arellano JL, Cabezas T, et al. Management of severe strongyloidiasis attended at reference centers in Spain. PLoS Med. 2018; February 23;12(2):e0006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igual-Adell R, Márquez V. D. Estrongiloidiasis: epidemiología, manifestaciones clínicas y diagnóstico. Experiencia en una zona endémica: la comarca de La Safor (Valencia). Enferm Infecc Microbiol Clin. 2007; (25): 38–44. [Google Scholar]
- 31.Pretel Serrano L, Page del Pozo MA, Ramos Guevara MR, Ramos Rincón JM, Martínez Toldos MC, Herrero Huerta F. Infestación por Strongyloides stercolaris en pacientes con enfermedad pulmonar obstructiva crónica en la Vega del Segura (Murcia). Presentación de tres casos. Rev Clin Esp. 2001; 201:109–110 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz Romero M, León Martínez MD, Muñoz Pérez MA, Altuna Cuesta A, Cano Sánchez A, Hernández Martínez J. Strongyloides stercoralys: una peculiar forma de exacerbación en la epoc. Arch Bronconeumol. 2008;44(8):451–3 [DOI] [PubMed] [Google Scholar]
- 33.Batista N, Davila MF, Gijón H, Pérez MA. Estrongiloidiasis en un paciente con el sìndrome de inmunodeficiencia adquirida. Enf Infecc Microbiol Clin. 1992;10(Agosto-Septiembre):431–2. [PubMed] [Google Scholar]
- 34.Llenas García J, Pacheco Tenza I, Lucas Dato A, Gonzalez Cuello, Garcia Lopez M, Ruiz Macia J.A et al. 0601 Estrongiloidiasis autóctona e importada en un hospital comarcal del levante español. Abstracts SEIMC 2018. Available from: http://www.elsevier.es/ficheros/congresos/congreso2018_eimc.pdf. Cited 5 August 2018
- 35.Tornero C, Orta N, Llopis M, Martinez M, Pariente M, Perea M et al. 0986. ¿Es la comarca de la Safor todavía un área endémica de estrongiloides? Abstracts SEIMC 2018. Available from: http://www.elsevier.es/ficheros/congresos/congreso2018_eimc.pdf. Cited 5 August 2018.
- 36.Corbacho Loarte MD, Abelenda Alonso G, de la Fuente Crespo E, Muñoz Valera MT, de Juan lvarez C, Merino Fernandez F.J. et al 807. Características epidemiológicas de los pacientes diagnosticados de estrongiloidiasis en el hospital universitario Severo Ochoa. Abstracts SEIMC 2017. Available from: https://www.seimc.org/contenidos/congresosyeventos/seimcanteriores/seimc-EIMC-2017.pdf Cited 5 August 2018
- 37.Garcia Garcia A, Navalpotro Rodriguez D, Tormo Palop N, Ramos Mart JL, Chanza Aviñon M, Ocete Mochon M.D. et al 203. ¿Es necesario descartar la infección por Strongyloides stercoralis en todo paciente que siga o inicie tratamiento inmunosupresor? Abstracts SEIMC 2011. Available from: https://www.seimc.org/contenidos/congresosyeventos/seimcanteriores/seimc-EIMC-2011.pdf. Cited 5 August 2018
- 38.Igual R, Domínguez V, Alonso C, Guna R. 611. Las parasitosis intestinales como causa de eosinofília. Abstracts SEIMC 2006. Available from: https://www.seimc.org/contenidos/congresosyeventos/seimcanteriores/seimc-EIMC-2006.pdf Cited 5 August 2018
- 39.Lozano Polo J, Rubio AV, Venero CS, Ogando VG. Strongyloides stercolaris y asma bronquial. Rev Clin Esp. 2005;205(10):519–20. [DOI] [PubMed] [Google Scholar]
- 40.Cremades Romero IA, Ricart Olmos, Estelles Piera, Pastor Guzman, Menendez Villanueva. Infestación en la comarca de la Safor. Med Clin (Barc). 1997;109:212–5. [PubMed] [Google Scholar]
- 41.Sanchis-Bayarri V, Gonzalez Cruz, Sanchis-Bayarri Lahoz. Infestación grave por Estrongiloides, síndrome paraneoplásico. Rev Diagn Biol. 1981;30:125–7. [Google Scholar]
- 42.Tirado MD, Gil M, Galiano J, Pardo F, Moreno R, del Busto AG et al. Insuficiencia respiratoria y renal en paciente con enfermedad pulmonar obstructiva crónica en tratamiento con corticoides. Enferm Infecc Microbiol Clin. 2002;20(8):401–2. [PubMed] [Google Scholar]
- 43.Nevado O, Salesa. Diarrea y eosinofilia en paciente con asma bronquial. Enferm Infecc Microbiol Clin. 1996;14(mayo):326–7. [PubMed] [Google Scholar]
- 44.Sampedro A, Riera JR, Junco P, Nieto R. Estrongiloidiasis en paciente inmunodeprimido. Rev Esp Enf Ap Digest. 1988;73(2):217–8. [PubMed] [Google Scholar]
- 45.Lopez Gallardo A, Diez Garcia F, Yelamos F, Orozco F. Strongyloides stercoralis y colitis ulcerosa. Enferm Infecc Microbiol Clin 1997;15:273. [PubMed] [Google Scholar]
- 46.Toldos M, Martín-Luengo, Serra, Artero, Segovia. Eosinofilia en un paciente bronquitico crónico. Enferm Infecc Microbiol Clin 1995;13:375–6 [PubMed] [Google Scholar]
- 47.Martínez-Pérez A, López-Velez R. Is Strongyloidiasis Endemic in Spain? PLoS Neg Trop Dis 2015May;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7(1):e2002 10.1371/journal.pntd.0002002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.