Abstract
Dorsal root ganglion stimulation is a fairly recent treatment that has emerged in chronic pain management, often used in cases involving patients with complex regional pain syndrome. We describe a 24-year-old woman with severe left hip pain secondary to avascular necrosis in whom multiple treatments failed, including oral analgesics and radiofrequency ablation. A dorsal root ganglion stimulator was implanted with significant pain relief.
Keywords: Avascular necrosis, dorsal column stimulation, dorsal root ganglion
Avascular necrosis is the destruction of bone tissue due to reduction in blood supply, leading to long-term effects. It is associated with trauma to the bone and extensive use of high-dose steroids. As the condition worsens, the affected joints will cause the individual to experience increasing pain. To our knowledge, this is the first reported case of the use of a dorsal root ganglion (DRG) stimulator on avascular necrosis of the hip.
Case report
A 24-year-old woman was seen in our clinic for severe left hip pain secondary to avascular necrosis developed after receiving high-dose steroids for treatment of transverse myelitis. The pain had been persistent for 2 years, did not radiate elsewhere, and was exacerbated by walking or standing for long periods. She described the pain as achy, sharp, and throbbing, rating it 9/10 in severity.
She had tried various forms of conservative therapy including duloxetine, pregabalin, and tramadol. Despite these therapies, she reported only minimal improvement in her pain. She continued use of amitriptyline, tramadol, and meloxicam. We introduced milnacipran to her regimen. Venlafaxine was added by her primary care physician. She did note improvement in her mood after these adjustments were made, but there was no improvement in her left hip pain.
We decided to perform a diagnostic block of the articular branches of the left obturator and femoral nerves using only local anesthetic. We made sure to avoid steroid injections, given the patient’s avascular necrosis. The patient subsequently reported 90% reduction in pain lasting about 2 weeks. We then performed radiofrequency ablation of the articular branches of the left obturator and femoral nerves; the patient subsequently reported a 60% reduction in pain for about 3.5 weeks. Having tried various therapies including oral medications, physical therapy, and radiofrequency ablation with minimal reduction in the patient’s hip pain, the decision was made to proceed with a DRG stimulator trial.
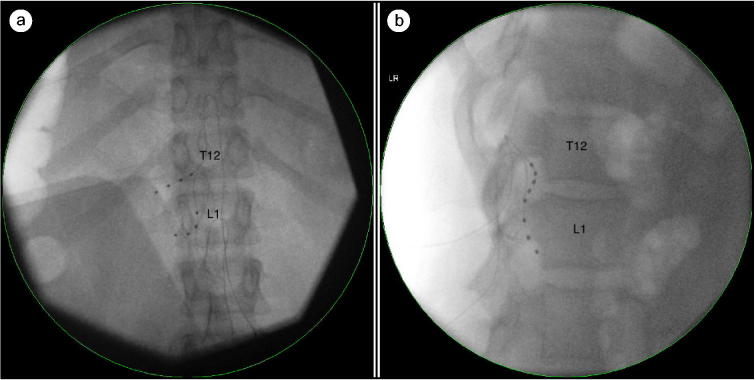
During the trial, leads were placed at the left T12 and L1 neuroforamen (Figure 1). Upon follow-up 6 days later, the patient reported a 95% reduction in pain and was deemed an appropriate candidate for permanent implantation. We elected to proceed with permanent implantation with placement of an implantable pulse generator the following day. The patient was seen 3 months after her DRG stimulator placement and still reported significant reduction in her pain. She noted progress toward functional goals, including an increased sense of well-being and the ability to walk without requiring a wheelchair or walker.
Figure 1.
The DRG stimulator leads placed at left T12 and L1 neuroforamen using fluoroscopy: (a) anteroposterior view and (b) lateral view.
Discussion
DRG stimulation is a treatment that may provide specific targeted relief; this may involve a particular dermatomal pattern for those with chronic pain.1,2 The DRG comprises primarily sensory cell bodies located in the neural foramen within the lateral portion of the epidural space.3,4 These cell bodies transduce sensory information and send signals to the spinal cord and the rest of the central nervous system.4 Injury to the DRG neurons causes them to become more excitable, leading to ectopic and pathological firing, which is a signature characteristic of neuropathic pain.4 Krames reported that direct stimulation of the DRG leads to a decrease in hyperexcitability in DRG neurons, therefore reducing pain.4
Dorsal column stimulation (DCS) has been considered a safe and effective treatment for chronic neuropathic pain and has been the mainstay treatment for patients with failed back surgeries and those who experience complex regional pain syndrome (CRPS). A prospective crossover cohort study comparing DCS with DRG stimulation in patients with CRPS confined to the knees found that a significant 83.3% of patients preferred DRG stimulation. It was postulated that this preference was due to the highly specific targeting capabilities of DRG stimulators when compared with DCS.2 In addition, DRG stimulators are less prone to lead migration within the epidural space with changes in the patient’s positioning. This gives DRG stimulators an advantage over DCS.5
Two case reports have also shown the effectiveness of DRG stimulators in treating localized neuropathic pain. The first case report involved a patient with right knee pain who had a three-lead DRG placed at L2–4. She experienced a significant drop in her pain score as well as improved mobility after DRG stimulator placement.1 The second case involved a patient with pelvic girdle pain with a 43% reduction in pain 6 months after DRG implantation.6
In addition, a retrospective review showed that 29 patients with groin pain had an average 70% reduction in pain, with about 82.6% of patients reporting more than 50% reduction in pain after DRG stimulator placement. DRG stimulation was found to provide long-lasting pain relief as well as positional stability and stimulation specificity, which is a difficult combination to achieve in other traditional nerve stimulation modalities.3,7
To our knowledge, this is the first report of successful use of DRG stimulation for the treatment of avascular necrosis–induced hip pain. In an age where there is an increase in opioid use, this modality can serve to reduce or even eliminate the need for chronic opioids in certain patient populations.
References
- 1.Van Bussel CM, Stronks DL, Hugyen FJ. Successful treatment of intractable complex regional pain syndrome type I of the knee with dorsal root ganglion stimulation: a case report. Neuromodulation. 2015;18(1):58–60. [DOI] [PubMed] [Google Scholar]
- 2.Van Bussel CM, Stronks DL, Hugyen FJ. Dorsal column stimulation vs. dorsal root ganglion stimulation for complex regional pain syndrome confined to the knee: patients’ preference following the trial period. Pain Pract. 2018;18(1):87–93. [DOI] [PubMed] [Google Scholar]
- 3.Schu S, Gulve A, ElDabe S, Baranidharan G, et al. . Spinal cord stimulation of the dorsal root ganglion for groin pain—a retrospective review. Pain Pract. 2015;15(4):293–299. doi: 10.1111/papr.12194. [DOI] [PubMed] [Google Scholar]
- 4.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18(1):24–32. doi: 10.1111/ner.12247. [DOI] [PubMed] [Google Scholar]
- 5.Van Buyten JP, Smet I, Liem L, et al. . Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain Pract. 2015;15(3):208–216. doi: 10.1111/papr.12170. [DOI] [PubMed] [Google Scholar]
- 6.Rowland DC, Wright D, Moir L, et al. . Successful treatment of pelvic girdle pain with dorsal root ganglion stimulation. Br J Neurosurg. 2016;30(6):685–686. doi: 10.1080/02688697.2016.1208810. [DOI] [PubMed] [Google Scholar]
- 7.Liem L, Russo M, Huygen FJ, et al. . A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 2013;16(5):471–482. doi: 10.1111/ner.12072. [DOI] [PubMed] [Google Scholar]