Abstract
Transcatheter aortic valve implantation (TAVI) is growing in utilization in the USA, and atrioventricular heart block is a common complication of the procedure. In patients with conduction system changes following TAVI, there are no clear guidelines for permanent pacing, leading to difficult clinical decisions on how long to leave temporary transvenous pacemakers in place. The aim of our study was to determine whether changes in electrocardiogram characteristics could predict the need for permanent pacing. A retrospective analysis was conducted of 209 consecutive TAVI patients seen from January 2012 to December 2015 at Baylor Heart and Vascular Hospital, Dallas. The baseline characteristics were similar between those who received a permanent pacemaker (PPM) within 7 days of the procedure (21.1%) and those who did not (78.9%); of those who did receive a PPM, 79.5% were implanted for complete heart block. The median (range) percentage change in the sum of QRS and PR was significantly higher in those who received a PPM (20.2%) than those who did not (7.1%) (P = 0.004). Using the percentage change in the sum of QRS and PR to predict PPM, the area under the curve was found to be 0.69. The optimal cutpoint was found to be 18.9% (sensitivity = 0.63, specificity = 0.73). Our study suggests that delay in the conduction system immediately following TAVI predicts the need for permanent pacing.
Keywords: Cutpoint, permanent pacemaker, sensitivity, transcatheter aortic valve implantation, transcatheter aortic valve replacement
Transcatheter aortic valve implantation (TAVI) is growing in utilization in the USA. The number of procedures increase from 22,248 over a three year period, 2011-2014, to an estimated 35,000 in 2016 (https://citoday.com/2017/06/pointcounterpoint-does-the-united-states-have-enough-tavr-centers/). Unfortunately, atrioventricular heart block is a common complication of the procedure, ranging from 20% to 30% of cases using the Medtronic CoreValve Revalving System (MCRS) and from 5% to 12% of cases receiving the Edwards SAPIEN Valve.1 In an analysis of the PARTNER trial, the need for a permanent pacemaker was associated with increased hospitalization and mortality at 1 year,2 although other studies have found no significant change in clinical outcomes.3 Studies have also found that left bundle branch block (LBBB) and a high burden of right ventricular pacing increased heart failure symptoms following TAVI.4 Various risk factors have been identified for heart block following TAVI, including age, male sex, left ventricular ejection fraction, left ventricular outflow tract diameter, mitral annular calcium, access route, implantation depth, balloon predilatation, valve size, atrial fibrillation, and preexisting conduction abnormalities.5,6 However, the significance of each of these factors varies widely from study to study and can be confounded by the various types of valves currently being implanted.
There are no clear guidelines on indications for permanent pacing following TAVI procedures. This makes periprocedural management of new conduction system abnormalities a difficult clinical question. At our institution, patients have a temporary transvenous pacemaker placed at the time of the procedure. In patients with changes on the electrocardiogram (ECG) following the procedure, it is often difficult to decide when to remove the temporary pacemaker and how long to observe the patient in the hospital. We undertook a detailed analysis of the ECGs of patients prior to and following TAVI to predict the need for permanent pacing. Our goal was to identify objective ECG findings, immediately following TAVI, that would predict the need for permanent pacing.
Methods
A total of 210 consecutive pacemaker-naïve patients who underwent TAVI between January 2012 and December 2015 at Baylor Heart and Vascular Hospital, Dallas, Texas, were evaluated for this study. All patients who received a permanent pacemaker (PPM) within 7 days of the valve procedure were included in the study. (Several patients who required permanent pacing qualified for implantable cardioverter defibrillators [ICD] and were included in the PPM group.) One patient with preexisting PPM was excluded from the study. Demographic details and medical history were abstracted from the electronic health records, and ECG characteristics were collected from the MUSE system. All valve types were included in this analysis, including the MCRS, Edwards SAPIEN, Medtronic Corevalve Evolut, and Boston Scientific Lotus valve. Of the 209 patients included in the analysis, 10 patients who had a PPM implanted between 1.7 months and 1.3 years after TAVI were considered as non-PPM (within 7 days of the procedure) for the analysis.
Data were collected on numerous variables previously described as influencing the need for permanent pacing, in addition to the ECG characteristics. The mean oversizing percentage was calculated as follows: (Area of the heart valve/Aortic annular area −1) × 100. The sum of the QRS and PR was the simple mathematical sum of the two numbers. Change in the ECG values prior to and following the procedure was calculated, and percentage change was also calculated for standardization purposes.
Postoperative ECGs were generally obtained within 1 to 2 hours of the procedure as part of our institutional protocol. If patients only had paced morphologies on their ECG, then telemetry was reviewed. If conducted beats were present on telemetry, then PR and QRS duration were recorded. If no conducted beats were recorded, then the patient was included in the preanalysis but excluded from the postanalysis (including evaluation of ECG differences). If patients were in atrial fibrillation, then no PR was recorded. All automated ECG intervals were confirmed by a cardiology fellow manually measuring the intervals using calipers.
Analyses were conducted using STATA 14.2. Continuous variables are presented as means/medians and categorical variables are presented as proportions. Chi-square/Fisher’s exact tests were employed to compare proportions and Wilcoxon rank sum/Student t tests for continuous variables as appropriate. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic performance of the pre- and post-TAVI ECG measurements for predicting the placement of a PPM in this cohort of patients. We further identified the optimal cutoff point for the percentage change in the sum of QRS + PR to predict the need for PPM; the optimal cutpoint was calculated based on the distance to (0, 1), where the point closest to the upper-left corner where 1 − Specificity = 0 and Sensitivity = 1 was identified.
This study was approved by the Baylor Health Care System institutional review board and funded by the Baylor Health Care System Foundation.
Results
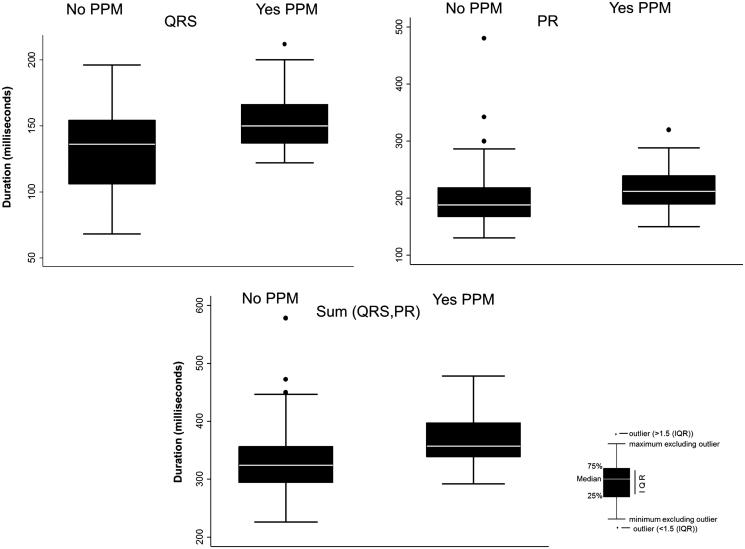
A total of 209 patients who underwent a TAVI procedure between 2012 and 2015 were included in this analysis. The median age of the cohort was 81 years (range, 33–94), and 118 (56.5%) were male. Forty-four patients (21.1%) had a PPM implanted within 7 days of the TAVI procedure. Most PPMs were placed for complete heart block or high-degree atrioventricular block (79.5%), followed by Mobitz 2 (6.8%), trifascicular heart block (6.8%), LBBB (2.3%), sick sinus syndrome (2.3%), and symptomatic bradycardia (2.3%). The comorbidities were similar between patients with and without a PPM (Table 1). The preprocedure ECG finding of a right bundle branch block (RBBB; P = 0.001) was greater in patients who required a PPM. Additionally, the sum of QRS + PR was noted to trend toward a higher value in patients who required a PPM, although this did not meet statistical significance (Table 2). Following the procedure, ECG values, including QRS duration (P = 0.0002), PR interval (P = 0.004), the sum of the PR + QRS (P < 0.001), LBBB (P < 0.001), and RBBB (P = 0.009) were found to be more common/higher in patients requiring a PPM.
Table 1.
Demographic characteristics and comorbidities of the 209 study patients
| Variable |
Pacemaker/ICD implant post-TAVI |
P value | |
|---|---|---|---|
| No (n = 165) | Yes (n = 44) | ||
| Median age (years), (range) | 80 (54–94) | 82 (33–92) | 0.53 |
| Gender | 0.96 | ||
| Female | 72 (44%) | 19 (43%) | |
| Male | 93 (56%) | 25 (57%) | |
| Diabetes mellitus | 62 (38%) | 20 (46%) | 0.34 |
| Hypertension | 151 (92%) | 39 (89%) | 0.56 |
| Coronary artery disease | 159 (96%) | 43 (98%) | NS |
| Atrial fibrillation | 49 (30%) | 18 (41%) | 0.16 |
| Mitral annular calcium | 145 (90%) | 40 (93%) | 0.77 |
ICD indicates implantable cardioverter defibrillator; TAVI, transcatheter aortic valve implantation; NS, not significant.
Table 2.
Clinical characteristics of the 209 study patients before and after TAVI
| Variable |
Pacemaker/ICD implant post-TAVI |
P value | |
|---|---|---|---|
| No (n = 165) | Yes (n = 44) | ||
| Pre-TAVI | |||
| Mean aortic annulus/LV outflow tract (mm) ± SD | 24.7 ± 2.8 | 25.1 ± 2.3 | 0.32 |
| Median preoperative ejection fraction (%) (range) | 60 (15–78) | 60 (20–70) | 0.99 |
| Median interventricular septum width (cm) (range) | 1.2 (0.6–3.0) | 1.2 (0.7–2.1) | 0.97 |
| Median LV end diastolic diameter (cm) (range) | 4.6 (0.9–6.8) | 4.5 (3.4–6.8) | 0.92 |
| Median LV end systolic diameter (cm) (range) | 3 (1.2–6.1) | 3 (1.4–4.8) | 0.88 |
| Median QRS duration (ms) (range) | 99 (70–188) | 108 (70–174) | 0.08 |
| Median PR interval (ms) (range) | 182 (118–440) | 181 (142–334) | 0.44 |
| Median sum of QRS and PR (ms) (range) | 284 (208–540) | 303 (218–438) | 0.06 |
| Left bundle branch block | 15 (9%) | 6 (14%) | 0.39 |
| Incomplete left bundle branch block | 0 | 3 (7%) | 0.009 |
| Right bundle branch block | 12 (7%) | 11 (25%) | 0.001 |
| Incomplete right bundle branch block | 1 (0.6%) | 0 | NS |
| Left anterior fascicular block | 6 (4%) | 1 (2%) | NS |
| Bifascicular block | 2 (1%) | 1 (2%) | 0.52 |
| Nonspecific intraventricular conduction delay | 0 | 1 (2%) | 0.21 |
| Balloon | 54 (33%) | 16 (36%) | 0.67 |
| Medications | |||
| Beta-blocker | 51 (31%) | 17 (39%) | 0.34 |
| Angiotensin-converting enzyme inhibitor | 30 (18%) | 5 (11%) | 0.28 |
| Angiotension II receptor blocker | 6 (4%) | 3 (7%) | 0.40 |
| HMG-CoA reductase inhibitor | 95 (58%) | 20 (46%) | 0.14 |
| Antiarrhythmic agents | 3 (2%) | 1 (2%) | 1.0 |
| Post-TAVI | |||
| Valve size (mm), median (range) | 29 (21–31) | 29 (23–31) | 0.25 |
| Interventricular septum width (cm), median (range) | 1.2 (0.6–4.1) | 1.3 (0.7–1.8) | 0.25 |
| LV end diastolic diameter (cm), median (range) | 4.7 (1.2–6.7) | 4.5 (2.4–6.1) | 0.25 |
| LV end systolic diameter (cm), median (range) | 3.2 (1.0–5.9) | 3.3 (1.5–5.0) | 0.93 |
| Median QRS duration (ms) (range) | 136 (68–196) | 150 (122–212) | <0.001 |
| Median PR interval (ms) (range) | 188 (130–480) | 212 (150–320) | 0.004 |
| Median sum of QRS and PR (ms) (range) | 324 (226–578) | 357 (292–478) | <0.001 |
| Left bundle branch block | 72 (45.9%) | 28 (82.4%) | <0.001 |
| Incomplete left bundle branch block | 3 (2%) | 0 | NS |
| Right bundle branch block | 8 (5%) | 6 (18%) | 0.009 |
| Incomplete right bundle branch block | 5 (3.2%) | 0 | 0.59 |
| Left anterior fascicular block | 5 (3%) | 2 (6%) | 0.34 |
| Left posterior fascicular block | 1 (0.6%) | 0 | NS |
| Bifascicular block | 1 (0.6%) | 2 (6%) | 0.08 |
| Nonspecific intraventricular conduction delay | 9 (6%) | 1 (3%) | NS |
| Balloon | 34 (21%) | 7 (16%) | 0.48 |
| Medications | |||
| Beta-blocker | 49 (30%) | 19 (43%) | 0.09 |
| Angiotensin-converting enzyme inhibitor | 36 (22%) | 9 (21%) | 0.85 |
| Angiotension II receptor blocker | 7 (4%) | 3 (7%) | 0.44 |
| HMG-CoA reductase inhibitor | 84 (51%) | 17 (39%) | 0.15 |
| Antiarrhythmic agents | 5 (3%) | 2 (5%) | 0.64 |
| Type of valve | 0.07 | ||
| Core valve | 112 (68%) | 36 (82%) | |
| Edwards SAPIEN | 23 (14%) | 1 (2%) | |
| Evolut core valve | 23 (14%) | 4 (9%) | |
| Lotus | 7 (4%) | 3 (7%) | |
| Mean oversizing percentage ± SD | 30.9 ± 23.4 | 30.4 ± 14.6 | 0.85 |
ICD indicates implantable cardioverter defibrillator; TAVI, transcatheter aortic valve implantation; LV, left ventricular; NS, not significant; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase.
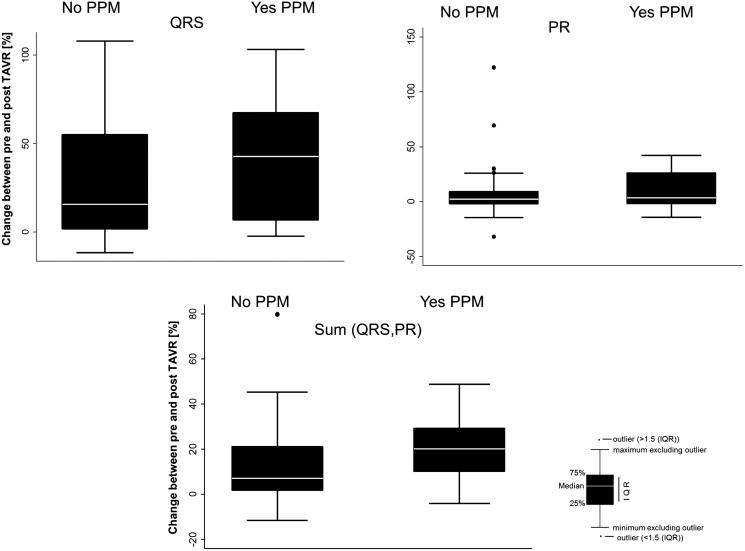
In addition to evaluating the pre- and post-ECG characteristics, we assessed whether changes in the QRS duration, PR interval, or their aggregate had an influence on a future PPM implantation (Table 3). The difference in the QRS duration (P = 0.03), the sum of the PR + QRS (P = 0.001), and the percentage change of each of these values were noted to be statistically significant in these two groups (Figures 1 and 2). Additionally, given that patients with RBBB prior to the procedure are at high risk, we conducted the statistical analysis excluding RBBB patients. There was no significant change in the findings. We also conducted a subgroup analysis including patients with a history of atrial fibrillation (n = 67, 32.1%). No significant differences were noted in the change in the QRS duration (post-pre) as well as in the sum of QRS and PR and percentage change in QRS and sum.
Table 3.
ECG characteristics of the 209 study patients before and after TAVI
| ECG characteristic |
Pacemaker/ICD implant post-TAVI |
P value | |
|---|---|---|---|
| No (n = 165) | Yes (n = 44) | ||
| Change in QRS duration (ms), median (range) | 18 (−12 to 84) | 52 (−3 to 99) | 0.03 |
| Change in PR interval (ms), median (range) | 4 (−66 to 154) | 7 (−30 to 68) | 0.16 |
| Change in sum of QRS and PR (ms), median (range) | 24 (−30 to 166) | 60 (−16 to 118) | 0.001 |
| Median change in QRS, % (range) | 15.6% (−11.8 to 108.1) | 42.8% (−2.3 to 103.1) | 0.04 |
| Median change in PR, % (range) | 2.4% (−32.0 to 122.2) | 3.7% (−14.0 to 41.9) | 0.17 |
| Median change in sum of QRS and PR, % (range) | 7.1% (−11.5 to 79.8) | 20.2% (−4.0 to 48.8) | 0.004 |
ECG indicates electrocardiographic; ICD, implantable cardioverter defibrillator; TAVI, transcatheter aortic valve implantation.
Figure 1.
Electrocardiographic intervals after transcatheter aortic valve implantation in patients with and without a permanent pacemaker (PPM).
Figure 2.
Change in electrocardiographic characteristics before and after transcatheter aortic valve implantation (TAVI) in patients with and without a permanent pacemaker (PPM).
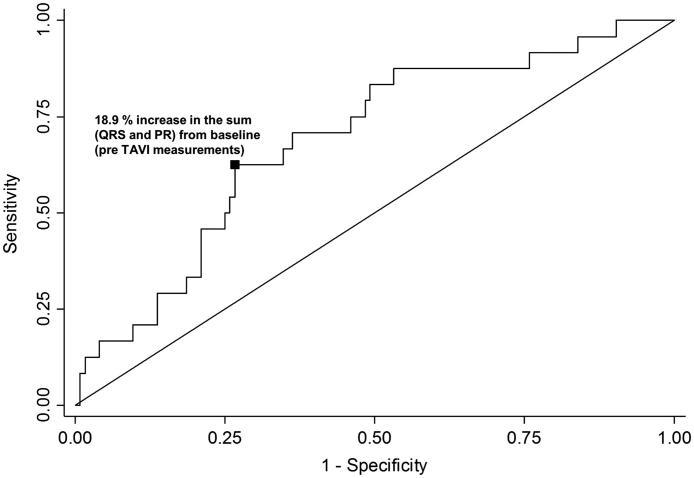
For the ROC analysis, we included 148 patients with a measurable PR interval and a measurable (nonpaced) QRS interval. By ROC analysis, the predictive value of the percentage change in the sum of the QRS + PR duration yielded an area under the curve of 0.69 (95% confidence interval [CI], 0.58–0.80) (Figure 3). It was similar when we excluded those patients with RBBB pre-TAVI (0.69; 95% CI, 0.56–0.81). Including only those patients with complete heart block, the area under the curve was 0.72 (95% CI, 0.59–0.84). With the detailed summary table, we found that values of −1.24 and 8.8 corresponded to sensitivities of 0.9 and 0.8, respectively. However, the specificity at these values was 0.16 and 0.5, respectively. By the nearest point calculation method, the optimal cutpoint was identified to be an 18.9% increase with the following decision statistics: sensitivity, 0.63; specificity, 0.73; positive likelihood ratio, 2.3; negative likelihood ratio, 0.5; and diagnostic odds ratio, 4.6.
Figure 3.
Optimal cutpoint for a permanent pacemaker with percentage change in sum of QRS and PR intervals. The area under the receiver operating characteristic curve is 0.69; sensitivity, 0.63; specificity, 0.73; positive likelihood ratio, 2.3; negative likelihood ratio, 0.5; diagnostic odds ratio, 4.6.
Discussion
This study resulted in several important findings. First, in agreement with other studies, preexisting RBBB seems to be the best ECG predictor of the need for a permanent pacemaker following TAVI. Second, a longer PR and QRS interval following TAVI is predictive of the need for a permanent pacemaker. Third, the pre- and post-TAVI difference in the QRS interval and the sum of the PR + QRS, expressed in either absolute numerical form or percentage change, are predictive of the need for a permanent pacemaker. Several publications have noted the importance of new LBBB following TAVI on the early requirement of PPM,7–9 with some studies showing no impact on clinic outcomes and others showing an increase in mortality.10 Given the known data about LBBB following implantation, it is not surprising we found that statistically significant changes in these values predicted the need for permanent pacing.
The generally accepted mechanism of atrioventricular block following TAVI is pressure on the native conduction system from the structure of the valve. This theory explains the higher rate of heart block seen with MCRS implantation compared with the Edwards SAPIEN valve, because the structure of the valve is larger and it is generally implanted deeper in the ventricle, closer to the conduction system. Given that the valves are generally placed at the level of the His bundle, we included the PR and QRS durations in our analysis. The PR interval typically represents a delay in the atrioventricular node; however, based on surface ECG, it is impossible to determine whether the PR delay occurs supra, intra, or infra Hisian.
Interestingly, none of our baseline characteristics, with the exception of RBBB, were found to be predictive of a PPM requirement. This is perhaps due to the inclusion of multiple types of valves in the study, although non-MCRS valves only represented a small number of the total cases. To our knowledge, this is the first publication that has evaluated numerical ECG intervals pre- and post-TAVI. We intend to conduct a prospective cohort study to validate this cutoff in TAVI patients to elucidate its clinical significance.
This study, as with any retrospective analysis, has inherent limitations, including being subject to confounding factors. Most patients in our study received the MCRS, which may limit the application of these findings to other valve types. Additionally, this was a single-center study, which may limit its generalizability to other patient populations. PPM were ultimately implanted, at the discretion of the physician caring for each patient, for complete heart block as well as high-degree atrioventricular block, which may introduce some bias, although an area under the curve calculation including only patients implanted for complete heart block was not significantly different. Further, we did not systematically collect information on the improvement in atrioventricular conduction before PPM implant. Some detailed measurements that may have significance were not included, including p wave duration, paced QRS duration, and left/right ventricular volumes, which may be areas to examine in further studies. Finally, our hospital is a tertiary referral center, so we did not have PPM follow-up data, because most patients follow up with their primary cardiologist across a tristate area. We attempted to collect these data, but too often they were not available.
In conclusion, our findings are consistent with the current understanding of the requirement for permanent pacing following TAVI. The statistically significant identification of QRS, PR + QRS, and the change in these values, both absolute and based on percentage, suggests that a delay in the conduction system immediately following TAVI predicted the physician’s decision to implant a PPM. Unfortunately, there was too much overlap in pre- and post-TAVI values of the two populations to allow for a specific Boolean metric (Figures 1 and 2). However, ROC analysis did determine important cutoffs in the percentage change of the PR + QRS of 8.8% (sensitivity 0.8, specificity 0.5) and 18.9% (sensitivity 0.63, specificity 0.73). Prospective studies are needed to validate these measurements, but this study does suggest that changes in PR, QRS, and PR + QRS, immediately post-TAVI, may be helpful in predicting the need for permanent pacing with the sensitivities and specificities detailed above. These data add to the understanding of the effects TAVI has on the native conduction system and need for permanent pacing.
Funding Statement
This study was supported by the Baylor Health Care System Foundation.
Conflicts of interest
R.C.S. is on the advisory board of Medtronic and Boston Scientific and is a global TAVR proctor; M.A. is on the speaker’s bureau for Medtronic, St. Jude Medical (Abbott), and Boston Scientific; other authors have no potential conflicts of interest to report.
References
- 1.Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64:129–140. doi: 10.1016/j.jacc.2014.04.033. PMID: 25011716. [DOI] [PubMed] [Google Scholar]
- 2.Nazif TM, Dizon JM, Hahn RT, et al. PARTNER Publications Office. Predictors and clinical outcomes of perm;anent pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:60–69. doi: 10.1016/j.jcin.2014.07.022. PMID: 25616819. [DOI] [PubMed] [Google Scholar]
- 3.Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233–1243. doi: 10.1161/CIRCULATIONAHA.113.005479. PMID: 24370552. [DOI] [PubMed] [Google Scholar]
- 4.Weber M, Brüggemann E, Schueler R, et al. Impact of left ventricular conduction defect with or without need for permanent right ventricular pacing on functional and clinical recovery after TAVR. Clin Res Cardiol. 2015;104:964–974. doi: 10.1007/s00392-015-0865-9. PMID: 25967154. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Selles M, Bramlage P, Thoenes M, Schymik G. Clinical significance of conduction disturbances after aortic valve intervention: current evidence. Clin Res Cardiol. 2015;104:1–12. doi: 10.1007/s00392-014-0739-6. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Tuzcu EM, Krishnaswamy A, et al. Transcatheter aortic valve replacement: current perspectives and future implications. Heart. 2015;101:169–177. doi: 10.1136/heartjnl-2014-306254. PMID: 25410500. [DOI] [PubMed] [Google Scholar]
- 7.Testa L, Latib A, De Marco F, et al. Clinical impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve revalving system. Circulation. 2013;127:1300–1307. doi: 10.1161/CIRCULATIONAHA.112.001099. PMID: 23443735. [DOI] [PubMed] [Google Scholar]
- 8.Nazif TM, Williams MR, Hahn RT, et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. 2014;35:1599–1607. doi: 10.1093/eurheartj/eht376. PMID: 24179072. [DOI] [PubMed] [Google Scholar]
- 9.Franzoni I, Latib A, Maisano F, et al. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the Corevalve versus the Edwards valve. Am J Cardiol. 2013;112:554–559. doi: 10.1016/j.amjcard.2013.04.026. PMID: 23726173. [DOI] [PubMed] [Google Scholar]
- 10.Houthuizen P, van der Boon RM, Urena M, et al. Occurrence, fate and consequences of ventricular conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention. 2014;9: 1142–1150. doi: 10.4244/EIJV9I10A194. PMID: 24273252. [DOI] [PubMed] [Google Scholar]