ABSTRACT
There is limited information on the effects of enteric pathogen on bone quality in rapidly growing broiler chicks. We examined tibia and femur attributes (length, diameter, relative weight of ash content [AC] to the BW, ash concentration [AP]) and serum bone-turnover markers including receptor activator of nuclear factor kappa-B ligand (RANKL) for resorption, alkaline phosphatase (ALP) for mineralization, and selected serum metabolites in 14-day-old broilers challenged with Eimeria. A total of 160 (80 males and 80 females) 1-day-old Ross × Ross 708 chicks were used. Based on BW, birds were placed within sex in cages (5 birds per cage) and fed chick starter diets to day 9 of age. On day 9, half of the cages were orally gavaged with 1 mL of Eimeria culture (100,000 oocysts of E. acervulina and 25,000 oocysts of E. maxima) and the other half (unchallenged control) received 1 mL 0.9% saline in distilled water. On day 14, 2 birds were randomly selected and necropsied for intestinal lesion score, blood, tibia, and femur samples. Data were analyzed in a 2 (challenged vs. unchallenged) × 2 (males vs. females) factorial arrangement. There was no interaction (P > 0.05) between Eimeria and sex on any measurement. Whereas there were no intestinal lesions in unchallenged birds, Eimeria resulted in lesion score (0 to 4) of 3.35, 2.59 and 0.11 in duodenum, jejunum and ileum, respectively. Eimeria challenge decreased (P < 0.05) tibia AC and AP by 10 and 8.2%, respectively but had no (P > 0.10) effect on femur attributes. Generally, males showed (P < 0.05) longer and wider bones with more AC compared with the female. Circulating serum RANKL concentration increased (P = 0.017) in response to Eimeria challenge and was negatively correlated with tibia AC (–0.731; P = 0.021). Our findings showed that Eimeria damage to the intestinal physiology had adverse effects on long bone attributes linked to increased resorption.
Keywords: Eimeria, Ca homeostasis, bone mineralization, osteoimmunology, broilers
INTRODUCTION
Coccidiosis is a prevalent poultry disease that results in significant economic losses to the global poultry industry (Chapman, 2014). Eimeria, the causative protozoan parasite, invades intestinal cells adversely affecting barrier function, digestive physiology and subsequently growth performance (Chapman, 2014; Kim et al., 2017). Enteric disturbances at this stage of a chick's life may cause an early reduction in growth and increased susceptibility to secondary infections (Chapman, 2014).
The peculiarity of the modern broiler strains is that the long bones grow 4-fold in length in comparison to 60-fold growth in body mass during the first 6 wk post-hatch and the mid-shaft diameter of long bone expands 3- to 5-fold during the same timeline (Applegate and Lilburn, 2002; Havenstein et al., 2003). This disproportionate rate of body mass accretion vs. the progress of skeletal maturation is seen as the genesis of the leg and gait disorders often observed in meat-type birds (Fleming, 2008). Severe defects greatly impair walking ability of birds leading to mortality from starvation and dehydration, but even mild deformities have been shown to cause discomfort or pain (Fleming, 2008). Different Eimeria species invade specific regions of the gastrointestinal tract in poultry (Chapman, 2014). For instance, E. acervulina invades duodenum and the upper half of the small intestine and other species such as E. maxima and E. necatrix invades jejunum extending towards the posterior part of the small intestines (Chapman, 2014). Because duodenum and upper jejunum are the major sites of mineral absorption, E. acervulina and E. maxima will have adverse effects on mineral digestion and absorption (Van Der Klis et al., 1990). Earlier research showed reduction in blood mineral and tibia mineralization (in terms of ash and Ca concentration) in birds challenged with E. acervulina (Turk, 1986; Ward et al., 1990). The consequence of this peculiarity is reduced skeletal development in young chick; however, there is dearth of data on the magnitude of Eimeria infection impact on the bone attributes in modern rapidly growing broiler genetics.
Skeletal system can be influenced by physical, nutritional, and physiological factors (Rath et al., 2000), but one of the most important regulators of bone dynamics and turnover is the immune system (D’Amelio and Sassi, 2016). The crosstalk between the immune system and bone had been unclear until the discovery of the receptor activator of nuclear factor kappa-B ligand (RANKL) which is vital for osteoclastogenesis (Lee and Choi, 2015). Deregulation of bone turnover by the immune system has been linked to inflammatory bone loss and postmenopausal osteoporosis (D’Amelio and Sassi, 2016). Many differences between male and female broilers have been reported, such as growth rate, deposition of muscle and bone minerals, nutrient requirements, and diversity of gastrointestinal microbiome (Han and Baker, 1993; Havenstein et al., 2003; Lumkins et al., 2008). One could therefore hypothesize an interaction between Eimeria and sex on long bone attributes. However, there is little information on effects of Eimeria challenge on long bone attributes in female and male broiler chicks. Thus, we aimed to study the effects of an Eimeria challenge on bone attributes, serum bone-turnover markers, and other selected serum metabolites in 14-day-old male and female chicks.
MATERIALS AND METHODS
Birds and Management
The experimental protocol was approved by the University of Guelph Animal Care Committee, and birds were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009). Day old chicks were obtained from 32-wk-old Ross × Ross 708 broiler breeder flock at the Arkell Poultry Research Station, University of Guelph. The broiler breeders were kept on floor pens (60 females and 10 males). A total of 250 fertile eggs were collected, weighed, individually marked, and subsequently stored at 4°C until incubation (within 7 d of collection). Eggs were incubated and hatched in a commercial grade incubator and hatcher (Nature Form, Jacksonville, FL) at the Arkell Poultry Research Station. On the day of hatch, chicks were sexed, 80 males and 80 females were wing tagged with a unique number, weighed individually, and transported to the brooding room. Five birds of the same sex were placed in a cage of 20″ × 30″ (Ford Dickson Inc., Mitchell, Ontario, Canada). Chicks were allowed access to feed and water for ad libitum consumption. Temperature was initially set at 32°C on d 1 and gradually diminished to 29°C by d 14. The lighting program was 23L:1D (20+ LUX) from 1 to 3 d, and subsequently 20L:4D.
Experimental Treatments and Procedures
The diets were formulated to meet nutrients Ross 708 broiler (Aviagen International, 2014) in a 2-phase feeding program. The starter (day 0 to 9) was based on corn, soybean meal, wheat, and pork meal: 3.0 Mcal/kg AME, 23.0% crude protein, 1.28% SID Lys, 0.95% SID Met + Cys, 0.86% SID Thr, 0.96% Ca, and 0.48% available P. Grower (d 9 to 14) was based on corn, soybean meal, wheat, pork meal and corn DDGS: 3.1 Mcal/kg AME, 21.50% crude protein, 1.15% SID Lys, 0.85% SID Met + Cys, 0.77% SID Thr, 0.87% Ca and 0.44% available P. On d 9, 16 cages (8 for each gender) out of 32 cages were orally gavaged with 1 mL of Eimeria culture (100,000 sporulated oocysts of E. acervulina and 25,000 sporulated oocysts of E. maxima) as described in Kim et al. (2017). The other 16 cages were designated unchallenged cages and received 1 mL of 0.9% saline in distilled water. The Eimeria culture and challenge protocols were provided by Dr John Barta of the Department of Pathobiology, University of Guelph. On d 14, 2 birds with average BW close to the cage average were bled via wing vein puncture and necropsied for left tibia and femur samples. To confirm effectiveness of the Eimeria challenge, intestinal lesion scores (duodenum, jejunum, ileum, ceca, and colon) were assessed blindly as described by Johnson and Reid (1970) using a scale of 0 (none) to 4 (high). Blood samples were allowed to clot at 4°C, centrifuged at 2,000× g for 30 min at 5°C, and serum stored at –20°C until required for analysis. The left tibia and femur were partially defleshed and frozen at –20°C until further analysis.
Samples Processing and Analyses
The bones were defleshed followed by measurement of the length and diaphyseal diameter at the thinnest (middle) of the shaft (diaphysis) of tibia and femur using a digital caliper (MasterCraft #058-6800-4, Vonore, TN). Bone samples were subsequently dried at 105ºC for 24 h, weighed and ashed at 600ºC for 12 h (Akbari Moghaddam Kakhki et al., 2018). The weight of ash was recorded. The concentration of RANKL and tumor necrosis factor (TNF)-α in serum was measured in duplicate using Competitive ELISA kits following recommended assay procedures (RANKL: ECKR0012 and TNF-α: ECKT0015; ABclonal, Woburn, MA). Antibodies for ELISA kits were rabbit polyclonal and validated using an immunogen of the specific chicken protein. The serum samples were further analyzed for alkaline phosphatase (ALP), Ca, P, total protein, globulin, glucose, aspartate transaminase (AST), and uric acid by photometric method at the Animal Health Laboratory (University of Guelph, Guelph, ON).
Calculations and Statistical Analyses
Bone weight and ash content (AC) measurements were expressed relative to BW (Akbari Moghaddam Kakhki et al., 2018). Data were tested for normality with UNIVARIATE plot normal procedure of SAS and subsequently subjected to a 2-way ANOVA in a 2 (males vs. females) × 2 (challenge vs. unchallenged) factorial arrangement using the General Linear Model procedure. Correlation between bone-turnover markers (RANKL, TNFα and ALP) and bone attributes was performed using CORR procedure. Significance was declared at P < 0.05.
RESULTS
There was no (P > 0.05) interaction between Eimeria and sex on BW (Figure 1). The average BW of birds was 331.9 and 326.9 g for challenged and unchallenged birds, respectively. Males were 18.2% (P < 0.001) heavier than females. The average lesion scores for challenged birds were 3.35 ± 0.80, 2.59 ± 0.72, and 0.11 ± 0.32 for duodenum, jejunum, and ileum, respectively. Lesions were not noted in unchallenged birds, and there were no (P > 0.05) differences between males and females on lesion scores.
Figure 1.
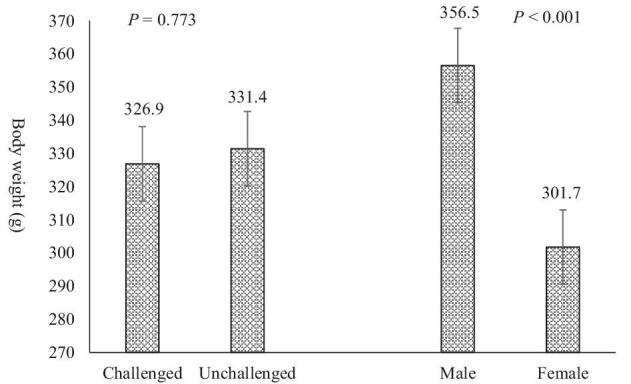
Effects of Eimeria challenge on body weight in 14-day-old male and female broilers. There was no interaction between Eimeria challenge and sex and main effect of Eimeria challenge (P > 0.05). The SEM was 11.14. Data are means of 40 birds per each treatment. Error bars indicate SEM.
Femur and Tibia Attributes
There was no (P > 0.05) interaction between Eimeria and sex or the main effect of Eimeria (P > 0.05) on femur attributes (Table 1). Femur AP tended (P = 0.09) to be reduced by Eimeria challenge. Male femur had (P > 0.05) longer and thicker diaphyseal diameter along with higher AC and AP than females. Similar to femur, there was no (P > 0.05) interactive effect between Eimeria and sex on tibia attributes (Table 1). However, a tendency (P = 0.06) was observed on tibia length in which case males tended to have longer tibia than females in the unchallenged group but not in the challenged group. Tibia AC and AP decreased (P < 0.05) by 10 and 8.2%, respectively, in response to Eimeria challenge. Tibia length, diameter, AP, and AC were higher (P < 0.05) in males compared to female chicks.
Table 1.
Effects of Eimeria challenge on femur and tibia attributes in 14-day-old male and female broilers.1
| Femur | Tibia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eimeria | Sex | Length (mm) | Diameter (mm) | Dry weight (g: g BW) | Ash content (g: g BW) | Ash (%) | Length (mm) | Diameter (mm) | Dry weight weight | Ash content (g: g BW) | Ash (%) |
| Challenged | Male | 43.00 | 4.00 | 0.190 | 0.064 | 33.45 | 57.06 | 3.66 | 0.316 | 0.089 | 32.64 |
| Challenged | Female | 41.24 | 3.89 | 0.181 | 0.056 | 30.64 | 55.39 | 3.42 | 0.250 | 0.072 | 29.17 |
| Unchallenged | Male | 44.32 | 4.17 | 0.190 | 0.066 | 34.72 | 59.33 | 3.85 | 0.261 | 0.091 | 35.11 |
| Unchallenged | Female | 40.89 | 3.81 | 0.184 | 0.058 | 31.68 | 53.75 | 3.54 | 0.268 | 0.084 | 31.63 |
| SEM | 0.701 | 0.109 | 0.006 | 0.002 | 0.654 | 0.935 | 0.104 | 0.030 | 0.004 | 1.648 | |
| Main Effects Eimeria | |||||||||||
| Challenged | 42.12 | 3.95 | 0.186 | 0.060 | 32.05 | 56.23 | 3.54 | 0.283 | 0.081b | 30.91b | |
| Unchallenged | 42.61 | 3.99 | 0.187 | 0.062 | 33.20 | 56.54 | 3.70 | 0.265 | 0.088a | 33.37a | |
| Sex | |||||||||||
| Male | 43.66a | 4.09a | 0.190 | 0.065a | 34.09a | 58.20a | 3.76a | 0.288 | 0.089a | 33.88a | |
| Female | 41.07b | 3.85b | 0.183 | 0.057b | 31.16b | 54.57b | 3.48b | 0.260 | 0.078b | 30.40b | |
| SEM | 0.506 | 0.081 | 0.009 | 0.002 | 0.470 | 0.675 | 0.078 | 0.021 | 0.002 | 1.185 | |
| Probabilities (P-value) | |||||||||||
| Challenge | 0.502 | 0.716 | 0.918 | 0.186 | 0.088 | 0.746 | 0.166 | 0.549 | 0.045 | 0.039 | |
| Sex | <0.001 | 0.049 | 0.259 | <0.001 | <0.001 | <0.001 | 0.018 | 0.335 | 0.004 | 0.044 | |
| Challenge × Sex | 0.244 | 0.300 | 0.752 | 0.837 | 0.862 | 0.057 | 0.714 | 0.234 | 0.181 | 0.998 | |
a,bValues with uncommon superscripts within each column are significantly different (P < 0.05).
1Data are means of 16 bone samples per each treatment.
Bone-turnover Markers, Serum Biochemistry, and Correlation Analyses
Intra-assay CV was 6.06 and 9.01% for RANKL and TNFα, respectively. There was no (P > 0.05) interaction between Eimeria and sex on the concentration of bone-turnover markers and serum biochemistry (Table 2). Eimeria challenge elevated the concentration of RANKL (P = 0.017) by 28.0%, while TNFα showed a tendency (P = 0.07) to increase in response to Eimeria challenge. Sex had no effect (P > 0.05) on concentration of TNFα and RANKL. Neither Eimeria challenge nor sex affected (P > 0.05) serum ALP, Ca, P, total protein, glucose, AST, and uric acid. There was a positive correlation (r = 0.859; P < 0.001) between serum TNFα and RANKL in challenged birds (Table 3). Negative correlation was observed between serum RANKL and ALP (r = –0.659; P = 0.043), tibia AC (r = –0.731; P = 0.021), and percentage (r = –0.717; P = 0.040) in challenged birds.
Table 2.
Effects of Eimeria challenge on serum bone-turnover markers and serum biochemistry in 14-day-old male and female broilers.1
| Bone-turnover markers | Serum biochemistry | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | TNFα2 (pg/mL) | RANKL3 (pg/mL) | ALP4 (U/L) | Ca (mmol/L) | P (mmol/L) | Total protein (g/L) | Globulin (mmo/L) | Glucose (mmol/L) | AST4 (U/L) | Uric acid (μmol/L) |
| Eimeria | ||||||||||
| Challenged | 92.68 | 14.64a | 25841 | 1.785 | 2.173 | 21.100 | 11.00 | 13.40 | 185.45 | 414.25 |
| Unchallenged | 86.21 | 11.44b | 28858 | 1.706 | 2.188 | 20.625 | 10.33 | 12.60 | 182.87 | 349.47 |
| Sex | ||||||||||
| Male | 86.21 | 11.32 | 27454 | 1.708 | 2.080 | 21.225 | 10.87 | 13.22 | 185.57 | 372.67 |
| Female | 92.28 | 13.81 | 24245 | 1.783 | 2.282 | 20.500 | 10.56 | 12.77 | 182.75 | 391.05 |
| SEM | 2.995 | 1.008 | 2790 | 0.101 | 0.108 | 0.510 | 0.292 | 0.438 | 9.714 | 38.946 |
| Probabilities (P value) | ||||||||||
| Challenge | 0.067 | 0.017 | 0.125 | 0.557 | 0.931 | 0.563 | 0.078 | 0.263 | 0.839 | 0.212 |
| Sex | 0.129 | 0.335 | 0.978 | 0.577 | 0.249 | 0.380 | 0.436 | 0.512 | 0.824 | 0.719 |
| Challenge × Sex | 0.894 | 0.211 | 0.467 | 0.931 | 0.712 | 0.523 | 0.463 | 0.212 | 0.933 | 0.983 |
a,bValues with uncommon superscripts within each column are significantly different (P < 0.05).
1Data are means of 10 serum samples per each treatment.
2Tumour necrosis factor α.
3Receptor activator of nuclear factor kappa-B ligand.
4Alkaline phosphatase.
5Aspartate transaminase.
Table 3.
Coefficient of correlation analyses between serum bone-turnover markers and bone attributes.1
| Item | TNFα2 | RANKL3 | ALP4 |
|---|---|---|---|
| Challenged birds | |||
| RANKL | 0.859** | ||
| ALP | –0.328 | –0.659* | |
| Tibia ash content (g: g BW) | –0.338 | –0.731* | 0.020 |
| Tibia ash percentage (%) | –0.110 | –0.717* | –0.158 |
| Femur ash content (g: g BW) | –0.005 | –0.158 | 0.083 |
| Femur ash percentage (%) | –0.011 | –0.123 | –0.081 |
| Unchallenged birds | |||
| RANKL | 0.017 | ||
| ALP | –0.163 | –0.318 | |
| Tibia ash content (g: g BW) | 0.089 | –0.142 | –0.205 |
| Tibia ash percentage (%) | –0.096 | –0.184 | 0.060 |
| Femur ash content (g: g BW) | 0.024 | 0.012 | 0.085 |
| Femur ash percentage (%) | –0.023 | –0.079 | –0.189 |
1 * indicates P < 0.05 and ** indicates P < 0.001.
2Tumour necrosis factor α.
3Receptor activator of nuclear factor kappa-B ligand.
4Alkaline phosphatase.
DISCUSSION
Bone integrity can be affected by enteric infection and stress subsequently leading to bone disorders such as osteomyelitis and osteonecrosis (Rath et al., 2000). Eimeria replicates within the intestinal wall of the chicken causing lesions (Chapman, 2014). Lesion locations vary depending on the species, but for the species used in the current study (E. acervulina and E. maxima) mainly target the duodenum and jejunum, respectively (Chapman, 2014). The observed lesion scores corroborate our previous studies employing similar challenge protocol in cage and floor housing (Kim et al., 2017; Leung et al., 2018). Our previous studies also demonstrated that lesion scores of more than 2 in duodenum and jejunum were accompanied with reduced villi height, downregulation of genes for digestive enzymes, and nutrient transporters and reduced nutrients utilization. In the current study, Eimeria challenge reduced mineral content in the tibia. It has been reported that by invading intestinal cells, Eimeria impairs mineral absorption and retention (Sakkas et al., 2018). In agreement with our results, Sakkas et al. (2018) observed a reduction in AC and AP in femur on day 6 post-challenge in response to inoculation with 7 × 103E. maxima oocysts in slow-growing and conventional broiler strains. In addition, they observed less mineralized tibia and femur in infected birds even though growth rates matched the non-infected counterparts during the recovery period. They concluded that bone development falls behind tissue accretion during Eimeria infection. The lack of response of femur attributes in response to Eimeria challenge in the current study might be attributed to the differences between strains and age of inoculation.
In poultry, there are physiologically distinct regions of a growing bone, each with its own unique developmental characteristics. The tibia has been extensively studied due to cellular sensitivity to numerous dietary deficiencies and because it is the region where the cartilage anomaly tibial dyschondroplasia most often occurs (Leach and Lilburn, 1992). Applegate and Lilburn (2002) demonstrated that the femur and tibia of broilers showed different patterns of development with the length of the femur as a function of BW reaching plateau at 1.5 kg (35 d of age), whereas the length of the tibia did not plateau through 43 d of age. These differences in growth pattern may have influenced observed responses in the tibia and femur in the current study. Sexual dimorphism among bone traits was apparent in the current study with males having greater values. In general, previous reports have shown that males have higher bone morphological traits such as longer tibia with greater volume, cortical, and marrow area (Rose et al., 1996) and compositional traits such as AC (Rath et al., 1999; Applegate and Lilburn, 2002; Charuta et al., 2013). However, the lack of interaction between Eimeria and sex suggested that the impact of Eimeria and sex on long bone attributes are independent.
Bone formation and remodeling are continuous processes associated with the activity of 5 different types of cells including bone resorbing cells; osteoclasts (OCs), osteoblasts (OBs), chondrocytes, osteocytes, and chondroclasts (Roux and Orcel, 2000). Parathyroid hormone acts as a promoter of bone resorption in order to maintain blood Ca concentration and has been linked with increased expression of RANKL in OBs (Proszkowiec-Weglarz and Angel, 2013). Two essential factors in osteoclastogenesis are macrophage colony-stimulating factor (M-CSF) and RANKL. The M-CSF is involved in the proliferation and differentiation of OC. The RANKL is expressed at the mRNA level in most organs such as spleen, skeletal muscle, brain, liver, kidney, and surface expression is detected in OCs (Sutton et al., 2015). The role of RANKL in osteoclastogenesis is associated with differentiation of OC (Lee and Choi, 2015), stimulation of OC activity (Roux and Orcel, 2000), and as a survival factor for mature OC (Hayward et al., 2006). There is a little information regarding activity pathway and role of RANKL in immune system in birds and mammals during parasite infection. It has been reported that RANK-RANKL is crucial in the priming of antiviral immunity and improving antigen transport to regional lymph nodes in mice infected with Herpes virus-type 1 (Klenner et al., 2015). In the current study, increased serum RANKL in response to the Eimeria challenge was correlated with the tibia mineral loss (P < 0.05). Lloyd et al. (2008) observed an acceleration in bone turnover and an increase in endocortical bone erosion surface in response to subcutaneous injections of RANKL in 10-wk-old female mice.
Several methods have been developed for quantitative and qualitative measurements of physiological proteins such as ELISA, western blot (WB), fluorescent antibody test etc. (Kim et al., 2001). Although ELISA quantifies specific protein in a matrix of various proteins and is preferred for screening many samples for the concentration of a specific protein, it may have cross-reactivity based on epitope which its antibody detects. Thus, one limitation of the current study is a lack of validation of ELISA by other confirmatory methods such as WB. Western blot, in comparison with ELISA, has higher specificity and is the most common method of confirming results from ELISA test (Bass et al., 2017). In retrospect, our future studies will consider confirmatory test.
Discovery of a common regulatory mechanism via RANKL in both the immune and bone system led to the evolution of the interdisciplinary field of osteoimmunology that studies the interaction between immune system and bone metabolism (D’Amelio and Sassi, 2016). The crosstalk between immune cells and bone is established by bone through provision of a local environment for differentiating hematopoietic cells and resorbing functionality of OCs (Hayward et al., 2006). Activation of the immune system results in an alteration in the formation and activity of OCs and OBs in response to cytokines such as the interleukin (IL)-1 family or TNF-α, hormones and mechanical load (D’Amelio and Sassi, 2016). Although, many of these studies have focused on rodent models, osteoimmunology is rarely studied in farm animals (D’Amelio and Sassi, 2016). Stimulation of the immune system alters the partitioning of nutrients away from growth in favor of supporting an immune response through increased protein degradation and reduced protein synthesis, enhanced basal metabolic rate, reduced bone mineralization, and increased release of hormones such as insulin, glucagon, and corticosterone (Klasing and Johnstone, 1991; Rath et al., 2000).
We did not observe an influence of Eimeria on serum on total protein, P, globulin, glucose, AST, and uric acid. Hamzic et al. (2015) observed significant changes of mean cellular volume, mean corpuscular hemoglobin, serum coloration, plasma protein, and albumin in broiler blood at 7 d post-inoculation by E. maxima (50,000 oocysts). In addition, they reported increase in α and β fractions of globulins, but not in γ fraction. Although we did not measure these parameters in the current study, total globulins tended to increase in response to the Eimeria challenge. Based on the distinctive incorporation of globulin fractions in immune responses, response of globulin fractions to Eimeria challenge is needed to be examined in future studies.
Mireles et al. (2005) observed a decrease in tibia weight, Ca, and breaking strength in 28-day-old broiler after multiple challenges on 15 and 23 d by 1 mg/kg BW of Escherichia coli lipopolysaccharide. They hypothesized that released TNFα, IL-1, and IL-9 increased bone resorption (Mireles et al., 2005). Produced pro-inflammatory cytokines such as IL-1, IL-6, and TNFα affect the differentiation process of cells (Klasing and Johnstone, 1991). It has been reported that TNFα increases bone resorption by stimulating OCs in rodent (Dewhirst et al., 1987) and mammals (Rath et al., 2000). In addition, expression of RANKL is induced in response to various inflammatory cytokines (i.e., IL-1, IL-6, and TNF-α), 1,25-dihydroxycholecalciferol, prostaglandin E-2, and lipopolysaccharide (Lee and Choi, 2015) which can confirm the positive correlation between TNFα and RANKL observed in the current study. It is worth noting that serum ALP as an indicator of bone formation activity (Christenson, 1997) and Ca were not influenced by Eimeria challenge. This can be attributed to the fact that serum Ca homeostasis is tightly controlled (Proszkowiec-Weglarz and Angel, 2013) and in the event of reduced intestinal mineral absorption, bone resorption is increased to sustain serum Ca.
Skeletal system deformities such as lameness are prevalent in the poultry industry. In modern broiler, significant skeletal growth, both in terms of bone length and width and mineral deposition, occurs in the first 2 wk of life (Angel, 2007). Eimeria challenge adversely affected intestinal digestive and absorptive capacity as indicated by lesion scores resulting in increased bone resorption. In addition, cross-regulation between the immune system and bone metabolism might be involved in disturbance of bone homeostasis in response to Eimeria challenge. Since the mechanisms that underlie bone turnover in response to different physiological condition is not well understood, more research should focus on the role of enteric disturbances and dietary interventions thereof to maintain early skeletal development more so as the industry is under pressure to reduce the use of antimicrobials and anticoccidials.
ACKNOWLEDGEMENTS
Authors appreciate technical assistance of Tanka Khanal, Emily Kim, and Conor Voth. Our appreciation is extended to Dr John Barta and his lab for the Eimeria culture and lesion scoring.
Notes
The research was funded by Ontario Ministry of Agriculture, Food and Rural Affairs and Natural Sciences and Engineering Research Council of Canada-Discovery program. R. Akbari Moghaddam Kakhki is a recipient of the Ontario Trillium Doctoral and Mary Edmunds Williams Scholarships.
REFERENCES
- Akbari Moghaddam Kakhki R., T. Heuthorst, Wornath-Vanhumbeck A., Neijat M., Kiarie E.. 2018. Medullary bone attributes in aged Lohmann LSL-lite layers fed different levels of calcium and top-dressed 25-hydroxy vitamin D3. Can. J. Anim. Sci. In press. 10.1139/CJAS-2018-0062. [DOI] [PubMed] [Google Scholar]
- Angel R. 2007. Metabolic disorders: limitations to growth of and mineral deposition into the broiler skeleton after hatch and potential implications for leg problems. J. Appl. Poult. Res. 16,138–149. [Google Scholar]
- Applegate T. J., Lilburn M. S.. 2002. Growth of the femur and tibia of a commercial broiler line. Poult. Sci. 81:1289–1294. [DOI] [PubMed] [Google Scholar]
- Aviagen International 2014. Nutrition Specifications Manual: Ross 708. Aviagen Ltd, Scotland, UK. [Google Scholar]
- Bass J. J., Wilkinson D. J., Rankin D., Phillips B. E., Szewczyk N. J., Smith K., Atherton P. J.. 2017. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 27:4–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCAC 2009. Guidelines on the Care and Use of Farm Animals in Research, Teaching and Testing. Pages 1–168. Canadian Council on Animal Care, Ottawa, ON, Canada: Accessed Oct. 2018. https://www.ccac.ca/Documents/Standards/Guidelines/Farm_Animals.pdf. [Google Scholar]
- Chapman H. 2014. Milestones in avian coccidiosis research: a review. Poult. Sci. 93:501–511. [DOI] [PubMed] [Google Scholar]
- Charuta A., Dzierzęcka M., Komosa M., Kalinowski Ł., Pierzchała M.. 2013. Age-and sex-related differences of morphometric, densitometric and geometric parameters of tibiotarsal bone in Ross broiler chickens. Folia. Biol. 61:211–220. [DOI] [PubMed] [Google Scholar]
- Christenson R. H. 1997. Biochemical markers of bone metabolism: an overview. Clin. Biochem. 30:573–593. [DOI] [PubMed] [Google Scholar]
- D’Amelio P., Sassi F.. 2016. Osteoimmunology: from mice to humans. Bone Key Rep. 5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E., Ago J. M., Peros W. J., Stashenko P.. 1987. Synergism between parathyroid hormone and interleukin 1 in stimulating bone resorption in organ culture. J. Bone Miner. Res. 2:127–134. [DOI] [PubMed] [Google Scholar]
- Fleming R. H. 2008. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 67:177–183. [DOI] [PubMed] [Google Scholar]
- Hamzic E., Bed’Hom B., Juin H., Hawken R., Abrahamsen M. S., Elsen J.-M., Servin B., Pinard-van Der Laan M.-H., Demeure O.. 2015. Large-scale investigation of the parameters in response to Eimeria maxima challenge in broilers. J. Anim. Sci. 93:1830–1840. [DOI] [PubMed] [Google Scholar]
- Han Y., Baker D. H.. 1993. Effects of sex, heat stress, body weight, and genetic strain on the dietary lysine requirement of broiler chicks. Poult. Sci. 72:701–708. [DOI] [PubMed] [Google Scholar]
- Hayward L. S., Richardson J. B., Grogan M. N., Wingfield J. C.. 2006. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. Gen. Comp. Endocrinol. 146:144–148. [DOI] [PubMed] [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A.. 2003. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1509–1518. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M.. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 28:30–36. [DOI] [PubMed] [Google Scholar]
- Klenner L., Hafezi W., Clausen B. E., Lorentzen E. U., Luger T. A., Beissert S., Kühn J. E., Loser K.. 2015. Cutaneous RANK–RANKL signaling upregulates CD8-mediated antiviral immunity during herpes simplex virus infection by preventing virus-induced langerhans cell apoptosis. J. Investig. Dermatol. 135:2676–2687. [DOI] [PubMed] [Google Scholar]
- Kim S.-J., Lee E.-Y., Oh M.-J., Choi T.-J.. 2001. Comparison of IHNV detection limits by IMS-RT-PCR, western blot and ELISA. Fish Aquatic Sci. 4:32–38. [Google Scholar]
- Kim E., Leung H., Akhtar N., Li J., Barta J., Wang Y., Yang C., Kiarie E.. 2017. Growth performance and gastrointestinal responses of broiler chickens fed corn-soybean meal diet without or with exogenous epidermal growth factor upon challenge with Eimeria1. Poult. Sci. 96:3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K. C., Johnstone B. J.. 1991. Monokines in growth and development. Poult. Sci. 70:1781–1789. [DOI] [PubMed] [Google Scholar]
- Leach R. M. Jr., Lilburn M. S.. 1992. Current knowledge on the etiology of tibial dyschondroplasia in the avian species. Poult. Sci. Rev. 4:57–65. [Google Scholar]
- Lee S. H., Choi Y.. 2015. Communication between the skeletal and immune systems. Osteoporosis Sarcopenia. 1:81–91. [Google Scholar]
- Lloyd S., Yuan Y., Kostenuik P., Ominsky M., Lau A., Morony S., Stolina M., Asuncion F., Bateman T. A.. 2008. Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif. Tissue Int. 82:361–372. [DOI] [PubMed] [Google Scholar]
- Leung H. A, R. Snyder Yitbarek, Patterson R, Barta J. R., Karrow N., Kiarie E.. 2018. Responses of broiler chickens to Eimeria challenge when fed a nucleotide-rich yeast extract. Poult. Sci. 10.3382/ps/pey533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkins B. S., Batal, A. B., Lee M.. 2008. The effect of gender on the bacterial community in the gastrointestinal tract of broilers. Poult. Sci. 87:964–967. [DOI] [PubMed] [Google Scholar]
- Mireles A., Kim S., Klasing K.. 2005. An acute inflammatory response alters bone homeostasis, body composition, and the humoral immune response of broiler chickens. Poult. Sci. 84:553–560. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Angel R.. 2013. Calcium and phosphorus metabolism in broilers: effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 22:609–627. [Google Scholar]
- Rath N., Balog J., Huff W., Huff G., Kulkarni G., Tierce J.. 1999. Comparative differences in the composition and biomechanical properties of tibiae of seven- and seventy-two-week-old male and female broiler breeder chickens. Poult. Sci. 78:1232–1239. [DOI] [PubMed] [Google Scholar]
- Rath N., Huff G., Huff W., Balog J.. 2000. Factors regulating bone maturity and strength in poultry. Poult. Sci. 79:1024–1032. [DOI] [PubMed] [Google Scholar]
- Rose N., Constantin P., Leterrier C.. 1996. Sex differences in bone growth of broiler chickens. Growth. Develop. Aging 60:49–59. [PubMed] [Google Scholar]
- Roux S., Orcel P.. 2000. Bone loss: Factors that regulate osteoclast differentiation-an update. Arthritis. Res. Ther. 2:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas P., Oikeh I., Blake D. P., Nolan M. J., Bailey R. A., Oxley A., Rychlik I., Lietz G., Kyriazakis I.. 2018. Does selection for growth rate in broilers affect their resistance and tolerance to Eimeria maxima? Vet. Parasitol. 258:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton K. M., Hu T., Wu Z., Siklodi B., Vervelde L., Kaiser P.. 2015. The functions of the avian receptor activator of NF-?B ligand (RANKL) and its receptors, RANK and osteoprotegerin, are evolutionarily conserved. Dev. Comp. Immunol. 51:170–184. [DOI] [PubMed] [Google Scholar]
- Turk D. 1986. Macroelements in the circulation of coccidiosis-infected chicks. Poult. Sci. 65:462–468. [DOI] [PubMed] [Google Scholar]
- Van Der Klis J. D., Verstegen M. W. A., De Wit W.. 1990. Absorption of minerals and retention time of dry matter in the gastrointestinal tract of broilers. Poult. Sci. 69:2185–2194. [DOI] [PubMed] [Google Scholar]
- Ward T., Watkins K., Southern L.. 1990. Interactive effects of sodium zeolite A (Ethacal®) and monensin in uninfected and Eimeria acervulina-infected chicks. Poult. Sci. 69:276–280. [DOI] [PubMed] [Google Scholar]


