ABSTRACT
The increase in antibiotic resistance is a global concern for human and animal health. Resistant microorganisms can spread between food-producing animals and humans. The objective of this review was to identify the type and amount of antibiotics used in poultry production and the level of antibiotic resistance in Escherichia coli isolated from broilers. Isolate information was obtained from national monitoring programs and research studies conducted in large poultry-producing regions: US, China, Brazil, and countries of EU—Poland, United Kingdom, Germany, France, and Spain.
The survey results clearly display the absence of a harmonized approach in the monitoring of antibiotics per animal species and the evaluation of resistances using the same methodology. There is no public long-term quantitative data available targeting the amount of antibiotics used in poultry, with the exception of France. Data on antibiotic-resistant E. coli are available for most regions but detection of resistance and number of isolates in each study differs among regions; therefore, statistical evaluation was not possible. Data from France indicate that the decreased use of tetracyclines leads to a reduction in the detected resistance rates. The fluoroquinolones, third-generation cephalosporins, macrolides, and polymyxins (“highest priority critically important” antibiotics for human medicine according to WHO) are approved for use in large poultry-producing regions, with the exception of fluoroquinolones in the US and cephalosporins in the EU. The approval of cephalosporins in China could not be evaluated. Tetracyclines, aminoglycosides, sulfonamides, and penicillins are registered for use in poultry in all evaluated countries. The average resistance rates in E. coli to representatives of these antibiotic classes are higher than 40% in all countries, with the exception of ampicillin in the US. The resistance rates to fluoroquinolones and quinolones in the US, where fluoroquinolones are not registered for use, are below 5%, while the average of resistant E. coli is above 40% in Brazil, China, and EU, where use of fluoroquinolones is legalized. However, banning of fluoroquinolones and quinolones has not totally eliminated the occurrence of resistant populations.
Keywords: antimicrobial, avian, E. coli, resistance, poultry
INTRODUCTION
The application of antimicrobials results in the emergence and spread of antimicrobial resistance, which is a cause of worldwide concern (Garcia-Migura et al., 2014). An antimicrobial agent is defined as a “naturally occurring, semi-synthetic or synthetic substance that exhibits antimicrobial activity (kills or inhibits the growth of microorganisms) at concentrations attainable in vivo. Anthelmintics and substances classed as disinfectants or antiseptics are excluded from this definition” (World Organisation for Animal Health, 2016). Although antimicrobial agents are active against bacteria, protozoa, viruses, and fungi, it is the antibacterial class that is of greatest interest for public health (Page and Gautier, 2012). Thus, the present review will exclusively focus on the antibacterial class of antimicrobial agents. The term antibiotic will be applied throughout this paper, as this term is widely used. This paper provides information on the antibiotic usage (AU) and antibiotic resistance (AR) in broilers. The term poultry is used in the paper if the sources cited do not clearly distinguish between the poultry and broilers. However, 87% of poultry production is broiler production (FAO, 2010).
Accelerated evolutionary trends toward AR are a major threat to human and animal health (Harbarth et al., 2015; World Health Organization, 2015; European Food Safety Authority and European Centre for Disease Prevention and Control, 2016). In addition to being essential for the treatment and prophylaxis of human infections, antibiotics are also widely applied in food-producing animals, which can serve as a reservoir of antibiotic-resistant bacteria and AR determinants that may be transferred to humans (Marshall and Levy, 2011). Subsequently, the effectiveness of antibiotics in humans decreases, resulting in treatment failures (Aarestrup et al., 2008; Mellata, 2013; European Food Safety Authority and European Centre for Disease Prevention and Control, 2016).
In recent decades, broilers have increased in relevance as a meat source. Data about AU and AR in Escherichia coli in broilers are shown for the largest broiler meat producers worldwide: the United States, Brazil, China, and countries of European Union—Poland, United Kingdom, Germany, France, and Spain. These regions count for approximately 60% of the total worldwide broiler production, as shown in Figure 1 (Association of Poultry Processors and Poultry Trade in the EU Countries, 2015; United States Department of Agriculture, 2016).
Figure 1.
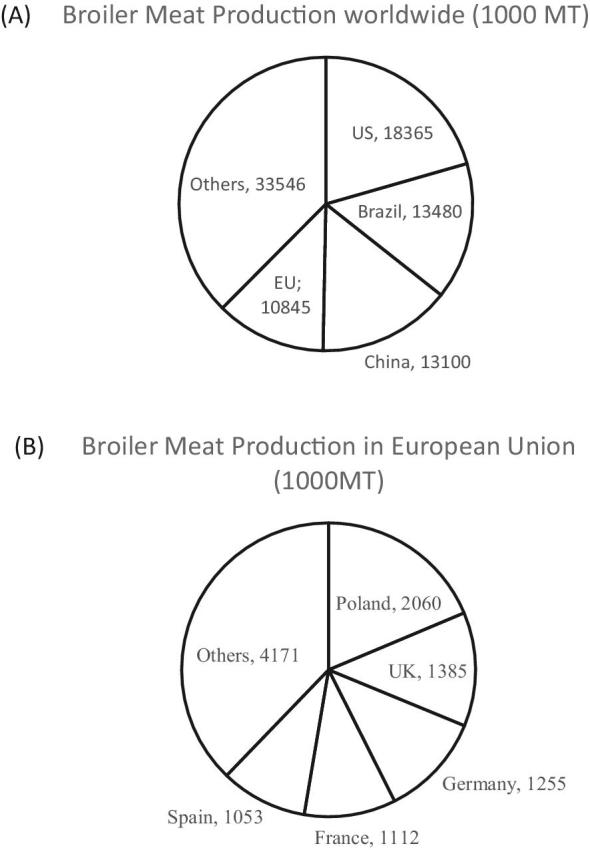
Broiler meat production in 1,000 metric tons (MT) by country worldwide (A) and in the European Union (Association of Poultry Processors and Poultry Trade in the EU Countries 2015; United States Department of Agriculture, 2016).
Broiler meat produced by these countries is exported globally. For example, broiler meat from Brazil reaches 142 countries (Ministry of Agriculture Livestock and Farming in Brazil, 2016a). The amount of exported broiler meat per country in 1,000 metric ton is as follows: Brazil, 4,090; US, 3,057; EU, 1,180; China 375 (United States Department of Agriculture, 2016). This review lists the governmental authorization and monitoring of antibiotics in use in poultry and the available data of AR in E. coli of broiler origin. Escherichia coli is regarded as indicator organism of AR for a wide range of bacteria (EFSA, 2008; Kaesbohrer et al., 2012). Data from the monitoring programs and available scientific literature about AR in E. coli from the US, Brazil, China, and the large poultry producers in the EU from 2000 to 2017 were considered. All sources were obtained through online database searches including the Web of Science, PubMed, Scopus, and Google using translations from Chinese, Portuguese, Polish, German, French, and Spanish.
The objective of this study was to identify the legalized antibiotics, the amounts thereof administered, and the level of AR monitored in E. coli isolated from broilers originating from the large poultry-producing regions such as US, China, Brazil, Poland, United Kingdom, Germany, France, and Spain. We hypothesize that the application of antibiotics leads to high and consistent resistance levels in E. coli isolates, whereas ban of some classes of antibiotics results in low resistance levels. This review is the first comprehensive evaluation of data recording the authorized antibiotics for poultry production combined with AR data in E. coli isolates in large poultry-producing regions.
USE OF ANTIBIOTICS
Antibiotics in poultry are generally administered to the entire flock and are used for the treatment of disease (therapy), disease prevention (methaphylaxis), and growth promotion (Poole and Sheffield, 2013). Antibiotic growth promoters were banned in the EU in 2006, in the US in 2017 and are currently allowed in Brazil and China (European Commission, 2005, AccessScience Editors, 2017). Antibiotic usage for disease prevention is permitted in all large poultry-producing countries. Antibiotics are applied for the treatment of intestinal infections such as colibacillosis, necrotic enteritis, and other diseases generally caused by Salmonella, E. coli, or Clostridium spp. These infections are a major concern among poultry leading to enormous economic losses (United States Department of Agriculture, 2015). The type and extent of AU differ from country to country based on the country's economy, and its level of development, animal husbandry, and the animal species (Archawakulathep et al., 2014). The method of administration and the volume of antibiotic used vary depending on the stage of production and the risk of disease (Rosengren et al., 2010). In general, there are different methods of monitoring AU: following the sales of antibiotics is one method, although long-term data for individual animal species are unavailable (with the exception of France); the detection of prescribed antibiotics per animal species; and the detection of AU in animals on farm level.
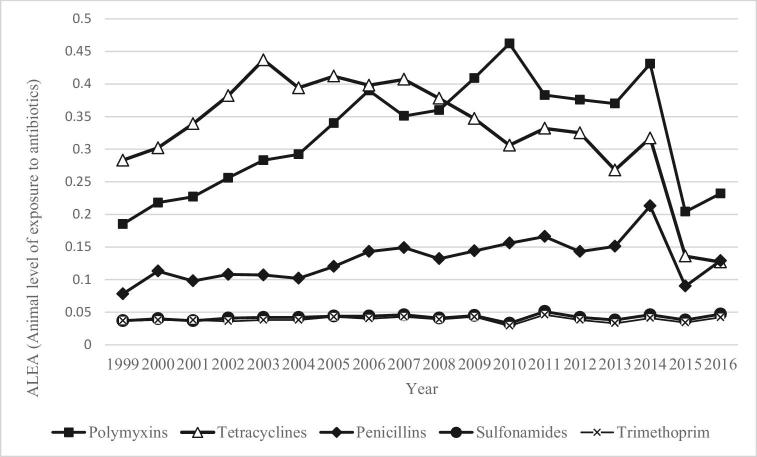
The French Agency for Veterinary Medicinal Products has monitored the sale of active antibiotic substances applied in poultry production since 1999. In 2016, 106 metric ton of active antibiotic substances were sold for use in poultry. This number represents 20% of all veterinary antibiotics and an average annual consumption of 47 mg of active ingredients per kilogram of chicken produced (French Agency for Food Environmental and Occupational Health & Safety and French Agency for Veterinary Medicinal Products, 2017). Figure 2 shows the changes in the sales of mainly applied antibiotic classes, expressed using the Animal Level of Exposure to Antimicrobials indicator for poultry between 1999 and 2016. In 2016, poultry herds were basically treated with polymyxins, penicillins, and tetracyclines, then with sulfonamides and trimethoprim (French Agency for Food Environmental and Occupational Health & Safety and French Agency for Veterinary Medicinal Products, 2017).
Figure 2.
Sales of antibiotics for use in poultry by class between 1999 and 2013 in ALEA (France), modified from French Agency for Food Environmental and Occupational Health & Safety and French Agency for Veterinary Medicinal Products (2017). ALEA = [Live weight treated]/[Total number of animals] × [Weight of adult animals or at slaughter].
No quantitative monitoring data of AU in broilers are currently available in most large poultry-producing countries. Only in the US, data on AU of medically important antibiotics per animal species are available for 2016, which do not provide the complete picture on AU in broilers as it does not include non-medically important antibiotics (US Food and Drug Administration, 2017). However, the list of antibiotics that are approved by regulatory agencies may provide an indication of the use of antibiotics in poultry production in every country considered in this report. National listings of all medical products that are approved for use for poultry in the US, Brazil, China, Poland, United Kingdom, Germany, France, and Spain were analyzed for active antibiotic substances that may be used in feed, water, or administered parenterally.
The WHO categorizes fluoroquinolones, third- and fourth-generation cephalosporins, macrolides, glycopeptides, and polymyxins as “highest priority critically important” antibiotics for human medicine due to the limited availability of alternatives for the treatment of bacterial infections. These antibiotics are the preferred option for the treatment of serious human infections (World Health Organization, 2011). The data in Table 1 show that fluoroquinolones, third-generation cephalosporins, macrolides, and polymyxins are approved for use in poultry in the largest poultry-producing countries, with the exception of fluoroquinolones in the US and cephalosporins in the EU. The FDA banned the use of enrofloxacin in poultry in the US in 2005 (US Food and Drug Administration, 2005).
Table 1.
Antibiotic substances approved for use in poultry by national regulative authorities in the US, Brazil, China, Poland, United Kingdom, Germany, France, and Spain based on national reports.1
| Antimicrobial class | Compound | US | BR | CN2 | PL | GB | DE | FR | ES |
|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | Apramycin | x | x | x | |||||
| Gentamicin | x | x | |||||||
| Hygromycin | x | ||||||||
| Kanamycin | x | ||||||||
| Neomycin | x | x | x | x | x | x | x | ||
| Spectinomycin | x | x | x | x | x | x | |||
| Streptomycin | x | x | |||||||
| Arsenical | Arsanilic acid | o | x | ||||||
| Nitarsone | o | ||||||||
| Roxarsone | o | x | |||||||
| ß-lactams—penicillins | Amoxicillin | x | x | x | x | x | x | x | |
| Ampicillin | x | x | x | x | |||||
| Benzlypenicillin | x | x | x | x | |||||
| Phenoxymethyl-penicillin | x | x | x | ||||||
| ß-lactams—1 g cephalosporins | Cefalexin | x | |||||||
| ß-lactams—3 g cephalosporins | Ceftiofur | x | x | ||||||
| Diaminopyrimidines | Ormethoprim | o | |||||||
| Trimethoprim | x | x | x | x | x | x | x | ||
| Fenicols | Florfenicol | x | x | ||||||
| Thiamphenicol | x | x | |||||||
| Fluoroquinolones | Ciprofloxacin | x | |||||||
| Difloxacin | x | x | |||||||
| Enrofloxacin | x | x | x | x | x | x | |||
| Flumequine | x | x | x | ||||||
| Norfloxacin | x | ||||||||
| Glycophospholipid | Bambermycin | x | x | ||||||
| Ionophores | Hainanmycin | x | |||||||
| Lasalocid | x | x | |||||||
| Maduramicin | x | x | x | ||||||
| Monensin | x | x | x | ||||||
| Narasin | x | x | |||||||
| Salinomycin | x | x | x | ||||||
| Semduramicin | x | x | |||||||
| Lincosamides | Lincomycin | x | x | x | x | x | x | x | |
| Macrolides | Erythromycin | x | x | x | x | ||||
| Tylosin | x | x | x | x | x | x | x | x | |
| Tilmicosin | x | x | x | x | x | x | |||
| Spiramycin | x | x | |||||||
| Tylvalosin | x | x | |||||||
| Kitasamycin | x | x | |||||||
| Orthosomycins | Avilamycin | x | x | ||||||
| Phosphonic acids | Fosfomycin | x | |||||||
| Pleuromutilins | Tiamulin | x | x | x | x | x | |||
| Polypeptides | Enramycin | x | x | ||||||
| Bacitracin | x | x | x | ||||||
| Polymyxmins | Colistin | x | x | x | x | x | x | x | x |
| Quinolone | Halquinol | x | |||||||
| Oxolinic acid | x | ||||||||
| Streptogramins | Virginiamycin | x | x | ||||||
| Sulfonamides | Phalysysulfathiazole | x | |||||||
| Sulfachlorpyrazine | x | x | |||||||
| Sulfachlorpyridazine | x | x | |||||||
| Sulfadiazine | x | x | x | ||||||
| Sulfaguanidine | x | ||||||||
| Sulfadimethoxine | x | x | x | x | x | x | |||
| Sulfadimidine | x | x | x | ||||||
| Sulfamerazine | x | ||||||||
| Sulfamethazine | x | x | |||||||
| Sulfamethoxazole | x | x | x | x | x | ||||
| Sulfamethoxypyridazine | x | x | |||||||
| Sulfanilamide | x | ||||||||
| Sulfaquinoxaline | x | x | x | x | x | ||||
| Sulfisoxazole | x | ||||||||
| Sulfomyxin | x | ||||||||
| Tetracyclines | Chlortetracycline | x | x | x | x | x | x | x | |
| Doxycycline | x | x | x | x | x | x | |||
| Oxytetracycline | x | x | x | x | x | x | x | ||
| Tetracycline | x | x | x | x | x | x | |||
| Thiostrepton 50S | Nosiheptide | x |
1Following national reports were used: US—US Food and Drug Administration 2016; Brazil—Ministry of Agriculture Livestock and Farming in Brazil 2008, 2014, 2016b; China—Ministry of Agriculture of People's Republic of China, 2001, 2013; Poland—The office for registration of medicinal products medical devices and biocidal products in Poland 2016; the United Kingdom—Veterinary Medicine Directorate UK 2016; Germany—German Federal Ministry of Health 2016; France—French Agency for Food Environmental and Occupational Health & Safety 2016; Spain—Spanish Agency of Medicines and Sanitary Products 2016.
2CN—the list of licensed antibiotics in China does not include parenterally administered antibiotics.
US—USA, BR—Brazil, CN—China, PL—Poland, GB—United Kingdom, DE—Germany, FR—France, ES—Spain, o—antibiotic was voluntary withdrawn by producers.
ANTIBIOTIC RESISTANCE IN E. COLI FROM BROILERS
The use of antibiotics in poultry production increases the selection pressure for antibiotic-resistant bacteria (Diarra and Malouin, 2014). Escherichia coli are commensal bacteria that are ubiquitous in animals and humans. Because of their widespread availability, monitoring of commensal bacteria allows the comparison of the selective pressure effects in all relevant populations and is considered useful as an early alert system, for tracking emerging resistance in livestock and possible spread to animal-derived food (EFSA, 2008). Due to this prevalence, they are widely accepted as indicator bacteria for AR in Gram-negative bacteria populations and serve as a model for studying the emergence of AR (Kaesbohrer et al., 2012). Additionally, E. coli as well as other bacteria of the commensal flora can form a reservoir of AR genes that may be transferred between bacterial species, including organisms capable of causing disease in both humans and animals. The effects of antibiotics used and the trends in the prevalence of AR in food-producing animals can be more accurately investigated in this indicator bacterium, than in food-borne pathogens (European Food Safety Authority, 2008). Reflecting the awareness of the AR problem and the need for research on the triggers that cause AR development and spreading, some countries established strategies for surveillance and monitoring programs that concern AR and its determinants. Usually, national monitoring studies publish data yearly and use the same criteria for the determination of antibiotic resistances (European Food Safety Authority and European Centre for Disease Prevention and Control, 2018; US Food and Drug Administration, 2018). However, there is no harmonized evaluation of AR in different monitoring programs, which makes the comparison between regions impossible.
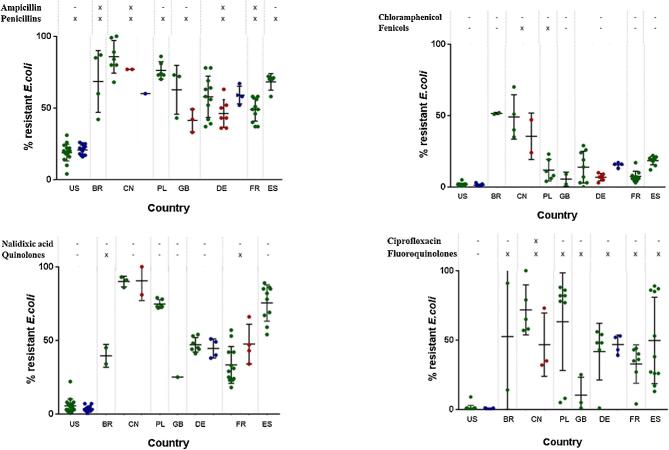
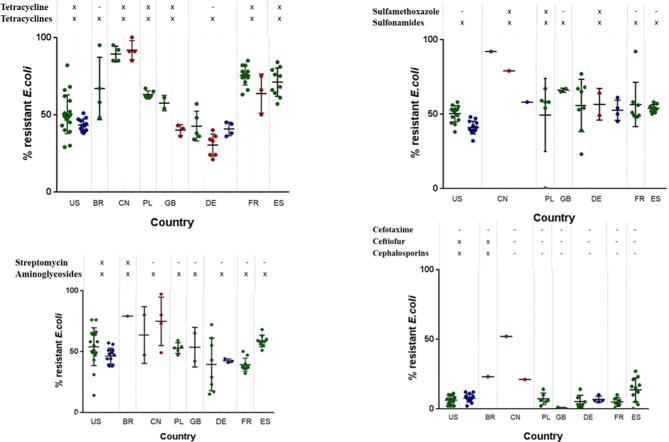
An overview of the prevalence of AR in E. coli is given for the US, Brazil, China, Poland, the United Kingdom, Germany, France, and Spain by using the results of their national monitoring programs as well as the scientific literature. Each scientific study evaluates AR using different methodology (and number of isolates); therefore, the statistical comparison is not possible. However, descriptive presentation of available data is possible. The source of AR data from each country is provided below in the description of each country. Additionally resistance rates and their evaluation from national monitoring systems of Poland, the United Kingdom, Germany, France, and Spain are presented in the section Supplementary material, due to the large volume of data. All cited resistance rates from 2000 to 2017 are presented with the support of GraphPad PRISM (2016). The outcome of the evaluation is the plot diagram (Figures 4 and 5), which is presented in the section overarching view.
Figure 4.
Resistance rates in E. coli to antibiotics from healthy animals (green dots), chicken retail meat (blue dots), and diseased chickens (red dots) detected within scientific studies or national monitoring programs. Each dot represents 1 study or data set in 1 yr. On the top of the figure, status of approval for the specific antibiotic tested for resistance (first line), the antimicrobial class (second line).
Figure 5.
Resistance rates in E. coli to antibiotics from healthy animals (green dots), chicken retail meat (blue dots), and diseased chickens (red dots) detected within scientific studies or national monitoring programs. Each dot represents 1 study or data set in 1 yr. On the top of the figure, status of approval for the specific antibiotic tested for resistance (first line), the antimicrobial class (second line).
Because of the harmonized sampling and detection of AR at the national levels, the AR results are shown in the form of figures, allowing the comparison of AR in E. coli over time. The data regarding AR in E. coli that is reported in scientific publications are presented in tabular format and includes the number of E. coli isolates that were tested and the average percentages of detected AR (Tables 2 and 3). If more than one set of data were indicated, data on the minimal and maximal AR rates in E. coli are additionally presented in the tables. Throughout the paper, the percentage of AR is defined as the percentage of resistant E. coli as proportion of the number of tested E. coli isolates.
Table 2.
Percentages of E. coli isolates from broilers in Brazil exhibiting resistance to antibiotics published in scientific literature based on Cardoso et al. (2002), Barros et al. (2012), Bezerra et al. (2016), Pessanha and Filho (2001), Stella et al. (2013), and Korb et al. (2015).
| Class | Compound | % res average | % res min | % res max | No. of studies | No. of isolates |
|---|---|---|---|---|---|---|
| Aminoglycosides | Gentamycin | 27 | 26 | 28 | 2 | 244 |
| Streptomycin | 79 | 1 | 91 | |||
| Cephalosporines | Cefalexin | 31 | 0 | 61 | 1 | 35 |
| Cefepime | 10 | 1 | 120 | |||
| Cefotaxime | 23 | 1 | 120 | |||
| Ceftazidime | 3 | 1 | 120 | |||
| Ceftiofur | 43 | 1 | 174 | |||
| Ceftriaxone | 24 | 1 | 120 | |||
| Cephalothin | 65 | 51 | 78 | 2 | 161 | |
| Fosfomycins | Fosfomycin | 29 | 10 | 45 | 3 | 360 |
| Lincosamides | Lincomycin | 100 | 100 | 100 | 1 | 35 |
| Macrolides | Azithromycin | 49 | 1 | 174 | ||
| Erytromycin | 97 | 1 | 91 | |||
| Nitrofurans | Nitrofurantoins | 13 | 1 | 120 | ||
| Penicillines | Amoxicillin | 65 | 50 | 84 | 2 | 101 |
| Ampicillin | 69 | 42 | 87 | 4 | 455 | |
| Phenicols | Chloramphenicol | 52 | 51 | 52 | 2 | 244 |
| Thiamphenicol | 51 | 25 | 77 | 1 | 35 | |
| Polymyxins | Polymyxin B | 1 | 1 | 174 | ||
| Quinolone | Ciprofloxacin | 53 | 14 | 91 | 2 | 294 |
| Enrofloxacin | 40 | 13 | 76 | 2 | 155 | |
| Nalidixic acid | 40 | 34 | 45 | 2 | 199 | |
| Norfloxacin | 59 | 38 | 76 | 2 | 101 | |
| Oxolinic acid | 88 | 1 | 66 | |||
| Tetracyclines | Chlortetracycline | 74 | 63 | 84 | 1 | 35 |
| Oxitetracycline | 81 | 62 | 100 | 1 | 35 | |
| Tetracycline | 67 | 48 | 95 | 4 | 455 | |
| Combination of compounds | Trimethoprim-Sulfamethoxazole | 60 | 27 | 100 | 6 | 556 |
% res—average value of percentages of antibiotic resistant E. coli found in referenced studies.
% res min—minimal value of percentages of antibiotic resistant E. coli found in referenced studies.
% res max—maximal value of percentages of antibiotic resistant E. coli found in referenced studies.
Table 3.
Percentages of E. coli isolates from broilers exhibiting resistance to antibiotics in China, based on Dai et al. (2008), Chen et al. (2014), Ho et al. (2011), Gai et al. (2015), Lei et al. (2010), Jiang et al. (2011), Lu et al. (2010), Wu et al. (2015), Yang et al. (2004), Yu et al. (2012), and Zhang et al. (2012a,b, 2014, 2016).
| Class | Compound | % res | % res min | % res max | No. of studies | No. of isolates |
|---|---|---|---|---|---|---|
| Aminoglycosides | Amikacin | 18 | 3 | 46 | 10 | 2,784 |
| Apramycin | 68 | 1 | 45 | |||
| Gentamycin | 50 | 9 | 82 | 11 | 2,302 | |
| Kanamycin | 59 | 24 | 97 | 5 | 951 | |
| Neomycin | 26 | 7 | 50 | 3 | 705 | |
| Spectinomycin | 26 | 15 | 42 | 3 | 403 | |
| Streptomycin | 71 | 47 | 97 | 6 | 1,480 | |
| Tobramycin | 14 | 1 | 389 | |||
| Carbapenems | Meropenem | 0 | 1 | 540 | ||
| Cephalosporines | Cefalexin | 8 | 5 | 11 | 2 | 553 |
| Cefazoline | 33 | 9 | 92 | 4 | 1,157 | |
| Cefotaxime | 37 | 21 | 52 | 2 | 627 | |
| Ceftazidime | 18 | 1 | 540 | |||
| Ceftiofur | 45 | 0 | 90 | 2 | 116 | |
| Ceftriaxone | 4 | 2 | 8 | 3 | 647 | |
| Cefalothin | 32 | 27 | 41 | 4 | 1,206 | |
| Fluoroquinolones | Difloxacin | 91 | 1 | 71 | ||
| Ciprofloxacin | 62 | 32 | 100 | 8 | 2,272 | |
| Enrofloxacin | 71 | 38 | 100 | 7 | 1,479 | |
| Gatifloxacin | 67 | 1 | 71 | |||
| Levofloxacin | 44 | 21 | 63 | 4 | 1,187 | |
| Norfloxacin | 53 | 21 | 100 | 5 | 1,322 | |
| Ofloxacin | 37 | 24 | 50 | 2 | 476 | |
| Orbifloxacin | 76 | 1 | 71 | |||
| Sarafloxacin | 100 | 1 | 71 | |||
| Sulfadimidine | 100 | 1 | 45 | |||
| Fosfomycins | Fosfomycin | 16 | 1 | 540 | ||
| Monobactams | Aztreonam | 10 | 1 | 540 | ||
| Nitrofurans | Nitrofurantoins | 3 | 1 | 540 | ||
| Penicillines | Amoxicillin | 54 | 1 | 389 | ||
| Ampicillin | 81 | 60 | 100 | 10 | 2,581 | |
| Piperacillin | 30 | 1 | 540 | |||
| Phenicols | Chloramphenicol | 44 | 24 | 69 | 6 | 1,854 |
| Florfenicol | 41 | 15 | 78 | 4 | 682 | |
| Quinolone | Nalidixic acid | 91 | 81 | 94 | 5 | 1,465 |
| Polymyxins | Colistin | 9 | 5 | 13 | 2 | 251 |
| Polymyxin B | 1 | 1 | 389 | |||
| Sulfonamides | Sulfamethoxazole | 76 | 58 | 92 | 3 | 294 |
| Sulfisoxazole | 83 | 1 | 87 | |||
| Doxycycline | 79 | 48 | 93 | 6 | 1,594 | |
| Tetracyclines | Tetracycline | 87 | 85 | 100 | 9 | 2,164 |
| Amoxicillin/clavulanate | 50 | 0 | 100 | 5 | 1,156 | |
| Combination of antimicrobials | Cefoperazone- Sulbactam | 51 | 1 | 373 | ||
| Trimethoprim- Sulfamethoxazole | 78 | 66 | 93 | 8 | 2,314 |
% res—average value of percentages of antibiotic resistant E. coli found in referenced studies.
% res min—minimal value of percentages of antibiotic resistant E. coli found in referenced studies.
% res max—maximal value of percentages of antibiotic resistant E. coli found in referenced studies.
The literature analyzed in this review uses different breakpoints to determine AR; additionally, resistance levels of E. coli were determined in the different time points. Therefore, any interpretation of these results should consider this issue/fact. Nevertheless, surveillance systems, together with scientific literature, provide valuable contribution for the overview of the occurrence of AR in the indicator organism E. coli combining this data with possible AU in large poultry-producing regions. The evaluation of data is included in the description of each country and it shows the need for global harmonized approach in the detection of AU and AR.
United States of America
Established in 1996, the National Antibiotic Resistance Monitoring System (NARMS) is a national public health surveillance system in the US. The United States Department of Agriculture (USDA) reports each year AR in E. coli isolates from retail raw chicken meat, caecal E. coli isolates from slaughtered animals, and isolates from processing plants collected as part of the Hazard Analysis Critical Control Point(HACCP). All data are available and easy to compare on NARMS interactive database (US Food and Drug Administration, 2018). NARMS uses similar methods and defined protocols, which supports the analysis. The monitoring of AR in the US shows similar resistance rates in E. coli from retail meat, slaughterhouse, and intestinal samples. The testing of AR from different sources to the same antibiotics makes this comparison possible. Antibiotic resistance rates in E. coli remain on the same level from 2000 to 2015, only resistance to streptomycin decreases from 78 to 46%. Independent from the source of the isolates, resistance rates of approximately 45% were detected for streptomycin, tetracycline, and sulfamethoxazole-sulfisoxazole. Antibiotic resistance percentages of E. coli from broilers in the US obtained by scientific publications show similar resistance rates for streptomycin and tetracycline as detected in the monitoring programs (Johnson et al., 2007; Smith et al., 2007; Zhang et al., 2011; Millman et al., 2013; Rothrock et al., 2016). However, this comparison cannot be statistically evaluated due to the different methodologies of AR determination in scientific studies and national monitoring. Streptomycin and tetracycline are approved for use in poultry. In contrast, sulfamethoxazole and sulfisoxazole are not licensed, but there are other sulfonamides that are approved and therefore may influence the resistance rates. Resistance rates to gentamicin (approved for use) and ampicillin (not approved, but penicillins approved) are approximately 40 and 20%, respectively. Consequently, detected higher resistance rates in E. coli may be driven by the use of antibiotics, but quantitative data on AU would be needed in order to confirm this hypothesis.
Brazil
Brazil does not have any central microbiology reference laboratory. Therefore, no regular AR-monitoring data are available (Rossi, 2011). An overview of the scientific publications that presents the percentages of AR in E. coli isolates from broilers in Brazil since 2001 is shown in Table 2.
In general, the low number of studies as well as use of different numbers of isolates in each study does not allow any proper conclusions about resistance rates, which shows the necessity of national monitoring. According to Table 2, the highest detected resistance rates of E. coli from broilers were identified in the study of Barros et al. (2012) for lincomycin, erythromycin, and oxolinic acid, with 100, 97, and 88% of resistant isolates, respectively. Lincomycin is registered for use in Brazil, while erythromycin and oxolinic acid are not allowed, but other representatives of macrolides (tylosin and tilmicosin) and quinolones (halquinol) are registered for use. Resistance rates to penicillins and ampicillins are around 75 and 65%, respectively. Representatives of both antibiotic classes may be used for poultry in Brazil. More studies found variations in the AR rates of E. coli from broilers to the antibiotic combination of sulfamethoxazole and trimethoprim. Hence, 1 study reports that 100% of the 174 isolates that were tested were resistant (Bezerra et al., 2016), while 3 other studies detected resistance rates of 66 and 68% in 66 and 91 isolates, respectively (Cardoso et al., 2002; Stella et al., 2013). Moreover, 2 additional studies present resistance rates of 27 and 28% in 70 and 120 tested isolates, respectively (Pessanha and Filho, 2001; Korb et al., 2015). The variation of resistance rated may be explained by different locations of studies as well as different times of detections.
China
There is no national monitoring of AR in E. coli isolates from poultry in China. Table 3 presents an overview of the percentages of AR in E. coli isolates from healthy and diseased broilers in China from available scientific literature from 2004 to 2017.
The results presented in Table 4 indicate that the AR rates in E. coli to sulfonamides and tetracyclines are around 80%, and 40% to phenicols. Representatives of all 3 antibiotic classes are approved for the use in poultry. The resistances vary in the ranges of 50 to 100% for quinolones, 30 to 80% for penicillins, 20 to 70% for aminoglycosides, and 4 to 45% for cephalosporins. Quinolones and penicillins are registered for the use in poultry, while cephalpsporins are not. However, it has to be taken into account that listed licensed antibiotics in China do not include parenterally administered antibiotics.
Table 4.
Percentage of antibiotic resistant E. coli isolated from broilers in selected European countries in 2016 (EFSA/ECDC, 2018).
| Antibiotic class | Compound | PL | GB | DE | FR | ES | Average |
|---|---|---|---|---|---|---|---|
| Number of isolates | 173 | 190 | 177 | 188 | 171 | ||
| Aminoglycosides | Gentamicin | 10 | 7 | 7 | 3 | 36 | 13 |
| ß-lactam cephalosporines | Cefotaxime | 3 | 0 | 1 | 4 | 9 | 3 |
| Ceftazidime | 3 | 0 | 1 | 2 | 8 | 3 | |
| ß-lactam penicillines | Ampicillin | 91 | 67 | 56 | 56 | 63 | 67 |
| Diaminopyrimidines | Trimethoprim | 62 | 43 | 38 | 47 | 37 | 45 |
| Fenicols | Chloramphenicol | 25 | 4 | 10 | 7 | 17 | 13 |
| Fluoroquinolone | Ciprofloxacin | 90 | 22 | 60 | 36 | 91 | 60 |
| Macrolides | Azithromycin | 5 | 0 | 2 | 0 | 11 | 4 |
| Polimyxmins | Colistin | 3 | 0 | 4 | 3 | 1 | 2 |
| Quinolone | Nalidixic acid | 78 | 21 | 45 | 34 | 88 | 53 |
| Sulfonamides | Sulfamethoxazole | 71 | 53 | 47 | 55 | 50 | 55 |
| Tetracyclines | Tetracycline | 73 | 44 | 28 | 62 | 61 | 54 |
| Tigecycline | 2 | 0 | 0 | 0 | 0 | 0 |
PL—Poland, GB—United Kingdom, DE—Germany, FR—France, ES- Spain.
Antibiotic susceptibility testing of 326 E. coli isolates from food animals collected in China over the last 4 decades showed that AR in E. coli in the country has increased since the 1970s (Song et al., 2010). Furthermore, an evaluation of 540 E. coli isolates from broilers showed that resistance to amikacin, ampicillin, aztreonam, ceftazidime, cefotaxime, cefalothin, chloramphenicol, ciprofloxacin, fosfomycin, levofloxacin, norfloxacin, nalidixic acid, piperacillin, and trimethoprim-sulfamethoxazole increased significantly from 1993 to 2013 (Chen et al., 2014). Liu et al. (2016) observed a major increase of the relevance of colistin resistance in E. coli in China due to the detection of the plasmid-mediated colistin resistance mechanism MCR-1.
European Union
The European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) jointly analyzed data submitted by EU Member States regarding AR in E. coli from broilers. The organizations presented the results in the annual “European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food”. Last report evaluates AR in bacteria isolated in 2016 (EFSA/ECDC, 2018). Antibiotics were selected based on their relevance to public health and/or their epidemiological relevance. Epidemiological cut-off values were used for the AR interpretation (European Food Safety Authority, 2008). France, Germany, Poland, and Spain have participated in the monitoring since 2008. An overview of the prevalence of resistance for the largest poultry-producing countries in the EU for 2015 is shown in Table 4.
In addition to this EU monitoring, Poland, the UK, Germany, and France have national AR-monitoring systems. Some data from national monitoring are used for EU monitoring. Thus, they correspond to each other. There is a variation in resistance rates in E. coli among the mentioned EU countries. Nevertheless, comparing the obtained averages of resistance, that of ampicillin (70%) was the highest one. Approximately 60% resistance rates were detected for ciprofloxacin, sulfamethoxazole, tetracycline, and nalidixic acid(European Food Safety Authority and European Centre for Disease Prevention and Control, 2018).
Poland
Poland began to implement a national monitoring program for AR in commensal E. coli isolates from broilers in 2009 (Wasyl et al., 2012). Antibiotic resistance levels to several antibiotics in E. coli isolates from broilers at slaughterhouse level from 2009 to 2014 are available (Wasyl et al., 2013; European Food Safety Authority and European Centre for Disease Prevention and Control, 2015, 2016). Data show high AR rates (from 70 to 90%) to ciprofloxacin, nalidixic acid, and ampicillin, and 50 to 70% to tetracycline, sulfamethoxazole, and streptomycin. Wasyl et al. (2013) detected increasing trends of ampicillin and cefotaxime resistance in the observed E. coli isolates.
United Kingdom
Antibiotic resistance data for E. coli isolated from broilers in the United Kingdom are available from 2 distinct AR-monitoring programs: the EU-monitoring and the clinical monitoring programs (Veterinary Medicines Directorate, 2015). The EU-monitoring program isolated E. coli from healthy broilers across the United Kingdom. The clinical monitoring program is a passive monitoring program. Its aim is the evaluation of AR in bacteria that are isolated from clinical samples of diseased animals to antibiotics of veterinary relevance. Both monitoring programs show high AR rates of E. coli to ampicillin and tetracycline. Bywater et al. (2004) and Randall et al. (2011) confirm the higher AR rates to ampicillin and tetracycline in E. coli in UK. An analysis of fluoroquinolone resistance in E. coli from feces samples of 68 broiler farms detected resistance to ciprofloxacin in 50% of these farms (Taylor et al., 2008).
Germany
The Federal Office of Consumer Protection and Food Safety reports on AR monitoring in Germany. Similar to the monitoring programs of the UK, 2 different surveillance systems exist in Germany. The first one (Reports on food safety-zoonoses monitoring) monitors healthy animals and the products thereof. The second system is the GERM Vet Report that contains data about the resistance of animal pathogens. Germany monitors the AR in E. coli isolates from intestinal samples of broilers, chicken meat, and diseased animals. Data from all systems show higher resistance rates to ampicillin and sulfamethoxazole than to other antibiotics (German Federal Office of Consumer Protection and Food Safety, 2012a, 2012b, 2014, 2015, 2016). For E. coli that were isolated from intestinal samples and retail meat, the resistance rates to ciprofloxacin, nalidixic acid, streptomycin, tetracycline, and trimethoprim are between 40 and 60%. Except for trimethoprim, these antibiotics are not allowed for use in broilers in Germany. However, other representatives of the corresponding antibiotic classes may be used. The resistance rates of E. coli from diseased chickens to ciprofloxacin are approximately 7%. It seems that the resistance to ciprofloxacin is lower in diseased animals than in healthy animals, and resistance to cephalosporins is higher in diseased animals than in healthy animals. However, the resistance rates must be measured over longer periods of time to confirm these differences.
France
France participates in the EU monitoring of AR in animals and presents data since 2004. The French Agency for Veterinary Medicinal Products (ANSES-ANMV) provides reports on the French surveillance network for AR in pathogenic bacteria of animal origin (RESAPATH). The RESAPATH presents the results of the monitoring of AR in E. coli from diseased hens and broilers that are treated by veterinarians as part of their regular clinical services. Additionally, AR data are available from the EU Summary reports (European Food Safety Authority and European Centre for Disease Prevention and Control, 2011, 2012, 2013, 2014, 2015, 2016, 2018).
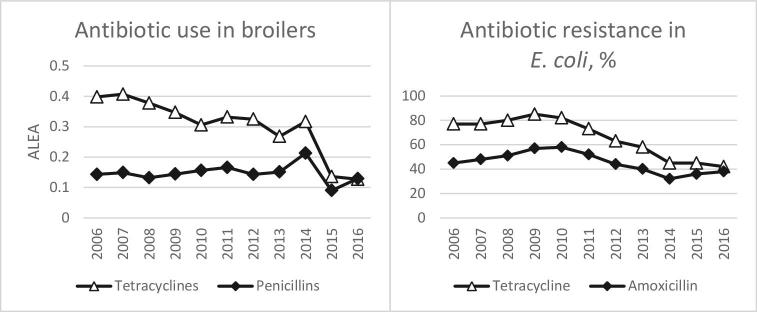
Tetracyclines and penicillins are approved for use in poultry in France. Due to the availability of the quantitative use of antibiotics in poultry, a comparison between antibiotic use and resistance is possible, as can be seen in Figure 3. The use of penicillins in France was stable over time and resistance level to amoxicillin (40%) was the same in 2006 and 2016, although there was an increase and decrease of resistance rates between 2006 and 2016. The decrease in the use of tetracycline between 2006 and 2016 was accompanied by a decrease of tetracycline-resistance rates in E. coli between 2006 and 2016. Thus, less use of tetracyclines may result in less resistance to those antibiotics. This hypothesis needs to be confirmed in other countries, and the availability of quantitative data of antibiotic use allows this comparison. In contrast, the use of polymyxins does not correspond with the resistance rates of colistin. The use of polymyxins is high in France, as it can be seen in Figure 2, but the resistance level to colistin is around 3%. Low colistin resistance levels in E. coli can be observed in large poultry-producing European countries. However, it needs to be taken into account that in vitro antimicrobial susceptibility testing for polymyxins is challenging and may be associated with high major errors (Bakthavatchalam et. al, 2018). Therefore, no conclusion can be made to the correlation of antibiotic use and resistance to colistin.
Figure 3.
Tetracycline and penicillins use in poultry and resistance in E. coli isolates from broilers in France. ALEA (Animal level of exposure to antibiotics) = Animal level of exposure to antibiotics.
Spain
The Spanish AR surveillance network “Red de Vigilancia de Resistencias Antibioticas en Bacterias de Origen Veterinario” (VAV) was formed in 1996 (Moreno, 2000). The VAV reported data on AR in E. coli that were taken from healthy broilers during the period from 1999 to 2005 (Ministry of Agriculture Fisheries and Food in Spain 2005, 2006). Additionally, AR data are available from the EU Summary reports (European Food Safety Authority and European Centre for Disease Prevention and Control, 2011, 2013, 2015, 2016, 2018).
The fluoroquinolones enrofloxacin and flumequine are allowed for use in poultry in Spain. The monitoring data show that AR to ciprofloxacin in E. coli increased from 17% in 2001 to 91% in 2016, and that of nalidixic acid from 60% in 2001 to 88% in 2014. Whether an increased use of the fluoroquinolones may have influenced this increase cannot be evaluated without the presence of quantitative AU data. Tetracycline and the penicillins are also registered for use in Spain. The AR resistance rates to tetracycline and ampicillin are approximately 70%. A decrease of resistance to tetracycline was observed between 1999 and 2016.
OVERARCHING VIEW AND RECOMMENDATIONS
Quantitative AU data for poultry are not available for most large poultry-producing countries. Availability of data for AR in E. coli is limited for Brazil. Scientific publications from all regions as well as national monitoring of USA and Europe use different methods for the determination of AR. Harmonized approach in detection of AR is of special importance to provide the global evaluation of data. However, the list of all approved antibiotics for poultry provides valuable qualitative data for all countries, which was combined with AR rates in E. coli and presented with the mean of a plot diagram. Data for AR rates from the US were used from the surveillance systems of E. coli isolates from retail raw chicken meat, caecal E. coli isolates from slaughtered animals, and isolates from processing plants collected as part of HACCP (US Food and Drug Administration, 2018). Additionally, data from the scientific publications on AR in E. coli from the US were also included. Data for AR rates in China and Brazil are included in Tables 2 and 3. Data for AR from large European poultry producers were used from European monitoring as well as available data from national resistance monitoring systems in Poland, the UK, Germany, France, and Spain from 2000 to 2017. All used data from national monitoring systems are available in section Supplementary material of this manuscript.
There is a representative amount of AR data from E. coli for some antibiotic classes such as aminoglycosides, penicillins, cephalosporins, fenicols, quinolones, fluoroquinolones, sulfonamides, and tetracyclines for all large poultry-producing countries. The resistance rates of these antibiotics are represented in plot diagrams by dots, as shown in Figures 4 and 5. Each dot on the diagram represents the resistance rate detected during 1 scientific study or a national monitoring program from 2000 to 2017.
Tetracyclines, aminoglycosides, sulfonamides, and penicillins are registered for use in poultry in all countries. The resistance rates in E. coli of broiler origin to representatives of these antibiotic classes, e.g., tetracycline, sulfamethoxazole, streptomycin, and ampicillin, are higher than 40% in all countries, with the exception of ampicillin resistance in the US. This outcome indicates that the use of these antibiotics in poultry results in high resistance rates; however, quantitative data on antibiotic use in poultry would be essentially needed for the confirmation. The resistance rates to fluoroquinolones and quinolones are lower in the US in comparison to other large poultry producers where the use of fluoroquinolones is allowed. These findings demonstrate the possibility to produce broilers without fluoroquinolones, which may result out in low resistance rates, but there was no elimination of the occurrence of resistant population.
Colistin, as a representative of the polymyxins, and tylosin, as representative of the macrolides, are both allowed for poultry use in all countries for oral treatment or injection solution, but there is only a limited amount of resistance data available. Due to the detection of plasmid-located colistin resistance genes in some countries, the assessment of resistance rates to this antibiotic would be essential.
There are several classes of antibiotics that are approved for use in poultry, but no E. coli AR are available for studied regions. Such classes are as follows: arsenicals, glycophospholipids, ionophores, lincosamides, orthosomycins, pleuromutilins, polypeptides, and streptogramins. It is important to note that most of the representatives of these antibiotic classes act against Gram-positive bacteria. However, the prevalence of resistance or resistance determinants in E. coli to some of these antibiotics was detected by Heir et al. (2004), Bonnet et al. (2009), Cervantes et al. (1994), and Hummel et al. (1979).
The above outlined evaluation and conclusions from review of AU and AR provide input for the monitoring of antibiotic use in poultry and underlie the need for harmonized global surveillance and detection of the AU and AR.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to Franz Waxenecker, Gerd Schatzmayer, Birgit Rabus, Xiangling Kong, Renata Reis, Anna Nowak from BIOMIN Holding GmbH, Kai Grathwohl, and Marcela Cardoso from SANPHAR Ltd, Rachel Trode from European University Institute for providing useful source of information on registration of antibiotics, and fruitful contribution during writing processes.
REFERENCES
- Aarestrup F. M., Wegener H. C., Collignon P.. 2008. Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev. Anti Infect. Ther. 6:733–750. [DOI] [PubMed] [Google Scholar]
- AccessScience Editors. 2017. U.S. bans antibiotics use for enhancing growth in livestock. McGraw-Hill Education; Accessed May 2018. 10.1036/1097-8542.BR0125171. [DOI] [Google Scholar]
- Archawakulathep A., Kim C. T. T., Meunsene D., Handijatno D., Abu Hassim H., Rovira H. R. G., Myint K. S., Baldrias L. R., Sothy M., Aung M., Wahyu N. H., Chea R., Boonmasawai S., Vannamahaxay S., Angkititrakul S., Collantes T. M. A., Van T. N., Punyapornwithaya V., Zakaria Z., Chuanchuen R.. 2014. Perspectives on antimicrobial resistance in livestock and livestock products in asean countries. Wetchasan Sattawaphaet 44:5–13. [Google Scholar]
- Association of Poultry Processors and Poultry Trade in the EU Countries. 2015. Annual Report 2015 of ASBL. Accessed May 2016. http://www.avec-poultry.eu/system/files/archive/new-structure/avec/Annual_Report/2015/Annual%20Report%202015.pdf. [Google Scholar]
- Bakthavatchalam Y. D., Pragasam A. K., Biswas I., Veeraraghavan B.. 2018. Polymyxin susceptibility testing, interpretative breakpoints and resistance mechanisms: An update. J. Gobal Antimicrob. Res. 12:124–136. [DOI] [PubMed] [Google Scholar]
- Barros M. R., da Silveira W. D., de Araujo J. M., Costa E. P., Oliveira A. A. D., Santos A., Silva V. A. S., Mota R. A.. 2012. Resistência antimicrobiana e perfil plasmidial de Escherichia coli isolada de frangos de corte e poedeiras comerciais no Estado de Pernambuco, Pesq. Vet. Bras. 32:405–410. [Google Scholar]
- Bezerra W. G. A., Silva I. I. N. G., Vasconcelos R. H., Machado D. N., Lopes E. S. P., Lima S. V. G., Teixeira R. S. C., Lima J. B., Oliveira F. B., Maciel W. C.. 2016. Isolation and antimicrobial resistance of Escherichia coli and Salmonella enterica subsp. enterica (O:6,8) in broiler chickens. Acta. Sci. Vet. 44:1–7. [Google Scholar]
- Bonnet C., Diarrassouba F., Brousseau R., Masson L., Topp E., Diarra M. S.. 2009. Pathotype and antibiotic resistance gene distributions of Escherichia coli isolates from broiler chickens raised on antimicrobial-supplemented diets. Appl. Environ. Microbiol. 75:6955–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R., Deluyker H., Deroover E., de Jong A., Marion H., McConville M., Rowan T., Shryock T., Shuster D., Thomas V., Valle M., Walters J.. 2004. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food-producing animals. J. Antimicrob. Chemother. 54:744–754. [DOI] [PubMed] [Google Scholar]
- Cardoso A. L. S. P., Tessari E. N. C., Castro A. G. M., Zanatta G. F.. 2002. Antimicrobial susceptibility evaluation of Escherichia coli strains of avian origin/Avaliação da susceptibilidade a antimicrobianos de cepas de Escherichia coli de origem aviária. 14ª Reunião Anual do Instituto Biológico, São Paulo 69:1–5. [Google Scholar]
- Cervantes C., Ramirez J. L., Silver S.. 1994. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 15:355–367. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang W. Q., Yin J. J., Zhang N., Geng S. Z., Zhou X. H., Wang Y. H., Gao S., Jiao X. N.. 2014. Escherichia coli isolates from sick chickens in China: changes in antimicrobial resistance between 1993 and 2013. Vet. J. 202:112–115. [DOI] [PubMed] [Google Scholar]
- Dai L., Lu L. M., Wu C. M., Li B. B., Huang S. Y., Wang S. C., Qi Y. H., Shen J. Z.. 2008. Characterization of antimicrobial resistance among Escherichia coli isolates from chickens in China between 2001 and 2006. FEMS Microbiol. Lett. 286:178–183. [DOI] [PubMed] [Google Scholar]
- Diarra M. S., Malouin F.. 2014. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. 2005. Ban on antibiotics as growth promoters in animal feed enters into effect. European Commisision Press Release Database. Accessed July 2018. http://europa.eu/rapid/press-release_IP-05-1687_en.htm. [Google Scholar]
- European Food Safety Authority. 2008. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA J. 2008:1–44. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2011. European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from animals and food in the European Union in 2009. EFSA J. 9:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2012. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2010. EFSA J. 10:2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2013. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA J. 11:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2014. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2012. EFSA J. 12:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2015. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J. 13:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2016. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J. 14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2018. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA J. 5182:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2010. Agribusiness handbook. Poultry meat and eggs. Accessed July 2018. http://www.documentcloud.org/documents/406299-fc39-agribusinesshandbook.html. [Google Scholar]
- French Agency for Food Environmental and Occupational Health & Safety. 2016. Index des Médicaments vétérinaires autorisés en France. Accessed July 2016. http://www.ircp.anmv.anses.fr/. [Google Scholar]
- French Agency for Food Environmental and Occupational Health & Safety and French Agency for Veterinary Medicinal Products. 2017. Sales survey of Veterinary Medicinal Products containing Antimicrobials in France - 2016. Accessed June 2018. https://www.anses.fr/en/system/files/ANMV-Ra-Antibiotiques2016EN.pdf. [Google Scholar]
- French Agency for Food Environmental and Occupational Health & Safety and French Agency for Veterinary Medicinal Products. 2016. RESAPATH French surveillance network for antimicrobial resistance in pathogenic bacteria of animal origin 2014. Annual Report. [Google Scholar]
- Gai W. Y., Wang J. W., Wang J., Cui Z. G., Qu Z. N., Cui J. H., Du X. L., Huang X. M., Zhao J. M.. 2015. Molecular classification and drug resistance analysis of Escherichia coli isolated from poultry in China. Int. J Clin. Exp. Med. 8:836–844. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Migura L., Hendriksen R. S., Fraile L., Aarestrup F. M., 2014. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: The missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 170:1–9, Accessed Aug. 2016. https://www.anses.fr/en/system/files/LABO-Ra-Resapath2014EN.pdf. [DOI] [PubMed] [Google Scholar]
- German Federal Office of Consumer Protection and Food Safety. 2012a. Berichte zur Resistenzmonitoringstudie 2008. Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien, Accessed Aug. 2016. https://www.bvl.bund.de/SharedDocs/Downloads/09_Untersuchungen/Archiv_berichte_Resistenzmonitoring/Bericht_Resistenzmonitoring_2008.html?nn=1401286. [Google Scholar]
- German Federal Office of Consumer Protection and Food Safety. 2012b. Berichte zur Resistenzmonitoringstudie 2009. Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien, Accessed Aug. 2016. https://www.bvl.bund.de/SharedDocs/Downloads/09_Untersuchungen/Archiv_berichte_Resistenzmonitoring/Bericht_Resistenzmonitoring_2008.html?nn=1401286. [Google Scholar]
- German Federal Office of Consumer Protection and Food Safety. 2014. BVL-Report 8.6. Berichte zur Resistenzmonitoringstudie Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien 2010/2011, Accessed Aug. 2016. https://www.bvl.bund.de/SharedDocs/Downloads/09_Untersuchungen/Archiv_berichte_Resistenzmonitoring/Bericht_Resistenzmonitoring_2009.html?nn=1401286. [Google Scholar]
- German Federal Office of Consumer Protection and Food Safety. 2015. BVL-Report 9.5. Berichte zur Resistenzmonitoringstudie. Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien 2011/2012, Accessed Aug. 2016. https://www.bvl.bund.de/DE/09_Untersuchungen/01_Aufgaben/03_Nationales%20Resistenz-Monitoring/Archiv_Berichte/Archiv_Resistenzmonitoring_node.html. [Google Scholar]
- German Federal Ministry of Health. 2016. Drug information for everyone. Accessed Sept. 2016. http://www.pharmnet-bund.de. [Google Scholar]
- German Federal Office of Consumer Protection and Food Safety. 2016. BVL-Report 10.5. Berichte zur Resistenzmonitoringstudie. Resistenzsituation bei klinisch wichtigen tierpathogenen Bakterien 2012/2013. [Google Scholar]
- GraphPad PRISM, Prism 7 for Windows, Version 7.02, September 13, 2016; 1992–2016GraphPad Software, Inc., San Diego, California, USA. [Google Scholar]
- Harbarth S., Balkhy H. H., Goossens H., Jarlier V., Kluytmans J., Laxminarayan R., Saam M., Van Belkum A., Pittet D.. 2015. Antimicrobial resistance: one world, one fight! Antimicrob. Resist. Infect. Control 4:49. [Google Scholar]
- Heir E., Lindstedt B. A., Leegaard T. M., Gjernes E., Kapperud G.. 2004. Prevalence and characterization of integrons in blood culture Enterobacteriaceae and gastrointestinal Escherichia coli in Norway and reporting of a novel class 1 integron-located lincosamide resistance gene. Ann. Clin. Microbiol. Antimicrob. 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. L., Chow K. H., Lai E. L., Lo W. U., Yeung M. K., Chan J., Chan P. Y., Yuen K. Y.. 2011. Extensive dissemination of CTX-M-producing Escherichia coli with multidrug resistance to 'critically important' antibiotics among food animals in Hong Kong, 2008-10. J. Antimicrob. Chemother. 66:765–768. [DOI] [PubMed] [Google Scholar]
- Hummel H., Piepersberg W., Bock A.. 1979. Analysis of lincomycin resistance mutations in Escherichia coli. Mol. Gen. Genet. 169:345–347. [DOI] [PubMed] [Google Scholar]
- Jiang H. X., Lu D. H., Chen Z. L., Wang X. M., Chen J. R., Liu Y. H., Liao X. P., Liu J. H., Zeng Z. L.. 2011. High prevalence and widespread distribution of multi-resistant Escherichia coli isolates in pigs and poultry in China. Vet. J. 187:99–103. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Sannes M. R., Croy C., Johnston B., Clabots C., Kuskowski M. A., Bender J., Smith K. E., Winokur P. L., Belongia E. A.. 2007. Antimicrobial drug-resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 13:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaesbohrer A., Schroeter A., Tenhagen B. A., Alt K., Guerra B., Appel B.. 2012. Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance. Zoonoses Public Health 59:158–165. [DOI] [PubMed] [Google Scholar]
- Korb A., de Nazareno E. R., Costa L. D., Nogueira K. D. S., Dalsenter P. R., Tuon F. F. B., Pomba M. C.. 2015. Tipagem molecular e resistência aos antimicrobianos em isolados de Escherichia coli de frangos de corte e de tratadores na Região Metropolitana de Curitiba, Paraná. Pesq. Vet. Bras. 35:258–264. [Google Scholar]
- Lei T., Tian W., He L., Huang X.-H., Sun Y.-X., Deng Y.-T., Sun Y., Lv D.-H., Wu C.-M., Huang L.-Z., Shen J.-Z., Liu J.-H.. 2010. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. Vet. Microbiol. 146:85–89. [DOI] [PubMed] [Google Scholar]
- Liu Y.-Y., Wang Y., Walsh T. R., Yi L.-X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., Yu L.-F., Gu D., Ren H., Chen X., Lv L., He D., Zhou H., Liang Z., Liu J.-H., Shen J.. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16:161–168. [DOI] [PubMed] [Google Scholar]
- Lu L., Dai L., Wang Y., Wu C., Chen X., Li L., Qi Y., Xia L., Shen J.. 2010. Characterization of antimicrobial resistance and integrons among Escherichia coli isolated from animal farms in Eastern China. Acta Trop. 113:20–25. [DOI] [PubMed] [Google Scholar]
- Marshall B. M., Levy S. B.. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24:718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M. 2013. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 10:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman J. M., Waits K., Grande H., Marks A. R., Marks J. C., Price L. B., Hungate B. A.. 2013. Prevalence of antibiotic-resistant E. coli in retail chicken: comparing conventional, organic, kosher, and raised without antibiotics. F1000Res. 2:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture Livestock and Farming in Brazil. 2016a. Especies Aves. Accessed Jan. 2016. http://www.agricultura.gov.br/animal/especies/aves. [Google Scholar]
- Ministry of Agriculture Livestock and Farming in Brazil. 2008. Tabela de aditivos antimicrobianos, anticoccidianos e agonistas com uso autorizado na alimentação animal. Accessed Apr. 16. http://www.agricultura.gov.br/animal/qualidade-dos-alimentos/aditivos-autorizados. [Google Scholar]
- Ministry of Agriculture Livestock and Farming in Brazil. 2014. Lista de produtos com licencas vigentes (04/2014). Accessed Oct. 2016. http://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/produtos-veterinarios/arquivos-comunicacoes-e-instrucoes-tecnicas/ProdutosVigentesAbril2014.pdf. [Google Scholar]
- Ministry of Agriculture of People's Republic of China. 2001. Announcement of MOA No. 168 (2001) Usage Specification Antibiotics in Feed. Accessed Mai16. http://jsvd.org.cn/forum.php?mod=viewthread&tid=13477&highlight=168. [Google Scholar]
- Ministry of Agriculture of People's Republic of China. 2013. Regulation of veterinary drugs. Nongye Bulletin No.1997. Accessed May 2016. http://www.moa.gov.cn/zwllm/tzgg/gg/201310/t20131010_3625542.htm. [Google Scholar]
- Ministry of Agriculture Livestock and Farming in Brazil. 2016b. Compendio de produtos veterinarios SINDAN. Accessed Oct. 2016. http://www.cpvs.com.br/cpvs/pesquisar.aspx. [Google Scholar]
- Ministry of Agriculture Fisheries and Food in Spain. 2005. Veterinary monitoring of antimicrobial resistance in Spain VAV 2004. [Google Scholar]
- Ministry of Agriculture Fisheries and Food in Spain. 2006. Veterinary monitoring of antimicrobial resistance in Spain 2005, Accessed Aug. 2016. https://www.visavet.es/data/VAV2004.pdf. [Google Scholar]
- Moreno M. 2000. Antibiotic resistance monitoring: the Spanish programme. Int. J. Antimicrob. Agents. 14:285–290, Accessed Aug. 2016. https://www.visavet.es/data/VAV2005.pdf. [DOI] [PubMed] [Google Scholar]
- Page S. W., Gautier P.. 2012. Use of antimicrobial agents in livestock. Rev. Sci. Tech. OIE 31:145–188. [DOI] [PubMed] [Google Scholar]
- Pessanha R. P., Filho P. P. G.. 2001. Uso de antimicrobianos como promotores de crescimento e resistência em isolados de Escherichia coli e de Enterobacteriaceae lactose-negativa da microflora fecal de frangos de corte. Arq. Bras. Med. Vet. Zootec. 53:111–115. [Google Scholar]
- Poole T., Sheffield C.. 2013. Use and misuse of antimicrobial drugs in poultry and livestock: mechanisms of antimicrobial resistance. Pak. Vet. J. 33:266–271. [Google Scholar]
- Randall L. P., Clouting C., Horton R. A., Coldham N. G., Wu G., Clifton-Hadley F. A., Davies R. H., Teale C. J.. 2011. Prevalence of Escherichia coli carrying extended-spectrum beta-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J. Antimicrob. Chemother. 66:86–95. [DOI] [PubMed] [Google Scholar]
- Rosengren L. B., Gow S. P., Scott W. J.. 2010. Antimicrobial use and resistance in pigs and chickens: a review of the science, policy, and control practices from farm to slaughter - executive summary. Can.J. Infect. Dis. Med. Microbiol. 21:123–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F. 2011. The challenges of antimicrobial resistance in Brazil. Clin. Infect. Dis. 52:1138–1143. [DOI] [PubMed] [Google Scholar]
- Rothrock M. J., Hiett K. L., Guard J. Y., Jackson C. R.. 2016. Antibiotic resistance patterns of major zoonotic pathogens from All-Natural, Antibiotic-Free, Pasture-Raised Broiler Flocks in the Southeastern United States. J. Environ. Qual. 45:593–603. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Drum D. J., Dai Y., Kim J. M., Sanchez S., Maurer J. J., Hofacre C. L., Lee M. D.. 2007. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl. Environ. Microbiol. 73:1404–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Ning Y. B., Shen J. Z., Fan X. Z., Zhang C. P., Yang C. H., Han J. F.. 2010. Investigation of integrons/cassettes in antimicrobial-resistant Escherichia coli isolated from food animals in China. Sci. China Life Sci. 53:613–619. [DOI] [PubMed] [Google Scholar]
- Spanish Agency of Medicines and Sanitary Products. 2016. Centro de Información online de Medicamentos Veterinarios de la AEMPS, CIMA Vet. Accessed Aug. 2016. https://cimavet.aemps.es/cimavet/medicamentos.do. [Google Scholar]
- Stella A. E., Vitor T. L., Gadelha D. F. B. G., Moreira C. N., Meirelles-Bartoli R. B., Oliveira A. F.. 2013. Escherichia coli resistente a antimicrobianos isolada de bovinos e aves/Antimicrobial drug resistant Escherichia coli from cattle and poultry. Ars. Vet. 29:14. [Google Scholar]
- Taylor N. M., Davies R. H., Ridley A., Clouting C., Wales A. D., Clifton-Hadley F. A.. 2008. A survey of fluoroquinolone resistance in Escherichia coli and thermophilic Campylobacter spp. on poultry and pig farms in Great Britain. J. Appl. Microbiol. 105:1421–1431. [DOI] [PubMed] [Google Scholar]
- The office for registration of medicinal products medical devices and biocidal products in Poland. 2016. List of Medical Products in 2016 / Urzędowy Wykaz Produktów Leczniczych 2016. Accessed Aug. 2016. http://www-old.urpl.gov.pl/urzedowy-wykaz-produktow-leczniczych-2016. [Google Scholar]
- United States Department of Agriculture. 2015. Economics of antibiotic use in U.S. livestock production. Accessed Jan. 2016. http://www.ers.usda.gov/media/1950577/err200.pdf. [Google Scholar]
- United States Department of Agriculture. 2016. Broiler meat production by country in 1000MT. Accessed Feb. 2016. http://www.indexmundi.com/Agriculture/?commodity=broiler-meat&graph=production. [Google Scholar]
- US Food and Drug Administration. 2005. FDA announces final decision about veterinary medicine. Accessed Mar. 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108467.htm. [Google Scholar]
- US Food and Drug Administration. 2016. FDA approved animal drugs products. Accessed Mar. 2016. http://www.accessdata.fda.gov/scripts/animaldrugsatfda/index.cfm?gb=2. [Google Scholar]
- US Food and Drug Administration. 2018. NARMS interactive data displays. Accessed June 2018. https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm416741.htm. [Google Scholar]
- US Food and Drug Administration. 2017. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals 2016. Accessed Jan. 2018. https://www.fda.gov/downloads/forindustry/userfees/animaldruguserfeeactadufa/ucm588085.pdf. [Google Scholar]
- Veterinary Medicines Directorate. 2015. UK veterinary antibiotic resistance and sales surveillance report 2014. Accessed Aug. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477788/Optimised_version_-_VARSS_Report_2014__Sales___Resistance_.pdf. [Google Scholar]
- Veterinary Medicine Directorate UK. 2016. Veterinary medicines directorate government services and information. Accessed Apr. 2016. http://www.vmd.defra.gov.uk/ProductInformationDatabase/. [Google Scholar]
- Wasyl D., Hasman H., Cavaco L. M., Aaresturp F. M.. 2012. Prevalence and characterization of cephalosporin resistance in nonpathogenic Escherichia coli from food-producing animals slaughtered in Poland. Microb. Drug. Resist. 18:79–82. [DOI] [PubMed] [Google Scholar]
- Wasyl D., Hoszowski A., Zajac M., Szulowski K.. 2013. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front. Microbiol. 4:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2011. Critically important antimicrobials for human medicine. Accessed Jan. 2017. http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf. [Google Scholar]
- World Health Organization. 2015. Global Action Plan on Antimicrobial Resistance. WHO, Geneva. [DOI] [PubMed] [Google Scholar]
- World Organisation for Animal Health – OIE. 2016. Terrestrial animal health code glossary. Accessed Oct. 2016. http://www.oie.int/index.php?id=169&L=0&htmfile=glossaire.htm#terme_antibiotique. [Google Scholar]
- Wu H., Xia S. B., Bu F. Y., Qi J., Liu Y. Q., Xu H.. 2015. Identification of integrons and phylogenetic groups of drug-resistant Escherichia coli from broiler carcasses in China. Int. J. Food Microbiol. 211:51–56. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Chen S., White D. G., Zhao S. H., McDermott P., Walker R., Meng J. H.. 2004. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J. Clin. Microbiol. 42:3483–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. G., Chu W. H., Wang H. F., Zhu W.. 2012. Antimicrobial susceptibility and virulence factors of Escherichia coli isolates obtained from faeces samples of chickens in east China. Afr. J. Microbiol. Res. 6:1591–1596. [Google Scholar]
- Zhang A. Y., He X. M., Meng Y., Guo L. J., Long M., Yu H., Li B., Fan L. Q., Liu S. L., Wang H. N., Zou L. K.. 2016. Antibiotic and disinfectant resistance of Escherichia coli isolated from retail meats in Sichuan, china. Microb. Drug Resist. 22:80–87. [DOI] [PubMed] [Google Scholar]
- Zhang J. Y., Massow A., Stanley M., Papariella M., Chen X., Kraft B., Ebner P.. 2011. Contamination rates and antimicrobial resistance in Enterococcus spp., Escherichia coli, and Salmonella isolated from "no antibiotics added''-labeled chicken products. Foodborne Pathog. Dis. 8:1147–1152. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wang C. G., Jiang G. E., Lv J. C., Zhong X. H.. 2012. Molecular epidemiological survey on aminoglycoside antibiotics-resistant genotype and phenotype of avian Escherichia coli in North China. Poult. Sci. 91:2482–2486. [DOI] [PubMed] [Google Scholar]
- Zhang T., Wang C. G., Lv J. C., Wang R. S., Zhong X. H.. 2012. Survey on tetracycline resistance and antibiotic-resistant genotype of avian Escherichia coli in North China. Poult. Sci. 91:2774–2777. [DOI] [PubMed] [Google Scholar]
- Zhang X. L., Li Y. X., Liu B., Wang J., Feng C. H., Gao M., Wang L. N.. 2014. Prevalence of veterinary antibiotics and antibiotic-resistant Escherichia coli in the surface water of a livestock production region in Northern China. PLoS One 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.