Abstract
BACKGROUND
A systematic literature review was conducted to determine whether US blacks and whites have differential blood pressure (BP) response to calcium channel blocker (CCB) monotherapy.
METHODS
Six published studies made up the final cohort of eligible articles. Multiple treatment groups within some studies led to a total of eight sets of estimates for BP reduction with a total of 6,851 white or nonblack participants and 3,371 black participants.
RESULTS
The pooled difference in systolic blood pressure (SBP) change between blacks and whites was –2.7 mm Hg (95% confidence interval (CI): –4.0, –1.3) with blacks having greater response. The difference in diastolic blood pressure (DBP) between blacks and whites was –0.4 mm Hg (95% CI: –1.0, 0.3) with blacks having greater response. Using a dichotomous outcome measure, whites were found to be just as likely as blacks to attain the DBP goal of <90 mm Hg or a 10 mm Hg or greater change (relative risk: 1.00 95% CI: 0.91, 1.11). In addition, examination of the continuous distribution of BP responses of whites and blacks showed over 90% overlap in treatment response.
CONCLUSION
Assessment of differential response to CCB monotherapy by race in published data depends on choice of outcome metric. Nonetheless, the results of this systematic review indicate that BP response is qualitatively similar in US blacks and whites, suggesting that patient race is not likely to offer any clinical utility for decisions about the likely effect of this antihypertensive therapy.
There has been much speculation over the past 40 years of hypertension research regarding differential effectiveness of therapeutic regimens as a function of patient’s racial identity.1 This has been a controversial literature because race as an epidemiologic quantity has an ambiguous status as a social grouping, ancestry marker and surrogate for myriad psychological and material disadvantages.2,3 Nonetheless, numerous studies have compared antihypertensive responses in various racial groups, with the focus in US studies being comparisons between so-called “blacks” (i.e., Americans with ancestry from sub-Saharan Africa) and “whites” (Americans with ancestry from Europe and the Middle East).4–7 The general paradigm of putative racial variations in therapeutic effectiveness is furthered by similar claims for treatment of other cardiovascular outcomes,8 and by the advent of the first Food and Drug Administration–approval for a race-specific therapy in 2005.9,10
The existing hypertension literature emphasizes a decreased effectiveness for blacks to monotherapy with drugs that modulate the renin–angiotensin–aldosterone system,11,12 which is interpreted as a consequence of blacks being a “low renin” population.13 Calcium channel blockers (CCBs) and diuretics do not inhibit the renin–angiotensin system, and this has therefore been one of the proposed explanations for observed findings that CCBs and diuretics are more effective than other therapies in black patients.14 The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) states that in blacks, CCBs or thiazide-type diuretics are more effective than angiotensin-converting enzyme inhibitors.14 Similarly, the guidelines from the European Society of Hypertension, the European Society of Cardiology, and the British Hypertension Society recommend CCBs and diuretics in black patients over β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor antagonists.15,16 Many published sources also compare efficacy across racial groups and suggest an equivalent or superior antihypertensive effect for CCBs in blacks compared to whites.17,18
A systematic review of black vs. white blood pressure (BP) reductions for multiple antihypertensive classes published by Sehgal in 2004 found a small differential for CCBs in the direction of a black advantage, but with substantial overlap between the BP response distributions of each racial group.19 However, clinical interest is focused not only on the effect of pharmacologic monotherapy on systolic (SBP) and diastolic blood pressure (DBP) change, but also the achievement of treatment goal. In the current review, we sought to complete an updated systematic review of clinical trials in which monotherapy with CCBs is compared in blacks and whites in the United States, especially in light of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial, whose results on race-specific reduction in BP became available after the last systematic review. We examined between-study variance more carefully than had been previously done, compared BP declines from baseline and achievement of previously defined treatment goals, and considered both the distribution of responses as well as the black-white “overlap” in treatment response as defined previously.19
METHODS
Selection of trials.
A systematic literature search was conducted on PubMed, EMBASE, and the Cochrane Clinical Trials Database. The following text words were used: CCBs, African American, African Americans, race, racial, black, blacks, white, whites, hypertension, BP and (individual drug names) amlodipine, bepridil, diltiazem, felodipine, isradipine, nicardipine, nifedipine, nimodipine, nisoldipine, or verapamil. In PubMed, the following Medical Subject Headings terms were added: CCBs, African Continental Ancestry Group, and European Continental Ancestry Group. Studies were included if they provided race-specific changes in BP for blacks and whites treated with a CCB. Studies were excluded if they recruited children, were published before 1990, were conducted outside the United States, involved combination therapy, or did not provide race-specific mean changes in SBP or DBP for both whites and blacks. The last search was conducted on 8 October, 2008. From the 775 articles identified, 36 did not provide information on CCBs, 18 involved combination therapy, 2 enrolled children, 166 did not provide race-specific estimates, 21 were non-US studies, 337 did not provide BP reductions from baseline, 147 did not provide BP reductions for both blacks and whites, and 37 articles reported results from a study already included in the analysis. Of the articles that met the selection criteria, 5 did not provide a measure of precision of the estimates (s.e., s.d., or CI). The corresponding authors were contacted for missing information, but one author was unreachable, and the others were unable to provide the additional information. Six published studies made up the final cohort of eligible articles. Multiple treatment groups within two studies led to a total of eight sets of estimates for BP reduction that included 6,851 whites (or “nonblacks”) and 3,371 blacks.
Data extraction.
After the establishment of exclusion and inclusion criteria, one investigator extracted the data. The race-specific mean reduction in SBP and DBPs from baseline and the corresponding standard errors or standard deviations were extracted. The black vs. white differences in mean reduction of SBP and DBP were also recorded. When the standard errors for the white–black differences were not provided, the standard errors were calculated using the following equation: standard error of white–black difference This equation is justified by the fact that the two samples are statistically independent, and therefore the covariance is 0 (although no assumption is imposed that the standard errors for blacks and whites are equal). We also extracted the necessary data to calculate the proportion of black and white patients reaching the BP target. The institutional review board at the University of North Carolina, Chapel Hill reviewed the protocol and determined that it did not require institutional review board’s approval because only published data with no patient identifiers were used.
Statistical methods.
Statistical analyses were conducted using STATA 9.0 (Stata, College Station, TX). Random effect estimates were calculated. The race-specific reductions in BP from baseline to post-treatment were pooled. The differences between the two race-specific BP reductions were also pooled, and we refer to this quantity as the white–black difference in treatment response. Publication bias was assessed using Begg’s and Egger’s tests, and the Duval and Tweedie nonparametric “trim and fill” method was utilized to account for the presence of publication bias. The presence of heterogeneity was assessed by examining the P value of Cochran’s Q statistic. The among-studies variance τ2 was also used to examine heterogeneity. The following study characteristics were examined in stratified analyses: type of drug group (Nondihydropyridines or dihydropyridines), age, percent female, and percent receiving antihypertensive medications, and treatment duration. Due to the relatively small number of trials, the validity of estimates controlling for multiple characteristics simultaneously would be questionable, and we therefore examined each study characteristic separately.
Using the well-known decomposition of the population variance shown in Eq. 1, sample sizes and standard deviations extracted from the individual articles were combined with the estimated pooled means in order to estimate the pooled variances.
| (1) |
Using the pooled means and the pooled standard deviations (square root of the variances), distributions of the decrement in BP by race were constructed. The authors plotted the distribution of BP response of whites and blacks together for comparison. Overlap in treatment response was calculated by summing the area under each curve in the overlapping region and expressing the sum as a percentage of the total area (see Supplementary Appendix A1 online)19.
RESULTS
Table 1 displays characteristics of the included studies. The estimates of race-specific BP reductions (i.e., the differences between the baseline and post-treatment measures) as well as the between-race differences in BP reduction were heterogeneous (P < 0.05). Stratified analyses indicated the estimates within the dihydropyridine drug group and the group of studies with no female participants were reasonably homogeneous. Within categories of all other study characteristics, at least some of the estimates were heterogeneous (see Supplementary Table S1 online).
Table 1 |.
Characteristics of trials
| No. of subjects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Randomized | Blinded | Whites or nonblacksa |
Blacks | Mean age | Percent female |
Treatment | Duration (weeks) |
Drug | Min-max dose (mg/day) |
| Krakoff (1990)21b | No | No | 803 | 304 | 54 | 32 | Monotherapy | 13–18 | Nifedipine± | 30–180 |
| Black (1992)27c | Yes | Yes | 53a | 34 | 55 | 33 | Monotherapy | 12 | Diltiazem | 40–120 |
| Black (1992)27c | Yes | Yes | 50a | 37 | 56 | 34 | Monotherapy | 12 | Isradipine | 2.5–10 |
| Weir (1992)28 | Yes | No | 35 | 33 | 54.1 | NA | Monotherapy | 2–8 | Nifedipine± | 30–120 |
| Materson (1993A)18d | Yes | Yes | 38 | 37 | NA | 0 | Monotherapy | 4–8 | Diltiazem± | 60–180 |
| Materson (1993B)18d | Yes | Yes | 52 | 53 | NA | 0 | Monotherapy | 4–8 | Diltiazem± | 60–180 |
| Kloner (1996)29 | No | No | 857 | 227 | 55.5 | 35 | Monotherapy | 4 | Amlodipine | 5–10 |
| Wright (2005)20b | Yes | Yes | 4,963a | 2,646 | 66.1 | 54.4 | Add-on therapy Starting with CCB |
52 | Amlodipine | 2.5–10 |
Study participants were nonblacks
Sustained-release.
Not included in pooled analysis.
Same rial, two drug groups.
Same trial, A = participants <60 years old B = participants ≥60 years old.
When the Wright20 and Krakoff21 studies were excluded, the pooled estimates of the white–black differences were no longer significantly heterogeneous. Despite the absence of significant heterogeneity in pooled white–black differences after exclusion of these two studies, race-specific reductions remained significantly heterogeneous. As a result, general trends are the focus of the current review, rather than relying only on a single overall effect estimate. Nonetheless, pooled race-specific changes in BP are also presented for completeness.
Blacks generally had similar though slightly higher baseline pressures than whites (see Supplementary Table S2 online). Table 2 presents treatment response by race for each study. White–black differences in BP decrement for each study are also presented on Table 2. All but one study reported blacks having greater mean reductions than whites, and pooled results also indicate greater reduction for blacks (Table 3). The white–black differences in SBP and DBP reduction were –2.7 mm Hg (95% CI: –4.0, –1.3) (Figure 1), and –0.4 mm Hg (95% CI: –1.0, 0.3) (Figure 2), respectively. The estimated among-study variance τ2 was essentially 0 for both pooled estimates, and tests of homogeneity indicated minimal variability in racial contrasts across studies. For white–black differences in SBP, the P value for Begg’s test was consistent with the null (P = 0.2), but Egger’s test was more suggestive of the possibility of additional unpublished studies (P = 0.03) (Table 3). To account for the possibility of publication bias, we imputed estimates using the Duval and Tweedie nonparametric “trim and fill” method. Nonetheless, estimates taking account of publication bias were generally similar, although slightly closer to the null. Moreover, for DBP there was considerably less evidence of publication bias (Begg’s test P = 0.3 and Egger’s test P = 0.7) (Table 3).
Table 2 |.
White–black differences in blood pressure response
| Race-specific reduction in blood pressure (mm Hg) | White–black difference in response (mm Hg) | |||||
|---|---|---|---|---|---|---|
| Systolic | Diastolic | |||||
| Author (year) | Whites | Blacks | Whites | Blacks | Systolic | Diastolic |
| Krakoff (1990)21a | 16.5 (15.5, 17.5) | 18.5 (17.0, 20.0) | 12.9 (12.4, 13.4) | 13.9 (13.0, 14.8) | –2.0 (–3.8, –0.2) | –1.0 (–2.0, 0.0) |
| Weir (1992)28 | 12.6 (8.4, 16.8) | 16.8 (11.2, 22.4) | 10.7 (9.6, 11.8) | 12.1 (10.2, 14.0) | –4.2 (–11.2, 2.8) | –1.4 (–3.6, 0.8) |
| Black (1992)27b | not reported | not reported | 12.1 (10.5, 13.7) | 13.0 (10.8, 15.2) | not reported | –0.9 (–3.6, 1.8) |
| Black (1992)27b | not reported | not reported | 13.6 (11.3, 15.9) | 11.4 (8.4, 14.4) | not reported | 2.2 (–1.6, 6.0) |
| Materson (1993A)18c | 11.0 (8.1, 13.9) | 14.0 (10.8, 17.2) | 13.0 (11.4, 14.6) | 14.0 (12.4, 15.6) | –3.0 (–7.3, 1.3) | –1.0 (–3.3,1.3) |
| Materson (1993B)18c | 12.0 (9.6, 14.4) | 15.0 (13.1, 16.9) | 14.0 (12.6, 15.4) | 15.0 (13.7, 16.3) | –3.0 (–6.1, 0.1) | –1.0 (–2.9, 0.9) |
| Kloner (1996)29 | 15.8 (15.0, 16.6) | 18.2 (16.7, 19.7) | 12.5 (12.1, 12.9) | 12.6 (11.9, 13.3) | –2.4 (–4.1, –0.7) | –0.1 (–0.9, 0.7) |
| Wright (2005)20a | 8.4 (7.9, 8.9) | 5.7 (5.0, 6.4) | 5.6 (5.3, 5.9) | 4.1 (3.7, 4.5) | 2.7 (1.8, 3.6) | 1.5 (1.0, 2.0) |
Values in parentheses are the 95% confidence intervals.
Not included in pooled analysis.
Same trial, two drug groups.
Same trial, A = participants <60 years old B=participants ≥60 years old.
Table 3 |.
Pooled estimates
| No. of studies |
Pooled estimate |
95% CI |
P for test of homogeneity |
τ2a |
P for Begg’s Test |
P for Egger’s test |
|
|---|---|---|---|---|---|---|---|
| Relative risk comparing proportion of blacks and whites (referent) reaching BP target of DBP <90 or a decline of >10 mm Hg | 4 | 1.0 | (0.9, 1.0) | 0.6 | 0.0 | 0.2 | 0.5 |
| White–black differences in SBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 4 | −2.7 | −4.0, −1.3) | >0.99 | 0.0 | 0.2 | 0.03 |
| Including Wright, Krakoff | 6 | −0.8 | (−3.3, 1.8) | <0.001 | 7.8 | 0.9 | 0.1 |
| White–black differences in DBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 6 | −0.4 | (−1.0, 0.28) | 0.6 | 0.0 | 0.3 | 0.7 |
| Including Wright, Krakoff | 8 | 0.2 | (−0.8, 1.1) | 0.001 | 1.0 | 0.6 | 0.1 |
| White SBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 4 | 13.1 | (10.2, 15.9) | <0.001 | 6.6 | 0.5 | 0.1 |
| Including Wright, Krakoff | 6 | 12.7 | (8.9, 16.6) | <0.001 | 22.2 | 0.9 | 0.5 |
| White DBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 6 | 12.5 | (11.6, 13.4) | 0.006 | 0.8 | 0.3 | 0.8 |
| Including Wright, Krakoff | 8 | 11.8 | (8.8, 14.8) | <0.001 | 18.4 | 0.8 | 0.2 |
| Black SBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 4 | 16.1 | (13.8, 18.4) | 0.02 | 3.3 | >0.99 | 0.5 |
| Including Wright, Krakoff | 6 | 14.6 | (8.6, 20.7) | <0.001 | 54.5 | 0.9 | 0.2 |
| Black DBP (mm Hg) | |||||||
| Excluding Wright, Krakoff | 6 | 13.2 | (12.2, 14.2) | 0.02 | 0.9 | 0.e | 0.8 |
| Including Wright, Krakoff | 8 | 12.0 | (8.0, 16.0) | <0.001 | 32.4 | 0.1 | 0.1 |
Among-study variance.
Figure 1|.
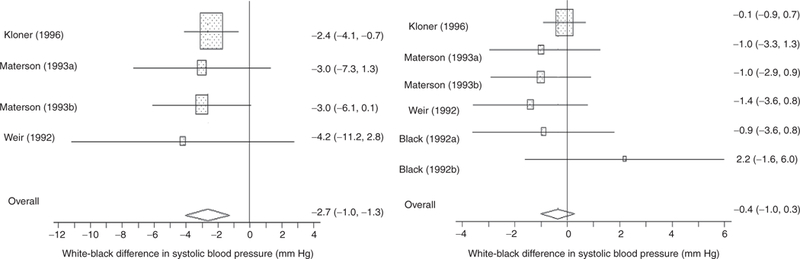
Systolic and diastolic blood pressure reduction from baseline: white–black differences (95% confidence interval).
Figure 2|.
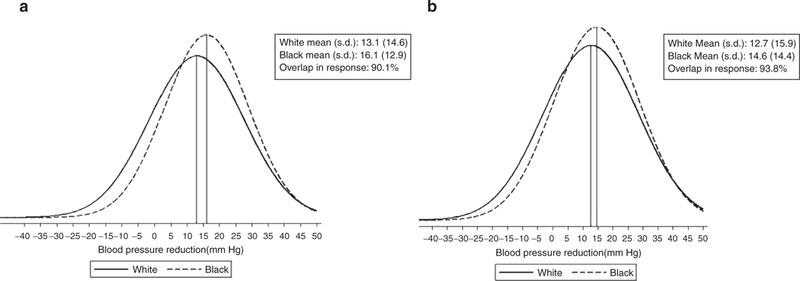
SBP reduction (a) excluding Wright20 and Krakoff21 and (b) including Wright20 and Krakoff.21
Figures 2 and 3 present distributions of treatment responses, with and without the Wright20 and Krakoff21 studies. As shown in Figures 2 and 3, exclusion of these two studies brings the white and black mean BP reduction closer to the null. As shown in the figures, there was almost complete overlap in the race- specific BP responses to CCB monotherapy. The overlap in SBP response is 93.8 and 90.1% in the reduced data set, whereas the comparable numbers for DBP response are 94.8 and 93.4%.
Figure 3|.
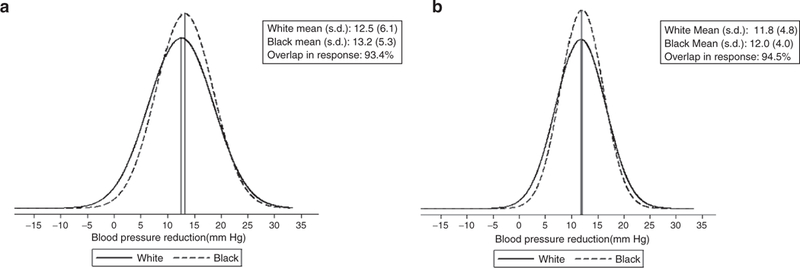
DBP reduction (a) excluding Wright20 and Krakoff21 and (b) including Wright20 and Krakoff.21
Considering the proportion of patients reaching a clinically defined BP goal, the trials were inconsistent on the direction of the racial disparity with some reporting greater efficacy among blacks and others reporting the opposite or equal response. In all studies, however, the proportions of whites and blacks reaching treatment goals were within 10% of each other, except one where they differed by 20% (Table 4). Pooled results indicated that whites were just as likely as blacks to attain the goal of DBP <90 mm Hg or DBP decrement of >10 mm Hg (relative risk = 1.0; 95% CI: 0.9, 1.0). There was little evidence of publication bias (Begg’s test P = 0.2, Egger’s test P = 0.5). The estimated between-studies variance τ2 was essentially 0, indicating that the estimates may be considered as homogeneous (Table 3). Overall, these results consistently support the conclusion that whites and blacks have similar likelihood of achieving the BP goal.
Table 4 |.
Proportion of subjects achieving blood pressure target
| No. of subjects | No. reached target | Response proportion | ||||||
|---|---|---|---|---|---|---|---|---|
| Author (year) | Duration (week) |
Target | Whites | Blacks | Whites | Blacks | Whites | Blacks |
| Krakoff (1990)21a | 13–18 | DBP <90 or decline of >10 | 790 | 301 | 591 | 244 | 0.75 | 0.81 |
| Weir (1992)28 | 2–8 | DBP <90 or decline of >10 | 35 | 33 | 30 | 26 | 0.86 | 0.79 |
| Materson (1993A)18b | 4–8 | DBP <90 | 39 | 36 | 17 | 23 | 0.44 | 0.64 |
| Materson (1993B)18b | 4–8 | DBP <90 | 52 | 53 | 33 | 34 | 0.63 | 0.64 |
| Kloner (1996)29 | 4 | DBP <90 or decline of >10 | 857 | 227 | 737 | 195 | 0.86 | 0.86 |
| Wright (2005)20a | 52 | SBP/DBP <140/90 | 4,960 | 2,646 | 2,862 | 1,336 | 0.58 | 0.51 |
Not included in pooled analysis.
Same trial, A = participants <60 years old B =participants ≥60 years old.
DISCUSSION
These results are relevant to the practical question of whether or not one should consider patient race when making clinical decisions about choice of antihypertensive therapy. In the case of CCB monotherapy for the treatment of hypertension, blacks generally experienced slightly greater declines in continuous-scale measures of BP. These results are similar to a previous meta-analysis which reported white–black differences in SBP and DBP response to CCB monotherapy of –2.4 mm Hg (95% CI: –3.4, –1.3) and –0.6 mm Hg (95% CI: –1.2, 0.0), respectively.19 As a dichotomous outcome of meeting BP goals, we found blacks and whites are equally likely to reach the defined target pressures. Nonetheless, regardless of the outcome definition, racial group provides at best a trivial amount of information about expected BP reduction for an individual patient. Although previous studies have argued that racial differences in renin–angiotensin system activity result in racial differences in drug response, differences in mean renin–angiotensin system activity are quite modest,22 and there is substantial overlap in activity between the racial groups.23 A statement about the mean response of a population of patients is not the same as a statement about the expected response of an individual patient, and the degree of overlap of the response distributions indicates that clinicians would learn very little about the likely response of an individual patient by considering them as drawn from the race-specific response distribution as opposed to the overall response distribution.
Our results are consistent with the recent review by Johnson et al., which found no obvious difference between blacks and whites in outcomes to various antihypertensive therapies and concluded that clinical decisions should not be based on race/ethnicity.24 Furthermore, the results of the International Verapamil-Trandolapril Study similarly found no differences in event rates for death, myocardial infarction, or stroke for whites, blacks, or Hispanics randomized to receive either a CCB or a β-blocker.25 The African American Study of Kidney Disease and Hypertension trial, which randomized black participants to receive either a β-blocker, CCB, or angiotensinconverting enzyme inhibitor, reported that DBP and SBP during follow-up were similar across the three drug groups.26 Although the racially homogeneous study population precludes examination of racial differences in outcomes, the African American Study of Kidney Disease and Hypertension results suggest that blacks have similar response to various antihypertensive therapies.
There are several limitations to this study. First, only one reviewer extracted the data. However, this is unlikely to result in substantial error as the extraction focused primarily on numerical data, with limited opportunity for subjectivity or interpretation. A complication in our analysis was the question of effect heterogeneity across studies. As the race-specific BP decrements were apparently heterogeneous, general trends were described rather than relying on a single uniform estimate. Pooled estimates were also provided, but could be considered as an over-simplification of the literature. In the case of estimating the white–black differences, we considered exclusion of two trials18,19 from some of the pooled analyses in order to minimize the differences in study design and provide estimates that were more homogeneous. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial reported by Wright et al.20 is by far the largest trial included in the meta-analysis and is one of the two trials excluded. This study differs in important ways from the others, including allowing individual clinicians to switch patients off of the assigned treatment or to assign additional medications if participants taking a CCB did not reach the BP goal of 140/90 mm Hg. In addition, although we extracted information for the shortest treatment duration available for Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial (52 weeks), the treatment duration for this trial was still considerably longer than the other studies (2–18 weeks). Furthermore, in all included studies, participants were given increasing doses of the study drug within a particular dosing range until the BP goal was achieved. Blacks and whites may have differed in the average dose received, especially in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack trial study where patients were treated by a myriad of individual clinicians. These or other unmeasured factors may account for the observed heterogeneity and racial differences in response. It would be unethical, however, to maintain hypertensive research subjects on an ineffective dose or regiment, which points out important limitations to randomized trials in studying this question. The heterogeneity of the results prevents us from providing a simple and definitive summary statement about racial differences in BP response.
It is also important to keep in mind that the black and white patient groups analyzed here certainly differed in characteristics other than racial identity. We were unable to explore whether socioeconomic, psychological or behavioral differences might have accounted for the racial difference found because none of the trials reported covariate information on these quantities. Racial difference in baseline BP or previous treatment is another source of variability that could explain differences in response (see Supplementary Table S2 online). Although racial differences in baseline BPs may partly explain the observed white–black difference in BP response for CCBs, it is unlikely to explain the entire difference. A previously published meta-analysis included studies in which blacks tended to have slightly higher baseline BP than whites, yet found whites to have greater BP reduction when treated with a angiotensin-converting enzyme inhibitor but lower BP reduction when treated with a CCB.19
We have extended previous studies of racial disparity in responsiveness to CCB monotherapy not only by including more recent studies, but also by examining several outcome measures. Moreover, our study makes a more careful assessment of between-studies variance than do previous publications, including a sensitivity analysis for the impact of removing two studies that may be incommensurate in some ways. By comparing race-specific BP reduction, estimating mean white–black differences in BP reduction, comparing the likelihood of patients in each race group reaching a specified BP target, constructing distributions of BP decrement by race, and calculating the overlap in the BP reduction distributions, we have comprehensively examined the literature to investigate comparative efficacy. However, due to the inherent limitations of our data, most notably the observed heterogeneity between studies, there may occur racial differences in BP response in either direction that were not reflected in our pooled analyses. Despite these caveats, however, it remains clear that there is extensive overlap in response between racial groups in all studies. The absence of quantitative evidence for substantial differences in BP response rules out a rational role for patients’ race in clinical decisions about use of CCB monotherapy.
Supplementary Material
Acknowledgment:
We thank Kathleen A. McGraw, MA MLS. This work was supported in part by a Robert Wood Johnson Foundation Investigator Award in Health Policy Research (J.S.K. and R.S.C.). The views expressed imply no endorsement by the Robert Wood Johnson Foundation.
Footnotes
Disclosure: The authors declared no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
References
- 1.Hall WD, Kong W. Hypertension in blacks: nonpharmacologic and pharmacologic therapy. Cardiovasc Clin 1991; 21:157–169. [PubMed] [Google Scholar]
- 2.Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol 2001; 154:291–298. [DOI] [PubMed] [Google Scholar]
- 3.Cooper RS, Kaufman JS. Race and hypertension: science and nescience. Hypertension 1998; 32:813–816. [DOI] [PubMed] [Google Scholar]
- 4.Wood AJ. Racial differences in the response to drugs--pointers to genetic differences. N Engl J Med 2001; 344:1394–1396. [DOI] [PubMed] [Google Scholar]
- 5.Wood AJ. Ethnic differences in drug disposition and response. Ther Drug Monit 1998; 20:525–526. [DOI] [PubMed] [Google Scholar]
- 6.Jamerson K, DeQuattro V. The impact of ethnicity on response to antihypertensive therapy. Am J Med 1996; 101:22S–32S. [DOI] [PubMed] [Google Scholar]
- 7.Bhopal R, Donaldson L. White, European, Western, Caucasian, or what? Inappropriate labeling in research on race, ethnicity, and health. Am J Public Health 1998; 88:1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1 351–1357. [DOI] [PubMed] [Google Scholar]
- 9.Carmody MS, Anderson JR. BiDil (isosorbide dinitrate and hydralazine): a new fixed-dose combination of two older medications for the treatment of heart failure in black patients. Cardiol Rev 2007; 15:46–53. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Fernandez A. BiDil for heart failure in black patients: implications of the U.S. Food and Drug Administration approval. Ann intern Med 2007; 146:52–56. [DOI] [PubMed] [Google Scholar]
- 11.Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann intern Med 2004; 141:614–627. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan NM, Flynn JT. Clinical Hypertension, 9th edn Lippincott Williams & Wilkins: Philadelphia, 2005. [Google Scholar]
- 13.Khan JM, Beevers DG. Management of hypertension in ethnic minorities. Heart 2005; 91:1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. <http://www.nhlbi.nih.gov.libproxy.lib.unc.edu/guidelines/hypertension/jnc7full.pdf>. Accessed 11 September 2008.
- 15.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P Redon J, Ruschitzka F, Tamargo J, van Zwieten P Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007; 28:1462–536. [DOI] [PubMed] [Google Scholar]
- 16.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, McG Thom S; British Hypertension Society. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens 2004; 18:139–185. [DOI] [PubMed] [Google Scholar]
- 17.Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr 2007; 18:241–247. [PMC free article] [PubMed] [Google Scholar]
- 18.Materson BJ, Reda DJ, Williams D. Lessons from combination therapy in Veterans Affairs Studies. Department of Veterans Affairs Cooperative Study Group on antihypertensive agents. Am J Hypertens 1993; 9:187S–191S. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal AR. Overlap between whites and blacks in response to antihypertensive drugs. Hypertension 2004; 43:566–572. [DOI] [PubMed] [Google Scholar]
- 20.Wright JT, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, Probstfield JL, Whelton PK, Habib GB. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005; 293:1595–1608. [DOI] [PubMed] [Google Scholar]
- 21.Krakoff LR, Bravo EL, Tuck ML, Friedman CP Nifedipine gastrointestinal therapeutic system in the treatment of hypertension. Results of a multicenter trial. The Modern Approach to the Treatment of Hypertension (MATH) Study Group. Am J Hypertens 1990; 3:318S–325S. [PubMed] [Google Scholar]
- 22.Havranek EP From black and white to shades of gray: race and renin–angiotensin system blockade. J Am Coil Cardiol 2008; 51:1872–1873. [DOI] [PubMed] [Google Scholar]
- 23.Sagnella GA. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens 2001; 15:17–25. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation 2008; 1 18:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816. [DOI] [PubMed] [Google Scholar]
- 26.Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASKtrial. JAMA 2002; 288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 27.Black HR, Lewin AJ, Stein GH, MacCarthy EP, Hamilton JH, Hamilton BP, Madias NE, Kochar MS, Abrams AP Isaacsohn JL. A comparison of the safety of therapeutically equivalent doses of isradipine and diltiazem for treatment of essential hypertension. Am J Hypertens 1992; 5:141–146. [DOI] [PubMed] [Google Scholar]
- 28.Weir MR, Lavin PT. Comparison of the efficacy and tolerability of Prinivil anc Procardia XL in black and white hypertensive patients. Clin Ther 1992; 14:730–739. [PubMed] [Google Scholar]
- 29.Kloner RA, Sowers JR, DiBona GF, Gaffney M, Wein M. Sex- and age-related antihypertensive effects of amlodipine. The Amlodipine Cardiovascular Community Trial Study Group. Ami J Cardiol 1996; 77:713–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


