Abstract
The transient receptor potential vanilloid isoform 4 (TRPV4) functions as polymodal transducer of swelling, heat, stretch, and lipid metabolites, is widely expressed across sensory tissues, and has been implicated in pressure sensing in vertebrate retinas. Although TRPV4 knockout mice exhibit a variety of mechano-sensory, nociceptive, and thermo- and osmoregulatory phenotypes, it is not known whether the transmission of light-induced signals in the eye is affected by the loss of TRPV4. We utilized field potentials, a measure of rod and cone signaling, to determine whether TRPV4 impacts on the generation and/or transmission of the photoreceptor light response and neurotransmission. Luminance intensity-response relationships were acquired in anesthetized wild-type and TRPV4−/− mice and evaluated for peak amplitude and implicit time under scotopic and photopic conditions. We found that the morphology of the outer retina is unaffected by the ablation of the Trpv4 gene. Calcium imaging of dissociated Müller glia showed that selective TRPV4 stimulation induces oscillatory calcium signals in adjacent rods. However, no differences in scotopic or photopic light-evoked signaling in the distal retina were observed in TRPV4−/− eyes, suggesting that TRPV4 signaling in healthy Müller cells does not modulate the transmission of light-evoked signals at rod and cone synapses.
Keywords: TRPV4, Müller glia, Photoreceptor
67.1. Introduction
TRPV4 is a polymodal channel widely expressed across the vertebrate bone, cartilage, muscle, skin and internal organs (lung, bladder, heart, kidney, oviduct), and the brain where it functions as a dominant neuronal and glial volume sensor (White et al. 2016). Its prominent expression across auditory, nociceptive, thermosensitive, mechanosensitive, and visual pathways (White et al. 2016; Redmon et al. 2017) is suggestive of important functional roles in sensory signaling. The strong expression levels of TRPV4 mRNA/protein in the cornea (Mergler et al. 2012), ciliary body (Jo et al. 2016), trabecular meshwork (Ryskamp et al. 2016), lens (Shahidullah et al. 2012), and retina (Ryskamp et al. 2011) predict that this polymodal nonselective cation channel contributes to the regulation of aqueous fluid transport, accommodation, ocular pressure sensing, endocannabinoid signaling, and/or volume regulation in the eye (Krizaj et al. 2014; Krizaj 2016); however whether and what roles TRPV4 plays in visual signaling is unknown. Here, we show that TRPV4 activation in Müller cells, radial glia that play a key role in retinal volume regulation and transretinal propagation of calcium waves (Jo et al. 2015; Phuong et al. 2016), induces a calcium response in adjacent photoreceptors. Moreover, we tested the hypothesis that TRPV4 activation modulates the light response in the outer retina by recording field potentials in wild-type and TRPV4−/− mice under scotopic and photopic conditions.
67.2. Materials and Methods
67.2.1. Animals
C57BL/6 J and TRPV4−/− mice were maintained in the university animal vivarium on a 12 h:12 h light-dark cycle and fed lab chow and water ad libitum. Animal handling and anesthetic procedures were approved by the University Institutional Animal Care committees and conform to National Institutes of Health guidelines. Intraocular pressure was elevated through injection of polystyrene microbeads, as described (Ryskamp et al. 2016).
67.2.2. Histology
Immunohistochemical experiments were conducted as described (Renteria et al. 2005; Jo et al. 2015). The primary antibodies were LS-C94498 anti-TRPV4 (Lifespan Biosciences) which showed appropriate immunoblot molecular weight and did not label TRPV4−/− sections (Ryskamp et al. 2011, 2016; Jo et al. 2015, 2016) and glial fibrillary acidic protein (GFAP) (Sigma), a well established marker of retinal gliosis.
67.2.3. Electroretinographic Analysis
Full-field ERGs were recorded as described (Duncan et al. 2006; Barabas et al. 2013). Briefly, 2–3-month-old C57BL/6 and TRPV4−/− mice were dark-adapted and anesthetized with ketamine/xylazine (90 mg/10 mg per kg body weight). The animals were placed on a heating pad, and a golden ERG electrode was placed on the cornea surface. Stimuli were in order of increasing luminance from 0.00025 to 79 cd.s/m2 (scotopic) and from 2.5 to 79 cd.s/m2 (photopic), and 2–12 traces were averaged. The photoflash unit was calibrated to deliver 2.5 cd.s/m2 at 0 dB.
67.2.4. Calcium Imaging
Calcium imaging followed the protocols described in Ryskamp et al. (2011; 2016).
67.3. Results
67.3.1. TRPV4 Is Localized to RGCs and Müller Glia
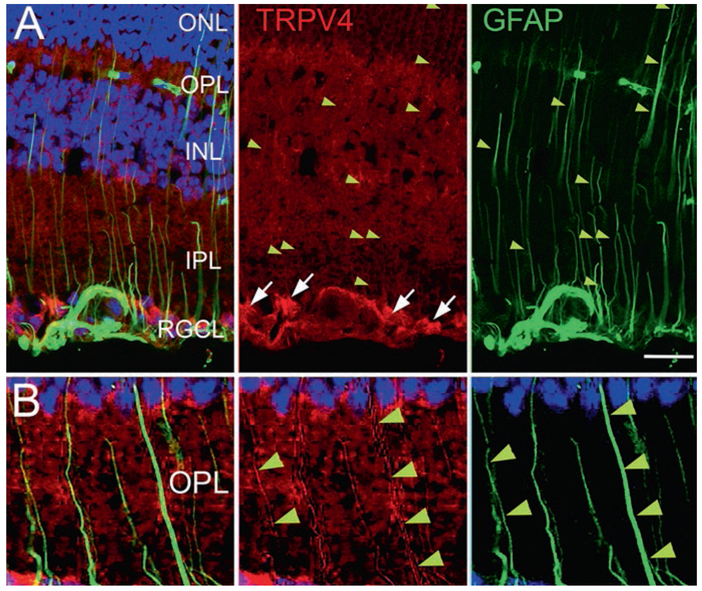
To better visualize the radial Müller processes, we double-labeled retinal sections from hypertensive eyes in which IOP was elevated with microbead injections, for TRPV4 and the gliotic marker GFAP. Expression of the TRPV4 channel was prominent in retinal ganglion cell layer (RGCs; somata in Fig. 67.1a marked by arrows) and Müller perikarya in the inner nuclear layer (INL). TRPV4 was also expressed in the transverse processes of Müller cells within the IPL and ONL (marked by yellow arrowheads in Fig. 67.1a, b). Its expression in the ONL and OPL suggests a potential role in the modulation of photoreceptor function.
Fig. 67.1.
(A) TRPV4 in the hypertensive retina colocalizes with the gliotic marker GFAP. The TRPV4 antibody labels RGC somata (white arrows) and radial processes of Müller cells (yellow arrowheads). Scale bar = 20 um. (B) A higher-resolution image from another section
67.3.2. TRPV4 Activation of Müller Cells Elevates [Ca2+]i in Adjacent Rod Photoreceptors
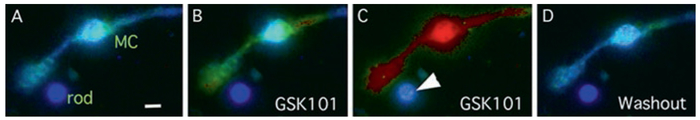
Stimulation of dissociated Müller glia with the selective TRPV4 agonist GSK1016790A triggered Ca2+ waves together with large sustained [Ca2+]i elevations across the endfoot, cell body and the apical process (Fig. 67.2; also Ryskamp et al. 2014). Occasionally, a rod soma was located close to the glial processes, and we observed that passage of the glial Ca2+ wave often correlated with [Ca2+]i elevations within the photoreceptor (Fig. 67.2c, arrowhead). TRPV4-dependent calcium signals were never observed in rods that were apart from Müller cells. They were typically transient and oscillatory, and disappeared upon agonist washout. We thus hypothesized that TRPV4-induced Ca2+ signals drive the release of a neuroactive substance from Müller processes (e.g., Seminario-Vidal et al. 2011), which led us to test whether the channel plays a modulatory role in light-induced signaling.
Fig. 67.2.
TRPV4-mediated calcium signals in Müller cell induced by the selective agonist GSK1016790A (GSK101) correlate with oscillatory increase in [Ca2+]i in the nearby rod soma (white arrowhead). (A) Dissociated Müller cell (MC) and rod photoreceptor in control saline show low [Ca2+]i levels. (B) GSK101 induces calcium wave propagation across Müller processes. (C) The [Ca2+]i increase in the endfoot process correlates with transient oscillatory [Ca2+]i elevations in the rod perikaryon (arrow). (D) Washout
67.3.3. Ablation of TRPV4 Has No Effect on the Amplitude and Kinetics of the Distal Light Response
Transmission of rod- and cone-mediated signals across the outer retina was evaluated with electroretinograms (ERGs) which represent the summed activity of photoreceptors and downstream bipolar cells (Masu et al. 1995; Barabas et al. 2013). We found that neither the kinetics nor sensitivity of ERG signals was altered in mice lacking TRPV4. Representative ERG traces of the scotopic light response are illustrated in Fig. 67.3a, and the cumulative average intensity-response data for a-wave and b-wave response components is shown in Fig. 67.1b, c. No obvious alterations in the amplitude and time course of a-wave, b-wave, and oscillatory potential components could be discerned in TRPV4 KO eyes. Photopic responses were induced by single flashes that followed 30 min light (30 cd s m−2) adaptation. Signals consisted of a low-amplitude a-wave (at 15 dB; generated from the small number of mouse cones) that was followed by the fast, cone-driven photopic b-wave with time-to-peak latencies and peak amplitudes that were indistinguishable between control and TRPV4−/− animals. These results demonstrate that TRPV4 channels do not modulate the light response in the outer retina, neither directly through the hypothesized Müller glial “light response” (Rillich et al. 2009) nor indirectly via putative gliotransmitters, released by light from apical Müller cell processes.
Fig. 67.3.
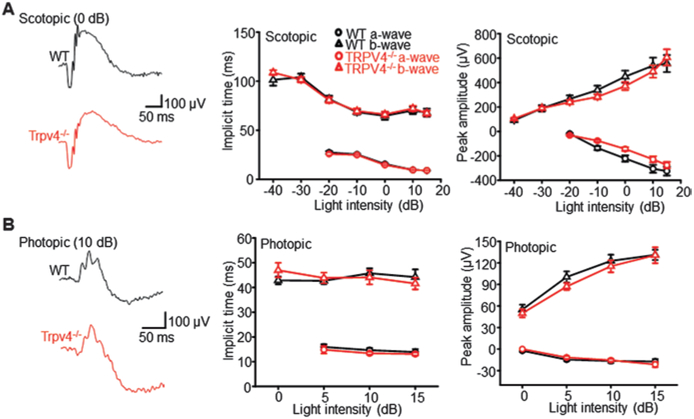
Ablation of TRPV4 has no effect on the onset kinetics, waveform, and amplitude of (a) scotopic and (b) photopic a- and b-waves. Wild-type (WT) traces are shown in black; TRPV4−/− traces are shown in red
67.4. Discussion
Although the phenotype of TRPV4−/− mice is mild compared to human mutations which may result in debilitating dysplasias and sensorimotor neuropathies (Nilius and Voets 2013), TRPV4−/− animals do show defective osmoregulation (White et al. 2016; Redmon et al. 2017), compromised mechanical and thermal hyperalgesia (Alessandri-Haber et al. 2004), nociception (Chen et al. 2007), visceral sensing (Brierley et al. 2008), and auditory transduction (Tabuchi et al. 2005). Here, we report that analysis of outer retinal light responses does not show any obvious visual signaling phenotypes in mouse retinas that lack TRPV4 channels. While this result might be anticipated given previous immunocytochemical localization of the channel to proximal retinal cell types that include RGCs and Müller glia (Ryskamp et al. 2015), photoreceptors could be influenced by TRPV4-induced Ca2+-dependent glio-transmitter release from radial glial processes: (i) Müller cells were reported to sustain both vesicular and nonvesicular release of gliotransmitters ATP and glutamate (Slezak et al. 2012; Newman 2015); (ii) TRPV4 activation drives ATP release in other cell types (Seminario-Vidal et al. 2011); and (iii) preliminary evidence shown in Fig. 67.2 suggests that pharmacological activation of TRPV4 elicits small oscillatory [Ca2+]i responses in adjacent, non-TRPV4-expressing photoreceptor cells. Nonetheless, the absence of observable ERG phenotype in TRPV4−/− eyes argues against a role for Müller glial TRPV4 channels in modulation of scotopic and photopic light responses in the healthy outer retina. The sensitivity, amplitude, and kinetics profiles of light-evoked ERG signals in TRPV4−/− eyes were indistinguishable from wild-type responses. We conclude that photoreceptor responses are not influenced by TRPV4-mediated gliotransmitter release under conditions that prevail in healthy retinas. However, recent reports suggest that TRPV4 activation in Müller glia could play more significant roles under pathological circumstances associated with retinal detachment, photoreceptor degeneration, ischemia, and increased intraocular pressure (Ryskamp et al. 2014; Taylor et al. 2016). Moreover, TRPV4 is likely to play important functions in pressure, stretch, temperature, and volume sensing in the inner retina, including modulation of the blood-retina barrier permeability, responsiveness of RGC dendrites to intraocular pressure, and force-dependent remodeling of the optic nerve head in diseases such as diabetic retinopathy, ischemia, and glaucoma (Ryskamp et al. 2011; Jo et al. 2015; Phuong et al. 2017; Krizaj 2016).
Acknowledgments
This study was supported by the NIH (R01EY022076, R01EY027920, P30EY014800), the University of Utah Neuroscience Initiative, Glaucoma Research Foundation, the Willard Eccles Foundation, Glaucoma Research Foundation, the Diabetes and Metabolism Research Center at the University of Utah and unrestricted support from Research to Prevent Blindness to the Moran Eye Institute at the University of Utah.
Contributor Information
Oleg Yarishkin, Department of Ophthalmology & Visual Sciences, Moran Eye Institute, Salt Lake City, UT, USA.
Tam T.T. Phuong, Department of Ophthalmology & Visual Sciences, Moran Eye Institute, Salt Lake City, UT, USA
Monika Lakk, Department of Ophthalmology & Visual Sciences, Moran Eye Institute, Salt Lake City, UT, USA.
David Križaj, Department of Ophthalmology & Visual Sciences, Moran Eye Institute, Salt Lake City, UT, USA; Department of Bioengineering, University of Utah, Salt Lake City, UT, USA; Department of Neurobiology & Anatomy, University of Utah, Salt Lake City, UT, USA david.krizaj@hsc.utah.edu.
References
- Alessandri-Haber N, Dina OA, Yeh JJ et al. (2004) Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci 24:4444–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas P, Liu A, Xing W et al. (2013) Role of ELOVL4 and very long-chain polyunsaturated fatty acids in mouse models of Stargardt type 3 retinal degeneration. Proc Natl Acad Sci U S A 110:5181–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA et al. (2008) Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134(7):2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Alessandri-Haber N, Levine JD (2007) Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4−/− mice. Mol Pain 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, Yang H, Doan T et al. (2006) Scotopic visual signaling in the mouse retina is modulated by high-affinity plasma membrane calcium extrusion. J Neurosci 26:7201–7211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Frye AM et al. (2016) Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci U S A 113(14):3885–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D (2016) Polymodal sensory integration in retinal ganglion cells. Adv Exp Med 854:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Ryskamp DA, Tian N et al. (2014) From mechanosensitivity to inflammatory responses:new players in the pathology of glaucoma. Curr Eye Res 39:105–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y et al. (1995) Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80(5):757–765 [DOI] [PubMed] [Google Scholar]
- Mergler S, Garreis F, Sahlmüller M et al. (2012) Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells. Histochem Cell Biol. 137:743–761 [DOI] [PubMed] [Google Scholar]
- Newman EA (2015) Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond Ser B Biol Sci 370:1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Voets T (2013) The puzzle of TRPV4 channelopathies. EMBO Rep. 14:152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TT, Yarishkin O, Križaj D (2016) Subcellular propagation of calcium waves in Müller glia does not require autocrine/paracrine purinergic signaling. Channels 10:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuong TTT, Redmon S, Yarishkin O et al. (2017) The permeability of human retinal microvascular endothelial cells is modulated by TRPV4-dependent modulation of cytoskeletal and cell-cell adhesion proteins. Journal of Physiology 595: 6869–6885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmon SN, Shibasaki Krizaj D (2017) Transient receptor potential cation channel subfamily V member 4 Encyclopedia of Signaling Molecules, 2nd Edition. Choi Sangdun, Ed. [Google Scholar]
- Rentería RC, Strehler EE, Copenhagen DR et al. (2005) Ontogeny of plasma membrane Ca2+ ATPase isoforms in the neural retina of the postnatal rat. Vis Neurosci. 22:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillich K, Gentsch J, Reichenbach A et al. (2009) Light stimulation evokes two different calcium responses in Müller glial cells of the guinea pig retina. Eur J Neurosci 29:1165–1176 [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P et al. (2011) The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci 31:7089–7101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM et al. (2014) Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci 34:15689–15700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Iuso A, Krizaj D (2015) TRPV4 channels link volume regulation, calcium homeostasis and inflammatory signaling in the retina. Channels 9:70–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Frye AM, Phuong TT et al. (2016) TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep 6:30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L, Okada SF, Sesma JI et al. (2011) Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 286:26277–26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak M, Grosche A, Niemiec A et al. (2012) Relevance of exocytotic glutamate release from retinal glia. Neuron 74:504–516 [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Mandal A, Delamere NA (2012) TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am J Physiol Cell Physiol. 302:C1751–C1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Suzuki M, Minzuno A et al. (2005) Hearing impairment in TRPV4 knockout mice. Neurosci Lett 382:304–308 [DOI] [PubMed] [Google Scholar]
- Taylor L, Arnér K, Ghosh F (2016) Specific inhibition of TRPV4 enhances retinal ganglion cell survival in adult porcine retinal explants. Exp Eye Res 154:10–21 [DOI] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L et al. (2016) TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 96:911–973 [DOI] [PubMed] [Google Scholar]