Abstract
Background:
Delivery of therapeutic agents directly through the round window (RW) offers promise for treating sensorineural hearing loss. However, hearing loss can result from the surgical approach itself, and the reasons for this are poorly understood. We examined the hearing loss following the three major steps involved with the RW approach to access the mouse cochlea: bullostomy, RW puncture, and RW injection.
Methods:
21 adult CBA/J mice underwent bullostomy alone; 10 underwent RW puncture, and 8 underwent RW injection with PBS with 5% glycerol. Auditory brainstem responses and otoscopy were performed preoperatively and up to six weeks postoperatively. Hair cells were stained and survival was assessed using immunofluorescence.
Results:
One week postoperatively, mice in all groups showed significant threshold shifts. Otoscopy revealed approximately half of all mice had middle ear effusion (MEE), with a higher incidence of effusion in the RW puncture and RW injection groups. Those with MEE had significant ABR threshold shifts, whereas those without MEE had minimal hearing loss. MEE persisted through six weeks in a majority of cases, but in those mice with MEE resolution, there was at least partial improvement in hearing. Immunohistochemistry showed minimal loss of hair cells in all animals.
Conclusion:
MEE is highly correlated with hearing loss in mice undergoing round window surgery. Otoscopy is an important adjunct to consider after ear surgery in mice, as MEE may contribute to post-surgical hearing loss.
Keywords: ear surgery, hearing loss, inner ear, middle ear, round window membrane, gene therapy.
INTRODUCTION
Surgical delivery of therapeutic agents directly into the cochlea has great potential for treatment of sensorineural hearing loss. Several animal studies have examined the feasibility of intra-cochlear drug delivery and cochlear gene therapy, and a few studies have reported some recovery of hearing after cochlear gene therapy [Akil et al., 2012; Askew et al., 2015; Chang et al., 2015; Kraft et al., 2013]. However, hearing loss can result from the surgical approach itself [Akil et al., 2012; Chien et al., 2015; Kawamoto et al., 2001; Okada et al., 2012; Wenzel et al., 2007]. The etiology of hearing loss associated with cochlear gene and drug delivery has not been well characterized to date.
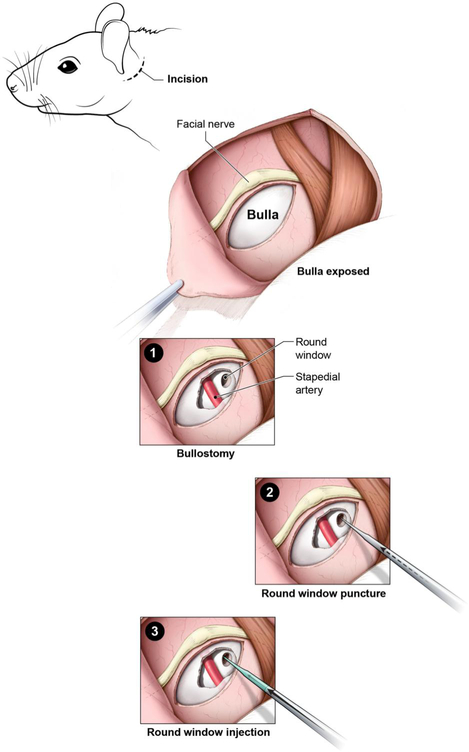
Various surgical approaches exist for inner ear gene delivery; these include cochleostomy, round window (RW) injection, canalostomy, and endolymphatic sac injection. While cochleostomy offers the most direct method of delivery into the endolymph, it is associated with higher rates of hearing loss [Akil et al., 2012; Chien et al., 2015; Wenzel et al., 2007]. The RW approach offers high transduction efficiency and possibly lower rates of hearing loss [Akil et al., 2012; Chien et al., 2015; Xia et al., 2012]. The canalostomy approach results in the least amount of hearing loss, but the pattern of viral transduction in the cochlea is variable [Kawamoto et al., 2001; Okada et al., 2012; Praetorius et al., 2003]. There has only been one study describing endolymphatic sac injection for gene therapy, and hearing results were not reported [Yamasoba et al., 1999]. Our previous study indicates that there are similar rates of hair cell transduction between the RW and cochleostomy approaches, with the RW approach causing less hearing loss at four weeks after surgery [Chien et al., 2015]. However, a large amount of variability was found in the post-surgical hearing in these animals, with some mice having no hearing loss and some having profound hearing loss. In this study, we examine the etiologies of hearing loss associated with the RW approach following the three major steps in this surgical technique: bullostomy, RW puncture, and RW injection (Figure 1). It is important to note that in order to perform RW puncture, bullostomy is necessary to expose the RW. Similarly, in order to perform RW injection, both bullostomy and RW puncture are necessary. Therefore, the three surgical steps are not mutually exclusive, but rather they are sequential steps involved in the surgical procedure.
Figure 1: Schematic of surgical steps for round window (RW) injection in mice.
A postauricular incision is made. The facial nerve is identified, and the bulla is exposed. 1) Bullostomy: An opening in the bulla is made, visualizing the stapedial artery and RW membrane. 2) RW puncture: The RW membrane is punctured with a glass micropipette. 3) RW injection: The glass micropipette is used to inject fluid (PBS with 5% glycerol) through the RW into the cochlea.
We hypothesized that the variability in postoperative hearing loss after gene therapy was due to middle ear effusion (MEE). In humans undergoing ear surgery, there is frequently a temporary conductive hearing loss that occurs right after surgery. This is caused by fluid and/or blood filling the middle ear space and may last for several weeks. ABR testing in mice does not distinguish between sensorineural and conductive hearing loss. Bone conduction testing is used to measure sensorineural hearing in humans, but it is difficult to implement in mice [Steel et al., 1987]. There is one report using tympanometry [Zheng et al., 2007] and another using DPOAE threshold changes [Qin et al., 2010] to distinguish conductive hearing loss, but neither of these methods have been standardized or replicated. Otoscopic examination of the eardrum, however, has been performed by multiple groups looking at animal models of otitis media, and it correlates very well with ABR threshold shifts [Kerschner et al., 2013; MacArthur et al., 2006; Plassmann and Kadel, 1991]. We utilized otoscopy to assess for MEE in animals after ear surgery. Our results suggest MEE is highly associated with hearing loss after each of the steps for RW surgery in mice.
METHODS
Animals
CBA/J mice of both sexes were obtained from the Jackson Laboratory. 39 adult mice aged P30-90 and weighing between 20-30 grams with normal ABR thresholds and clear tympanic membranes on otoscopy preoperatively were included. 21 underwent bullostomy alone, while 10 underwent bullostomy followed by RW puncture, and 8 underwent bullostomy, RW puncture, and RW injection with PBS with 5% glycerol (common carrier solution for viral gene delivery). All mice were followed for at least 1 week postoperatively, with a predetermined subset of mice followed for 4-6 weeks postoperatively. Mice with clear tympanic membranes and no ABR threshold shifts were euthanized 4 weeks after surgery. Those with persistent effusion were observed for 2 more weeks with additional otoscopy and a final ABR at 6 weeks. All animal procedures were approved by the NIDCD Animal Care and Use Committee.
Auditory Brainstem Response Recording
Mice were anesthetized by an intraperitoneal injection of 0.3750 mg/kg dexmedetomidine (Zoetis, Florham Park, NJ) and 56 mg/kg ketamine (Putney, Portland, ME) and placed on a thermostatic heating pad maintained at 37.0° C using an ATC-1000 temperature controller (World Precision Instruments, Sarasota, FL) in a sound-insulated chamber. Subdermal needle electrodes were inserted at the vertex and test-ear mastoid with a ground under the contralateral ear. Stimulus generation and ABR recordings were completed using an RZ6 Multi I/O Processor and BioSigRz v.5.1 software (both from Tucker-Davis Technologies, Gainesville, FL). ABR thresholds were measured at 8, 16, and 32 kHz using 3-ms, Blackman-gated tone pips presented at 29.9/s with alternating stimulus polarity. At each stimulus level, 512 to 1024 responses were averaged. Thresholds were determined by visual inspection of the waveforms and were defined as the lowest stimulus level at which any wave could be reliably detected. A minimum of two waveforms was obtained at the threshold level to ensure repeatability of the response.
Surgical Procedures
Mice were anesthetized with isoflurane gas (Baxter, Deerfield, IL) through a nose cone at a flow rate of 0.5 L/min. The mouse was placed on a heating pad maintained at 37.0° C using a TC-1000 temperature controller (CWE, Ardmore, PA). The left pinna was taped forward for retraction, and an operating microscope (Carl Zeiss, Jena, Germany) was used at this point. The region posterior to the left ear was trimmed with scissors and disinfected with 70% ethanol. A post-auricular incision was made using small scissors. The soft tissues were bluntly dissected to expose the bulla. A small hole was created in the bulla with a 25-gauge needle and enlarged with forceps to expose the RW membrane. This procedure will be referred to as bullostomy (Figure 1, panel 1), and 21 mice underwent this procedure alone. In the RW puncture group, bullostomy was followed by puncture of the RW membrane using a glass micropipette with an approximately 10μm tip; the needle tip remained in place for one minute and was then removed (Figure 1, panel 2). Entry into the scala tympani was confirmed by visualization of perilymph. For the RW injection group, bullostomy and RW puncture were performed as described above, and the glass micropipette was attached to a Nanoliter Microinjection System (World Precision Instruments, Sarasota, FL), which was used to inject 46 nL per second of 1x PBS with 5% glycerol through the RW, for a total of 7 injections (total volume of 322 nL) (Figure 1, panel 3). After both RW puncture and RW injection, the glass micropipette was removed, and the bulla was covered with a small piece of muscle. The incision was closed with 5-0 Vicryl (Ethicon, Somerville, NJ) sutures. The mice received 3 doses of subcutaneous ketoprofen (5 mg/kg/day) (Zoetis, Florham Park, NJ) and 1 week of sulfamethoxazole-trimethoprim suspension (Hi-Tech Pharmacal, Amityville, NY) dissolved in drinking water at 12 mg/mL and 2.4 mg/mL, respectively, postoperatively.
Otoscopic Examination
Mice were anesthetized with isoflurane gas (as above) either as a stand-alone procedure or at the time of surgery. The outer ear canal and tympanic membrane (TM) were examined under the operating microscope at 2.5x magnification using two forceps to gently spread and hold open the pinna to obtain a complete view of the TM. The procedure lasted about 3 minutes. Otoscopy was performed on both ears immediately before and after surgery, and then at one-week intervals up to 4-6 weeks postoperatively. Photos were taken using DMCap software and an HD Video Capture Device (both from Diamond Multimedia, Canoga Park, CA) connected to the operating microscope. Normal otoscopy was defined as a clear TM through which the mucosa of the middle ear and a well-defined malleus were readily observed. Effusion was defined as the presence of any substance medial to the TM interfering with this view (e.g., amber fluid, pus, blood, or bubbles). Otoscopy results were recorded before performing before ABR to avoid bias, and images were reviewed by a senior otolaryngology resident who was blinded to the ABR results.
Cochlear Dissections and Immunofluorescence
Mice were euthanized with CO2 followed by decapitation per protocol, and their cochleas were isolated and dissected. The dissected cochleas were perfused with 4% paraformaldehyde (PFA) through a small hole in the apex and also through the oval and round windows. They were incubated overnight at 4°C in PFA. After fixation, the cochleae were rinsed with 1x PBS and subsequently immersed in a 1:1 solution of 5% EDTA and 1x PBS for 4-5 days. When the cochleas were completely decalcified, they were washed with 1x PBS. Each cochlea was carefully microdissected into apical, middle, and basal turns and placed in blocking solution composed of 0.02 g/mL bovine albumin, 0.8% normal goat serum, and 0.4% Triton X-100 (all from Sigma-Aldrich, St. Louis, MO) dissolved in 1x PBS for 2 hours. The cochleas were stained with the primary antibody mouse anti-myosin 7A (Santa Cruz Biotechnology, Dallas, TX) at a dilution of 1:250 in blocking solution, followed by the secondary antibody Alexa Fluor 546 conjugated goat anti-mouse IgG (Life technologies, Eugene, OR) at a dilution of 1:500 in 1x PBS. Phalloidin-647 (Life technologies, Eugene, OR) at a dilution of 1:50 in 1x PBS was applied for 30 minutes to label filamentous actin. Finally, the cochleas were washed with 1x PBS and mounted on glass slides with Fluoromount G (SouthernBiotech, Birmingham, AL). 10x and 40x images of each cochlear turn were taken using an LSM 780 confocal microscope (Carl Zeiss, Jena, Germany).
Statistical Analyses
All statistical tests were performed in Microsoft Excel (Microsoft, Redmond, WA). Paired t-tests were used to compare ABR thresholds for the same mouse at different time-points. One-way analysis of variance (ANOVA) was used to compare threshold shifts among the three groups. χ2 test was used to compare the incidence of effusion in the combined RW puncture and RW injection groups with the bullostomy group. All other comparisons of threshold shifts were made using unpaired, two-tailed t-tests assuming equal variance. P values less than 0.05 were considered statistically significant.
RESULTS
Hearing loss is similar after bullostomy, RW puncture, or RW injection
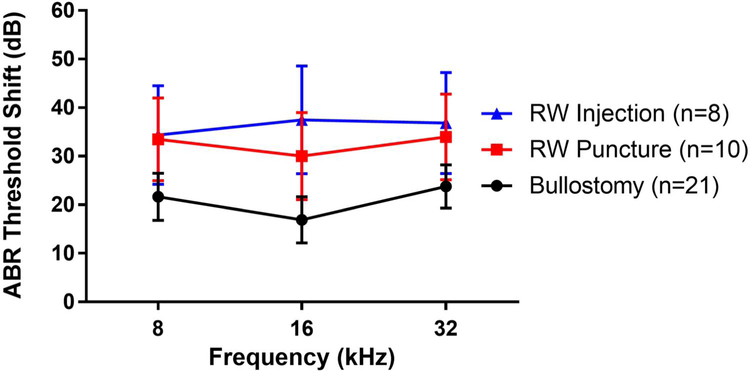
At one week postoperatively, all three groups had statistically significant ABR threshold shifts compared to their preoperative thresholds (Figure 2). Mice that underwent bullostomy alone had 17-24 dB threshold shifts (p=0.002). Those that underwent bullostomy followed by RW puncture had 30-34 dB threshold shifts (p=0.004). Those that underwent bullostomy, RW puncture, and RW injection had threshold shifts of 34-38 dB (p=0.01). Although the hearing loss was greater in the RW puncture and RW injection groups, the differences in hearing loss among these groups were not statistically significant at any frequency (one-way ANOVA, p=0.13 to 0.35).
Figure 2: Hearing loss (ABR threshold shifts) one week after surgery.
Mice that underwent bullostomy alone had 17-24 dB threshold shifts. Mice that underwent bullostomy followed by RW puncture had 30-34 dB threshold shifts. Mice that underwent bullostomy, RW puncture, and RW injection had 34-38 dB threshold shifts. No significant differences in hearing loss were found among the groups at any frequency (one-way ANOVA, p=0.13 to 0.35).
Hearing loss is associated with middle ear effusion
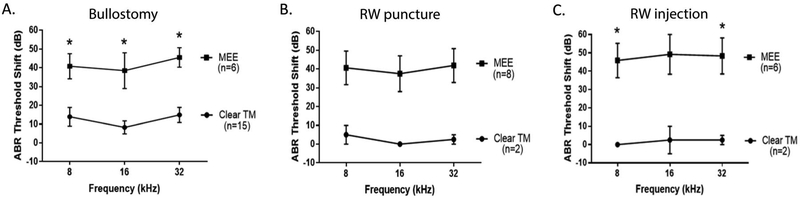
Otoscopic examination was clear on all mice preoperatively and immediately postoperatively. At one week postoperatively, approximately half of all surgical ears had MEE. Examples of postoperative MEEs and clear TMs are shown in Figure 3 for each of the three groups. There was a significantly higher incidence of effusion in the RW puncture (8/10 mice, 80%) and RW injection (6/8 mice, 75%) groups, while only 6/21 mice (29%) in the bullostomy group had MEE postoperatively (χ2 test, p=0.002).
Figure 3: Postoperative otoscopic findings demonstrating middle ear effusion (MEE) vs. clear tympanic membrane (TM).
A. Representative images of otoscopy from mice that underwent bullostomy alone and had either an amber effusion (top) or a normal, clear TM with a well-defined malleus (bottom) (scale bar = 1 mm). B. Representative images of otoscopy from mice that underwent RW puncture and had either an amber effusion (top) or a normal, clear TM with a well-defined malleus (bottom) (scale bar = 1 mm). C. Representative images of otoscopy from mice that underwent RW injection and had either an amber effusion (top) or a normal, clear TM with a well-defined malleus (bottom) (scale bar = 1 mm).
We further analyzed the ABR data by using the presence or absence of MEE to divide the animals in each group. In the bullostomy group, mice with MEE had significantly greater threshold shifts than those without MEE (p=0.001 to 0.008 across all frequencies) (Figure 4A). In the RW puncture group, mice with MEE had greater threshold shifts than those without MEE with an overall trend towards statistical significance (p=0.070 to 0.096) (Figure 4B). In the RW injection group, mice with MEE had significantly greater threshold shifts at 8 kHz (p=0.036) and 32 kHz (p=0.043) with a trend towards significance at 16 kHz (p=0.059) (Figure 4C). Of note, only two animals in each of the RW puncture and RW injection groups had clear otoscopic findings (no MEE) at one week postoperatively, which may explain the lack of statistical significance when comparing their ABR threshold shifts to those with MEE.
Figure 4: Postoperative hearing loss at one week is associated with the presence or absence of effusion.
A. In the bullostomy group, mice with MEE had a greater ABR threshold shift one week postoperatively compared to those without MEE (clear TM). Asterisks designate statistical significance (p<0.05). B. In the RW puncture group, mice with MEE had a greater ABR threshold shift one week postoperatively compared to those without MEE (clear TM), with a trend towards statistical significance. C. In the RW injection group, mice with MEE had a greater ABR threshold shift one week postoperatively compared to those without MEE (clear TM). Asterisks designate statistical significance (p<0.05).
Resolution of effusion results in partial improvement in hearing
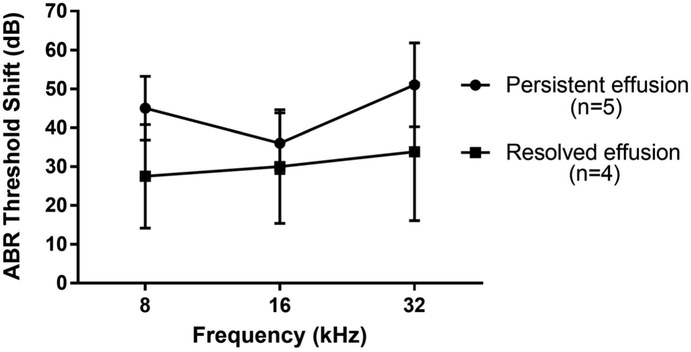
In order to determine if resolution of MEE resulted in improved hearing sensitivity after surgery, we followed a subset of animals for up to six weeks after surgery (n=8, 4, and 6 mice from the bullostomy, RW puncture, and RW injection groups, respectively). At 1 week postoperatively, 0/8 (0%) of the mice in the bullostomy subset had MEE, while 4/4 (100%) of the RW puncture subset and 5/6 (83%) of the RW injection subset had MEE. Of those with MEE, 1/4 (25%) in the RW puncture subset and 3/5 (60%) in the RW injection subset demonstrated resolution of MEE as evidenced by clear otoscopic findings starting two weeks after surgery. These mice demonstrated an average of 6-17 dB improvement in their hearing when compared to those with persistent MEE, with a trend towards statistical significance at all frequencies (p=0.08 to 0.13) (Figure 5). The remainder of the mice with MEE maintained their effusion through six weeks postoperatively with no improvement in hearing.
Figure 5: Resolution of effusion results in partial improvement in hearing.
Mice with resolved effusion demonstrated less hearing loss at 4 weeks postoperatively when compared to those with persistent effusion, though this was not statistically significant.
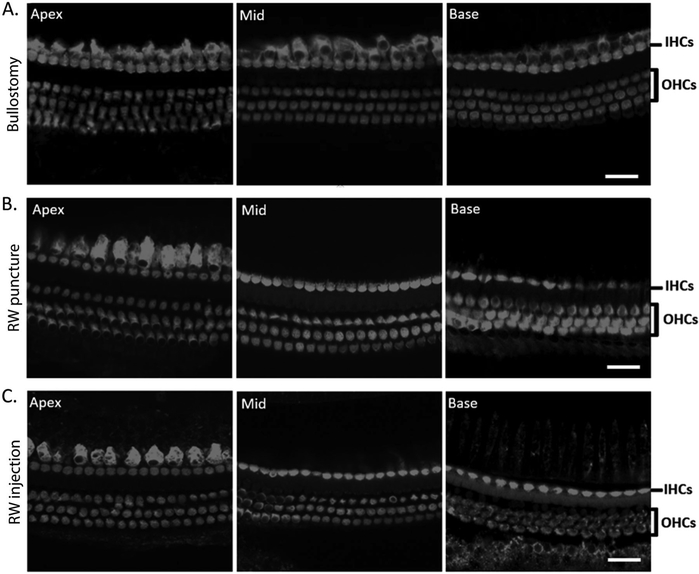
Hair cells are intact after bullostomy, RW puncture, or RW injection
To assess for the integrity of sensory epithelium in the cochlea after RW surgery, the inner and our hair cells were examined using confocal microscopy. Inner and outer hair cells were preserved throughout the entire cochlea in all specimens, even in mice with hearing loss. Representative examples of cochleas from mice that had MEE and hearing loss in the bullostomy, RW puncture, and RW injection groups are shown in Figures 6A, 6B, and 6C, respectively.
Figure 6:
Hair cells are intact in mice with effusion and hearing loss after bullostomy, RW puncture, or RW injection. A. Representative images of a cochlea from a mouse with effusion following bullostomy alone. Inner hair cells (IHCs) and outer hair cells (OHCs) are intact in all three cochlear turns (scale bar = 20 μm). B. Representative images of a cochlea from a mouse with effusion following RW puncture. IHCs and OHCs are intact in all three cochlear turns (scale bar = 20 μm). C. Representative images of a cochlea from a mouse with effusion following RW injection. IHCs and OHCs are intact in all three cochlear turns (scale bar = 20 μm).
DISCUSSION
There is great potential for treating sensorineural hearing loss with direct cochlear delivery of therapeutic agents, particularly with viral-mediated gene therapy [Geleoc and Holt, 2014; Muller and Barr-Gillespie, 2015]. However, iatrogenic hearing loss after surgery has contributed to inconsistency in hearing outcomes both in our experience and in the literature [Akil et al., 2012; Chien et al., 2015; Kawamoto et al., 2001; Okada et al., 2012; Wenzel et al., 2007]. Of the various approaches to the cochlea, the RW approach appears to offer the best balance between maximizing transduction efficiency and minimizing surgical hearing loss [Akil et al., 2012; Chien et al., 2015; Xia et al., 2012], so this approach was selected to be further investigated.
The causes of hearing loss associated with the RW approach used to deliver gene therapy to the cochlea in mice are not well characterized. Here, the individual steps of RW injection were examined in order to determine the effect of each step on hearing outcome. One week after surgery, there was significant hearing loss in mice that underwent bullostomy alone, indicating that hearing loss after RW surgery was not necessarily caused by trauma to the cochlea. Moreover, hearing loss was highly associated (p=0.001 to 0.008) with MEE on otoscopy, suggesting that the hearing loss was conductive, rather than sensorineural. Additional evidence that post-surgical hearing loss was conductive in these mice comes from 1) the fact that the inner ears in these animals had not been violated, and 2) our finding that their inner and outer hair cells were intact. We suspect the source of effusion was likely serous transudate from the surrounding tissues after surgical manipulation. Bleeding was probably not the main source, as the color of the effusion was usually amber, not red.
There was slightly greater hearing loss after RW puncture and RW injection compared to bullostomy alone, but this difference was not statistically significant. Furthermore, minimal difference in hearing loss was found between the RW puncture and RW injection groups, suggesting that the fluid injection was not traumatic in itself, although the volume injected (322 nL) was relatively large compared to the perilymphatic volume, which is only about 620 nL in mice [Thorne et al., 1999]. Some of the injected fluid is thought to go through the cochlear aqueduct or displace the endolymph in the scala media, but some of it may also leak out through the puncture site [Stover et al., 1999]. There was, in fact, a significantly higher percentage of animals with MEE in the RW puncture (80%) and RW injection (75%) groups than in the bullostomy group (29%) (χ2 test, p=0.002). The higher rate of effusion in the RW puncture and RW injection groups may have been due to the leakage of CSF into the middle ear space after puncturing the RW. Since the inner ear was violated, sensorineural hearing loss cannot be ruled out in these animals; however, there were four mice with no hearing loss after RW puncture or RW injection, and these mice were the only ones without MEE on otoscopy. Similar to the bullostomy group, we did not observe inner or outer hair cell loss in those mice with hearing loss in these two groups.
In addition to our finding that hearing loss was highly associated with MEE, we found that animals with resolution of MEE had at least partial improvement in their hearing (6-17 dB), though this did not reach statistical significance. It is important to consider that there may be hearing loss due to other reasons besides MEE. There were four mice in the bullostomy group that had hearing loss without effusion, suggesting inadvertent damage to the ossicles or alteration to the resonance properties of the bulla, which amplifies low-frequency sounds in rodents [Plassmann and Kadel, 1991]. In those cases where the RW was punctured or injected, it is possible that there was a concurrent sensorineural hearing loss due to an unhealed RW (resulting in persistent perilymphatic fistula), or trauma, inflammation, or infection within the cochlea itself. Cochlear dissection and immunohistochemistry, however, revealed that both inner and outer hair cells were preserved in all turns, suggesting that these outcomes, if present, were not traumatic enough to result in hair cell death.
The duration of MEE was somewhat variable. In the subset of mice followed for 4-6 weeks, an effusion was found to either resolve by 2 weeks (n=4) or persist through 6 weeks (n=5). It is unknown whether or not these effusions would have spontaneously resolved over a longer period of time. This variability in the recovery from effusion may be related to the characteristics of the fluid (e.g., viscosity) or underlying Eustachian tube dysfunction. Interestingly, two of these 18 mice developed MEE in their right ears (the non-operated side) 2 weeks after surgery, which suggests that there is a low baseline incidence of MEE, another factor that may need to be taken into account when evaluating hearing outcomes in mice.
The presence of absence of MEE may explain some of the hearing outcomes seen in other studies after inner ear surgery in mice, and has implications for the delivery of therapeutics into the inner ear in general. Of note, we have also observed similar middle ear effusion and ABR threshold shift after round window surgery in mice with C57BL/6 background, suggesting these findings are not mouse strain specific. In our prior gene therapy experiment comparing the RW and cochleostomy approaches, there was an average of a 25-40 dB threshold shift after 1 week with either approach, which persisted in the RW injection group and worsened in the cochleostomy group after 4 weeks [Chien et al., 2015]. Two other studies also found large threshold shifts after cochleostomy and viral injection [Akil et al., 2012; Kawamoto et al., 2001], as did another study even after injection of control buffer [Wenzel et al., 2007]. Our current data indicate that it is likely many of these mice had MEE at least partially contributing to the hearing loss. Several studies describe minimal hearing loss after RW injection, but these were all performed in neonatal mice, whose developing ears may more readily recover from RW trauma and often do not require a bullostomy [Akil et al., 2012; Askew et al., 2015; Okada et al., 2012; Xia et al., 2012]. Finally, minimal to no hearing loss has been found after canalostomy in adult mice, likely because direct trauma to the cochlea is avoided and the bulla is not violated in this approach, thus reducing the opportunity for MEE to develop [Kawamoto et al., 2001; Praetorius et al., 2003]. However, otoscopy was not performed in conjunction with ABR acquisition in any of these studies.
Future studies may examine the potential sources of post-surgical MEE and ways to prevent or resolve it. Since we suspect some of the effusion may originate from puncturing the RW and leakage of perilymph postoperatively, investigation into methods of effectively sealing the RW puncture site may reveal whether this reduces the rate of effusion and hearing loss, as suggested by another study [Akil et al., 2015]. Advances in distinguishing between conductive and sensorineural hearing loss in mice will also be helpful in clarifying the etiologies of postoperative hearing loss. In the meantime, our results highlight the importance of otoscopy as an adjunctive measure when performing otologic surgeries in mice. Otoscopy should be performed to check for effusion in operated ears before assessing hearing outcomes, and hearing results should be evaluated in light of the otoscopic findings.
CONCLUSION
Round window delivery of viral gene therapy directly into the cochlea offers great promise for treating sensorineural hearing loss, but the potential for iatrogenic, surgically induced hearing loss must be kept in mind. By investigating the individual steps of the surgery and by correlating hearing outcomes with otoscopic findings, we found that hearing loss after round window surgery was largely caused by middle ear effusion, and that resolution of effusion led to an improvement in hearing. Even though this study focused on investigating the mechanism of hearing loss following round window gene delivery in mice, the results have implications for inner ear therapeutic delivery in general. Otoscopy is an important adjunct to perform after ear surgery in mice, as middle ear effusion may contribute to post-surgical hearing loss.
Acknowledgements:
We thank Dr. Carmen Brewer and Dr. Carter Van Waes for their comments on the manuscript. We thank the NIDCD animal facility for providing care to mice that underwent ear surgeries. We thank Dr. Tracy Fitzgerald for providing help with auditory testing.
Source(s) of Funding: National Institutes of Health (NIH) / National Institute on Deafness and Other Communication Disorders (NIDCD) intramural research grants DC00082-02 (W.W.C.) and DC00079-04 (L.L.C).
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
REFERENCES
- Akil O, Rouse SL, Chan DK, Lustig LR: Surgical method for viraNy mediated gene delivery to the mouse inner ear through the round window membrane. Journal of visualized experiments : JoVE 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR: Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012;75:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P, Holt JR: Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med 2015;7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Wang J, Li Q, Kim Y, Zhou B, Wang Y, Li H, Lin X: Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med 2015;7:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, McDougald DS, Roy S, Fitzgerald TS, Cunningham LL: Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. The Laryngoscope 2015. [DOI] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR: Sound strategies for hearing restoration. Science 2014;344:1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Oh SH, Kanzaki S, Brown N, Raphael Y: The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther 2001;4:575–585. [DOI] [PubMed] [Google Scholar]
- Kerschner JE, Hong W, Taylor SR, Kerschner JA, Khampang P, Wrege KC, North PE: A novel model of spontaneous otitis media with effusion (OME) in the Oxgr1 knock-out mouse. International journal of pediatric otorhinolaryngology 2013;77:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Hsu C, Brough DE, Staecker H: Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. The Laryngoscope 2013;123:992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Hefeneider SH, Kempton JB, Trune DR: C3H/HeJ mouse model for spontaneous chronic otitis media. The Laryngoscope 2006;116:1071–1079. [DOI] [PubMed] [Google Scholar]
- Muller U, Barr-Gillespie PG: New treatment options for hearing loss. Nat Rev Drug Discov 2015;14:346–365. [DOI] [PubMed] [Google Scholar]
- Okada H, Iizuka T, Mochizuki H, Nihira T, Kamiya K, Inoshita A, Kasagi H, Kasai M, Ikeda K: Gene transfer targeting mouse vestibule using adenovirus and adeno-associated virus vectors. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2012;33:655–659. [DOI] [PubMed] [Google Scholar]
- Plassmann W, Kadel M: Low-frequency sensitivity in a gerbilline rodent, Pachyuromys duprasi. Brain, behavior and evolution 1991;38:115–126. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Baker K, Weich CM, Plinkert PK, Staecker H: Hearing preservation after inner ear gene therapy: the effect of vector and surgical approach. ORL J Otorhinolaryngol Relat Spec 2003;65:211–214. [DOI] [PubMed] [Google Scholar]
- Qin Z, Wood M, Rosowski JJ: Measurement of conductive hearing loss in mice. Hearing research 2010;263:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel KP, Moorjani P, Bock GR: Mixed conductive and sensorineural hearing loss in LP/J mice. Hearing research 1987;28:227–236. [DOI] [PubMed] [Google Scholar]
- Stover T, Yagi M, Raphael Y: Cochlear gene transfer: round window versus cochleostomy inoculation. Hearing research 1999;136:124–130. [DOI] [PubMed] [Google Scholar]
- Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW Jr., Gewalt SL: Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. The Laryngoscope 1999;109:1661–1668. [DOI] [PubMed] [Google Scholar]
- Wenzel GI, Xia A, Funk E, Evans MB, Palmer DJ, Ng P, Pereira FA, Oghalai JS: Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2007;28:1100–1108. [DOI] [PubMed] [Google Scholar]
- Xia L, Yin S, Wang J: Inner ear gene transfection in neonatal mice using adeno-associated viral vector: a comparison of two approaches. PLoS One 2012;7:e43218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y: Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther 1999;10:769–774. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Tong YC, Alagramam KN, Yu H: Tympanometry assessment of 61 inbred strains of mice. Hearing research 2007;231:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]