Abstract
Bone marrow microenvironment mediated downregulation of BCL6 is critical for maintaining cell quiescence and modulating therapeutic response in B-cell acute lymphoblastic leukemia (ALL). In the present study, we have performed a high throughput cell death assay using BCL6 knockdown REH ALL cell line to screen a library of FDA-approved oncology drugs. In the process, we have identified a microtubule inhibitor, cabazitaxel (CAB), and a RNA synthesis inhibitor, plicamycin (PLI) as potential anti-leukemic agents. CAB and PLI inhibited cell proliferation in not only the BCL6 knockdown REH cell line, but also six other ALL cell lines. Furthermore, combination of CAB and PLI had a synergistic effect in inhibiting proliferation in a cytarabine-resistant (REH/Ara-C) ALL cell line. Use of nanoparticles for delivery of CAB and PLI demonstrated that the combination was very effective when tested in a co-culture model that mimics the in vivo bone marrow microenvironment that typically supports ALL cell survival and migration into protective niches. Furthermore, exposure to PLI inhibited SOX2 transcription and exposure to CAB inhibited not only Mcl-1 expression but also chemotaxis in ALL cells. Taken together, our study demonstrates the utility of concomitantly targeting different critical regulatory pathways to induce cell death in drug resistant ALL cells.
Keywords: B-cell acute lymphoblastic leukemia, Bone marrow microenvironment, Minimal residual disease, Drug resistance, Nanoparticles, Cabazitaxel, Plicamycin, Co-culture model
1. Introduction
B-cell acute lymphoblastic leukemia (ALL) manifests itself as an accumulation of poorly differentiated malignant lymphoid cells within the bone marrow (BM), resulting in the disruption of normal hematopoiesis [1]. BM is also the most common site of disease relapse, contributed by minimal residual disease (MRD), a major factor associated with poor prognosis and mortality [2]. Unlike T-cell lymphoblastic leukemia, MRD in ALL has been less completely studied and its etiology still remains to be more clearly delineated [3]. However, there is sufficient evidence that the maintenance of MRD is due, in part, to the ability of the BM microenvironment to provide sanctuary to the ALL cells allowing malignant cells to survive, even in the presence of chemotherapy [4,5]. Interestingly, MRD cells became sensitive following release from their BM niche, emphasizing the crucial role of the BM microenvironment in contributing to therapeutic resistance [6]. Within the BM niche, in addition to often being non-responsive to chemotherapy, ALL cells have been described as quiescent with ‘stem cell like’ characteristics [6–8]. Hence, in order to consider strategies to eradicate MRD, the ability of ALL cells to migrate to the BM niche has to be considered in addition to the quiescent and drug resistant phenotype acquired when resident within the niche.
We previously developed an in vitro co-culture model of ALL cells with either primary human-derived BM stromal cells or osteoblasts (components of the BM niche) [9]. From this co-culture we characterized a drug resistant sub-population of leukemic cells referred to as “phase dim” (PD), based on their lack of light refraction coincident with their migration beneath adherent layers of stroma or osteoblasts. The PD tumor cells are used to model cells that contribute to MRD in vivo based on phenotypic similarities [9]. Using this niche-based co-culture model, we have reported that primary ALL samples, or ALL cells in co-culture with the BM cellular components, have reduced BCL6 expression in the PD cell population [10]. Furthermore, reduction in BCL6 resulted in disruption of cell cycle progression, with cyclin D3-dependent accumulation of cells in the G0/G1 phase. The importance of BCL6 in maintaining cell quiescence, drug resistance and the resulting MDR phenotype was further validated in vivo by demonstrating significant event free survival in mice treated with a combination of caffeine (stabilizer of BCL6) and cyatarabine (Ara-C) when compared to mice treated with Ara-C alone [10]. BCL6 has also been shown to be a master regulator of glycolysis by directly repressing the overall gene program of the glycolytic pathway [11]. Not surprisingly, we have shown that drug resistant PD ALL cells, characterized by reduced expression of BCL6, demonstrate increased glycolysis coincident with upregulation of several molecules that modulate the metabolic pathway, including hexokinase II [9,10]. Based on these observations we screened for drugs that induce death in leukemic cells with diminished BCL6, with the intent to identify agents that could be tested for efficacy in targeting MRD in ALL.
In the present study, we have successfully screened a library of FDA-approved oncology drugs in a BCL6 knockdown ALL cell line and identified cabazitaxel (CAB) and plicamycin (PLI) as potential candidates that could target and eliminate drug resistant leukemic cells. We further validated the anti-leukemic activity of CAB and PLI in six ALL cell lines and demonstrated that part of the anti-leukemic activity was attributed to cell cycle arrest. Furthermore, to show activity in low expressing BCL6 cells, we demonstrated synergism of the CAB/PLI combination in a cytarabine resistant REH cell line (REH/Ara-C) and our co-culture model. Collectively our observations suggest this combination therapy, with inhibition of chemotaxis and downstream modulation of SOX2 and Mcl-1, warrants further evaluation in settings that are refractory to traditional chemotherapy.
2. Materials and methods
2.1. Cell culture and chemicals
The development of doxycycline-inducible REH BCL6 knockdown cells and its comparative REH scrambled stable cells has been previously published [10]. SUPB15 (ATCC #CRL-1929) and JM1 (ATCC #CRL-10423) were purchased and maintained in RPMI 1640 containing 10% FBS, 0.05 mM β-mercapto-ethanol and 1X streptomycin/penicillin antibiotics. REH (ATCC #CRL-8286), NALM1 (ATCC #CRL-1567), NALM6 (DSMZ ACC #128), BV173 (DSMZ ACC#20), RS4 (ATCC #CRL-1873) and SD1 (DSMZ ACC#366) were purchased and maintained in RPMI 1640 containing 10% FBS and 1X streptomycin/penicillin antibiotics. Human osteoblasts (HOB) was purchased from PromoCell (Cat No: C-12720, Hiedelberg, Germany) and cultured according to the vendor’s recommendations. All the ALL cell lines were authenticated by short tandem repeat (STR) analysis (University of Arizona Genetic Core, Tuscon, Az) and maintained in 6% CO2 in normoxia at 37 °C. Primary immune cells CD3+ T cells, CD19+ B cells, peripheral blood mono-nuclear cells (PBMC) and bone marrow mononuclear cells (BMMC) were all purchased from ALLCELLS and maintained in Lymphocyte growth medium −3 (Lonza, Cat No: CC-3211) containing 10% FBS and 1X streptomycin/penicillin antibiotics. De-identified primary human ALL cells were obtained from the West Virginia University Health Sciences Center and West Virginia University Cancer Institute Biospecimen Processing Core. CAB and PLI were purchased from Fisher Scientific (Cat No: 501014444 and 50488911, respectively) and stored at −80 °C as a 10 mM stock.
2.2. High throughput screening assay
FDA-approved oncology drug set VII was obtained from NCI/NIH in 96 well plate format with each well containing 20 μl of 10 mM drug stock. REH BCL6 knockdown and scrambled cells were treated with 1 μg/ml of doxycycline for 24 h (to induce shRNA expression) and then cultured in a 96 wells plate at 50,000 cells per well. Drugs were added in triplicate at log concentrations with the lowest concentration of0.1 μM and the highest concentration of 100 μM. The cells were incubated for 72 h in a humidified atmosphere under 5% CO2 at 37 °C following which the cell viability and the IC50 was measured and analyzed as described below.
2.3. Cell viability assay
ALL cells were grown in 96-well plates at 50,000 cells per well and treated with CAB or PLI at indicated concentrations. Cell viability was measured 72 h post-treatment using a cell counting kit according to the manufacturer’s instructions (Dojindo Molecular Technologies Inc., Cat No: CK04). Briefly, 10 μl of the assay reagent was added to each well and incubated for 2 h at 37 °C in normoxia, after which the plates were read on a BioTek Cynergy 5 plate reader at 450 nm absorbance. Untreated cells were used as controls. IC50 and combination index (CI) was analyzed using compusyn (www.combosyn.com).
2.4. Cell cycle analysis
ALL cells at 106 cells/ml were treated with either 3 nM of CAB or 15 nM of PLI for 24 h. Post-treatment the cells were washed with phosphate buffered saline (PBS) and fixed using 70% ethanol for 30 min at 4 °C. After incubation, the cells were washed with PBS and re-suspended in a staining buffer containing 50 μg/ml of propidium iodide (PI) and 100 μg/ml of RNase A. Cells were incubated for 30 min in the staining buffer, followed by wash and analysis of the DNA content using flow cytometry.
2.5. Long-term co-culture and isolation of leukemic cell population
Long-term co-culture conditions have been previously described [9]. Briefly, 1 million REH cells were seeded on 85% confluent HOB layer and maintained in 5% O2. The co-culture was fed every 4 days and on the 12th day in culture, REH cells were isolated for western blotting. The leukemic cell population that were in suspension and not interacting with the osteoblasts were removed and spun down and designated as suspended cells (S). The REH cells which are buried under the HOB were separated by size exclusion with G10 sephadex after vigorous washing. These buried REH cells were designated as phase dim cells (PD) and have been previously described to be representative of MRD [10,12].
2.6. Nanoparticle drug delivery system
The nanoparticles were prepared as described earlier [13]. Briefly, 2 mg of CAB or PLI were dissolved in 500 microliters of acetone containing 20 mg PEG-PLGA-COOH and added dropwise into a constantly stirring 25 mM of MES (pH 5). The organic solvent was allowed to evaporate in a fume hood and the nanoparticles (NP) were activated for 1 h using a 25 mM solution of MES containing 20 mg NHS and 20 mg EDC (pH 5). The quality of the resultant NP was analyzed using NanoSight NS300 (Malvern Instruments Ltd, UK).
2.7. Cell death analysis
12-day co-culture experiments were carried out as described above in a 24-well plate. On day 10, the cells were treated with either CAB, PLI, combination of CAB and PLI (C + P) or Ara-C. After 48 h (day 12), the live ALL cells were counted using trypan blue dye exclusion method and the % viability was calculated.
2.8. Hemosphere assay
SD1 cells were plated at 100,000 cells/well and allowed to form spheroids in a 96 well plate. The formation of spheroids was visualized and confirmed using light microscope and then treated with 15 nM of PLI for 72 h. Post-treatment, the drug effects on the spheroids were captured using a Leica camera attached to a Leica DMIL LED microscope.
2.9. Western blots
REH cell pellet was re-suspended in RIPA buffer and vortexed for 15 min followed by centrifugation at 4 °C at 20,000 g to collect the protein extract. Protein content was determined by using BCA method and resolved on SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked (TBS with 5% Milk and 0.05% Tween-20) and probed with primary antibody and horseradish peroxidase conjugated secondary antibody. Signals were visualized with chemiluminescence reagents using an imager. Antibodies for BCL6, CDKN1B, Mcl-1, Bcl-2 and Bcl-xL were purchased from Cell Signaling (Cat No: 5650, 3688, 9426, 2872 and 2764 respectively) and GAPDH antibody was purchased from Fisher Scientific (Cat No: 10R-G109a).
2.10. Chemotaxis assay
Transwell with 5 μm pore size (Corning, Cat No: 3421) were used for the assay and the chemoattractant was either SDF-1 [100 ng/ml] or adherent HOB (placed in the bottom well of the transwell). REH cells were treated with 2 nM CAB for 1 h and then plated on the transwell inserts and allowed to migrate towards the bottom for 4 h. The migrated cells were counted using a flow cytometer.
2.11. Statistical analysis
All the experiments were carried out in triplicates in three individual experiments unless otherwise stated. All the data was represented as Mean ± SEM and p < 0.05 was considered as statistically significant. Statistical significance between groups was determined using one-way ANOVA followed by a post-hoc Tukey’s test.
3. Result
3.1. High throughput screening identifies cabazitaxel and plicamycin as potential anti-leukemic agents
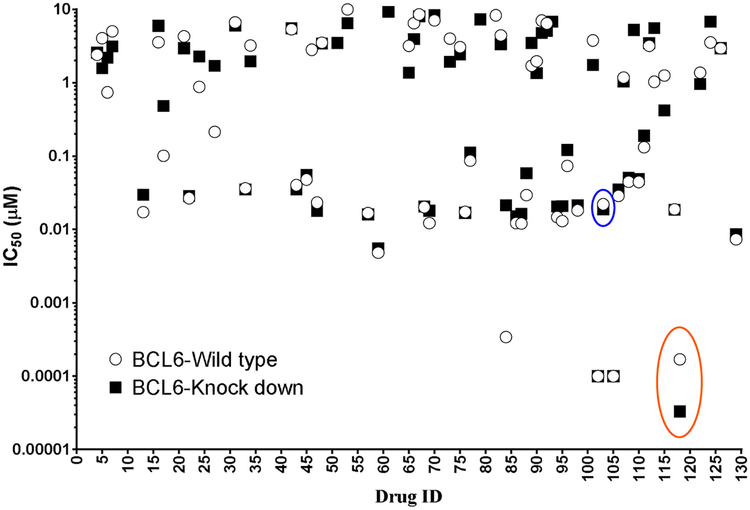
A total of 129 FDA-approved oncology drugs were screened for its selective anti-leukemic activity in REH cells with knockdown BCL6 compared to REH cells with expression of BCL6 comparable to parental REH cells. The inducible knockdown of BCL6 after doxycycline treatment was confirmed by western blotting (Suppl. 1). As seen in Fig. 1, 66 drugs from the library had IC50 values less than 10 μM and out of this, cabazitaxel (CAB) (red circle) and plicamycin (PLI) (blue circle) showed increased sensitivity in BCL6 knockdown cells compared to the cells expressing parental levels of BCL6. Importantly, a more thorough concentration-response analysis demonstrated that CAB had an IC50 value of 1 nM in BCL6 knockdown REH cells compared to 3.25 nM in the parental REH cell line and at the same time PLI had an IC50 value of8.95 nM in BCL6 knockdown cells compared to 21.9 nM in the parental cells (Fig. 2). To confirm that the anti-leukemic activity of CAB and PLI extended to other ALL cell lines, irrespective of their molecular bio-genesis, we utilized six different ALL cell lines (BV173, JM1, NALM1, NALM6, RS4 and SUPB15). As seen in Fig. 3A & C, CAB and PLI decreased cell viability in a concentration dependent manner in all the cell lines tested. The efficacy of the drugs in inhibiting viability exhibited a very tight spectrum with the CAB being most effective in BV173 cells at a low concentration (IC50 = 1.8 nM) with higher concentrations required in NALM1 (IC50 = 4.94 nM). PLI was most effective in NALM6 (IC50 = 15.72 nM) and the RS4 cell line being notably resistant (IC50 = 37.26 nM) (Fig. 3B & D).
Fig. 1.
High-throughput screening analysis in BCL6 knockdown cells. REH cell lines with doxycycline-inducible BCL6 shRNA (BCL6-Knock down) or scrambled shRNA (BCL6-Wild type) were treated with 1 μg/ml of doxycycline for 24 h. Following treatment, the cells were washed and plated in a 96 well plate at 50,000 cells/well and treated with 129 FDA-approved oncology drugs in a 4-point log concentration. The cell viability at the end of 72 h was measured as described in the methods section. IC50 values were measured and plotted.
Fig. 2.
Concentration-dependent curve for cabazitaxel and plicamycin. REH cell lines with doxycycline-inducible BCL6 shRNA (BCL6-Knock down) or REH parental cells were treated with 1 μg/ml of doxycycline for 24 h. Following treatment, the cells were washed and treated with a 9-point concentration of either cabazitaxel or plicamycin (A & C). Cell viability was measured at the end of 72 h and the resulting concentration-curves were used to calculate the IC50 values using Compusyn (B & D). The data was represented as Mean ± SEM of a study performed in triplicate at least three independent times. *P < 0.05, when compared to BCL6-Wild type.
Fig. 3.
Anti-leukemic activity of cabazitaxel and plicamycin. ALL cell lines were plated at 50,000 cells/well in a 96 well cell culture plate and treated with indicated concentrations of either cabazitaxel (A) or plicamycin (C). 72 h post-treatment the cell viability was measured as described in methods. IC50 values were calculated from the concentration curves for cabazitaxel (B) and plicamycin (D) using the compusyn software. The data was represented as Mean ± SEM of a study performed in triplicates at least three independent times.
3.2. Combination therapy of cabazitaxel and plicamycin shows synergism in drug resistant ALL cell line
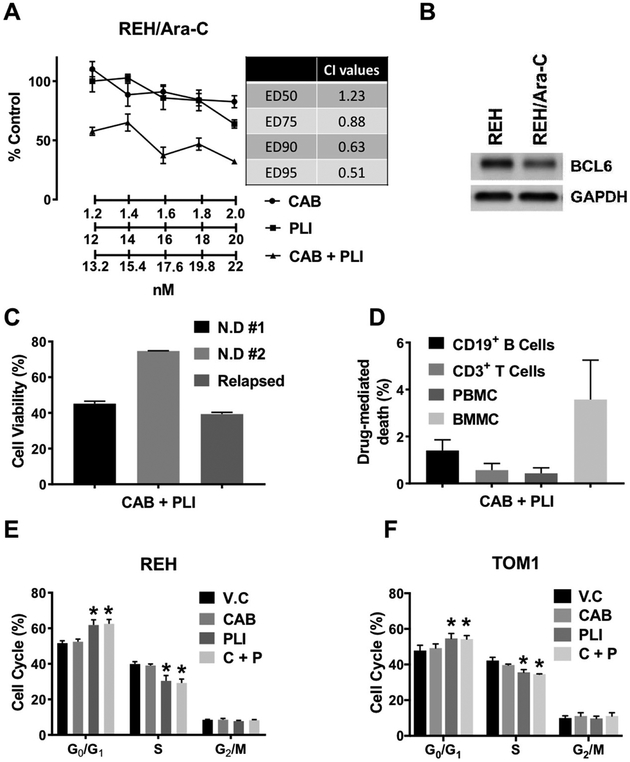
Since, our previous experiment showed a different profile of sensitivity of CAB and PLI in the ALL cell lines, we decided to test the efficacy of the combination in (i) Ara-C resistant REH cell line, REH/Ara-C (Suppl. 2), (ii) primary ALL samples and (iii) primary normal immune cells. As seen in Fig. 4A, at the doses tested, while CAB and PLI were unable to inhibit proliferation when treated alone, the combination of CAB and PLI synergistically inhibited proliferation as assessed by combination index of less than 1 at effective concentrations of the combination that decreased viability by 75, 90 and 95% (Fig. 4A). Interestingly, BCL6 expression in the REH/Ara-C cell lines where lower compared to its parental REH cell lines (Fig. 4B). Next, we tested the activity of the combination in two newly diagnosed and one relapsed primary ALL patient samples. As seen in Fig. 4C, the combination of CAB and PLI demonstrated activity at a single combination dose in all the three primary cell lines. However, the extent of its activity varied, with the combination being most effective in the relapsed patient ALL cells (40% cell viability). Furthermore, the activity of the combination was selective for leukemic cells, as the combination did not significantly change the viability of normal immune cells like CD3+ T cells, CD19+ B cells or normal peripheral mononuclear cells and bone marrow mononuclear cells (Fig. 4D). To further elucidate the activity of CAB, PLI and the combination in leukemic cells, we performed a cell cycle analysis. As seen in Fig. 4E & F, while CAB had no effect on the cell cycle, PLI significantly increased the number of cells in G0 that was inversely proportional to a significant decrease of cells in S. This effect of the PLI was maintained in the combination in both REH and TOM1 cell lines and correlated with an increase in the expression of CDKN1B in these cells after treatment (Suppl. 3).
Fig. 4.
Combination therapy of cabazitaxel and plicamycin in leukemic cells. (A) REH/Ara-C cells were plated at 50,000 cells/well in a 96 well cell culture plate and treated with indicated concentrations of either cabazitaxel (CAB), plicamycin (PLI) or combination of cabazitaxel and plicamycin (CAB + PLI). 72 h post-treatment the cell viability was measured as described in methods. Combination index (CI) values were calculated from the concentration curves for the combination using the compusyn software (inset table). (B) REH and REH/ARA-C cells were plated at 106 cells/ml and after 48 h processed for western blotting as described in the method section. (C) Primary ALL cells were plated at 50,000 cells/well in a 96 well cell culture plate and treated with combination of cabazitaxel and plicamycin (CAB + PLI). 72 h post-treatment the cell viability was measured as described in methods. (D) Normal primary immune cells were pated at 106 cells/ml in a 24 well plate and then treated with combination of CAB + PLI for 24 h. The cells were then processed for flow cytometry using a Live/Dead stain following the manufacturer’s instructions (ThermoFisher Scientific, Cat No: L34963). REH (E) and TOM1 (F) cells were treated with either cabazitaxel (CAB), plicamycin (PLI) or combination of cabazitaxel and plicamycin (CAB + PLI). 24 h post-treatment the cell cycle analysis was performed as described in methods. The data was represented as Mean ± SEM of a study performed in triplicate at least three independent times. (*P < 0.05, when compared to V.C treated group).
3.3. Combination therapy of nanoparticle-encapsulated cabazitaxel and plicamycin targets the drug resistant ALL cell population in co-culture
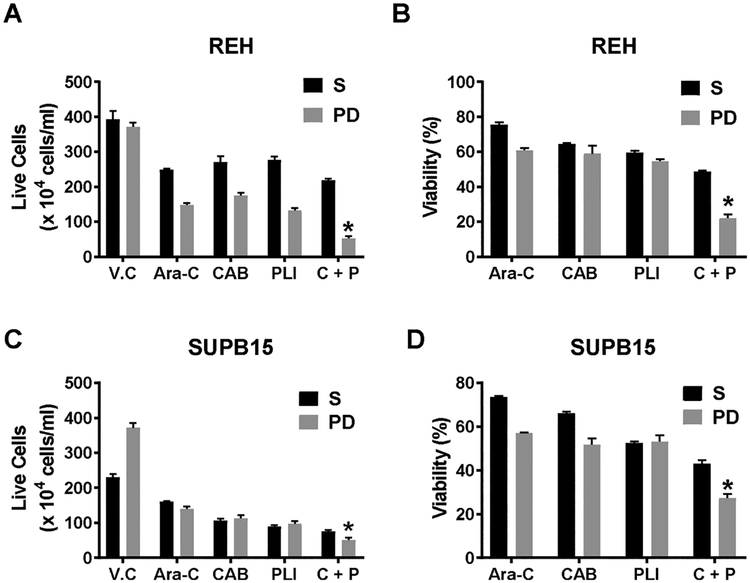
We had previously reported the utility of our co-culture model in studying the drug resistant cell population called PD cells that mimic the properties of MRD found in the BM microenvironment [9]. In the present study, we first tested the uptake of rhodamine-loaded nanoparticles (NP) in our co-culture model. As seen in Supplementary data 4, the NP preferentially were taken up by leukemic cells when compared to the HOB cells. Next, we tested the treatment of nanoparticle encapsulated CAB, PLI or their combination in the co-culture of ALL cells (either REH or SUPB15) with HOB. After 48 h of treatment, we observed that the single treatment of CAB or PLI were similar in activity in reducing the live cell population of the cells in suspension (S) and the drug resistant phase dim (PD) cells in both REH and SUPB15 co-cultures. Interestingly, the combination of the CAB and PLI reduced the live cell population statistically significantly in the PD cell population of both REH and SUPB15 (Fig. 5A & C). Similarly, the combination treatment reduced the viability of the REH PD cells to 22.2% and SUPB15 PD cells to 27.28% compared to the untreated PD cells (Fig. 5B & D).
Fig. 5.
Anti-leukemic activity of the combination therapy of cabazitaxel and plicamycin in co-culture. REH (A & B) and SUPB15 (C & D) were grown in a co-culture with osteoblast (HOB) in a 24 well plate for 12 days. On day 10 in co-culture, the cells were treated with either 3 nM of cabazitaxel (CAB) or 15 nM of Plicamycin (PLI) or their combination (C + P). All drugs were delivered in a nanoparticle encapsulated formulation. At the end of treatment, the live cells were counted, using trypan blue dye exclusion method, in suspension (S) and phase dim (PD) population when in co-culture using REH (A) and SUPB15 (C). The total cells and the live cell population was used to calculate the % viability in REH (B) and SUPB15 (D). The data is presented as Mean ± SEM and is a representative of a study performed in triplicate and conducted three independent times. *P < 0.05, when compared to all treatment groups.
3.4. Plicamycin shows anti-leukemic activity in a hemosphere assay
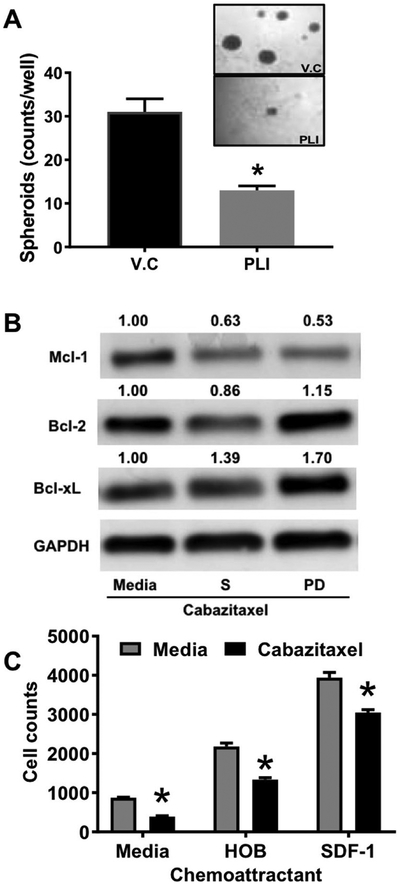
To determine the relevance of SOX2 expression in primary ALL samples, we performed real-time RT-PCR and determined that SOX2 was overexpressed three ALL patient samples compared to REH cells (Suppl. 5A). In order to mimic SOX2 overexpression in leukemic cells, we performed a hemosphere assay and showed up-regulation of the stem cell factor, SOX2 (Suppl. 5B). To investigate, if PLI manifests its anti-leukemic activity by inhibition of SOX2 transcription, we tested the activity of PLI in a hemosphere assay. After confirmation of hemosphere formation, the same were treated with 15 nM of the PLI. After 72 h of treatment, quantitation of the spheres using light microscopy showed that the PLI was very efficient in disrupting pre-formed SD1 hemosphers (13 spheroids in PLI treated vs. 31 spheroids in control) (Fig. 6A).
Fig. 6.
Mechanism of action of plicamycin and cabazitaxel in leukemic cells. (A) SD1 cells were plated at 100,000 cells/well and the spheroids were visualized using light microscope after 4 days in culture. After spheroid formation confirmation the cells were treated with 15 nM of plicamycin (PLI) or vehicle(V.C). After 72 h of treatment, the resulting hemosphere images were captured and counted. (B) REH cells were maintained in media alone or in co-culture with oseteoblast for 12 days. The cells were treated with cabazitaxel (3 nM) for 2 days and at the end of treatment, the different sub-populations were isolated and subjected to western blotting. GAPDH was used as loading control and the blots are representative of an experiment performed two independent times. (C) REH cells were plated on the insert and allowed to migrate towards the bottom well containing no chemoattractant (media), 100 ng/ml of SDF-1 or osteoblast (HOB). The number of migrated cells were enumerated using flow cytometry after 4 h. The data is presented as Mean ± SEM and is a representative of a study performed in triplicates and conducted three independent times. *P < 0.05, when compared to all untreated cells.
3.5. Cabazitaxel inhibits Mcl-1 expression and chemotaxis in ALL
Anti-apoptotic protein Mcl-1 expression and chemotaxis have both been shown to be regulated by microtubules [14,15]. To determine the relevance of Mcl-1 expression in primary ALL samples, we performed western blotting analysis and determined that it was expressed in three primary ALL patient samples (Suppl. 6A). Interestingly, Mcl-1 was overexpressed in the drug resistant PD cell population in our co-culture (Suppl. 6B). Next we determined the effect of CAB on Mcl-1 expression in ALL cells in co-culture (S & PD) compared to ALL cells grown in media (M). As seen in Fig. 6B. CAB (3 nM) selectively decreased the Mcl-1 in PD cells compared to cells in suspension (S) in the co-culture or cells grown in media (M). Surprisingly, this decrease in Mcl-1 was accompanied by an increase in Bcl-xL expression while Bcl-2 expression remained relatively unchanged. Finally, we performed a chemotaxis assay and demonstrated that pre-treatment with 2 nM of CAB significantly reduced migration of REH towards the chemoattractant (both SDF-1 and HOB).
4. Discussion
Eradication of MRD in leukemic patients is a major challenge impeding complete ablation of the disease. Factors affecting progress include; (i) inability of the standard of care to specifically target and induce death of refractory cells that comprise MRD within the BM and(ii) high cost of drug discovery coupled with a high rate of attrition of successful drugs that make it to the clinic. In the present study, we have used our model of MRD to screen for already FDA-approved oncology drugs. Identified hits, in our screening, were then compared to reported studies that used ex vivo and in vivo models of ALL to test for drug efficacy [16,17]. Using this approach, we have identified CAB and PLI as potential combination therapy for use in targeting MRD. Since, the ADME profile and mechanism of action of both these drugs has already been established [18–21], it allows for accelerated progression of these drugs through the pre-clinical stages of development for use in ALL patients.
PLI is an aureolic acid-type polyketide produced by Streptomyces strain that has been used clinically in the treatment of testicular cancer and Ewing sarcoma [22–24]. The main mode of anti-tumor activity of PLI was found to be inhibition of RNA synthesis and specifically its activity on targeting SOX2+ spheroids has been very well characterized [25–27]. In the present study, we utilized a hemoshpere assay that enriches for SOX2+ ALL cells to confirm PLI’s potential to influence tumor cells in a SOX2-associated manner.
CAB is semisynthetic taxane specifically developed to overcome taxane resistance and it acts through microtubule inhibition causing apoptosis by microtubule stabilization [28]. CAB is used in the clinic for the treatment of prostate cancer and breast cancer [29–31]. In the present study we observed that CAB mediated death in drug resistant ALL cells correlated with its ability to inhibit Mcl-1 and chemotaxis. Mcl-1 overexpression has been shown to be critical for ALL survival [32–34], and microtubule disruption has been shown to induce Mcl-1 degradation [35]. One of the interesting finding in our study was the compensatory overexpression of Bcl-xL and it would be relevant to study the combination of CAB with a specific Bcl-xL inhibitor in future studies. At the same time, soluble factor gradients driving chemotaxis play an important role in leukemic niche development [36,37]. Importantly, leukemia propagating cells have been shown to rebuild their niche in response to chemotherapy [38]. Taken together, our data suggests that in addition to microtubule inhibition, degradation of Mcl-1 and chemotaxis, all together encompasses the full spectrum of CAB’s anti-leukemic activity.
CAB and PLI have a well-characterized toxicity profile reported from multiple trials [39,40]. Neutropenia is the dose limiting toxicity during CAB therapy [29,30] with hemorrhagic and hepatotoxicity associated with PLI [41,42]. NPs are shown to reduce systemic toxicity and also demonstrate preferential uptake by cancer cells [43,44]. With these toxicities in mind we incorporated a NP formulation for drug delivery into our model to establish the potential for targeted drug delivery in future studies, based on the ease of conjugating antibodies on the surface of these PEG-PLGA derived NP [45,46]. This is not intended to suggest targeting in an overly simplified manner, but rather that the approach has viability moving forward as one mechanism to reduce toxicity.
5. Conclusion
In summary, we have shown the anti-leukemic activity of CAB and PLI in; (i) ALL cell lines driven by different oncogenic signals, (ii) an ALL cell line with acquired drug resistance (REH/Ara-C) and primary ALL patient samples, and (iii) ALL cells with de novo drug resistance (co-culture model). Taken together, the CAB and PLI combination comprises a new therapeutic strategy for treating relapsed leukemic disease that is refractory to the present standard of care.
Supplementary Material
Acknowledgements
Funding was provided by the Alexander B. Osborn Hematopoietic Malignancy and Transplantation program, WV CTR-IDEA NIH 1U54 GM104942, CoBRE P30 GM103488, WV-INBRE P20 GM103434 (LFG) and NIH WVU stroke CoBRE P20 GM109098 (WJG).
Abbreviations:
- ALL
acute lymphoblastic leukemia
- BM
bone marrow
- MRD
minimal residual disease
- CAB
cabazitaxel
- PLI
plicamycin
- NP
nanoparticles
- PD
phase dim
Footnotes
Publisher's Disclaimer: NOTICE WARNING CONCERNING COPYRIGHT RESTRICTIONS
Publisher's Disclaimer: The copyright law of the United States [Title 17, United States Code] governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the reproduction is not to be used for any purpose other than private study, scholarship, or research. If a user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use,” that use may be liable for copyright infringement. This institution reserves the right to refuse to accept a copying order if, in its judgement, fullfillment of the order would involve violation of copyright law. No further reproduction and distribution of this copy is permitted by transmission or any other means.
Declaration of interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.leukres.2018.08.002.
References
- [1].Jabbour E, O’Brien S, Konopleva M, Kantarjian H, New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia, Cancer 121 (15) (2015) 2517–2528. [DOI] [PubMed] [Google Scholar]
- [2].Bailey LC, Lange BJ, Rheingold SR, Bunin NJ, Bone-marrow relapse in paediatric acute lymphoblastic leukaemia, Lancet Oncol. 9 (9) (2008) 873–883. [DOI] [PubMed] [Google Scholar]
- [3].Kunz JB, Rausch T, Bandapalli OR, Eilers J, Pechanska P, Schuessele S,Assenov Y, Stutz AM, Kirschner-Schwabe R, Hof J, Eckert C, von Stackelberg A,Schrappe M, Stanulla M, Koehler R, Avigad S, Elitzur S, Handgretinger R,Benes V, Weischenfeldt J, Korbel JO, Muckenthaler MU, Kulozik AE, Pediatric T-cell lymphoblastic leukemia evolves into relapse by clonal selection, acquisition of mutations and promoter hypomethylation, Haematologica 100 (11) (2015) 1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manabe A, Murti KG, Coustan-Smith E, Kumagai M, Behm FG, Raimondi SC,Campana D, Adhesion-dependent survival of normal and leukemic human B lymphoblasts on bone marrow stromal cells, Blood 83 (3) (1994) 758–766. [PubMed] [Google Scholar]
- [5].Murti KG, Brown PS, Kumagai M, Campana D, Molecular interactions between human B-cell progenitors and the bone marrow microenvironment, Exp. Cell Res 226 (1) (1996) 47–58. [DOI] [PubMed] [Google Scholar]
- [6].Ebinger S, Ozdemir EZ, Ziegenhain C, Tiedt S, Castro Alves C, Grunert M,Dworzak M, Lutz C, Turati VA, Enver T, Horny HP, Sotlar K, Parekh S,Spiekermann K, Hiddemann W, Schepers A, Polzer B, Kirsch S, Hoffmann M,Knapp B, Hasenauer J, Pfeifer H, Panzer-Grumayer R, Enard W, Gires O,Jeremias I, Characterization of rare, dormant, and therapy-resistant cells in acute lymphoblastic leukemia, Cancer Cell 30 (6) (2016) 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lutz C, Woll PS, Hall G, Castor A, Dreau H, Cazzaniga G, Zuna J, Jensen C, Clark SA, Biondi A, Mitchell C, Ferry H, Schuh A, Buckle V, Jacobsen SW,Enver T, Quiescent leukaemic cells account for minimal residual disease in childhood lymphoblastic leukaemia, Leukemia 27 (5) (2013) 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilson K, Case M, Minto L, Bailey S, Bown N, Jesson J, Lawson S, Vormoor J,Irving J, Flow minimal residual disease monitoring of candidate leukemic stem cells defined by the immunophenotype, CD34+CD38lowCD19+ in B-lineage childhood acute lymphoblastic leukemia, Haematologica 95 (4) (2010) 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Slone WL, Moses BS, Evans R, Piktel D, Martin KH, Petros W, Craig M,Gibson LF, Modeling chemotherapy resistant leukemia in vitro, J. Vis. Exp (108) (2016) e53645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Slone WL, Moses BS, Hare I, Evans R, Piktel D, Gibson LF, BCL6 modulation of acute lymphoblastic leukemia response to chemotherapy, Oncotarget 7 (17) (2016) 23439–23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW,Krishnamoorthy V, Weinmann AS, Bcl-6 directly represses the gene program of the glycolysis pathway, Nat. Immunol 15 (10) (2014) 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moses BS, Evans R, Slone WL, Piktel D, Martinez I, Craig MD, Gibson LF, Bone marrow microenvironment niche regulates miR-221/222 in acute lymphoblastic leukemia, Mol. Cancer Res 14 (10) (2016) 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carroll RT, Bhatia D, Geldenhuys W, Bhatia R, Miladore N, Bishayee A,Sutariya V, Brain-targeted delivery of Tempol-loaded nanoparticles for neurological disorders, J. Drug Target 18 (9) (2010) 665–674. [DOI] [PubMed] [Google Scholar]
- [14].Chu R, Alford SE, Hart K, Kothari A, Mackintosh SG, Kovak MR,Chambers TC, Mitotic arrest-induced phosphorylation of Mcl-1 revisited using two-dimensional gel electrophoresis and phosphoproteomics: nine phosphorylation sites identified, Oncotarget 7 (48) (2016) 78958–78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ganguly A, Yang H, Sharma R, Patel KD, Cabral F, The role of microtubules and their dynamics in cell migration, J. Biol. Chem 287 (52) (2012) 43359–43369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jones L, Carol H, Evans K, Richmond J, Houghton PJ, Smith MA, Lock RB, A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the Pediatric Preclinical Testing Program, Leukemia 30 (11) (2016) 2133–2141. [DOI] [PubMed] [Google Scholar]
- [17].Frismantas V, Dobay MP, Rinaldi A, Tchinda J, Dunn SH, Kunz J, Richter-Pechanska P, Marovca B, Pail O, Jenni S, Diaz-Flores E, Chang BH, Brown TJ, Collins RH, Uhrig S, Balasubramanian GP, Bandapalli OR, Higi S, Eugster S,Voegeli P, Delorenzi M, Cario G, Loh ML, Schrappe M, Stanulla M, Kulozik AE, Muckenthaler MU, Saha V, Irving JA, Meisel R, Radimerski T, Von Stackelberg A, Eckert C, Tyner JW, Horvath P, Bornhauser BC, Bourquin JP, Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia, Blood 129 (11) (2017) e26–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morrison RK, Brown DE, Oleson JJ, A toxicologic study of mithramycin, Toxicol. Appl. Pharmacol 11 (3) (1967) 468–481. [DOI] [PubMed] [Google Scholar]
- [19].Vrignaud P, Semiond D, Benning V, Beys E, Bouchard H, Gupta S, Preclinical profile of cabazitaxel, Drug Des. Dev. Ther 8 (2014) 1851–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Galsky MD, Dritselis A, Kirkpatrick P, Oh WK, Cabazitaxel, Nat. Rev. Drug Discov 9 (9) (2010) 677–678. [DOI] [PubMed] [Google Scholar]
- [21].Fox KR, Howarth NR, Investigations into the sequence-selective binding of mithramycin and related ligands to DNA, Nucleic Acids Res. 13 (24) (1985) 8695–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kofman S, Perlia CP, Economou SG, Mithramycin in the treatment of metastatic Ewing’s sarcoma, Cancer 31 (4) (1973) 889–893. [DOI] [PubMed] [Google Scholar]
- [23].Kennedy BJ, Torkelson JL, Long-term follow-up of stage III testicular carcinoma treated with mithramycin (plicamycin), Med. Pediatr. Oncol 24 (5) (1995) 327–328. [DOI] [PubMed] [Google Scholar]
- [24].Curreri AR, Ansfield FJ, Mithramycin-human toxicology and preliminary therapeutic investigation, Cancer Chemother. Rep 8 (1960) 18–22. [PubMed] [Google Scholar]
- [25].Yarbro JW, Kennedy BJ, Barnum CP, Mithramycin inhibition of ribonucleic acid synthesis, Cancer Res. 26 (1) (1966) 36–39. [PubMed] [Google Scholar]
- [26].Vanner RJ, Remke M, Gallo M, Selvadurai HJ, Coutinho F, Lee L, Kushida M,Head R, Morrissy S, Zhu X, Aviv T, Voisin V, Clarke ID, Li Y, Mungall AJ, Moore RA, Ma Y, Jones SJ, Marra MA, Malkin D, Northcott PA, Kool M, Pfister SM, Bader G, Hochedlinger K, Korshunov A, Taylor MD, Dirks PB, Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma, Cancer Cell 26 (1) (2014) 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh DK, Kollipara RK, Vemireddy V, Yang XL, Sun Y, Regmi N, Klingler S, Hatanpaa KJ, Raisanen J, Cho SK, Sirasanagandla S, Nannepaga S, Piccirillo S,Mashimo T, Wang S, Humphries CG, Mickey B, Maher EA, Zheng H, Kim RS,Kittler R, Bachoo RM, Oncogenes activate an autonomous transcriptional regulatory circuit that drives glioblastoma, Cell Rep. 18 (4) (2017) 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vrignaud P, Semiond D, Lejeune P, Bouchard H, Calvet L, Combeau C, Riou JF,Commercon A, Lavelle F, Bissery MC, Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumors, Clin. Cancer Res 19(11) (2013) 2973–2983. [DOI] [PubMed] [Google Scholar]
- [29].Mita AC, Denis LJ, Rowinsky EK, Debono JS, Goetz AD, Ochoa L,Forouzesh B, Beeram M, Patnaik A, Molpus K, Semiond D, Besenval M,Tolcher AW, Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors, Clin. Cancer Res 15 (2) (2009) 723–730. [DOI] [PubMed] [Google Scholar]
- [30].Pivot X, Koralewski P, Hidalgo JL, Chan A, Goncalves A, Schwartsmann G,Assadourian S, Lotz JP, A multicenter phase II study of XRP6258 administered as a 1-h i.v. Infusion every 3 weeks in taxane-resistant metastatic breast cancer patients, Ann. Oncol 19 (9) (2008) 1547–1552. [DOI] [PubMed] [Google Scholar]
- [31].de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G,Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO,Investigators T, Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial, Lancet 376 (9747) (2010) 1147–1154. [DOI] [PubMed] [Google Scholar]
- [32].Koss B, Morrison J, Perciavalle RM, Singh H, Rehg JE, Williams RT,Opferman JT, Requirement for antiapoptotic MCL-1 in the survival of BCR-ABL B-lineage acute lymphoblastic leukemia, Blood 122 (9) (2013) 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aries IM, Hansen BR, Koch T, van den Dungen R, Evans WE, Pieters R, den Boer ML, The synergism of MCL1 and glycolysis on pediatric acute lymphoblastic leukemia cell survival and prednisolone resistance, Haematologica 98 (12) (2013) 1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stam RW, Den Boer ML, Schneider P, de Boer J, Hagelstein J, Valsecchi MG,de Lorenzo P, Sallan SE, Brady HJ, Armstrong SA, Pieters R, Association of high-level MCL-1 expression with in vitro and in vivo prednisone resistance in MLL-rearranged infant acute lymphoblastic leukemia, Blood 115 (5) (2010) 1018–1025. [DOI] [PubMed] [Google Scholar]
- [35].Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, Belmont LD, Kaminker JS, O’Rourke KM, Pujara K, Kohli PB, Johnson AR, Chiu ML, Lill JR, Jackson PK, Fairbrother WJ,Seshagiri S, Ludlam MJ, Leong KG, Dueber EC, Maecker H, Huang DC,Dixit VM, Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7, Nature 471 (7336) (2011) 110–114. [DOI] [PubMed] [Google Scholar]
- [36].Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA, Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells, Science 322 (5909) (2008) 1861–1865. [DOI] [PubMed] [Google Scholar]
- [37].Mohle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H,Brugger W, Kanz L, Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia, Br. J. Haematol 110 (3) (2000) 563–572. [DOI] [PubMed] [Google Scholar]
- [38].Duan CW, Shi J, Chen J, Wang B, Yu YH, Qin X, Zhou XC, Cai YJ, Li ZQ,Zhang F, Yin MZ, Tao Y, Mi JQ, Li LH, Enver T, Chen GQ, Hong DL, Leukemia propagating cells rebuild an evolving niche in response to therapy, Cancer Cell 25 (6) (2014) 778–793. [DOI] [PubMed] [Google Scholar]
- [39].Pean E, Demolis P, Moreau A, Hemmings RJ, O’Connor D, Brown D, Shepard T,Abadie E, Pignatti F, The European Medicines Agency review of cabazitaxel (Jevtana(R)) for the treatment of hormone-refractory metastatic prostate cancer: summary of the scientific assessment of the committee for medicinal products for human use, Oncologist 17 (4) (2012) 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Archimbaud E, Troncy J, Sebban C, Guyotat D, Devaux Y, French M,Moriceau M, Viala JJ, Fiere D, Phase II trial of plicamycin and hydroxyurea in acute myelogenous leukemia, Cancer Chemother. Pharmacol 25 (3) (1989) 223–225. [DOI] [PubMed] [Google Scholar]
- [41].Monto RW, Talley RW, Caldwell MJ, Levin WC, Guest MM, Observations on the mechanism of hemorrhagic toxicity in mithramycin (NSC 24559) therapy, Cancer Res. 29 (3) (1969) 697–704. [PubMed] [Google Scholar]
- [42].Green L, Donehower RC, Hepatic toxicity of low doses of mithramycin in hypercalcemia, Cancer Treat. Rep 68 (11) (1984) 1379–1381. [PubMed] [Google Scholar]
- [43].Liu L, Ye Q, Lu M, Lo YC, Hsu YH, Wei MC, Chen YH, Lo SC, Wang SJ, Bain DJ, Ho C, A new approach to reduce toxicities and to improve bioavail-abilities of platinum-containing anti-cancer nanodrugs, Sci. Rep 5 (2015) 10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Costa EC, Gaspar VM, Marques JG, Coutinho P, Correia IJ, Evaluation of nanoparticle uptake in co-culture cancer models, PLoS One 8 (7) (2013) e70072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gholizadeh S, Kamps J, Hennink WE, Kok RJ, PLGA-PEG nanoparticles for targeted delivery of the mTOR/PI3kinase inhibitor dactolisib to inflamed endothelium, Int. J. Pharm (2017), 10.1016/j.ijpharm.2017.10.032. [DOI] [PubMed] [Google Scholar]
- [46].Aggarwal S, Gupta S, Pabla D, Murthy RS, Gemcitabine-loaded PLGA-PEG immunonanoparticles for targeted chemotherapy of pancreatic cancer, Cancer Nanotechnol. 4 (6) (2013) 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.