Abstract
The anticonvulsant vigabatrin (VGB; SabrilR) irreversibly inhibits GABA transaminase to increase neural GABA, yet its mechanism of retinal toxicity remains unclear. VGB is suggested to alter several amino acids, including homocarnosine, β-alanine, ornithine, glycine, taurine, and 2-aminoadipic acid (AADA), the latter a homologue of glutamic acid. Here, we evaluate the effect of VGB on amino acid concentrations in mice, employing a continuous VGB infusion (subcutaneously implanted osmotic minipumps), dose-escalation paradigm (35–140 mg/kg/d, 12 days), and amino acid quantitation in eye, visual and prefrontal cortex, total brain, liver and plasma. We hypothesized that continuous VGB dosing would reveal numerous hitherto undescribed amino acid disturbances. Consistent amino acid elevations across tissues included GABA, β-alanine, carnosine, ornithine and AADA, as well as neuroactive aspartic and glutamic acids, serine and glycine. Maximal increase of AADA in eye occurred at 35 mg/kg/d (41 ± 2 nmol/g (n=21, vehicle) to 60 ± 8.5 (n=8)), and at 70 mg/kd/d for brain (97 ± 6 (n=21) to 145 ± 6 (n=6)), visual cortex (128 ± 6 to 215 ± 19) and prefrontal cortex (124 ± 11 to 200 ± 13; mean ± SEM; p<0.05), the first demonstration of tissue AADA accumulation with VGB in mammal. VGB effects on basic amino acids, including guanidino- species, suggested the capacity of VGB to alter urea cycle function and nitrogen disposal. The known toxicity of AADA in retinal glial cells highlights new avenues for assessing VGB retinal toxicity and other off-target effects.
Keywords: GABA, GABA-transaminase, vigabatrin, amino acid, 2-aminoadipic acid, visual cortex, prefrontal cortex, eye
1. Introduction
Anticonvulsants represent diverse pharmaceuticals whose therapeutic goal is the suppression of excessive neuronal firing during seizure (Rogawski and Bazil 2008). Further, many anticonvulsants possess the potential to decrease the spread of seizure within the brain (Kaminski et al 2014). Typically, anticonvulsant drugs will block voltage-gated sodium and calcium channels with the ultimate objective of reducing the release of excitatory glutamic acid (Fekete et al 2009). However, other targets for anticonvulsants can include enhancement of the inhibitory GABAergic system, including GABAA receptors and GABA transporters (Treven et al 2015). Nonetheless, the exact mechanism of action for many anticonvulsant agents remains unknown, although their efficacy is appreciated (e.g., topiramate) (Palpacuer et al 2018).
The mechanism of action of vigabatrin (VGB; 4-aminohex-5-enoic acid; SabrilR, Fig. 1), directly impacting the GABA metabolic pathway by irreversible inhibition of GABA-transaminase (GABA-T; Rey et al 1990) (Fig. 1), is unique among antiepileptic drugs. VGB is a first-line intervention in infantile spasms (Hussain et al 2018), and it is employed in other convulsive disorders including refractive complex partial seizures, treatment resistant epilepsy, secondary generalized seizures, and others (van der Poest Clement et al 2018). Whereas VGB has been in use in Europe and Canada for decades, it was not approved in the United States until ~2009, primarily related to reports of visual field defects (Riikonen et al 2015). The mechanism by which VGB selectively affects the visual system has not been clearly defined (Shields and Pellock 2011; Tugcu et al 2017; Peng et al 2017; Yang et al 2012), while other studies suggest that VGB does not display an increased association with visual field abnormalities (Schwarz et al 2016). More recently, VGB has been associated with white matter spongiosis and restricted globus pallidus diffusion (Pearl et al 2018; Trindade et al 2018).
Fig. 1. Metabolic interrelationships of GABA metabolism.
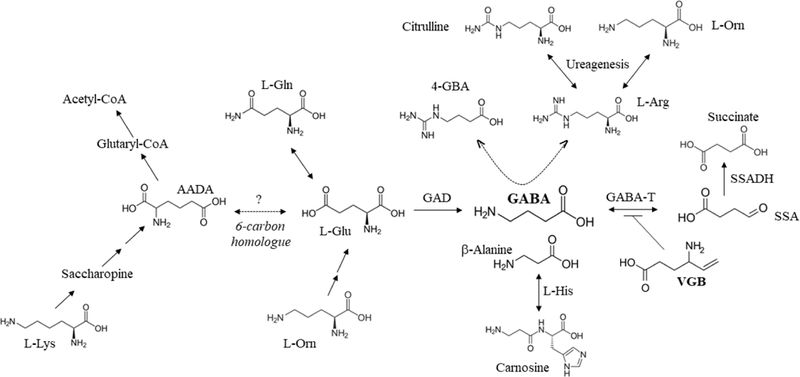
The major inhibitory neurotransmitter, GABA is produced from glutamic acid (which can interconvert with glutamine (gln)) in a reaction catalyzed by glutamic acid decarboxylase (GAD). Its catabolism is catalyzed by two sequential steps, the first GABA-transaminase (GABA-T), which produces succinic semialdehyde (SSA) that is subsequently oxidized to succinic acid by succinic semialdehyde dehydrogenase (SSADH). GABA-T is the target of vigabatrin (VGB) which results in its irreversible inactivation. A number of intermediates shown to be dysregulated by chronic VGB administration in mice (current study) are also depicted. Carnosine is the L-histidine dipeptide of β-alanine. The production of the GABA analogue, 4-guanidinobutyrate (4-GBA; not measured in this study but shown to be increased in patients with SSADH deficiency (Jansen et al 2006)), is postulated to derive from condensation of GABA with arginine (L-arg) catalyzed by arginine amidinotransferase in the pathway of creatine metabolism (Walters et al 2019). The cycle of ureagenesis encompasses arginine, citrulline (cit) and ornithine (orn). As well, there is non-mammalian evidence that ornithine can produce glutamic acid in a multistep reaction sequence (Brosnan 2000). The catabolism of lysine (lys) generates 2-aminoadipic acid (AADA) through the pathway of saccharopine metabolism, ultimately generating glutaryl-CoA and eventually acetyl-CoA. AADA is a 6-carbon homologue of glutamic acid, and its exact mechanism of generation during VGB administration, is unknown.
As for any therapeutic, VGB might manifest both on- and off-target toxicity. An example of the former includes VGB’s capacity to elevate GABA, homocarnosine, β-alanine, and potentially 4-guanidinobutyrate (Fig. 1; Grove et al 1981; Schechter 1989; Jansen et al 2006; 2008), the latter a derivate of arginine and GABA. All of these intermediates are predicted to arise via GABA-T inhibition, and their role, if any, in the ocular toxicity of VGB remains unknown (Hosking and Hilton 2002; Vogel et al 2017a; 2017b). As well, VGB use may associate with a number of amino acid alterations, including ornithine, glycine, taurine and the glutamate homologue, 2-aminoadipic acid (AADA) (Yu and Eldred 2005; Sorri et al 2010; Tao et al 2016; Vallat et al 1996). Whether these amino acid alterations are on- or off-target is also unresolved, but might be addressed through comparison of GABA-transaminase siRNA to VGB application.
The catabolic pathway of GABA to succinic acid (Fig. 1), thereby priming the Krebs cycle, occupies a central role in central nervous system metabolism. Accordingly, here we have undertaken a metabolomics approach focused on evaluating amino acid levels in mice treated with increasing levels of VGB via continuous administration. Constant dosing obviates the time-dependent parameter associated with VGB’s irreversible inhibition of GABA-T (t1/2 VGB 5–8 hours), and ensures that our amino acid profiles represent “steady-state” conditions. Our central hypothesis posited that chronic VGB intervention would result in multiple amino acid alterations in eye and visual cortex (VC), potentially providing novel clues to VGB’s selective ocular toxicity. As control regions, we also assessed comprehensive amino acids in prefrontal cortex (PFC), brain (post-VC and PFC excision), liver, and plasma. Refinement of the on- and off-target effects of VGB on the visual system, most likely in a cell line of ocular origin, may help to delineate pharmacotherapeutic approaches to mitigate this toxicity and significantly extend the utility of this important anticonvulsant agent.
2. Materials and Methods
Preparation and purification of VGB, as well as subcutaneous infusion of VGB to mice, has been recently described (Walters et al 2019). In brief, C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were bred in-house to obtain required cohort size (n=6–8 per dose group, male only), and were 8–10 weeks of age and 20.8–26.1 g in weight. Final animal numbers for study analyses included vehicle (n=21, representing a pool of animals across all studies), 35 mg/kg/d (n=8), 70 mg/kg/d (n=6) and 140 mg/kg/d (n=8). Osmotic minipumps, model 2002 (Alzet, Cupertino, CA) were prepared 18–24 hours prior to surgery. The minipumps deliver drug at a constant flow rate of 0.5 µL/hour for up to 14 days. To achieve the desired delivery concentrations of 35, 70 and 140 mg/kg/d VGB (based upon an average mouse weight of 25 grams), pumps were filled with vehicle (PBS) or 73, 146 and 291 mg/mL VGB solution in PBS, respectively. All procedures were approved by the Washington State University Institutional Animal Care and use Committee (Protocols ASAF 4232–42 and 6134), and consistent with the National Institutes of Health guide for the care and use of laboratory animals.
Animals were randomly assigned to vehicle or drug cohorts. Loading of pumps, surgical implantation, and duration of use (12 days) has been described (Walters et al 2019). Tissues isolated for analysis included intact eye, liver, prefrontal cortex (PFC, including both gray and white matter), visual cortex (VC), the remainder of the intact brain, and plasma. Retina was not isolated from intact eye for these studies. For isolation of PFC and VC, regions were identified and dissected according to the method of Spijker (2011), employing the stereotactic coordinates outlined by Paxinos and Franklin (1996). Blood was collected by cardiac puncture using a heparanized syringe, and plasma obtained following low-speed centrifugation. Following sacrifice, tissues were rapidly removed onto an ice-cold glass plate, washed with ice-cold PBS, weighed and flash-frozen in liquid nitrogen. Tissues were stored at – 80 ° C until analysis. For quantitative amino acid analysis, tissues were homogenized on ice in 0.1 M perchloric acid, followed by sonication and centrifugation to remove cellular debris. Amino acids were quantified by UPLC (Waters UPLC® MassTrak™ Amino Acid Analysis (AAA) Solution) as described (Vogel et al 2017). VGB was quantified by liquid-chromatography tandem mass spectrometry employing 2H4-gabapentin as described (Walters et al 2019). GraphPad Prism (version 8.0; San Diego, CA) was used for data and statistical analyses, the latter using one-way ANOVA with post-hoc analysis and significance set at p<0.05.
3. Results
3.1. Eye
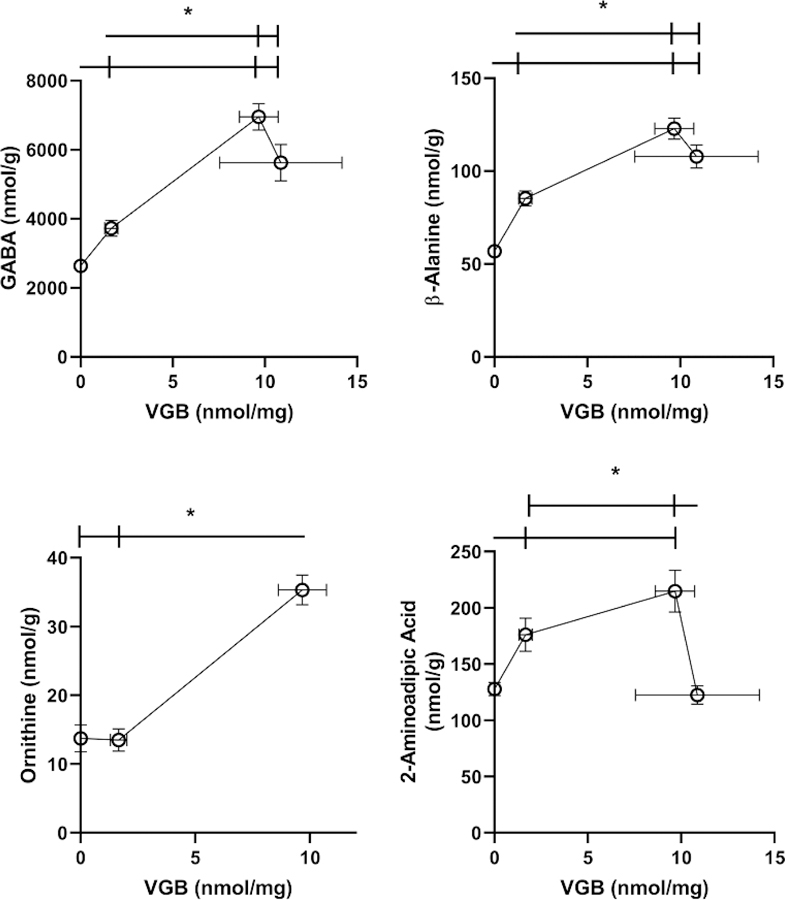
VGB eye levels increased with dose in an approximately linear fashion. Amino acids whose tissue concentration in eye was impacted by VGB are displayed in Fig. 2 and Table 1. For GABA and β-ala, increasing VGB levels essentially revealed saturation, while ornithine (orn) and 2-aminoadipic acid (AADA) showed roughly linear increases with VGB dose (orn data not available for the high VGB dose due to interference). Excitatory amino acids, including aspartic and glutamic acids (asp, glu) only revealed significant differences at the lowest VGB dose. This low-dose effect was also observed for other dysregulated amino acids, including glycine (gly), alanine (ala), serine (ser), lysine (lys), phosphoethanolamine (PEA) and ethanolamine (EA). Conversely, both cysteine (cys) and citrulline (cit) only demonstrated increased content with the highest dose of VGB (Table 1). All other amino acids (total of 27 analytes quantified) were unaffected by VGB administration (data not shown).
Fig. 2. Selected amino acids in eye as a function of vigabatrin dose.
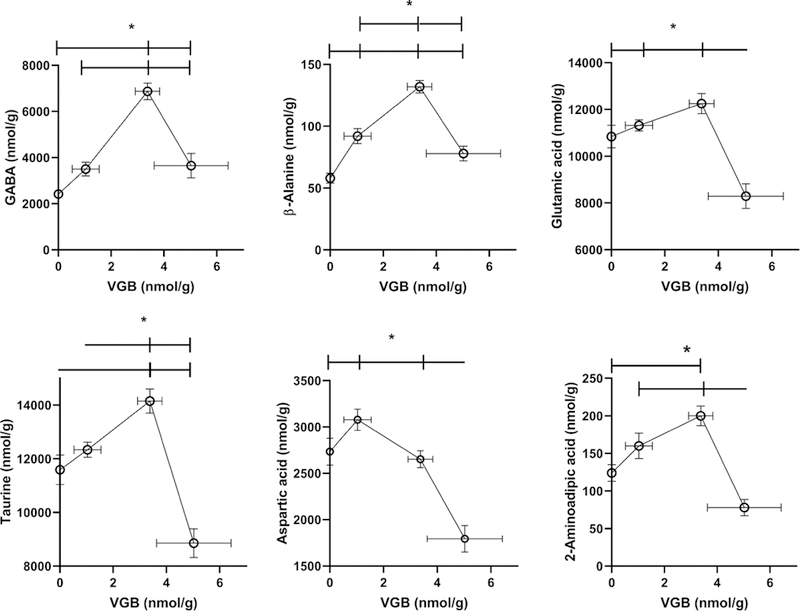
Amino acids included GABA, β-alanine, ornithine, 2-aminoadipic acid, aspartic and glutamic acids. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set at p<0.05). For ornithine, linear regression analysis revealed a statistically significant slope (r2=0.2624; p<0.01). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8). Data was not available for ornithine with high-dose VGB due to interference.
Table 1.
Amino acid content in eye as a function of increasing vigabatrin administered subcutaneously
| Description | Metabolite | Vehicle | 35 mg/kg/d | 70 mg/kg/d | 140 mg/kg/d |
|---|---|---|---|---|---|
| Drug | Vigabatrin | - | 6 (1) | 16 (1) | 29 (1) |
| Drug-Related | Carnosine | 9 (1) | nd | nd | 15 (2) |
| Neutral Amino Acids | Glycine | 575 (16) | 728 (76)a | 576(16) | 618 (19) |
| Alanine | 530 (12) | 671 (77)a | 576 (16) | 541 (17) | |
| Serine | 220 (7) | 281 (30)a | 250 (16) | 249 (12) | |
| Cysteine | 1.3 (1) | nd | nd | 23 (1)a | |
| Basic Amino Acids | Lysine | 306 (12) | 398 (55)a | 300 (9) | 299 (6) |
| Citrulline | 9 (1) | nd | nd | 13 (2)a | |
| Amines | Phosphoethanolamine | 547 (18) | 735 (92)a | 576 (16) | 591 (12) |
| Ethanolamine | 46 (2) | 61 (7)a | 43 (2) | 44 (3)b |
Legend: nmol/g tissue (mean ± (SEM))
p<0.05 compared to vehicle
p<0.05 between 35 and 140 dosing.
Abbreviations: nd, not detected. As an irreversible inhibitor of GABA-transaminase (GABA-T), vigabatrin is predicted to elevate both GABA and β-alanine (the latter a substrate for GABA-T). C arnosine is the L-histidine dipeptide of β-alanine. Animal numbers (n) were: n=21 for vehicle; n=8 for 35 mg/kg/d; n=6 for 70 mg/kg/d; and n=8 for 140 mg/kg/d.
3.2. Visual Cortex
VGB visual cortex levels increased with dose, plateauing between 70 and 100 mg/kg/d. Amino acids whose tissue concentration in visual cortex (VC) was impacted by VGB are displayed in Fig. 3 and Table 2. As for eye, the content of GABA and β-ala revealed saturation with VGB dose, and a waning effect with high-dose VGB. Similar effects were observed for AADA, but the high-dose effect was even more pronounced (Fig. 3) and returned AADA levels to that seen with vehicle. Levels of carnosine (carn), the dipeptide of β-ala and L-histidine (his), revealed increased levels in VC with higher dose VGB. For orn (Fig. 3), as well as threonine (thr), gly and ser, maximal levels were observed only at intermediate (70 mg/kg/d) dosing (Table 2). Conversely, the effect on serine was a significant decrease at the high-dose (140 mg/kg/d; Table 2). A similar decrease was observed for arginine (arg) with low dose (35 mg/kg/d; Table 2). All other amino acids were unaffected by VGB administration (data not shown).
Fig. 3. Selected amino acids in visual cortex as a function of vigabatrin dose.
Amino acids shown included GABA, β-alanine, ornithine and 2-aminoadipic acid. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set at p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
Table 2.
Amino acid content in visual cortex as a function of increasing vigabatrin administered subcutaneously
| Description | Metabolite | Vehicle | 35 mg/kg/d | 70 mg/kg/d | 140 mg/kg/d |
|---|---|---|---|---|---|
| Drug | Vigabatrin | - | 1.7 (0.4) | 9.7 (1.0) | 10.9 (3.3) |
| Drug-Related | Carnosine | 55 (3) | 65 (3) | 76 (5)a | 78 (4)a |
| Neutral Amino Acids | Glycine | 1331 (42) | 1443 (68)b | 1825 (69)a | 1337 (65)c |
| Alanine | 1075 (93) | 911 (42) | 1876 (54)d | 874 (34) | |
| Serine | 1111 (31) | 1059 (42) | 1026 (25) | 954 (44)a | |
| Threonine | 475 (34) | 478 (39) | 619 (45)c | 373 (43) | |
| Basic Amino Acids | Arginine | 152 (7) | 126 (6)e | 157 (8) | 169 (14) |
Legend: nmol/g tissue (mean ± (SEM))
p<0.05 compared to vehicle
p<0.05 between 35 and 140 dosing
p<0.05 between 70 and 140 dosing
p<0.05 compared to all other dosing (including vehicle)
p<0.05 between 35 and 140 dosing. As an irreversible inhibitor of GABA-transaminase (GABA-T), vigabatrin is predicted to elevate both GABA and β-alanine (the latter a substrate for GABA-T). Carnosine is the L-histidine dipeptide of β-alanine. Animal numbers (n) were: n=21 for vehicle; n=8 for 35 mg/kg/d; n=6 for 70 mg/kg/d; and n=8 for 140 mg/kg/d.
3.3. Prefrontal Cortex
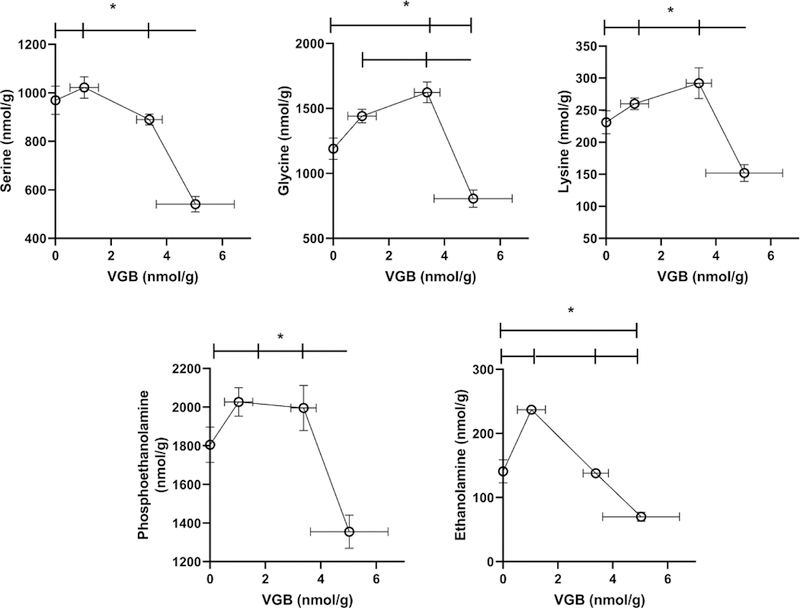
VGB prefrontal cortext levels increased with dose in an approximate linear fashion. For prefrontal cortex (PFC), every amino acid evaluated was impacted by VGB administration. For amino acids predicted to be impacted by VGB (GABA, β-ala, carn), concentrations revealed approximately linear increases with VGB administration until high-dose, at which time contents returned roughly to the level of vehicle (Figs. 4, 5 and Table 3). A similar effect was observed for taurine (tau), AADA, glutamic acid (glu), lys and gly (Figs. 4 and 5). For aspartic acid (asp) and ser, the content in PFC with low-dose VGB was not significantly different from vehicle, but was decreased with further VGB increase, and a similar high-dose effect with VGB was observed for phosphoethanolamine (PEA) and ethanolamine (EA; Fig. 2).
Fig. 4. Selected amino acids in prefrontal cortex as a function of vigabatrin dose.
Amino acids shown included GABA, β-alanine, glutamic and aspartic acids, taurine and 2-aminoadipic acid. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
Fig. 5. Additional selected amino acids in prefrontal cortex as a function of vigabatrin dose.
Amino acids shown included serine, glycine, lysine, phosphoethanolamine and ethanolamine. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
Table 3.
Amino acid content in prefrontal cortex as a function of increasing vigabatrin administered subcutaneously
| Description | Metabolite | Vehicle | 35 mg/kg/d | 70 mg/kg/d | 140 mg/kg/d |
|---|---|---|---|---|---|
| Drug | Vigabatrin | - | 1 (0.5) | 3.4 (0.5) | 5 (1.4) |
| Drug-Related | Carnosine | 84 (7) | 94 (5) | 161 (34)a,b | 100 (18) |
| Neutral Amino Acids | Asparagine | 76 (4) | 77 (3) | 77 (2) | 53 (4)d |
| Glutamine | 5014 (300) | 5379 (210) | 5287 (60) | 3172 (218)d | |
| Threonine | 454 (45) | 494 (35) | 583 (49) | 230 (41)a,f | |
| Alanine | 1041 (130) | 804 (38) | 1996 (117)a,b | 479 (27)a,c | |
| Proline | 115 (7) | 121 (4) | 134 (6) | 73 (6)d | |
| Basic Amino Acids | Histidine | 95 (7) | 100 (5) | 95 (6) | 53 (4)d |
| Arginine | 142 (9) | 153 (5) | 160 (9) | 98 (7)d | |
| Ornithine | 20 (2) | 17 (2) | 34 (2)a,b | int | |
| Large Neutral Amino Acids | Tyrosine | 76 (6) | 74 (5) | 69 (5) | 47 (4)a |
| Methionine | 58 (4) | 53 (5) | 58 (5) | 39 (3)a | |
| Valine | 94 (8) | 104 (8) | 103 (7) | 50 (4)d | |
| Isoleucine | 38 (4) | 40 (3) | 40 (2) | 18 (1)d | |
| Leucine | 88 (7) | 101 (4) | 95 (7) | 47 (4)a,f | |
| Phenylalanine | 70 (6) | 76 (3) | 75 (5) | 37 (3)d | |
| Tryptophan | 27 (3) | 38 (4)a | 17 (1)b | 16 (1)e |
Legend: nmol/g tissue (mean ± (SEM))
p<0.05 compared to vehicle
p<0.05 between 35 and 70 dosing
p<0.05 between 70 and 140 dosing
p<0.05 compared to all other dosing (including vehicle)
p<0.05 between 35 and 140 dosing
p<0.05 compared to both 35 and 70 dosing. Abbreviations: int, interference. As an irreversible inhibitor of GABA-transaminase (GABA-T), vigabatrin is predicted to elevate both GABA and β-alanine (the latter a substrate for GABA-T). Carnosine is the L-histidine dipeptide of β-alanine. Animal numbers (n) were: n=21 for vehicle; n=8 for 35 mg/kg/d; n=6 for 70 mg/kg/d; and n=8 for 140 mg/kg/d.
For PFC, high-dose VGB significantly decreased the large neutral amino acids (LNAAs; including tyrosine (tyr), methionine (met), valine (val), isoleucine (ile), leucine (leu), phenylalanine (phe) and tryptophan (trp) (Table 3). The high-dose effect of VGB was also observed for neutral amino acids (asparagine (asn), glutamine (gln), thr, ala, proline (pro)), as well as for the basic amino acids histidine (his) and arg (Table 3). It should be noted that some literature citations consider both his and gln as LNAAs (Pardridge and Oldendorf 1975). Finally, in a situation similar to VC, both ala and orn showed significant increases with intermediate VGB dosing. The data of Figs. 4 and 5, coupled with Table 3, highlights the total of 27 amino acids quantified in all tissues.
Brain (following removal of VC and PFC)
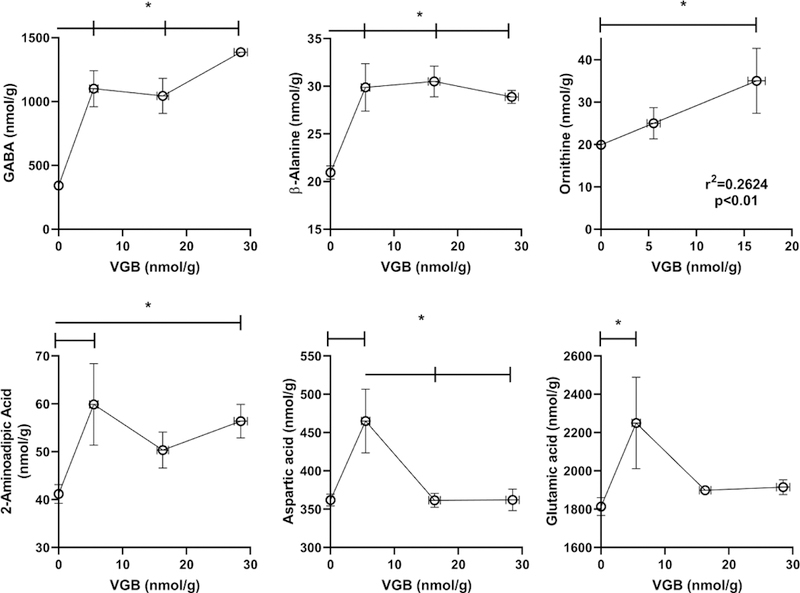
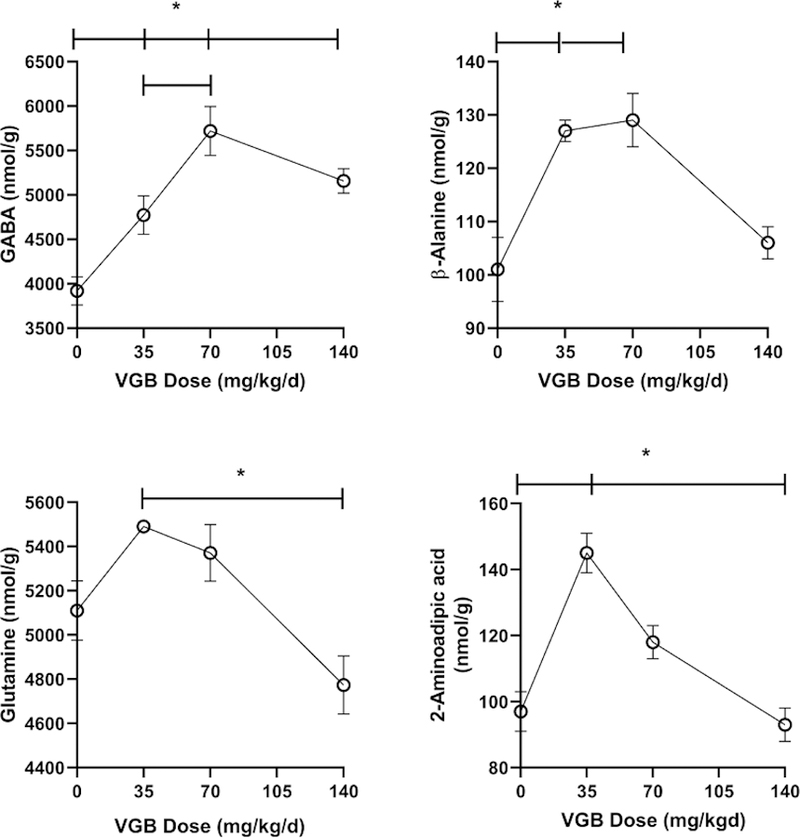
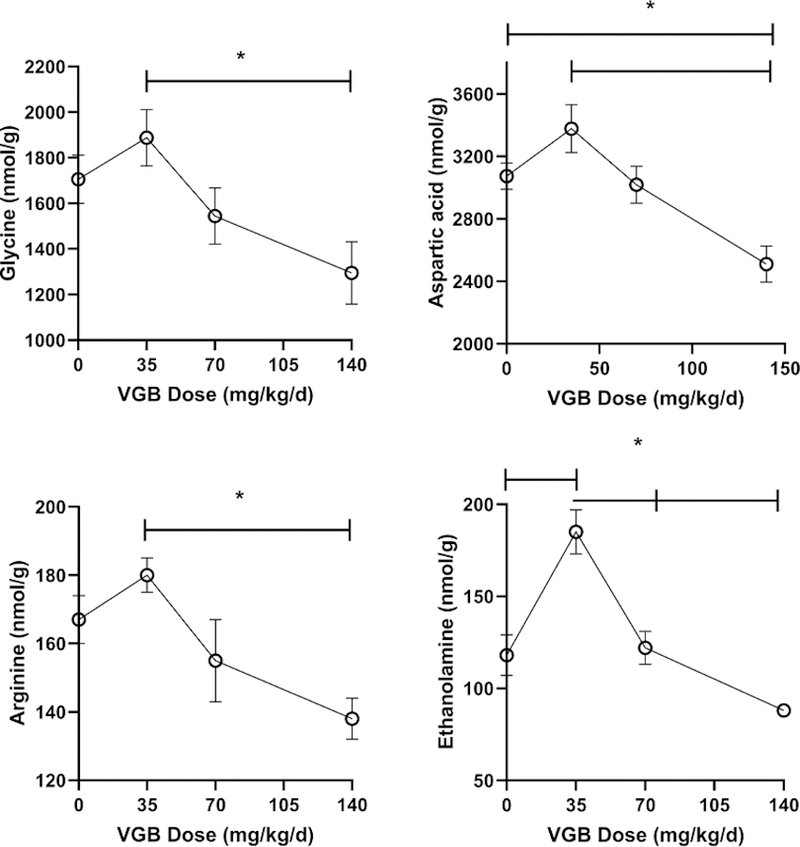
VGB brain levels increased with dose (35 mg/kg/d, 0.4 ± 0.1 nmol/g, mean ± SEM; 140 mg/kg/d, 6.8 ± 1.2 nmol/g). VGB data for the 70 mg/kg/d dose was unavailable, necessitating presentation of data in Figs. 6 and 7 for VGB as dose, and not actual concentration (x-axis). Amino acids were quantified in the remainder of brain homogenate, absent the dissected regions of VC and PFC (Figs. 6 and 7). Overall, brain extract revealed the fewest alterations with VGB administration, perhaps a reflection of the admixing of many different brain subsections. For both GABA and β-ala, high-dose VGB again revealed waning effects, with a similar effect for AADA and gln (Fig. 6). A similar high-dose effect was observed for gly, asp, arg and EA (Fig. 7), and there was an significant increase at low-dose VGB only for EA. All other amino acids were unaffected by VGB administration (data not shown).
Fig. 6. Selected amino acids in brain (without prefrontal and visual cortex) as a function of vigabatrin dose.
Amino acids shown include GABA, β-alanine, glutamine and 2-aminoadipic acid. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8). For both Figs. 6 and 7, the actual tissue VGB concentration for the 70 mg/kg/d dose was unavailable, and thus we presented x-axes values as dose, not concentration.
Fig. 7. Additional selected amino acids in brain (without prefrontal and visual cortex) as a function of vigabatrin dose.
Amino acids shown include glycine, aspartic acid, arginine and ethanolamine. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
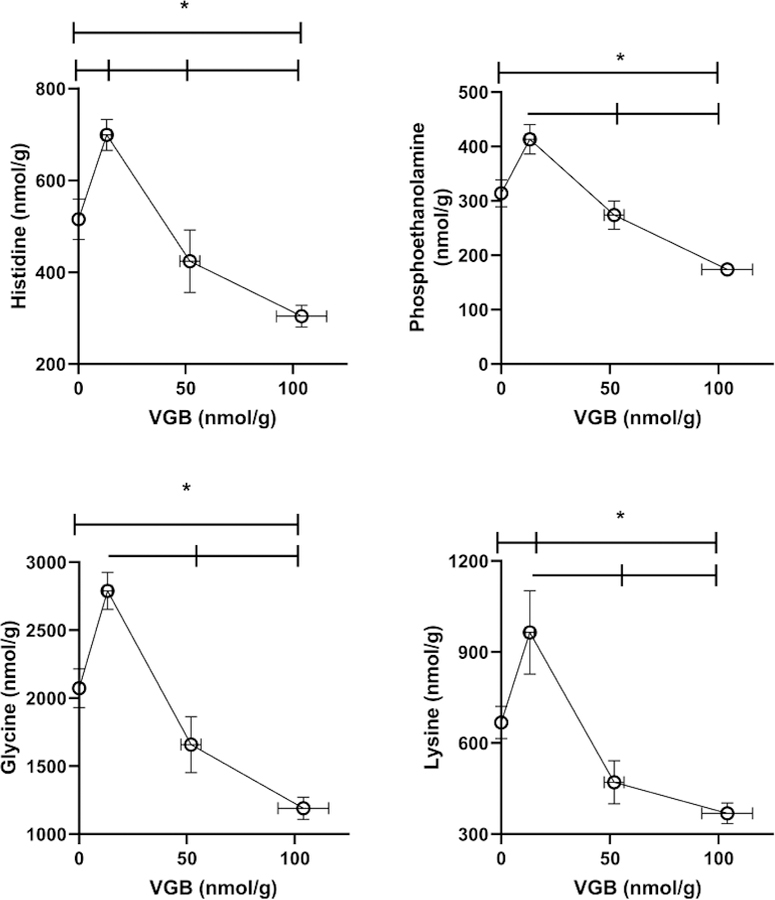
3.4. Liver
In the liver VGB levels increased with dose in a supra dose-proportional manner. As predicted, both GABA and β-ala were increased with increasing VGB administration, however the effect waned with increasing VGB for β-ala and appeared to move toward saturation for GABA (Fig. 8). For several other amino acids, including tau, gln, PEA, lys, gly and his, low dose VGB led to a significant elevation which significantly waned with intermediate and high-dose administration (Figs. 8, 9). This waning effect was also observed with a number of neutral amino acids (asparagine (asn), ser, thr, ala, pro) and acidic asp (Table 4). As was the case for the LNAAs in PFC, including tyr, met, val, ile, leu and phe, the effects of intermediate and high-dose VGB resulted in significant decreases in liver (Table 4). All other amino acids were unaffected by VGB administration (data not shown).
Fig. 8. Selected amino acids in liver as a function of vigabatrin dose.
Amino acids shown include GABA, β-alanine, taurine and glutamine. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
Fig. 9. Additional selected amino acids in liver as a function of vigabatrin dose.
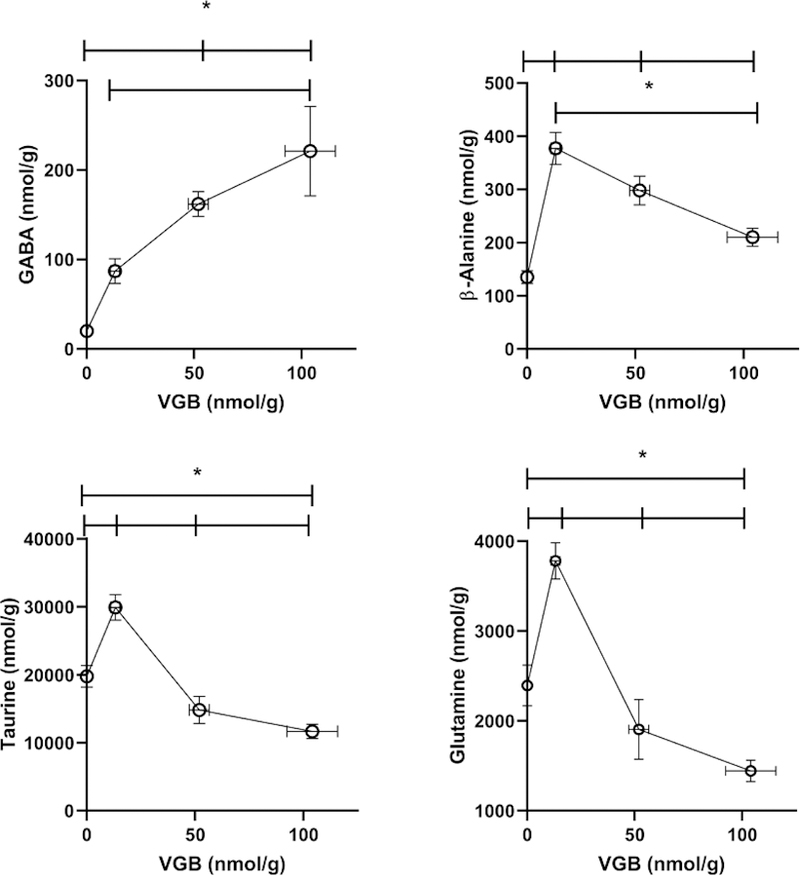
Amino acids shown include histidine, phosphoethanolamine, glycine and lysine. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
Table 4.
Amino acid content in liver as a function of increasing vigabatrin administered subcutaneously
| Description | Metabolite | Vehicle | 35 mg/kg/d | 70 mg/kg/d | 140 mg/kg/d |
|---|---|---|---|---|---|
| Drug | Vigabatrin | - | 13 (2) | 52 (5) | 104 (12) |
| Neutral Amino Acids | Asparagine | 203 (20) | 292 (26)a | 99 (10)a,c | 136 (19)e |
| Serine | 438 (46) | 583 (50) | 259 (34)b | 289 (42)e | |
| Threonine | 333 (31) | 461 (37) | 205 (25)b | 229 (30)e | |
| Alanine | 3325 (229) | 4693 (311)a | 2457 (213)b | 2342 (259)e | |
| Proline | 287 (18) | 359 (35) | 254 (58) | 224 (21)e | |
| Acidic Amino Acids | Glutamic acid | 1039 (114) | 1421 (165) | 575 (76)b | 659 (51)e |
| Large Neutral Amino Acids | Tyrosine | 207 (19) | 310 (25)a | 103 (13)b | 136 (13)e |
| Methionine | 135 (13) | 177 (15) | 54 (5)a,b | 108 (16)e | |
| Valine | 332 (26) | 444 (46) | 265 (48)b | 215 (15)e | |
| Isoleucine | 178 (13) | 275 (35)d | 137 (26) | 123 (10) | |
| Leucine | 507 (182) | 668 (61) | 311 (44)b | 408 (53)e | |
| Phenylalanine | 199 (17) | 260 (20) | 113 (13)a,b | 153 (23)e |
Legend: nmol/g tissue (mean ± (SEM))
p<0.05 compared to vehicle
p<0.05 between 35 and 70 dosing
p<0.05 between 70 and 140 dosing
p<0.05 compared to all other dosing (including vehicle)
p<0.05 between 35 and 140 dosing. Animal numbers (n) were: n=21 for vehicle; n=8 for 35 mg/kg/d; n=6 for 70 mg/kg/d; and n=8 for 140 mg/kg/d.
3.5. Plasma
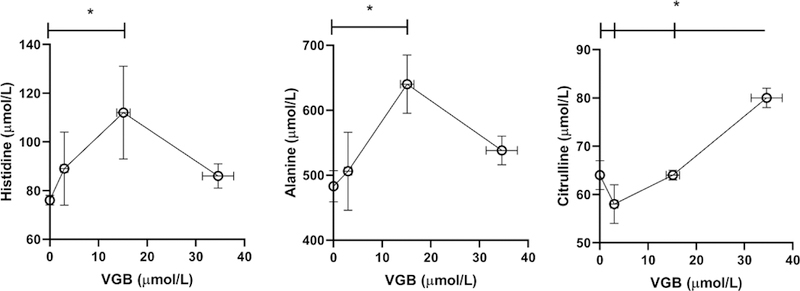
VGB plasma levels increased with dose (35 mg/kg/d, 3 ± 0.8 mM, mean ± SEM; 70 mg/kg/d, 15 ± 1.4; 140 mg/kg/d, 34.6 ± 3.2). With the exception of his, ala and citrulline (cit), all amino acids in plasma were within normal limits when comparing VGB against vehicle. Similar to other tissues studied, both his and ala increased with increasing VGB, and waned with high-dose VGB administration (Fig. 10). The converse was observed for cit, which showed a near linear increase with VGB administration.
Fig. 10. Selected amino acids in plasma as a function of vigabatrin dose.
Amino acids shown include histidine, alanine and citrulline. Statistical analysis employed a one way ANOVA with post-hoc analysis (significance set a p<0.05). Data presented as mean ± SEM for both x and y-values. Animal numbers included vehicle (n=21), 35 (n=8), 70 (n=6) and 140 mg/kg/d (n=8).
4. Discussion
Pharmacological investigations comparable to the present study frequently omit measurement of drug level. Nonetheless, Pitkanen and colleagues (1999) evaluated the effect of VGB administration on status epilepticus-induced neuronal damage employing a pump infusion paradigm in rat (2 months, 75 mg/kg/d). Sera VGB, quantified by HPLC, was ~ 35–54 mM (days 2–14), while GABA content in hippocampus, also measured by HPLC, was ~ 4–5 mmol/g tissue. In our analyses, plasma VGB levels were ~ 15–35 mM (70–140 mg/kg/d, 12 days), and GABA content in VC and PFC (70 mg/kg/d) was ~ 7 mmol/g tissue. Despite obvious study differences (rat vs. mouse; plasma vs. sera; hippocampus vs. VC and PFC; HPLC vs. mass spectrometry methodology), the consistency of analyte measures suggests that VGB pharmacokinetics/tissue distribution were comparable with previous studies conducted using the continuous infusion dose paradigm we employed.
The most consistent amino acid elevations demonstrated in all tissues were GABA, β-alanine, carnosine and AADA. The literature predicts increase of the first two with VGB (Grove et al 1981; Petroff et al 1998), and carnosine elevation is reasonable since it is the histidine dipeptide of β-alanine (Blancquaert et al 2016). However, AADA has never been demonstrated in mammalian tissues in association with VGB intake, although patients consuming VGB have shown 2-aminoadipic aciduria (Vallat et al 1996) which may be associated with mutations in DHTKD1 (dehydrogenase E1 and transketolase domain containing 1; Danhauser et al 2012). Patients with 2-aminoadipic aciduria do not manifest ocular features, however (OMIM 204750). Conversely, AADA has shown toxicity toward both astrocytes and Muller cells (Brown and Kretzschma 1998; Karlsen et al 1982), the latter the predominant form of glial cell in the vertebrate retina. Conversely, AADA has the capacity to suppress the development of form deprivation myopia via intravitreal administration in guinea pigs (Mao and Liu 2013). The six carbon homologue of glutamate (Fig. 1), AADA may exert its toxicity via enhanced release or transport of excitatory glutamate, or disruption of photoreceptors (Shen et al 2011; Pannicke et al 1994). Further exploration of the role of AADA, if any, in VGB ocular toxicity would appear warranted.
Our study demonstrated that VGB exerts numerous tissue alterations of neuroactive amino acids, including aspartic and glutamic acids, as well as serine and glycine. In the light/dark state, the signaling between retinal bipolar, ganglion, and amacrine cells (the latter the primary GABAergic cells of retina) is achieved via a series of graded potentials involving cyclic GMP (guanosine monophosphate) (Bloomfield and Dacheux 2001; Tanaka and Tachibana 2013). Glutamate and glycine are intimately involved in these potentials, as are D-serine (Gustafson et al 2015) and aspartic acid (Bloomfield and Dowling 1985). Interestingly, serine racemase is expressed in vertebrate retina (Stevens et al 2003), and our system for amino acid determination would not differentiate D- from L-serine. Abnormalities in aspartic and glutamic acids were consistent in eye and PFC, but not in VC. Conversely, consistent alterations of glycine, alanine and serine across the majority of tissues surveyed may simply reflect some degree of disruption of intermediary metabolism by VGB.
VGB has been shown to inhibit pyridoxine-5’-phosphate oxidase (PNPO), an enzyme involved in the reactivation of pyridoxal-5’-phosphate (PLP; vitamin B6) (An et al 2004). The latter is a coenzyme in all mammalian transamination reactions. If this inhibition is occurring in our study, the off-target effect of VGB to disrupt PLP reactivation could well explain many of the amino acid elevations we documented, and especially in PFC, in which every amino acid was dysregulated to some degree. An off-target role for VGB in PNPO inhibition, or perhaps transporters involved in amino acid movement (Nohr et al 2014), would also serve to explain the waning effect of high-dose VGB in many tissues (VC, brain and liver), and prominently in PFC. As well, the presence of elevated 2-AADA, a component of the lysine degradative pathway associated with PNPO deficiency, raises the possibility that pyridoxine (or pyridoxal-5’-phosphate) might have a protective role for VGB ocular toxicity. Assuming VGB off-target effects are exerted via a competitive mechanism, high levels might well be predicted to lead to waning effects. Conversely, low to medium doses of VGB might exert inhibitory effects while higher doses may conceivably either activate the enzyme or induce its expression. Irreversible GABA-T inhibition (Fig. 1) would also disrupt GABA shunt activity (Sills et al 2003), which utilizes 2-oxoglutarate as nitrogen acceptor from GABA to produce glutamic acid. This effect may also explain the alterations of glutamic acid-glutamine recycling by elevated GABA (Yang and Shen 2009). All of these potential phenomenona would require experimental evaluation, most likely in cultured cell models.
Previous work indicated that taurine and ornithine may have a role in the ocular toxicity of VGB (Sorri et al 2010; Gaucher et al 2012; Rasmussen et al 2014). In the current study, we did observe a consistent effect of VGB on ornithine in eye and VC, but not for taurine. The lack of effect on taurine suggests that it may not manifest a prominent protective role in VGB toxicity, but until we investigate amino acid parameters in isolated retina, this question will remain to be addressed. Indeed, we found a number of interesting findings for basic amino acids in the presence of VGB. At least at low-dose, VGB resulted in a significant reduction of arginine in VC, which would be consistent with the generation of 4-guanidinobutyrate from GABA (Fig. 1). Additionally, high-dose VGB significantly lowered histidine in PFC, consistent with the formation of homocarnosine from GABA conjugating to histidine. Although homocarnosine was not measured, it has been shown to be increased with VGB administration (Petroff et al 1998; 1999). In addition, there was a very consistent dose-response effect of VGB on citrulline levels in plasma, one of the few changes in plasma for any amino acid, and a finding hitherto unreported with the use of VGB in a continuous administration paradigm.
Perusal of Fig. 1 reveals a significant number of basic amino acids whose levels are altered during chronic VGB treatment, including lys, AADA, and gln, in addition to the guanidino species orn, cit, arg and 4-GBA (the latter not characterized in the current study; Walters et al 2019). The structural similarities of these species with VGB is apparent and may provide clues to many of its off-target effects. With regard to guanidino species, there are unique transporters for these compounds across the blood-brain and blood-cerebrospinal fluid barriers, and some of these accumulated guanidino species may elicit epileptic discharges (Tachikawa and Hosoya 2011). Further, alterations of guanidino species, especially arg, could suggest alterations in nitric oxide levels, a gaseous neurotransmitter shown to stimulate GABA release in retina while inhibiting release of glycine (Yu and Eldred 2005). Finally, there was a tissue-specific effect of VGB on large neutral amino acids in both liver and VC, including tyr, met, val, ile, leu, phe and trp. These species, in addition to gln and his, move from the peripheral circulation across the blood brain barrier via LAT-1, the large neutral amino acid transporter (Vogel et al 2013). Although VGB has not previously been reported to have an effect on LAT-1, this possibility seems plausible in relation to the current data, and the capacity of the LAT-1 to translocate the antiepileptic gabapentin (Furugen et al 2017).
5. Conclusions
We have documented significant alterations of amino acid content in the eye, VC, brain, liver, PFC and plasma with continuous VGB administration to mice over 12 days. Like GABA, many amino acids (glycine, β-alanine, serine, aspartic and glutamic acids) are neuroactive, potentially inducing an imbalance in excitatory vs. inhibitory potentiation. This is the first identification of the glutamate homologue, AADA, in mammalian tissues associated with VGB intake. The known toxicity of AADA in glial cells, an important component of retina, provides new avenues for assessing VGB ocular toxicity. The effect of VGB on basic amino acids may point to the capacity of VGB to disrupt urea cycle function and nitrogen disposal. Future studies will investigate amino acids in retina, as well as aqueous and vitreous humors, using a continuous VGB administration paradigm.
HIGHLIGHTS.
Continuous vigabatrin dosing induces amino acid alterations in mouse tissues
Altered neuroactive amino acids indicate imbalanced excitation/inhibition
2-aminoadipate, a glial cell toxin, increases with VGB intake in surveyed tissues
Basic amino acid changes suggest disrupted urea cycle activity/nitrogen disposal
Ours is the first metabolomic analysis of VGB toxicity reported in the mouse
Acknowledgments
*This work was supported by the National Institutes of Health National Eye Institute [Grant R01 EY027476]. Racemic vigabatrin (VU30118553) was provided by the Vanderbilt Institute of Chemical Biology, Chemical Synthesis Core, Vanderbilt, University, Nashville, TN 37232–0412
Abbreviations:
- GABA
γ-aminobutyric acid
- GABA-T
γ-aminobutyrate transaminase
- β-ala
β-alanine
- VGB
vigabatrin
- gly
glycine
- ala
alanine
- ser
serine
- cys
cysteine
- lys
lysine
- cit
citrulline
- PEA
phosphoethanolamine
- EA
ethanolamine
- his
histidine
- asp
aspartic acid
- glu
glutamic acid
- orn
ornithine
- arg
arginine
- thr
threonine
- gln
glutamine
- carn
carnosine
- AADA
2-aminoadipic acid
- 4-GBA
4-guanidinobutyric acid
- VC
visual cortex
- PFC
prefrontal cortex
- LNAA
large neutral amino acid
Footnotes
Declaration of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An SJ, Park SK, Hwang IK, Choi SY, Kwon OS, Won MH, Kang TC (2004) Vigabatrin inhibits pyridoxine-5’-phosphate oxidase, not pyridoxal kinase in the hippocampus of seizure prone gerbils. Neurochem Int 44: 133–7. [DOI] [PubMed] [Google Scholar]
- Blancquaert L, Baba SP, Kwiatkowski S, Stautemas J, Stegen S, Barbaresi S, Chung W, Boakye AA, Hoetker JD, Bhatnagar A, Delanghe J, Vanheel B, Veiga-da-Cunha M, Derave W, Everaert I (2016) Carnosine and anserine homeostasis in skeletal muscle and heart is controlled by β-alanine transamination. J Physiol 594: 4849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dowling JE (1985) Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol 53: 699–713. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF (2001) Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–84. [DOI] [PubMed] [Google Scholar]
- Brosnan JT (2000) Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 130(4S Suppl): 988S–90S. [DOI] [PubMed] [Google Scholar]
- Brown DR, Kretzschmar HA (1998) The glio-toxic mechanism of alpha-aminoadipic acid on cultured astrocytes. J Neurocytol 27: 109–18. [DOI] [PubMed] [Google Scholar]
- Danhauser K, Sauer SW, Haack TB, Wieland T, Staufner C, Graf E, Zschocke J, Strom TM, Traub T, Okun JG, Meitinger T, Hoffmann GF, Prokisch H, Kölker S (2012) DHTKD1 mutations cause 2-aminoadipic and 2-oxoadipic aciduria. Am J Hum Genet 91: 1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete A, Franklin L, Ikemoto T, Rózsa B, Lendvai B, Sylvester Vizi E, Zelles T (2009) Mechanism of the persistent sodium current activator veratridine-evoked Ca elevation: implication for epilepsy. J Neurochem 111: 745–56. [DOI] [PubMed] [Google Scholar]
- Furugen A, Ishiguro Y, Kobayashi M, Narumi K, Nishimura A, Hirano T, Iseki K (2017) Involvement of l-type amino acid transporter 1 in the transport of gabapentin into human placental choriocarcinoma cells. Reprod Toxicol 67: 48–55. [DOI] [PubMed] [Google Scholar]
- Gaucher D1, Arnault E, Husson Z, Froger N, Dubus E, Gondouin P, Dherbécourt D, Degardin J, Simonutti M, Fouquet S, Benahmed MA, Elbayed K, Namer IJ, Massin P, Sahel JA, Picaud S (2012) Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells. Amino Acids 43: 1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J, Schechter PJ, Tell G, Koch-Weser J, Sjoerdsma A, Warter JM, Marescaux C, Rumbach L (1981) Increased gamma-aminobutyric acid (GABA), homocarnosine and beta-alanine in cerebrospinal fluid of patients treated with gamma-vinyl GABA (4-amino-hex-5-enoic acid). Life Sci 28: 2431–9. [DOI] [PubMed] [Google Scholar]
- Gustafson EG, Stevens ES, Miller RF (2015) Dynamic regulation of D-serine release in the vertebrate retina. J Physiol 593: 843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking SL, Hilton EJ (2002) Neurotoxic effects of GABA-transaminase inhibitors in the treatment of epilepsy: ocular perfusion and visual performance. Ophthalmic Physiol Opt 22: 440–7. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Schmid E, Peters JM, Goyal M, Bebin EM, Northrup H, Sahin M, Krueger DA, Wu JY; Tuberous Sclerosis Complex Autism Center of Excellence Network (2018) High vigabatrin dosage is associated with lower risk of infantile spasms relapse among children with tuberous sclerosis complex. Epilepsy Res 148: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EE, Verhoeven NM, Jakobs C, Schulze A, Senephansiri H, Gupta M, Snead OC and Gibson KM (2006) Increased guanidino species in murine and human succinate semialdehyde dehydrogenase (SSADH) deficiency. Biochim Biophys Acta 1762: 494–498. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Rogawski MA, Klitgaard H (2014) The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics 11: 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen RL, Pedersen OO, Schousboe A, Langeland A (1982) Toxic effects of DL-alpha-aminoadipic acid on müller cells from rats in vivo and cultured cerebral astrocytes. Exp Eye Res 35: 305–11. [DOI] [PubMed] [Google Scholar]
- Mao JF, Liu SZ (2013) Mechanism of the DL-alpha-aminoadipic acid inhibitory effect on form-deprived myopia in guinea pig. Int J Ophthalmol 6: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr MK, Frølund S, Holm R, Nielsen CU (2014) Pharmacokinetic aspects of the anti-epileptic drug substance vigabatrin: focus on transporter interactions. Ther Deliv 5: 927–42. [DOI] [PubMed] [Google Scholar]
- Palpacuer C, Duprez R, Huneau A, Locher C, Boussageon R, Laviolle B, Naudet F (2018) Pharmacologically controlled drinking in the treatment of alcohol dependence or alcohol use disorders: a systematic review with direct and network meta-analyses on nalmefene, naltrexone, acamprosate, baclofen and topiramate. Addiction 113: 220–237. [DOI] [PubMed] [Google Scholar]
- Pannicke T, Stabel J, Heinemann U, Reichelt W (1994) alpha-Aminoadipic acid blocks the Na(+)-dependent glutamate transport into acutely isolated Müller glial cells from guinea pig retina. Pflugers Arch 429: 140–2. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Oldendorf WH (1975) Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta 401: 128–36. [DOI] [PubMed] [Google Scholar]
- Paxinos G and Franklin K (2012) The Mouse Brain in Stereotaxic Coordinates, 4th Edition, Academic Press, Cambridge, MA. [Google Scholar]
- Pearl PL, Poduri A, Prabhu SP, Harini C, Goldstein R, Atkinson RM, Armstrong D and Kinney H (2018) White matter spongiosis with vigabatrin therapy for infantile spasms. Epilepsia 59: e40–e44. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhao Y, Hu W, Hu Y, He Y and Zhou Y (2017) Reduction of retinal nerve fiber layer thickness in vigabatrin-exposed patients: A meta-analysis. Clin Neurol Neurosurg 157: 70–75. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Mattson RH, Behar KL, Hyder F, Rothman DL (1998) Vigabatrin increases human brain homocarnosine and improves seizure control. Ann Neurol 44: 948–52. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Nissinen J, Jolkkonen E, Tuunanen J, Halonen T (1999) Effects of vigabatrin treatment on status epilepticus-induced neuronal damage and mossy fiber sprouting in the rat hippocampus. Epilepsy Res 33: 67–85. [DOI] [PubMed] [Google Scholar]
- Rasmussen AD, Truchot N, Pickersgill N, Thale ZI, Rosolen SG, Botteron C (2015) The effects of taurine on vigabatrin, high light intensity and mydriasis induced retinal toxicity in the pigmented rat. Exp Toxicol Pathol 67: 13–20. [DOI] [PubMed] [Google Scholar]
- Rey E, Pons G, Richard MO, Vauzelle F, D’Athis P, Chiron C, Dulac O, Beaumont D, Olive G (1990) Pharmacokinetics of the individual enantiomers of vigabatrin (gamma-vinyl GABA) in epileptic children. Br J Clin Pharmacol 30: 253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R, Rener-Primec Z, Carmant L, Dorofeeva M, Hollody K, Szabo I, Krajnc BS, Wohlrab G, Sorri I (2015) Does vigabatrin treatment for infantile spasms cause visual field defects? An international multicentre study. Dev Med Child Neurol 57: 60–7. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Bazil CW (2008) New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels. Curr Neurol Neurosci Rep 8: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter PJ (1989) Clinical pharmacology of vigabatrin. Br J Clin Pharmacol 27 Suppl 1: 19S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MD, Li M, Tsao J, Zhou R, Wu YW, Sankar R, Wu JY and Hussain SA (2016) A lack of clinically apparent vision loss among patients treated with vigabatrin with infantile spasms: The UCLA experience. Epilepsy Behav 57(Pt A): 29–33. [DOI] [PubMed] [Google Scholar]
- Shen W, Zhang J, Chung SH, Hu Y, Ma Z, Gillies MC (2011) Submacular DL-alpha-aminoadipic acid eradicates primate photoreceptors but does not affect luteal pigment or the retinal vasculature. Invest Ophthalmol Vis Sci 52: 119–27. [DOI] [PubMed] [Google Scholar]
- Shields WD, Pellock JM (2011) Vigabatrin 35 years later - from mechanism of action to benefit-risk considerations. Acta Neurol Scand Suppl 192: 1–4. [DOI] [PubMed] [Google Scholar]
- Sills GJ, Butler E, Forrest G, Ratnaraj N, Patsalos PN, Brodie MJ (2003) Vigabatrin, but not gabapentin or topiramate, produces concentration-related effects on enzymes and intermediates of the GABA shunt in rat brain and retina. Epilepsia 44: 886–92. [DOI] [PubMed] [Google Scholar]
- Sorri I, Brigell MG, Mályusz M, Mahlamäki E, de Meynard C and Kälviäinen R (2010) Is reduced ornithine-δ-aminotransferase activity the cause of vigabatrin-associated visual field defects? Epilepsy Res 92: 48–53. [DOI] [PubMed] [Google Scholar]
- Spijker S (2011) Neuroproteomics: Dissection of rodent brain regions. In Neuromethods, Li KW, ed., pp 13–26, Totowa, NJ: Humana Press. [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF (2003) D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A 100: 6789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa M, Hosoya K (2011) Transport characteristics of guanidino compounds at the blood-brain barrier and blood-cerebrospinal fluid barrier: relevance to neural disorders. Fluids Barriers CNS 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tachibana M (2013) Independent control of reciprocal and lateral inhibition at the axon terminal of retinal bipolar cells. J Physiol 591: 3833–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Yang J, Ma Z, Yan Z, Liu C, Ma J, Wang Y, Yang Z and Huang YF (2016) The vigabatrin induced retinal toxicity is associated with photopic exposure and taurine deficiency: an in vivo study. Cell Physiol Biochem 40: 831–846. [DOI] [PubMed] [Google Scholar]
- Treven M, Koenig X, Assadpour E, Gantumur E, Meyer C, Hilber K, Boehm S, Kubista H (2015) The anticonvulsant retigabine is a subtype selective modulator of GABAA receptors. Epilepsia 56: 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade RAR, Wainstein B, Campos LG, Pérez JA, Bianchin MM, Vedolin LM and Duarte JA (2018) Globus pallidus restricted diffusion associated with vigabatrin therapy. Arq Neuropsiquiatr 76: 127–128. [DOI] [PubMed] [Google Scholar]
- Tuğcu B, Bitnel MK, Kaya FS, Güveli BT and Ataklı D (2017) Evaluation of inner retinal layers with optic coherence tomography in vigabatrin-exposed patients. Neurol Sci 38: 1423–1427. [DOI] [PubMed] [Google Scholar]
- Vallat C, Rivier F, Bellet H, Magnan de Bornier B, Mion H, Echenne B (1996) Treatment with vigabatrin may mimic alpha-aminoadipic aciduria. Epilepsia 37: 803–5. [DOI] [PubMed] [Google Scholar]
- van der Poest Clement EA, Sahin M, Peters JM (2018) Vigabatrin for Epileptic Spasms and Tonic Seizures in Tuberous Sclerosis Complex. J Child Neurol 33: 519–524. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Arning E, Wasek BL, Bottiglieri T, Gibson KM (2013) Non-physiological amino acid (NPAA) therapy targeting brain phenylalanine reduction: pilot studies in PAHENU2 mice. J Inherit Metab Dis 36: 513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Schmidt MA, Wisor JP and Gibson KM (2017a) mTOR inhibition mitigates molecular and biochemical alterations of vigabatrin-induced visual field toxicity in mice. Pediatr Neurol 66: 44–52.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Pearl PL and Gibson KM (2017b) Aberrant mTOR signaling and disrupted autophagy: The missing link in potential vigabatrin-associated ocular toxicity? Clin Pharmacol Ther 101: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Arning E, Bottiglieri T, Gibson KM (2017c) Multicompartment analysis of protein-restricted phenylketonuric mice reveals amino acid imbalances in brain. J Inherit Metab Dis 40: 227–235. [DOI] [PubMed] [Google Scholar]
- Walters DC, Jansen EEW, Ainslie GR, Salomons GS, Brown MN, Schmidt MA, Roullet JB, Gibson KM (2019) Preclinical tissue distribution and metabolic correlations of vigabatrin, an antiepileptic drug associated with potential use-limiting visual field defects. Pharmacol Res Perspect 7(1): e00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shen J (2009) Elevated endogenous GABA concentration attenuates glutamate-glutamine cycling between neurons and astroglia. J Neural Transm (Vienna) 116: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Naumann MC, Tsai YT, Tosi J, Erol D, Lin CS, Davis RJ and Tsang SH (2012) Vigabatrin-induced retinal toxicity is partially mediated by signaling in rod and cone photoreceptors. PLoS One 7: e43889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Eldred WD (2005) Nitric oxide stimulates gamma-aminobutyric acid release and inhibits glycine release in retina. J Comp Neurol 483: 278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


