Abstract
Heart failure with preserved ejection fraction (HFpEF) is a complex syndrome with an increasingly recognized heterogeneity in pathophysiology. Exercise intolerance is the hallmark of HFpEF, and appears to be caused by both cardiac, as well as peripheral abnormalities in the arterial tree and skeletal muscle. Mitochondrial abnormalities can significantly contribute to impaired oxygen utilization, and the resulting exercise intolerance in HFpEF. We review key aspects of the complex biology of this organelle, the clinical relevance of mitochondrial function, the methods that are currently available to assess mitochondrial function in humans, and the evidence supporting a role for mitochondrial dysfunction in the pathophysiology of HFpEF. We also discuss the role of mitochondrial function as a therapeutic target, some key considerations for the design of early-phase clinical trials using agents that specifically target mitochondrial function to improve symptoms in patients with HFpEF, and ongoing trials with mitochondrial agents in HFpEF.
Keywords: heart failure with preserved ejection fraction, mitochondrial function, magnetic resonance imaging, exercise intolerance
Introduction
Heart failure is a complex and heterogeneous clinical syndrome that results in significant morbidity and mortality for patients and a heavy societal and economic burden. According to NHANES data, between 2011 and 2014, there were 6.5 million Americans over the age of 20 living with heart failure. The prevalence is projected to increase by a further 46% to over 8 million adults by 2030.1 Approximately half of patients with heart failure have heart failure with preserved ejection fraction (HFpEF).2 Epidemiologic data indicate that the prevalence of HFpEF is increasing over time as our population ages and epidemics of obesity, diabetes and metabolic syndrome worsen.2 Despite numerous trials over the last few decades, at present there are no evidence-based available pharmacologic therapies that significantly impact outcomes in HFpEF.
An incomplete understanding of the pathophysiologic underpinnings of HFpEF represents a significant barrier to therapeutic advances in the field. Difficulties stem from heterogeneity within the HFpEF population, with research suggesting multiple unique phenotypes rather than a unified disease state.3 The presence of distinct phenotypes complicates the design of clinical trials and may reduce the likelihood of successful outcomes, as relatively indiscriminate enrollment may result in blunted average responses to interventions that target specific pathologic processes found in certain phenotypic subgroups.
Despite this phenotypic heterogeneity, exercise intolerance is common to all patients with HFpEF and is the primary driver of morbidity and reduced quality of life in this population.4 The normal physiologic adaptation to exercise involves a delicate systemic coordination between the cardiac pump, the respiratory system, and the arterial system, with the goal of delivering inhaled oxygen to mitochondria in skeletal and cardiac muscle. This oxygen can then be used by the mitochondria to generate adenosine triphosphate (ATP) and in turn, fuel locomotion, ventilation and cardiac contraction and relaxation. Peripheral abnormalities can therefore impact the threshold at which a diseased heart exhibits frank failure, and mitochondrial abnormalities (whether due to disease states directly impacting the mitochondria, deconditioning, or both) may impact both peripheral muscle (skeletal, respiratory) and myocardial function.
Peak exercise oxygen consumption (VO2), a widely validated measure of exercise capacity, is consistently reduced in studies of HFpEF patients5, indicating dysfunction in the oxygen delivery and utilization system. According to the Fick equation, peak VO2 is the product of cardiac output (CO) and arteriovenous oxygen (A-VO2Diff) difference, a marker of peripheral oxygen extraction. As such, a reduction in peak VO2 can be a consequence of reduced delivery of oxygen to the systemic circulation by the heart or reduced peripheral utilization of oxygen.6
It had classically been thought that exercise intolerance and reduced VO2 in HFpEF is primarily due to inadequate augmentation of cardiac output in response to exercise. Early evidence supported this theory and showed that patients with HFpEF had an inability to increase end-diastolic volume or stroke volume in response to exercise7 or exhibited impaired chronotropic reserve.8 However, more recent studies have demonstrated that peripheral dysfunction is also an important cause of exercise intolerance. Haykowsky et al. found that reduced cardiac output during exercise accounted for only 50% of the reduction in peak VO2 in HFpEF, with reduced arterio-venous oxygen difference (A-VO2Diff) accounting for the other half.5 In fact, the strongest predictor of peak VO2 was change in A-VO2Diff between resting and peak exercise. Similarly, Bhella et al. showed that that indices of cardiac reserve were not impaired in well-compensated HFpEF patients compared to healthy controls during exercise.9 A meta-analysis of six randomized controlled studies further demonstrated that, while exercise training significantly improved cardiorespiratory fitness in patients with HFpEF, resting LV function was largely unchanged, suggesting that the improved fitness may have been derived from changes in the periphery.10 Furthermore, in one study that simultaneously assessed cardiac output and VO2 during exercise, it was found that exercise training improved peak VO2 predominantly via increased A-VO2Diff during exercise, rather than increased cardiac output,11 indicating a peripheral rather than a central hemodynamic basis for the benefit of this intervention.
In sum, these studies indicate that peripheral abnormalities are important mediators of exercise intolerance in HFpEF. Among these abnormalities, mitochondrial dysfunction appears to be an important pathophysiologic contributor. Current heart failure therapies, including beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists, appear to lead to a nonspecific decrease in mitochondrial reactive oxygen species production.12 However, direct targeting of mitochondrial function as a therapeutic intervention has to date been insufficient explored. This review provides a discussion of mitochondrial abnormalities in HFpEF, currently available methods to assess mitochondrial function in humans, the role of mitochondrial function as a therapeutic target, key considerations for the design of early-phase clinical trials using mitochondrial-targeting agents, and completed or ongoing clinical trials that using novel therapies that target mitochondria.
Skeletal Muscle Mitochondrial Abnormalities in HFpEF
There is an increasing body of literature suggesting significant structural and functional abnormalities of skeletal muscle in patients with HFpEF. Kitzman et al. reported a shift towards fewer slow-twitch type I fibers (which have greater mitochondrial density and oxidative capacity), as well as a reduced capillary to fiber ratio in biopsy tissue from the vastus lateralis muscle among older patients with HFpEF. Both of these abnormalities were significantly related to peak VO2 on multivariate analysis.13 It was also shown that, compared with healthy controls, patients with HFpEF have a reduced percent of total and lean leg mass on DEXA scanning. Importantly, peak VO2 indexed to lean body mass was significantly reduced in HFpEF, indicating a reduced oxygen utilization even after accounting for the reduced skeletal muscle mass.14 While the above findings were important in illustrating significant skeletal muscle abnormalities in HFpEF, they did not directly assess mitochondrial content or oxidative capacity.
Mitochondrial function was directly assessed by Bowen et al. in a rat model of HFpEF.15 In this study, female salt-sensitive rats were separated into three groups: low-salt diet, high-salt diet or high-salt with treadmill exercise training. Diaphragm muscle biopsy from high-salt diet rats, who developed a HFpEF-like phenotype, demonstrated a fiber type shift from fast (type II) to slow (type I) twitch, with impaired mitochondrial respiration, indicating electron transport chain dysfunction. Interestingly, soleus muscle biopsy did not demonstrate a change in fiber-type ratio, but did demonstrate reduced activity of citrate synthase, a key Krebs cycle enzyme that has been well validated as a proxy for mitochondrial content and oxidative capacity.16 Exercise training attenuated the reduction of both soleus citrate synthase activity and diaphragmatic mitochondrial respiration. This rat model may not recapitulate the key features of skeletal muscle dysfunction in HFpEF, since human HFpEF has been shown to exhibit a shift towards fewer slow-twitch type I fibers in vastus lateralis muscle, as discussed above. Additionally, a biphasic pattern of response to pressure overload (an early increase in mitochondrial oxidative phosphorylation, with a subsequent decline that coincides with transition from cardiac hypertrophy to HF) has been reported in models of HFrEF; however, whether this pattern occurs in the transition to human HFpEF is unknown.17
Molina et al. subsequently studied the expression of important mitochondrial proteins (as markers of mitochondrial content and oxidative capacity) among elderly HFpEF patients. Mitochondrial content in vastus lateralis muscle tissue (as indicated by expression of porin, a mitochondrial membrane protein) was significantly reduced (46% lower) in the HFpEF group compared to sedentary healthy controls.18 HFpEF subjects also had a significantly lower expression of both citrate synthase and mitofusin 2, a key regulator of mitochondrial fusion. Both porin and mitofusin 2 expression exhibited a moderate significant direct correlation with peak VO2. These studies made a convincing case for the presence of skeletal muscle mitochondrial dysregulation in HFpEF, but further research was needed to assess the impact of such abnormalities on energetics during exercise.
Novel advanced imaging techniques provided additional insights into dysfunctional skeletal muscle energetics and further demonstrated the link between mitochondrial dysregulation and exercise intolerance. Recently, Weiss et al. compared 12 patients with HFpEF with 11 healthy controls, using31P magnetic resonance spectroscopy (MRS) during a plantar flexion exercise stress test to assess skeletal muscle energetics. They found that, while resting MRS measurements were not significantly different from healthy controls, patients with HFpEF exhibited very early and rapid depletion of high-energy phosphates during exercise, and significantly decreased maximal mitochondrial oxidative capacity.19 Whereas it is tempting to interpret this as evidence of intrinsic mitochondrial dysfunction, it should be noted that in vivo measurements of oxidative capacity interrogate the integrated function of the oxygen transport chain, including local oxygen diffusion capacity and intrinsic mitochondrial function. Therefore, impaired microvascular vasodilation and/or microvascular rarefaction (which can directly impact oxygen diffusion capacity), in addition to intrinsic mitochondrial dysfunction, could cause or contribute to these observations.
Whereas patients with HF can exhibit skeletal muscle deconditioning as a result of chronically reduced physical activity, it is important to note that the skeletal myopathy seen in HFpEF, and more broadly in heart failure, cannot be fully explained by deconditioning. While deconditioning likely contributes to HF myopathy, disuse atrophy causes an opposite fiber type shift than that reported in human HFpEF; deconditioning typically results in preferential decrease in type II fibers.20
In summary, available data demonstrates abnormalities in skeletal muscle mitochondrial content and structure, and a significant energetic impairment during exertion in HFpEF. This suggests that mitochondrial dysfunction may contribute to exercise intolerance in HFpEF patients and introduces a potential novel therapeutic target in this condition. The etiology and time-course leading to mitochondrial dysfunction in HFpEF is unclear; possible etiologies include chronic elevation of sympathetic tone, oxidative stress, overexpression of pro-inflammatory cytokines, or factors associated with the metabolic syndrome known to be associated with mitochondrial abnormalities, such as insulin resistance.21, 22 Given the potential role of this organelle in the pathophysiology of HFpEF, there is significant interest in novel therapeutics that directly target mitochondria; however, there are important aspects of mitochondrial biology, function and phenotyping that need to be considered when designing and interpreting early-phase mechanistic clinical trials with agents that impact mitochondrial function in HFpEF.
Cardiac Muscle Mitochondrial Abnormalities in HFpEF
There is also evidence that cardiomyocyte mitochondria in HFpEF have structural and energetic abnormalities. The proposed pathophysiology involves an increase in reactive oxygen species and mitochondrial damage, which results in a mismatch between ATP production and demand while also activating downstream signaling pathways that can result in cardiac remodeling, inflammation and diastolic dysfunction.23 A mouse-model of cardiac hypertrophy and diastolic dysfunction induced by abdominal aortic banding showed an overall decrease in cardiac mitochondrial metabolism and ATP production.24 A recent study in rats utilized a high fat, high sucrose diet to induce metabolic heart disease, characterized by left ventricular hypertrophy and diastolic dysfunction. Hearts isolated from rats with metabolic heart disease showed decreased rate of ATP synthesis measured by31P- magnetic resonance spectroscopy.
Abnormal myocardial mitochondrial energetics have also been demonstrated in human subjects using non-invasive imaging techniques. A study by Phan, et al., used31P-magnetic resonance spectroscopy at rest to evaluate in vivo myocardial energetics in 37 subjects with HFpEF and 20 control subjects. They found that subjects with HFpEF had significantly reduced energy reserves, as indicated by creatine phosphate/adenosine triphosphate (PCr/ATP) ratio.25 Of note, like in peripheral muscle, in vivo measurements of oxidative capacity may be impacted by factors that govern the oxygen transport upstream of the mitochondrial respiratory chain, including myocardial microvascular rarefaction, which is a feature of HFpEF.26 The relative causal impact of cardiac vs. skeletal mitochondrial dysfunction on exercise intolerance is unclear, but it is likely that therapeutic agents that improve mitochondrial function will likely enhance both cardiac function and peripheral oxygen utilization.
Comparison with HFrEF
Patients with HFrEF have been shown to have a number of skeletal muscle abnormalities that have also been shown in HFpEF, including reductions in type I oxidative fibers, citrate synthase activity, mitochondrial volume and content, and capillary to fiber ratio.27, 28
However, despite these similarities, there are differences in mitochondrial abnormalities between patients with HFpEF and HFrEF. Hunter et al. utilized mass spectrometry to quantify levels of 63 circulating metabolites among patients with HFpEF, HFrEF, and no HF. They found that long chain acyl carnitine levels, a marker of impaired fatty acid oxidation, were elevated in all subjects with heart failure; however, they were significantly higher in HFrEF than in HFpEF.29 In addition to this metabolic difference, prior studies indicate a greater degree of impairment in peripheral mitochondria in patients with HFpEF. As mentioned above, Weiss et al. used31P MRS imaging to show that subjects with HFpEF deplete PCr more rapidly than subjects with HFrEF during plantar flexion exercise.19 Additionally, a study utilizing invasive hemodynamics during cardiopulmonary exercise testing demonstrated that patients with HFpEF have more impaired peripheral oxygen extraction compared to patients with HFrEF.30
Methods for the Assessment of Mitochondrial Function
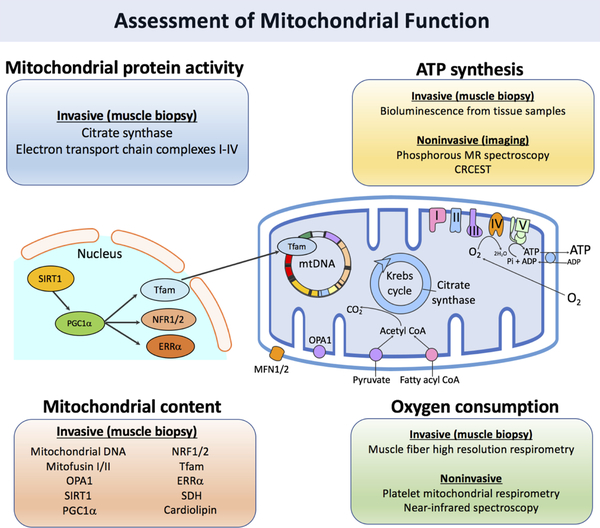
A variety of techniques can be applied to interrogate several aspects of mitochondrial biology, including mitochondrial biogenesis (content and turnover dynamics), ATP synthesis and mitochondrial oxygen consumption/utilization, as schematized in Figure 1 and Table 1.
Figure 1. Utilizing multiple aspects of mitochondrial biology for the assessment of functional changes.
Table 1.
Methods for the assessment of mitochondrial function
| Technique | Mitochondrial site | Invasiveness |
|---|---|---|
| Mitochondrial biogenesis | ||
| Transmission electron microscopy | Peripheral, cardiac | Biopsy |
| Enzyme activity | Peripheral, cardiac | Biopsy |
| Mitochondrial protein content | Peripheral, cardiac | Biopsy |
| Mitochondrial DNA content | Peripheral, cardiac | Biopsy |
| Proteome turnover dynamics | Peripheral, cardiac | Biopsy |
| ATP synthesis | ||
| Bioluminescence | Peripheral, cardiac | Biopsy |
| Phosphorous magnetic resonance spectroscopy | Peripheral, cardiac | Non-invasive (magnetic resonance) |
| Creatine chemical exchange saturation transfer (CrCEST) | Peripheral | Non-invasive (magnetic resonance imaging) |
| Oxygen consumption | ||
| High resolution respirometry | Peripheral | Biopsy (muscle), or non-invasive (serum leukocytes or platelets) |
| Near-infrared spectroscopy (NIRS) | Peripheral | Non-invasive |
Assessment of Mitochondrial biogenesis
Mitochondrial biogenesis is the process by which mitochondrial size and number are increased to escalate capacity for ATP production. Stimulating mitochondrial biogenesis in skeletal muscle to improve the bio-energetic capacity during exercise, is thus an important goal for novel therapeutics. During mitochondrial biogenesis, an increase in mitochondrial content as well as the dynamic remodeling of the mitochondrial proteome occurs. Turnover and removal of aged or damaged mitochondria occurs through mitophagy; this process involves induction of general autophagic mechanisms with selective priming of mitochondria for removal.31
Mitochondrial content:
Mitochondrial volume and content have been assessed using transmission electron microscopy of muscle biopsy samples for decades. Indeed, there is considerable evidence that mitochondrial content assessed in this manner is reduced in heart failure and correlates with exercise capacity. However, this technique is time-consuming, requires expensive equipment, and does not provide information regarding mitochondrial function.
Biomarkers isolated from muscle tissue samples are often used as surrogate measures of mitochondrial content. Activity of citrate synthase, which catalyzes the first reaction of the citric acid cycle, is the most commonly used biomarker for mitochondrial function and is thought to be the most reliable indicator of mitochondrial content.18 Activity of this enzyme is measurable via a commercially available spectrophotometric assay kit (Sigma-Aldrich, St. Louis MO, USA). Similar assays for activities of electron transport chain complexes I-IV, including cytochrome c oxidase, have also been developed.32
In addition to enzyme activity, expression of various mitochondrial structural proteins and transcription factors can provide an estimate of mitochondrial content; commonly used proteins include mitofusin I/II18, silent information regulator 1 (SIRT1)33, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α)34, nuclear respiratory factor 1/2 (NRF1/2), estrogen-related receptor alpha (ERRα)35, and succinate dehydrogenase36. Protein expression can be measured directly with Western blotting or through measurement of mRNA transcripts with real-time polymerase chain reaction (PCR).33
The content of cardiolipin, a phospholipid found only in the mitochondrial inner membrane, appears to be an excellent marker of mitochondrial content.16 Mitochondrial DNA (mtDNA) content can be quantified as well via polymerase chain reaction (PCR). Levels of mtDNA have been shown to increase in response to exercise training proportionally to citrate synthase and mitochondrial volume density37; however, recent evidence suggests that it may not be a reliable indicator of mitochondrial content.16
Mitochondrial turnover dynamics:
In addition to measuring fixed concentrations of mitochondrial proteins, assessment of dynamic changes in the mitochondrial transcriptome and proteome allow for a more specific and temporally-resolved assessment of mitochondrial biogenesis in response to stimuli. Mitochondrial proteins are continually remodeled with frequent turnover, allowing for quality control of proteins and optimal mitochondrial function.38 The synthesis and turnover rates of mitochondrial proteins thus reflect the overall quality of the mitochondria as a whole. However, mitochondrial proteins are particularly susceptible to damage by reactive oxygen species (ROS), given that most ROS are generated in the mitochondria during oxidative phosphorylation.39 As such, it becomes even more important to ensure that turnover continues to occur during pathologic states like heart failure. Indeed, it is thought that accumulation of protein damage from ROS and decreased mitochondrial protein turnover are key aspects of the pathophysiology of heart failure.40
It is possible to assess dynamic mitochondrial proteome-wide changes through measuring changes in the rates of protein synthesis or turnover. Novel stable isotope labeling techniques with high-resolution mass spectrometry have allowed for global assessments of mitochondrial proteome dynamics. Heavy water (2H2O) labeling has been used to assess turnover rates of mitochondrial proteins in a rat model, finding dramatic variations in half-lives; half-lives ranging from hours to months were calculated for the 458 mitochondrial proteins that were characterized.41 This same technique applied to a rat model of heart failure found bidirectional changes in half-lives of cardiac mitochondrial proteins, with several proteins involved in fatty acid oxidation, electron transport chain (ETC) function, and ATP synthesis showing increased turnover and other oxidative proteins showing decreased turnover.42 This approach has been extended to humans in vivo, with a study by Wang et al. showing the safety of a2H2O administration protocol for large scale tracking of serum proteome dynamics.43 The potential of this technique for characterizing patients with HFpEF and their response to novel therapeutics requires further study.
Assessment of ATP synthesis
In addition to evaluating changes in mitochondrial biogenesis, changes in the functional ability of mitochondria to generate ATP can be measured ex vivo and in vivo.
Ex vivo measurements of mitochondrial ATP synthesis can be performed via bioluminescence in mitochondria isolated from tissue samples.44 In brief, fresh tissue is sampled through percutaneous needle muscle biopsy and mitochondria are separated rapidly by centrifugation and suspended in a reaction mixture, which contains firefly luciferin-luciferase ATP-monitoring reagent, substrates for oxidation and ADP. ATP molecules produced by mitochondria react with firefly luciferase and create light signal proportional to the concentration of ATP in solution. The quantity of photons produced is then quantified using a luminometer. Whereas this technique is intuitive, ex vivo measurements of mitochondrial ATP synthesis obtained via skeletal muscle biopsy may not reflect their function in vivo, which depends on integrated oxygen transport and diffusion mechanisms. In addition, the need for single or serial skeletal muscle biopsies represents a barrier to subject participation in clinical trials.
A number of novel non-invasive imaging methods for measuring oxidative capacity and ATP production have been well described as markers of in vivo mitochondrial function. Unlike studies of mitochondria explanted from tissue biopsy, these imaging methods allow for in vivo, serial and real-time measurements of oxidative phosphorylation capacity during or immediately after exercise.
Magnetic resonance spectroscopy (MRS) can detect31P, which is commonly found in high-energy intracellular substrates. This requires MRI phosphorus coils (i.e., coils “tuned” to detect31P signal), which are different from standard clinical hydrogen coils. Exercise protocols can be performed while subjects are inside the magnet, causing demand for ATP that is met by breakdown of phosphocreatine (PCr). After depletion of PCr and cessation of exercise, oxidative phosphorylation within the muscle leads to ATP production. The recovery rate of PCr after exercise depletion reflects generation of ATP and can be used to calculate the maximum in vivo mitochondrial oxidative capacity (ATPmax).45
Phosphorous spectroscopy has been extensively validated through comparison with other markers of mitochondrial function. ATPmax measured with31P MRS has been correlated with gold-standard in vitro measures of mitochondrial respiration from muscle biopsy. Lanza et al. first provided this evidence in a study that used high-resolution respirometry of mitochondria isolated from muscle biopsy to show agreement between in vitro organelle oxygen consumption and in vivo oxidative capacity.46 Coen et al. expanded on these observations by comparing in vivo ATPmax with respiration from permeabilized muscle fibers.47 31P MRS can also be performed during exercise and recovery for dynamic assessment of mitochondrial capacity with high temporal resolution in stressed skeletal muscle (Figure 2A). As detailed above, Weiss et al. utilized this technique to show that subjects with HFpEF deplete PCr more rapidly than subjects with HFrEF and healthy controls during plantar flexion exercise; they also showed poor oxidative capacity in these subjects through assessment of PCr recovery rates.19 The main limitations of31P MRS are its poor spatial resolution, its low signal-to-noise ratio, and the lack of wide availability of phosphorus coils.
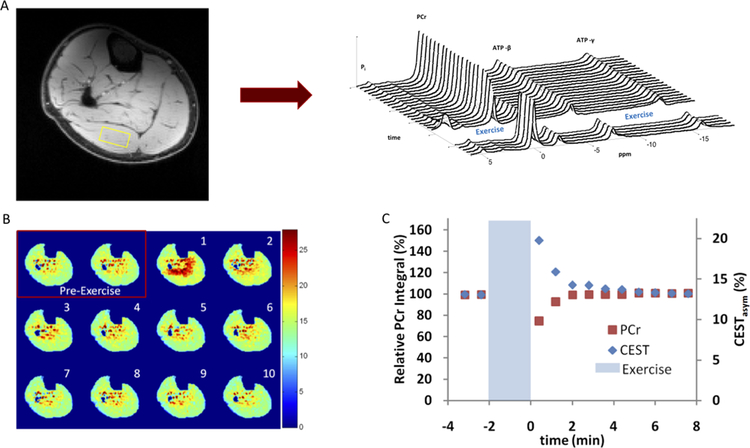
Figure 2. Utilizing phosphorous MRS and CrCEST for the quantification of oxidative capacity in lower limbs during exercise.
Panel A shows a representative image from a phosphorous MR spectroscopy study of the lower limb. The phosphorous spectra are analyzed for each voxel before and after exercise, allowing quantification of phosphocreatine recovery as a marker of oxidative capacity. Panel B shows an example image from a CrCEST study with pre-exercise baseline and post-exercise muscle-group specific increase in free creatine signal and subsequent decay during recovery. Panel C provides a comparison of signal recovery to baseline during the post-exercise period in both phosphorous MRS and CrCEST. Reproduced from Kogan, et al.100
Creatine chemical exchange saturation transfer (CrCEST) is a highly innovative novel MRI technique that addresses various limitations of31P MRS and allows for assessments of muscle oxidative capacity with high spatial (anatomic) resolution, high signal-to-noise ratio, and the use of hydrogen coils. Figure 2B shows an example of a CrCEST acquisition. CrCEST utilizes the magnetic saturation of creatine hydrogens, which are continuously exchanged (and transferred) to water molecules. This technique can perform fast, spatially-resolved measurements of creatine concentrations in tissue, and thus determine the kinetics of rate of free creatine recovery after exercise, which is a mirror image of PCr recovery (Figure 2C).48 Debrosse et al. utilized CrCEST in a study of subjects with genetic mitochondrial diseases and found a significantly prolonged rate of free creatine decline, consistent with poor mitochondrial oxidative function.49
Assessment of Mitochondrial Oxygen Consumption
Mitochondrial respiration involves a series of cellular processes that convert energy stored in macronutrients into ATP, utilizing oxygen as the final electron acceptor of the electron transport chain. Rates of oxygen consumption thus reflect mitochondrial capacity for ATP synthesis. High-resolution respirometry allows for the assessment of oxygen consumption in intact or permeabilized cells or isolated mitochondria. Unlike static measurements (such as concentrations of enzymes, DNA, RNA and various signaling molecules), respirometry allows for dynamic measurements of mitochondrial function at the level of the organelle. Respiration is measured by a temperature-controlled device, known as an oxygraph, that measures changes in oxygen tension inside a sealed incubation chamber; oxygen consumption is then derived from the change in oxygen concentration.50 Oxygen consumption can also be measured after the addition of various oxidative substrates. Notably, to ensure mitochondrial structural integrity, respiratory function must be measured on fresh tissue and not on frozen samples. Coen et al. reported that ex vivo oxygen consumption of permeabilized fibers obtained from vastus lateralis muscle biopsy, as measured by respirometry, significantly correlated with peak VO2 and maximal ATP production capacity (as measured by31P MRS) in older adults.47
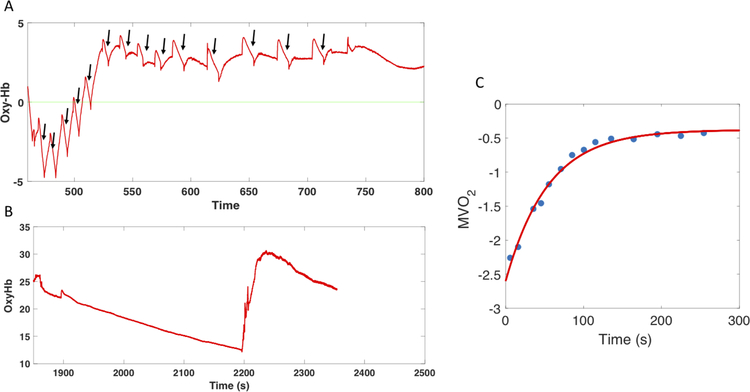
An interesting low-cost non-invasive method for the assessment of skeletal muscle mitochondrial function was developed by Ryan et al using near-infrared spectroscopy (NIRS).51 NIRS is a non-invasive minimal-risk method, which measures changes in optical absorption among the oxy and deoxy fractions of hemoglobin and myoglobin in tissue. These can be used to assess local oxygen consumption. When NIRS is used in tandem with repeated intermittent arterial occlusions via a cuff inflation, oxygen delivery is interrupted and the decline in tissue oxygenation measured by NIRS is considered a function of oxygen consumption. The rate of oxygen consumption can be assessed at various time points after a local exercise transient (such as repeated handgrip contractions with NIRS interrogation of the forearm), and the rate at which the consumption rate recovers to baseline can be quantified (Figure 3). This technique has been reported to be reproducible and was cross-validated with31P-MRS assessments in a study of healthy individuals after short-duration plantar flexion exercise.52 However, NIRS is significantly limited by depth and fat attenuation. Figure 3 demonstrates an example of a forearm NIRS intermittent occlusion study in a patient with HFpEF.
Figure 3. Use of Near Infrared Spectroscopy for the assessment of skeletal muscle oxygen consumption.
Panel A shows measurements of oxygenated hemoglobin during intermittent occlusions post-exercise (to calculate individual slopes, indicated by arrows). This signal is calibrated according to an ischemic occlusion (panel B). A subsequent exponential fit of the slopes (panel C) allows for the measurement of oxygen consumption (MVO2).
Assessment of cardiac mitochondrial function
Many of the above described techniques have been utilized for the assessment of mitochondrial function in the heart. However, these measurements present unique challenges when compared to the equivalent studies in skeletal tissue. Foremost among these is the difficulty of obtaining routine endomyocardial biopsy samples for research purposes. Given the invasiveness of obtaining heart tissue, most studies have utilized tissue that was obtained for clinical purposes or explanted hearts after transplantation.
Due to these barriers to obtaining cardiac tissue, significant attention has been paid to utilizing non-invasive imaging for assessments of cardiac metabolism.31P magnetic resonance imaging has been used in prior studies for assessment of myocardial mitochondrial function; unfortunately, data collected using older 3T MRI coils showed a relative low sensitivity, leading to poor spatial resolution and long acquisition times.53 More recent studies using 7T coils have shown a higher signal-to-noise ratio and more precise quantification of31P spectra.54 CrCEST has been applied for the assessment of myocardial mitochondrial function in an animal model of myocardial infarction, but significant technical development is required before it can be utilized in humans.55
Considerations for Early Phase Clinical Trial Design with Mitochondrial Agents in HFpEF
Treatment duration
Mitochondrial biology has important implications for clinical trial design. In particular, the kinetics of maximum effect and return to baseline after a pharmacologic intervention should be carefully considered.
Time Course of the Onset of Drug Effects
The goal of achieving a near-maximal effect prior to the end-point read outs (“on” effects) is paramount in deciding the duration of the active treatment phase in both cross-over and parallel arm early-phase trials. The goal of treatment with mitochondrial agents in HFpEF is to improve exercise capacity by increasing mitochondrial biogenesis and function. Mitochondrial biogenesis involves both an increase in mitochondrial content and remodeling of the mitochondrial proteome. It is well established in the literature that sustained exercise protocols increase mitochondrial content.56 Recent data indicate that exercise-induced adaptations in the mitochondrial transcriptome and proteome occur within days of stimuli, indicating that mitochondrial biogenesis can occur much earlier than previously believed.
A recent study of the mitochondrial proteome in human skeletal muscle indicated extensive remodeling in response to exercise in as little as 7–14 days, suggesting increased mitochondrial biogenesis and increased oxidative capacity; as an example, citrate synthase activity increased by 35% at 7 days and subsequently plateaued.57 Citrate synthase activity has been shown to be highly correlated and concordant with mitochondrial content and cristae density.16 A subsequent analysis of mitochondrial transcriptional regulation in response to short-term exercise demonstrated elevations of both mRNA and protein content of established regulators of mitochondrial biogenesis, including PGC-1α after 1 day and ERRα after three days.58 Increased levels of citrate synthase, cytochrome c, and cytochrome c oxidase subunit IV expression were found within 3–7 days following start of exercise training. Elevation of mitofusin-2 and transcription factor A mitochondrial factor mRNA expression was seen throughout training, though no change in protein content was measured.
The above data suggests that treatment for as little as one to two weeks could be feasible to see initial effects on mitochondrial function; it is unclear, however, when changes in mitochondrial content/function and particularly, their effects on whole-system aerobic capacity and other relevant endpoints such as quality of life, would reach a sustained steady state. Additionally, the HFpEF patient population may have a more limited capacity to stimulate mitochondrial biogenesis requiring treatment over longer time periods than non-HF patients. Given such uncertainties, it seems prudent to consider treatment durations of at least several (>4–6) weeks if feasible.
Washout of mitochondrial effects
In addition to the half-life of specific drugs, the longevity of newly formed mitochondria (which in turn determine the time-period required for mitochondrial density to return to baseline after discontinuation of therapy), is a key factor to consider, particularly when cross-over designs are being contemplated. Unfortunately, data regarding the mitochondrial half-life in various organisms and tissue types are scarce, and to our knowledge, no data are available in HFpEF patients. It is however clear than even within the same organism, mitochondrial half-lives differ greatly by organ/tissue type, as shown in Table 2. Further research is needed regarding the dynamics of mitochondrial turnover in human HFpEF. Given this uncertainty, results from crossover studies evaluating mitochondrial-targeting agents should be interpreted in the context of the limitations of this study design.
Table 2.
Overview of estimated mitochondrial protein half-lives
Strategies for modulation of mitochondrial function
Stimulation of mitochondrial biogenesis
Human and animal model data suggest that mitochondrial biogenesis is reduced in heart failure. The exact mechanisms behind this are unclear; however, evidence points to both downregulation of the PGC1-α pathway or defective mitochondrial DNA replication. Accumulating evidence suggests that stimulation of mitochondrial biogenesis is possible through activation of adenosine monophosphate-activated kinase (AMPK) and the nitric oxide/soluble guanylyl cyclase/cyclic guanosine monophosphate pathways.
AMPK has been shown to induce mitochondrial biogenesis through direct phosphorylation of PGC1-α production63. It also appears that AMPK coordinates mitochondrial biogenesis through epigenetic regulation of nuclear genes involved in nucleosome remodeling.64 A number of current therapies indirectly target AMPK and have cardioprotective effects, including metformin, telmisartan, thiazolidinediones and statins.65–67 Metformin, in particular, appears to reduce progression of HF in animal models and is currently being tested in an upcoming study of functional capacity and mean pulmonary artery pressures in subjects with HFpEF (NCT03629340; Table 3).68 Direct AMPK activators are in various stages of development, including 5-aminoimidazole-4-carboxamide riboside (AICAR), A-769662, and PT-1.69 However, development of AMPK activators is complicated by the heterogeneous expression and effects of its various subunits and isoforms. For example, gain-of-function mutations in the γ2 subunit of AMPK appears to induce familial hypertrophic cardiomyopathy.66
Table 3.
Ongoing and completed clinical trials testing the efficacy of agents that may impact mitochondrial function in HFpEF
| Drug (Study Acronym) |
Status at time of publication | Study Design | Patient population | Study Phase | Mitochondrial mechanism of Action | Relevant Study Endpoints | Results |
|---|---|---|---|---|---|---|---|
| Elamipretide | Completed (NCT02814097) | Randomized, double-blinded, placebo controlled, parallel-arm | Target enrollment 46 | 2 | Szeto-Schiller peptide that binds to cardiolipin on inner mitochondrial membrane and prevents conversion of cytochrome c into peroxidase, stabilizes electron transport chain, lowers reactive oxygen species levels.70 | Primary: change in E/e’ at rest and exercise measured with echocardiography after 4 weeks of daily subcutaneous study drug/placebo injection | Pending |
|
Resveratrol (contained in grape-seed
extract) (GRAPEVINE-HF) |
Completed (NCT01185067) | Randomized, double-blinded, placebo controlled, crossover | Target enrollment 15 | 1 | AMPK and SIRT1 mediated activation of nitric oxide synthase and PGC1-α-induced mitochondrial biogenesis.71, 72. | Primary: brachial artery flow-mediated dilation after 6 weeks of oral study
drug/placebo Secondary: maximal exercise capacity and oxygen consumption |
Pending |
|
Neladenoson bialanate
(PANACHE) |
Ongoing (NCT03098979) | Randomized, double-blinded, placebo controlled, parallel-arm | Target enrollment 288 | 2 | Adenosine A1 receptor agonist that leads to improved mitochondrial function via reduced opening of mitochondrial permeability transition pore (mPTP).73 | Primary: absolute change in 6-minute walk distance after 20 weeks of oral study drug/placebo | Pending |
| Inorganic nitrate-rich beetroot juice | Completed (NCT01919177) | Randomized, double-blinded, placebo controlled, crossover design | 17 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | Primary: change in peak exercise VO2 after single dose of inorganic
nitrate Secondary: change in skeletal muscle mitochondrial oxidative capacity measured with near-infrared spectroscopy |
Single dose (18 mmol) of nitrate-rich beetroot juice increased peak VO2, increased exercise cardiac output and reduced arterial wave reflections, with a non-significant trend towards improved mitochondrial oxidation.75 In this trial, the reduction in arterial wave reflections was the main predictor of improvement in peak VO2. |
| Potassium nitrate (KNO3) | Completed (NCT02256345) | Randomized, double-blinded, placebo-controlled | KNO3: 9, placebo: 3 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | The main objective of this study was to assess the safety, population-specific pharmacokinetics and dose-response effects with sustained administration of KNO3. Efficacy endpoints included the change in peak VO2 (primary), exercise capacity and quality of life. Mitochondrial function was assessed with NIRS. | KNO3 was safe and demonstrated favorable pharmacokinetics for sustained administration in this population. Non-significant trend towards improvement in peak VO2, with significant improvements in exercise duration, and Kansas City Cardiomyopathy Questionnaire total symptom and functional status scores. There was no evidence of improvement in mitochondrial oxidative function. |
|
Potassium nitrate (KNO3CK OUT HFPEF) |
Ongoing, actively recruiting (NCT02840799) | Randomized, double-blinded, placebo controlled, crossover design | Target enrollment 76 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | Co-primary endpoints: change in total work performed and peak VO2 during a maximal
effort exercise test, after 6 weeks of oral treatment. Secondary: various mechanistic endpoints, including free creatine recovery kinetics measured with CrCEST. |
Pending. |
| Intravenous Sodium Nitrite | Completed (NCT 01932606) | Randomized, double-blinded, placebo controlled, parallel-arm | NaNO2: 14, placebo: 14 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | Primary: change in pulmonary capillary wedge pressure (PCWP) during exercise | After single intravenous dose of study drug, significant improvement of exercise PCWP by nitrite vs. placebo.76 |
| Inhaled Sodium Nitrite | Completed (NCT02262078) | Randomized, double-blinded, placebo controlled, parallel-arm | NaNO2: 13, placebo: 13 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | Primary: change in pulmonary capillary wedge pressure (PCWP) during exercise | After single inhaled dose of study drug, significant improvement of exercise PCWP by nitrite vs. placebo and reduction of resting PCWP.77 |
|
Inhaled (Nebulized) Sodium Nitrite (INDIE-HFpEF) |
Completed (NCT02742129) | Randomized, double-blinded, placebo controlled, crossover design | 105 | 2 | Key non-mitochondrial mechanisms of action (such as vascular effects) have been demonstrated, but mitochondrial effects are also possible (reduced mitochondrial proton leak, stimulation of biogenesis via cGMP, improved efficiency of oxidative phosphorylation).74 | Primary: change in peak VO2 after 4 weeks of study drug/placebo | Three times daily dosing of inhaled sodium nitrate (NaNO2) did not result in a change in exercise capacity vs. placebo (P=0.27). Final results unpublished; initial results recently presented.78 The short half-life of nitrite (~35 minutes) from this delivery system may have contributed to neutral results with sustained administration. Oral potassium nitrate (being tested in KNO3CKOUT HFpEF) has a much longer half-life (~6–8 hours). |
| Ubiquinol (active coenzyme Q10) | Not yet recruiting (NCT03133793) | Randomized, double-blinded, placebo controlled, parallel-arm | Target enrollment 276 | 2 | Mitochondrial inner membrane electron transport chain cofactor.79 | Primary: change in health status defined by Kansas City Cardiomyopathy Questionnaire after 12
weeks of oral study drug/placebo Secondary: change in mitochondrial adenosine triphosphate (ATP) production measured using serum lactate/ATP ratio |
Pending |
|
Ranolazine (RALI-DHF) |
Completed (NCT01163734) | Randomized, double-blinded, placebo controlled, parallel-arm | Ranolazine: n=12; placebo: n=8. | 2 | Partial inhibitor of fatty acid oxidation.80 | Primary: change in hemodynamic parameters (LVEDP, PCWP, time-constant of relaxation tau) after 30-minute infusion of study drug/placebo | Improved LVEDP, pulmonary capillary wedge pressure. No change in relaxation parameters.81 |
| Epicatechin | Completed (NCT02068040) | Non-randomized, open-label, single group | 7 | 1 | Nitric oxide-mediated stimulation of mitochondrial biogenesis.82 | Primary: change in peak exercise VO2, skeletal muscle metabolism (measured with magnetic resonance spectroscopy) after 3 months of oral study drug | Unpublished |
| Perhexiline | Completed(NCT00839228) | Randomized, double-blinded, placebo controlled, parallel-arm | 70 | 2 | Inhibition of mitochondrial fatty acid oxidation.83 | Primary: change in peak exercise VO2 after 3 months of oral study drug | Unpublished |
|
Vericiguat (VITALITY-HFpEF) |
Ongoing (NCT03547583) | Randomized, double-blinded, placebo-controlled, parallel-arm | Target enrollment: 735 | 2 | Stimulation of soluble guanylate cyclase. | Primary: change in Kansas City Cardiomyopathy Questionnaire score after 24 weeks of oral study
drug. Secondary: change in six-minute walk test. |
Pending |
|
IW-1973 (CAPACITY-HFpEF) |
Ongoing (NCT03254485) | Randomized, double-blinded, placebo-controlled, parallel-arm | Target enrollment: 184 | 2 | Stimulation of soluble guanylate cyclase. | Primary: incidence of treatment-emergent adverse events, chance in peak
VO2 Secondary: change in six-minute walk test, ventilatory efficiency |
Pending |
SIRT 1 = sirtuin 1, PGC1-α = Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, cGMP = cyclic guanosine monophosphate, VO2 = oxygen consumption, NIRS = near infrared spectroscopy, CrCEST = creatine chemical exchange saturation transfer, LVEDP = left ventricular end-diastolic pressure
Nitric oxide, through a cyclic guanosine monophosphate dependent (cGMP) pathway, has also been shown to activate mitochondrial biogenesis.84 Nitric oxide signaling can be increased through two pathways: direct modulation of soluble guanylyl cyclase (sGC), which synthesizes cGMP, and through targeting of the endogenous nitrate-nitrite-NO pathway. Although pre-clinical and early phase studies were promising, increasing NO through the soluble guanylyl cyclase system with phosphodiesterase-5 inhibitors was not effective in improving clinical or exercise status in patients with HFpEF.85 Vericiguat, a direct sGC stimulator, appeared to exert positive effects on quality of life in the SOCRATES PRESERVED trial, an effect that is now being tested in a larger trial (VITALITY-HFpEF; NCT03547583).86 The CAPACITY-HFpEF trial is testing another sGC stimulator, IW-1973 (NCT03254485) (Table 3). However, these trials are not assessing the mechanism of any potential effect of sGC stimulation on clinical outcomes. Of note, these agents could impact symptoms through several non-cardiac effects, including improvements in conduit artery function, which is highly NO-dependent.87
Targeting the nitrate-nitrite-NO pathway also appears to be an efficacious means for increasing nitric oxide levels. Despite encouraging results with single-dose administration trials of inhaled nitrite,88 the recent INDIE-HFpEF phase IIb trial did not demonstrate a benefit of this agent on quality of life or aerobic capacity. The very short half-life of this agent (~35 minutes) may have substantially limited any potential efficacy in this agent, which was administered three times daily. In contrast to inorganic nitrite, orally-administered inorganic nitrate demonstrates a longer half-life and favorable pharmacokinetics for sustained administration.89 The use of oral inorganic nitrate has shown promise in single and repeated-dose administration in early phase studies, and a larger phase IIb trial (KNO3CK OUT HFpEF) is ongoing.89, 90 (Table 3). Of note, it is likely that the effect of inorganic nitrate on exercise capacity is mediated by arterial, rather than mitochondrial effects since reductions in arterial wave reflections were the main correlate of increases in peak VO2 with this agent.75
Resveratrol, a polyphenol found in red wine, has interestingly been shown to stimulate mitochondrial biogenesis through both AMPK and nitric oxide-dependent mechanisms.71, 72 Animal model data suggests that resveratrol can reduce cardiac dysfunction and improve mitochondrial biogenesis in hypertension-mediated heart failure.91 A study of 40 human subjects post-myocardial infarction demonstrated efficacy in improvement of diastolic function.92 Resveratrol has been studied in HFpEF with a completed study assessing the effect of grape seed extract on endothelial function, however, the results have not yet been published (Table 3).
Reduction of oxidative stress
Elevated and pathologic reactive oxygen species production has been implicated in a number of cardiometabolic disorders, including heart failure.93 However, trials of antioxidant therapies in HF have resulted in varying levels of success.
MitoQ, a lipophilic quinol that accumulates in the mitochondrial matrix and scavenges ROS, has been extensively studied as a mitochondrial antioxidant. A recently published study showed that in rats with pressure-overload induced heart failure, MitoQ reduced hydrogen peroxide levels and improved mitochondrial respiration.94 A human study of patients with chronic hepatitis C showed a significant improvement in hepatic function without severe side effects.95 However, the efficacy of MitoQ may be limited as its uptake into the mitochondria relies on an intermembrane potential difference, which is reduced in heart failure.66
Szeto-Schiller (SS) peptides are small amino acid sequences that are rapidly taken up by mitochondria due to their high affinity for cardiolipin, a phospholipid found in the inner mitochondrial membrane. The SS-31 variant of SS peptides (also called elamipretide or Bendavia), in particular, has shown benefit as a cardioprotective antioxidant with reduced mitochondrial ROS production and reduces maladaptive remodeling.70 There is also evidence that elamipretide improves skeletal muscle function and exercise capacity.96 Results from long-term phase II efficacy studies of Elamipretide in humans are pending.
Other antioxidant therapies include free radical scavengers and superoxide dismutase mimetics like mitoTEMPO and EUK8/EUK132.97, 98 Maintenance of mitochondrial iron homeostasis and reduction of iron content is also thought to result in inhibition of free radical formation and reduced oxidative stress; targeted therapies of targeted mitochondrial iron chelators like deferiprone and idebenone have undergone human testing and led to partial reversal of cardiomyopathy in patients with Freidrich’s ataxia.99
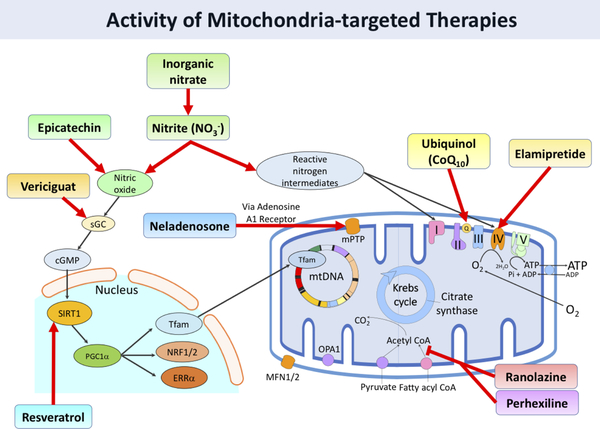
CONCLUSION
Current evidence supports mitochondrial function as an important factor contributing to the pathophysiology of HFpEF. As a result, there are a number of ongoing or completed early phase clinical trials of agents that seek to improve mitochondrial function in this population (Table 3) by acting on a number of different aspects of mitochondrial function (Figure 4). However, these trials largely utilize endpoints that indirectly assess the improvement of mitochondrial function through downstream effects on exercise capacity. While exercise capacity is a clinically important outcome, it may be vital to better assess how these novel therapeutics impact mitochondrial oxidative function, and whether dysfunction in other organ systems become limiting in the presence of substantially improved mitochondrial function. Direct measures of mitochondrial function have the potential to determine whether the pharmacologic effects of new drugs mimic those seen in animal models, and to enhance our understanding of how (and in whom) to target mitochondrial dysfunction in HFpEF. As described above, there are numerous measures of mitochondrial function that can be utilized in human studies, allowing for assessments of mitochondrial function at a level of detail never before possible.
Figure 4. Activity of mitochondria-targeted therapies.
However, more studies are needed to clarify both the role of abnormal mitochondrial function in HFpEF and the impact of mitochondrial therapies on morbidity and mortality in this patient population. Additionally, future studies will be needed to clarify the extent of mitochondrial pathology among the various phenotypic subgroups of patients with HFpEF. As these studies are being planned and conducted, it will be vital to consider the unique biology of the mitochondria and how we can best stimulate this powerhouse of the cell to improve the lives of patients with HFpEF.
Supplementary Material
Acknowledgments
Funding information: JAC is supported by NIH grants R56HL-124073–01A1 and R01 HL 121510–01A1. DPK is supported by NIH grants R01 HL058493 and R01 HL128349.
Disclosures: J.A.C. has received consulting honoraria from BMS, OPKO Healthcare, Fukuda Denshi, Microsoft, Merck, Ironwood Pharmaceuticals, Sanifit, Bayer, Akros Pharma and Vital Labs. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda-Denshi, Bristol-Myers Squibb, Microsoft and CVRx Inc., and device loans from AtCor Medical. J.A.C. is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. D.P.K. is a paid scientific consultant for Pfizer, Amgen, and Sanofi. Other authors have no disclosures
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL and Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC and Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haykowsky MJ and Kitzman DW. Exercise physiology in heart failure and preserved ejection fraction. Heart Fail Clin. 2014;10:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM and Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD and Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH and Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the Frank-Starling mechanism. Journal of the American College of Cardiology. 1991;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC and Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 9.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR and Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M and Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J and Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowlton AA, Chen L and Malik ZA. Heart failure and mitochondrial dysfunction: the role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. Journal of cardiovascular pharmacology. 2014;63:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM and Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J and Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Bronstad E, Rognmo O, Mangner N, Linke A, Schuler G, Silva GJ, Wisloff U, Adams V and Optimex Study G. Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail. 2015;17:263–72. [DOI] [PubMed] [Google Scholar]
- 16.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW and Dela F. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. The Journal of physiology. 2012;590:3349–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrepper A, Schwarzer M, Schöpe M, Amorim PA and Doenst T. Biphasic response of skeletal muscle mitochondria to chronic cardiac pressure overload—Role of respiratory chain complex activity. Journal of molecular and cellular cardiology. 2012;52:125–135. [DOI] [PubMed] [Google Scholar]
- 18.Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, Haykowsky MJ, Brubaker PH and Kitzman DW. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC: Heart Failure. 2016;4:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss K, Schär M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, Golozar A, Steinberg A, Gerstenblith G and Russell SD. Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circulation: Heart Failure. 2017;10:e004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ and Gheorghiade M. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J-a, Wei Y and Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circulation research. 2008;102:401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai D-F, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF and Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. Journal of the American College of Cardiology. 2011;58:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M and Lopaschuk GD. Cardiac Insulin-Resistance and Decreased Mitochondrial Energy Production Precede the Development of Systolic Heart Failure After Pressure-Overload HypertrophyClinical Perspective. Circulation: Heart Failure. 2013;6:1039–1048. [DOI] [PubMed] [Google Scholar]
- 25.Phan TT, Abozguia K, Shivu GN, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P and Ashrafian H. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. Journal of the American College of Cardiology. 2009;54:402–409. [DOI] [PubMed] [Google Scholar]
- 26.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ and Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drexler H, Riede U, Münzel T, König H, Funke E and Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MJ, Green HJ and Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. [DOI] [PubMed] [Google Scholar]
- 29.Hunter WG, Kelly JP, McGarrah III RW, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR and Muehlbauer MJ. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. Journal of the American Heart Association. 2016;5:e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS and Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circulation: Heart Failure. 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding W-X and Yin X-M. Mitophagy: mechanisms, pathophysiological roles, and analysis. 2012. [DOI] [PMC free article] [PubMed]
- 32.Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C and Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR and Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine. 2007;4:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiler M, Bowen TS, Rolim N, Dieterlen MT, Werner S, Hoshi T, Fischer T, Mangner N, Linke A, Schuler G, Halle M, Wisloff U and Adams V. Skeletal Muscle Alterations Are Exacerbated in Heart Failure With Reduced Compared With Preserved Ejection Fraction: Mediated by Circulating Cytokines? Circ Heart Fail. 2016;9:e003027. [DOI] [PubMed] [Google Scholar]
- 35.Ranhotra HS. Estrogen-related receptor alpha and mitochondria: tale of the titans. Journal of receptor and signal transduction research. 2015;35:386–90. [DOI] [PubMed] [Google Scholar]
- 36.Massie BM, Simonini A, Sahgal P, Wells L and Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. Journal of the American College of Cardiology. 1996;27:140–145. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Hiatt WR, Barstow TJ and Brass EP. Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. European journal of applied physiology and occupational physiology. 1999;80:22–27. [DOI] [PubMed] [Google Scholar]
- 38.Rooyackers OE, Adey DB, Ades PA and Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proceedings of the National Academy of Sciences. 1996;93:15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.X’avia C, Black CM, Lin AJ, Ping P and Lau E. Mitochondrial protein turnover: methods to measure turnover rates on a large scale. Journal of molecular and cellular cardiology. 2015;78:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammerling BC and Gustafsson ÅB. Mitochondrial quality control in the myocardium: cooperation between protein degradation and mitophagy. Journal of molecular and cellular cardiology. 2014;75:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim T-Y, Wang D, Kim AK, Lau E, Lin AJ, Liem DA, Zhang J, Zong NC, Lam MP and Ping P. Metabolic labeling reveals proteome dynamics of mouse mitochondria. Molecular & Cellular Proteomics. 2012;11:1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekar KC, Li L, Dabkowski ER, Xu W, Ribeiro RF, Hecker PA, Recchia FA, Sadygov RG, Willard B and Kasumov T. Cardiac mitochondrial proteome dynamics with heavy water reveals stable rate of mitochondrial protein synthesis in heart failure despite decline in mitochondrial oxidative capacity. Journal of molecular and cellular cardiology. 2014;75:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Liem DA, Lau E, Ng DC, Bleakley BJ, Cadeiras M, Deng MC, Lam MP and Ping P. Characterization of human plasma proteome dynamics using deuterium oxide. Proteomics Clin Appl. 2014;8:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Short KR, Nygren J, Barazzoni R, Levine J and Nair KS. T 3 increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and-3. American Journal of Physiology-Endocrinology And Metabolism. 2001;280:E761–E769. [DOI] [PubMed] [Google Scholar]
- 45.Kumar V, Chang H, Reiter DA, Bradley DP, Belury M, McCormack SE and Raman SV. Phosphorus-31 Magnetic Resonance Spectroscopy: A Tool for Measuring In Vivo Mitochondrial Oxidative Phosphorylation Capacity in Human Skeletal Muscle. JoVE (Journal of Visualized Experiments). 2017:e54977–e54977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanza IR, Bhagra S, Nair KS and Port JD. Measurement of human skeletal muscle oxidative capacity by 31P‐MR spectroscopy: a cross‐validation with in vitro measurements. Journal of Magnetic Resonance Imaging. 2011;34:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE and Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kogan F, Hariharan H and Reddy R. Chemical exchange saturation transfer (CEST) imaging: description of technique and potential clinical applications. Current radiology reports. 2013;1:102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeBrosse C, Nanga RPR, Wilson N, D’Aquilla K, Elliott M, Hariharan H, Yan F, Wade K, Nguyen S and Worsley D. Muscle oxidative phosphorylation quantitation using creatine chemical exchange saturation transfer (CrCEST) MRI in mitochondrial disorders. JCI insight. 2016;1:e88207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanza IR and Nair KS. Functional Assessment of Isolated Mitochondria In Vitro. Methods in enzymology. 2009;457:349–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan TE, Erickson ML, Brizendine JT, Young HJ and McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985). 2012;113:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan TE, Southern WM, Reynolds MA and McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. Journal of Applied Physiology. 2013;115:1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudsmith LE and Neubauer S. Magnetic resonance spectroscopy in myocardial disease. JACC: Cardiovascular Imaging. 2009;2:87–96. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers CT, Clarke WT, Snyder C, Vaughan JT, Neubauer S and Robson MD. Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla. Magnetic resonance in medicine. 2014;72:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haris M, Singh A, Cai K, Kogan F, McGarvey J, DeBrosse C, Zsido GA, Witschey WR, Koomalsingh K and Pilla JJ. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature medicine. 2014;20:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoppeler H, Luthi P, Claassen H, Weibel ER and Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Archiv : European journal of physiology. 1973;344:217–32. [DOI] [PubMed] [Google Scholar]
- 57.Egan B, Dowling P, O’Connor PL, Henry M, Meleady P, Zierath JR and O’Gorman DJ. 2-D DIGE analysis of the mitochondrial proteome from human skeletal muscle reveals time course-dependent remodelling in response to 14 consecutive days of endurance exercise training. PROTEOMICS. 2011;11:1413–1428. [DOI] [PubMed] [Google Scholar]
- 58.Egan B, O’connor PL, Zierath JR and O’gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One. 2013;8:e74098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menzies RA and Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–9. [PubMed] [Google Scholar]
- 60.Price JC, Guan S, Burlingame A, Prusiner SB and Ghaemmaghami S. Analysis of proteome dynamics in the mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF, Jr., Walsh K, Previs SF, Sadygov RG, Willard B and Stanley WC. Assessment of cardiac proteome dynamics with heavy water: slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2013;304:H1201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hickson RC and Rosenkoetter MA. Separate turnover of cytochrome c and myoglobin in the red types of skeletal muscle. The American journal of physiology. 1981;241:C140–4. [DOI] [PubMed] [Google Scholar]
- 63.Jäger S, Handschin C, Pierre JS- and Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proceedings of the National Academy of Sciences. 2007;104:12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S and Shyy JY-J. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal. 2017;10:eaaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Silljé HH and de Boer RA. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301:H459–H468. [DOI] [PubMed] [Google Scholar]
- 66.Bayeva M, Gheorghiade M and Ardehali H. Mitochondria as a therapeutic target in heart failure. Journal of the American College of Cardiology. 2013;61:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng X, Luo Z, Ma L, Ma S, Yang D, Zhao Z, Yan Z, He H, Cao T and Liu D. Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR‐δ/AMPK pathway. Journal of cellular and molecular medicine. 2011;15:1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Yong Ji S, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R and Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circulation research. 2009;104:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Yang G, Kim Y, Kim J and Ha J. AMPK activators: mechanisms of action and physiological activities. Experimental & molecular medicine. 2016;48:e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S and Zhang K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circulation: Heart Failure. 2016;9:e002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi S and Nakashima Y. Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. British Journal of Nutrition. 2012;107:774–780. [DOI] [PubMed] [Google Scholar]
- 72.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD and Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. American journal of hypertension. 2010;23:192–196. [DOI] [PubMed] [Google Scholar]
- 73.Dinh W, Albrecht-Kupper B, Gheorghiade M, Voors AA, van der Laan M and Sabbah HN. Partial Adenosine A1 Agonist in Heart Failure. Handb Exp Pharmacol. 2017;243:177–203. [DOI] [PubMed] [Google Scholar]
- 74.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO and Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–59. [DOI] [PubMed] [Google Scholar]
- 75.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC and Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–80; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borlaug BA, Koepp KE and Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 77.Borlaug BA, Melenovsky V and Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection FractionNovelty and Significance. Circulation research. 2016;119:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borlaug BA. Inorganic Nitrate Delivery to Improve Exercise Capacity in Heart Failure with Preserved Ejection Fraction: The INDIE Trial. Oral presentation at: American College of Cardiology Scientific Session; 2018; Orlando, Florida. [Google Scholar]
- 79.DiNicolantonio JJ, Bhutani J, McCarty MF and O’Keefe JH. Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart. 2015;2:e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCormack JG, Barr RL, Wolff AA and Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–42. [DOI] [PubMed] [Google Scholar]
- 81.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, Lang C, Wachter R, Edelmann F and Hasenfuss G. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC: Heart Failure. 2013;1:115–122. [DOI] [PubMed] [Google Scholar]
- 82.Ramirez-Sanchez I, Rodriguez A, Moreno-Ulloa A, Ceballos G and Villarreal F. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diab Vasc Dis Res. 2016;13:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fillmore N, Mori J and Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clementi E and Nisoli E. Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2005;142:102–110. [DOI] [PubMed] [Google Scholar]
- 85.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA and Mann DL. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. Jama. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pieske B, Maggioni AP, Lam CS, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD and Scalise A-V. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. European heart journal. 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC Jr, Roessig L, Stasch JP, Solomon SD and Paulus WJ. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. Journal of the American Heart Association. 2013;2:e000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borlaug BA. Inorganic Nitrite Delivery to Improve Exercise Capacity in HFpEF (INDIE-HFpEF) Oral presentation at: American College of Cardiology; 2018; Orlando, Florida. [Google Scholar]
- 89.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias P-T, Ischiropoulos H, Townsend RR and Margulies KB. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chirinos JA and Zamani P. The nitrate-nitrite-NO pathway and its implications for heart failure and preserved ejection fraction. Current heart failure reports. 2016;13:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rimbaud S, Ruiz M, Piquereau J, Mateo P, Fortin D, Veksler V, Garnier A and Ventura-Clapier R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PloS one. 2011;6:e26391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K and Szabados E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clinical hemorheology and microcirculation. 2012;50:179–187. [DOI] [PubMed] [Google Scholar]
- 93.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. The Journal of clinical investigation. 2005;115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Junior RFR, Dabkowski ER, Shekar KC, Hecker PA and Murphy MP. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radical Biology and Medicine. 2018;117:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA and Murphy MP. The mitochondria‐targeted anti‐oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver international. 2010;30:1019–1026. [DOI] [PubMed] [Google Scholar]
- 96.Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS and Marcinek DJ. Mitochondrial‐targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging cell. 2013;12:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang HL, Sedlic F, Bosnjak Z and Nilakantan V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radical Biology and Medicine. 2010;49:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawakami S, Matsuda A, Sunagawa T, Noda Y, Kaneko T, Tahara S, Hiraumi Y, Adachi S, Matsui H and Ando K. Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Circulation Journal. 2009;73:2125–2134. [DOI] [PubMed] [Google Scholar]
- 99.Velasco-Sánchez D, Aracil A, Montero R, Mas A, Jiménez L, O’Callaghan M, Tondo M, Capdevila A, Blanch J and Artuch R. Combined therapy with idebenone and deferiprone in patients with Friedreich’s ataxia. The Cerebellum. 2011;10:1–8. [DOI] [PubMed] [Google Scholar]
- 100.Kogan F, Haris M, Singh A, Cai K, Debrosse C, Nanga RPR, Hariharan H and Reddy R. Method for high‐resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magnetic resonance in medicine. 2014;71:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.