Supplemental Digital Content is available in the text.
Summary:
Reconstruction of maxilla defects has remained one of the most challenging problems in craniomaxillofacial reconstruction because it typically requires harvesting and grafting of autologous bone, which poses limitations related to the difficulties in accurately reconstructing the defected bone and the highly prolonged duration of surgery. We employed tissue-engineered, patient-specific, 3-dimensional (3D)-printed biodegradable scaffolds for maxillofacial bone reconstruction in patients with complex maxillary defects after surgical removal of cancer. A customized polycaprolactone (PCL) scaffold was designed and fabricated for each patient. For this purpose, we used computer-aided design and manufacturing combined with 3D printing technology. The patients implanted with the PCL scaffolds were followed up for up to 2 years with careful evaluation of morphological changes in the face. We confirmed that the patient-specific 3D-printed PCL scaffold effectively filled the maxillary defect and promoted regeneration of the deficient tissue while remaining stable in the body for a relatively long period. Employing customized tissue-engineered scaffolds built using the patient’s computed tomography data and an extrusion-based 3D printing system is safe and clinically feasible, helping create and maintain improved morphological features of the face, which represents the most important aspect from the perspective of the patients.
INTRODUCTION
Among the traditional surgical approaches for maxillofacial bone reconstruction, autologous bone grafting is the most widely used.1 However, autologous bone grafting requires harvesting bone from an unaffected area and inevitably causes donor-site morbidity.2–5
The emergence of 3-dimensional (3D) printing has helped overcome challenges related to obtaining and shaping the graft or implant. In fact, there have been various attempts to fabricate customized medical artificial implants using 3D printing technology.6,7 However, most implantable materials used thus far are not biodegradable, which substantially increases the risk of inflammation, infection, and even implant protrusion.8,9 To address these limitations, current efforts aim to facilitate regeneration or reconstruction of the defect area using 3D-printed scaffolds made of biodegradable and clinically safe polymers. Recently, 3D-printed, patient-tailored splints made of the biocompatible and biodegradable polymer polycaprolactone (PCL) were used to prevent airway stenosis in infants with tracheobronchomalacia.10,11
In this context, we describe the fabrication and implantation of patient-specific, 3D-printed PCL scaffolds with fully interconnected pores for complicated craniomaxillofacial reconstruction.
PATIENTS AND METHODS
Patient Characteristics
Patient 1 was a 43-year-old woman who had undergone left radial maxillectomy for minor salivary cancer in the left maxillary sinus and hard palate, with immediate reconstruction using a fibular osteocutaneous free flap. Patient 2 was a 45-year-old man who had been diagnosed with squamous cell carcinomas in the left maxillary sinus 8 years before presenting to our department. The patient had undergone radical maxillectomy and partial mandibulectomy, which resulted in severe facial deformity. Patient 3 was an 18-year-old man who had received right partial maxillectomy for chondrosarcoma 9 years before presenting to our department; the procedure had resulted in a medial maxilla defect on the right side of the face.
This study was approved by the Institutional Review Board and Ethics Committee of our center (approval No. KC14DNME0751). The 3D-printed scaffold was manufactured according to Good Manufacturing Practice provisions [registration No. KTC-ABB-170177, approved by the South Korea Ministry of Food and Drug Safety (MFDS) and was registered as a medical device in South Korea (registration No. 14–1337, approved by the MFDS].
Designing the Customized Scaffolds
Customized PCL scaffolds were designed using commercial 3D medical image editing software (Materialise Mimics; Materialise NV, Leuven, Belgium). The patient’s head was scanned using a computed tomography (CT) device, with a slice thickness of 1 mm. The side with the normal bony orbit was mirrored to the midsagittal plane, which was defined according to standardized bony landmarks (see figure, Supplemental Digital Content 1, which demonstrates designing the customized implant, http://links.lww.com/PRSGO/A906). Finally, patient-specific scaffolds were fabricated using a 3D-printing system (Fig. 1A).
Fig. 1.
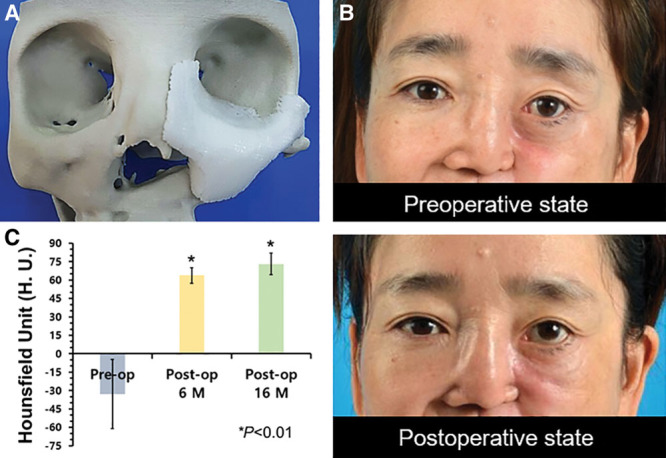
A, Evaluation of the patient-specific scaffold before reconstruction of a maxillary defect. The custom-designed 3D model of the scaffold is shown mounted on the model of the cranium generated using computer-aided design. B, Clinical photographs (preoperative and postoperative states) taken during the follow-up period. C, The calculated increase in HU as an indicator of tissue density. All of the calculations were based on CT image analysis of data collected preoperatively (Pre-op) and postoperatively (Post-op) at various intervals (in months, M). The bar height represents the mean, while the error bar indicates the SD.
Fabrication of 3D-printed PCL Scaffolds
For use as tissue-regeneration scaffolds, fully customized scaffolds made of medical-grade PCL (Evonik Industries, Essen, Germany) were fabricated using the in-house 3D printer.12,13 This process was performed in a facility with Good Manufacturing Practice certification (T&R Biofab Co. Ltd., Seoul, Korea) approved by the MFDS of Korea.
Surgical Procedures
The PCL scaffold customized for each patient was inserted under general anesthesia. The maxillary defect was exposed through a simple incision. The implant was inserted, and the incision was closed without using an additional flap or tissue transfer (see video, Supplemental Digital Content 2, which demonstrates the surgical process for Patient 3. This video is available in the “Related Videos” section at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A907).
Video Graphic 1.
See video, Supplemental Digital Content 2, which demonstrates the surgical process of patient 3. This video is available in the “Related Videos” section at PRSGlobalOpen.com or at http://links.lww.com/PRSGO/A907.
CT Image Analysis
The follow-up CT data were converted into 2D- or 3D-reconstructed images. The pre- and postoperative 3D images were overlapped to analyze the increase in soft-tissue volume after surgery.
Statistical Analysis
The difference between mean values was evaluated using Student’s t test, in which a P value < 0.01 was considered to indicate significance.
RESULTS
Clinical Outcomes
The implanted scaffolds were well maintained during follow-up, without the occurrence of any complications, such as wound dehiscence or infection. Eye height with an acceptable degree of symmetry (compared with that noted preoperatively) was maintained throughout the follow-up period (Fig. 1B). The presence of neo-tissue ingrowth can be seen in Supplemental Digital Content 3 (see figure, Supplemental Digital Content 3, which shows examples of the surgical outcomes for the patients, http://links.lww.com/PRSGO/A908).
CT-based Quantitative Analysis after Reconstruction
Overlapping the pre- and postoperative CT images revealed that reconstructive surgery achieved an increase of 2,711 mm3 in patient 1 at 16 months postoperatively (Fig. 1C). Measurement of Hounsfield unit values on CT images indicated that, tissue density (ie, the HU values) in the area where the scaffold was implanted increased significantly. In the preoperative state, the mean HU value was -76.269. After scaffold implantation, the HU values at 6 and 16 months postoperatively were 63.7825 and 73.0488, respectively.
DISCUSSION
By adjusting the parameters of the 3D printing–based fabrication method, the customized scaffolds can be designed to have thoroughly interconnected 3D pores. In the present study, the scaffolds were fabricated with a reference pore size of 900 μm to promote tissue ingrowth. Follow-up evaluation revealed that the pores had indeed become filled with soft tissue, and the facial contour was well maintained. Considering that no signs of infection were observed in any of the patients after scaffold implantation, we believe that fusion with existing native tissue occurred successfully due to early vascularization and tissue filling within the pores.
PCL scaffolds, which have a degradation period of 2–3 years, are suitable for use as biocompatible tissue-engineered scaffolds and the induction of tissue regeneration, although they have a limited capacity to induce bone tissue ingrowth.14–16 Indeed, our CT-based analysis revealed increased tissue density, thus confirming soft-tissue ingrowth. It did not, however, indicate that bone tissue had regenerated within the PCL scaffold. To promote bone tissue regeneration, future studies should consider using a different material, such as blended PCL/beta-tricalcium phosphate (powder type) with an adequate pore size and seeding of relevant cells.13,17,18
CONCLUSIONS
Complicated craniomaxillofacial defects can be reconstructed efficiently using 3D-printed, patient-specific scaffolds. We reported that customized, tissue-engineered scaffolds could be successfully fabricated using the patients’ CT data and an extrusion-based 3D printing system. Moreover, biodegradable PCL polymer promoted regeneration of deficient tissue while remaining stable in the body for a relatively extended period.
Supplementary Material
Footnotes
Published online 13 November 2018.
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2017R1A6A1A03015562). This work was also supported by ICT Consilience Creative Program funded by the Institute for Information & communications Technology Promotion (IITP) (No. 2017-0-01982).
Hyun Ho Han, Jin-Hyung Shim, and Hyungseok Lee contributed equally as co-first authors to this article.
Chung Hwan Baek, Jong-Won Rhie, and Dong-Woo Cho contributed equally as corresponding authors to this article.
Clinical Trial Registration information provided: Name of Trial database registered – Clinical Research Information Service (CRIS). Registration Number and date registered – KCT0002711 and 2014/11/20.
Disclosure: Dr. Shim and Dr. Yun are currently employed by T&R Biofab Inc. in addition to their work at the Korea Polytechnic University. All other authors declare no competing interests. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Baino F. Biomaterials and implants for orbital floor repair. Acta Biomater. 2011;7:3248. [DOI] [PubMed] [Google Scholar]
- 2.Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18:S77. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Rhim R, Li L, et al. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009;9:886. [DOI] [PubMed] [Google Scholar]
- 4.Corney J, Hieu L, Zlatov N, et al. Medical rapid prototyping applications and methods. Assembly Automation 2005;25:284. [Google Scholar]
- 5.Metzger MC, Hohlweg-Majert B, Schwarz U, et al. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e1. [DOI] [PubMed] [Google Scholar]
- 6.Nazimi AJ, Yusoff MM, Nordin R, et al. Use of polyetheretherketone (PEEK) in orbital floor fracture reconstruction—a case for concern. J Oral Maxillofac Surg Med Pathol. 2015;27:536. [Google Scholar]
- 7.Esses SJ, Berman P, Bloom AI, et al. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. Am J Roentgenol. 2011;196:W683. [DOI] [PubMed] [Google Scholar]
- 8.Younis I, Gault D, Sabbagh W, et al. Patient satisfaction and aesthetic outcomes after ear reconstruction with a Branemark-type, bone-anchored, ear prosthesis: a 16 year review. J Plast Reconstr Aesthet Surg. 2010;63:1650. [DOI] [PubMed] [Google Scholar]
- 9.Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615. [DOI] [PubMed] [Google Scholar]
- 10.Morrison RJ, Hollister SJ, Niedner MF, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med. 2015;7:285ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zopf DA, Hollister SJ, Nelson ME, et al. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368:2043. [DOI] [PubMed] [Google Scholar]
- 12.Pati F, Shim J-H, Lee J-S, et al. 3D printing of cell-laden constructs for heterogeneous tissue regeneration. Manuf Lett. 2013;1:49. [Google Scholar]
- 13.Shim JH, Yoon MC, Jeong CM, et al. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed Mater. 2014;9:065006. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Mei L, Song C, et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735. [DOI] [PubMed] [Google Scholar]
- 15.Yeo A, Rai B, Sju E, et al. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: an in vitro and in vivo study. J Biomed Mater Res A. 2008;84:208. [DOI] [PubMed] [Google Scholar]
- 16.Azam A, Laflin KE, Jamal M, et al. Self-folding micropatterned polymeric containers. Biomed Microdevices. 2011;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim JH, Huh JB, Park JY, et al. Fabrication of blended polycaprolactone/poly(lactic-co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng Part A. 2013;19:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim JH, Moon TS, Yun MJ, et al. Stimulation of healing within a rabbit calvarial defect by a PCL/PLGA scaffold blended with TCP using solid freeform fabrication technology. J Mater Sci Mater Med. 2012;23:2993. [DOI] [PubMed] [Google Scholar]


