Abstract
Joint attention behaviors include initiating one's own and responding to another's bid for joint attention to an object, person, or topic. Joint attention abilities in autism are pervasively atypical, correlate with development of language and social abilities, and discriminate children with autism from other developmental disorders. Despite the importance of these behaviors, the neural correlates of joint attention in individuals with autism remain unclear. This paucity of data is likely due to the inherent challenge of acquiring data during a real‐time social interaction. We used a novel experimental set‐up in which participants engaged with an experimenter in an interactive face‐to‐face joint attention game during fMRI data acquisition. Both initiating and responding to joint attention behaviors were examined as well as a solo attention (SA) control condition. Participants included adults with autism spectrum disorder (ASD) (n = 13), a mean age‐ and sex‐matched neurotypical group (n = 14), and a separate group of neurotypical adults (n = 22). Significant differences were found between groups within social‐cognitive brain regions, including dorsal medial prefrontal cortex (dMPFC) and right posterior superior temporal sulcus (pSTS), during the RJA as compared to SA conditions. Region‐of‐interest analyses revealed a lack of signal differentiation between joint attention and control conditions within left pSTS and dMPFC in individuals with ASD. Within the pSTS, this lack of differentiation was characterized by reduced activation during joint attention and relative hyper‐activation during SA. These findings suggest a possible failure of developmental neural specialization within the STS and dMPFC to joint attention in ASD. Hum Brain Mapp 34:2511–2523, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: social, fMRI, superior temporal sulcus, medial prefrontal cortex
INTRODUCTION
Joint attention is the process by which two people actively and intentionally coordinate their attention to a third thing (i.e., object, person, or topic of conversation). In a joint attention dyad, one person initiates a bid for joint attention while the other responds to the initiator's bid. Both people actively coordinate and share their attention with each other and the object. The beginnings of joint attention abilities (e.g., detection of mutual gaze and response to shifts in attention) emerge early in infancy [Farroni et al., 2007; Hood et al., 1998], while an understanding of the intentional coordination of attention between two people emerges within the second year of life [Brooks and Meltzoff, 2002; Tomasello et al., 2005; Woodward, 2003]. This seemingly simple behavior is a powerful social learning tool. Coordinated joint attention provides the infant with a means to learn about her world from others, for example the name of an object or the function of a new toy. As such, this behavior is fundamental to early word learning, and correlates with later language and social cognitive abilities [Baldwin and Moses, 2001; Morales, 2006, 1998; Nelson et al., 2008].
The link between language and social‐cognitive abilities is particularly relevant to autism, a neurodevelopmental disorder characterized by impairments in both of these domains. Indeed, joint attention disabilities have been posited to be a pivotal deficit in autism [e.g., Charman, 2003; Mundy and Crowson, 1997]. Proficiency in joint attention can be used diagnostically to discriminate between typically developing children, children with autism, and children with mental retardation [Bruinsma et al., 2004; Charman, 2003; Mundy, et al., 2007; Mundy and Newell, 2007]. Impairments in children with autism are seen in both initiating joint attention (IJA) and responding to joint attention (RJA), although RJA may eventually appear more typical in later childhood [review, Mundy and Newell, 2007]. Early delays in IJA and RJA behaviors, in children with autism spectrum disorder (ASD), correlate with later deficits in language and social abilities [e.g., Anderson et al., 2007; Delinicolas and Young, 2007; Sigman and McGovern et al., 2005; Toth et al., 2006].
Despite the importance of joint attention, the neurobiology underlying joint attention deficits in ASD remains unclear. Using MRI measures, Mosconi et al. 2011 found amygdala volume to be significantly correlated with joint attention abilities at 4 years of age [Mosconi et al., 2009]. However, the specificity of amygdala involvement cannot be determined given that the authors did not examine other brain regions and that structure–function correlations cannot reveal regions that are actively recruited during joint attention episodes. Using fMRI or event‐related potential (ERP) measures, a small handful of studies of autism have focused on the component processes of RJA in older children and adults, especially monitoring other people's gaze directions. In many of these studies, participants with ASD show abnormal neural responses to videos, animations, or static images depicting gaze shifts. For example, individuals with ASD show less discrimination between shifting gaze and a spatial arrow when they are used as distractors in a spatial attention task [Greene et al., 2011; Vaidya et al., 2011]. Individuals with ASD also showed a reduced response in the right temporal parietal junction (TPJ) and right insula to direct gaze, as compared to averted, in an fMRI study [Pitskel et al., 2011] and a reduced differentiation between direct and averted gaze over occipito‐temporal regions in an ERP study [Senju et al., 2005]. When watching an animation of a gaze shift towards, or away from, a flashing target, individuals with ASD did not show the typical enhanced response in superior temporal sulcus (STS) for gaze shifts away from the target [Pelphrey et al., 2005]. The authors interpret the typical enhanced response to incongruent shifts as evidence of processing the intention behind the character's gaze shift; the absence of this pattern may indicate that individuals with ASD do not spontaneously consider the intentions behind gaze shifts. In sum, individuals with ASD show reduced processing of another person's gaze, specifically for aspects of gaze that could affect joint attention, including recognition of direct gaze (often the first step in a bid for joint attention) and integration of the gaze shift with environmental targets.
These findings give hints into brain mechanisms underlying difficulties with joint attention in individuals with autism. However, no previous study has explicitly examined the online recruitment of brain regions during a full joint attention event in individuals with autism. Further, no study has examined the neural mechanisms involved in online episodes of IJA in autism. Examining both initiating and RJA in autism is critical given several lines of evidence that these two behaviors may be dissociable [Mundy et al., 2007]. First, behavioral studies in typical infants reveal only weak correlations between the development of IJA and RJA behaviors and IJA and RJA differentially correlate with later language development [Mundy and Newell, 2007; Mundy et al., 1990, 2007]. Second, impairments in IJA may be greater than RJA in autism [Mundy et al., 2009] Third, distinct neural systems may be recruited for IJA and RJA [Mundy and Newell, 2007; Schilbach et al., 2010]. One reason for this gap in studies investigating IJA is that having participants initiate joint attention episodes while in an fMRI scanner presents a technical challenge. Here, we address this challenge using a method we developed [Redcay et al., 2010] in which participant and experimenter can interact in a real‐time social interaction within the scanner via dual video‐feed technology.
The current study examined brain responses during both initiating and responding to a bid for joint attention during a face‐to‐face interactive game. In joint attention trials, the participant and an experimenter oriented to and shared attention to an object in the corner of a screen. In solo attention (SA) trials, participants oriented to a corner of the screen but did not share attention with the experimenter. Based on previous research in neurotypical participants (NT), we predicted that the posterior superior temporal sulcus (pSTS) and medial prefrontal cortex (MPFC) would show a greater response during both initiating and responding to bids for joint attention than during the SA conditions [Materna et al., 2008; Redcay et al., 2010; Schilbach et al., 2010; Williams et al., 2005]. Second, we predicted that individuals with autism would show a reduced difference between joint attention and SA control conditions. Third, given behavioral findings that impairments in IJA are longer lasting than impairments in RJA, we predicted more atypical patterns of brain activation during IJA in ASD individuals.
METHODS
Participants
All participants gave informed written consent as approved by the Massachusetts Institute of Technology (MIT) Committee on the Use of Humans as Experimental Subjects (COUHES) and conducted in accordance with the Declaration of Helsinki. Participants received monetary compensation for participation in this study. Participants were excluded if they had history of neurological or psychiatric disorder (other than autism), an IQ less than 90, or any contraindication for MRI safety (e.g., unapproved metal in the body). Participants completed the Autism Quotient (AQ) [Woodbury‐Smith et al., 2005]. NT with a score greater than 26 were excluded from the study [Woodbury‐Smith et al., 2005].
Autism spectrum disorder participants
Eighteen adults with high‐functioning ASD participated in this experiment. An autism behavioral therapist (PLM), who had 9 years of experience with children and adults with autism and who was trained and reliable on administration and scoring of the Autism Diagnostic Observation Schedule (ADOS), conducted a Module 4 ADOS with the participants [Lord et al., 2000] (scores are reported in Table 1). Participants met criteria for autism or spectrum with a score of at least 2 on the Communication subscale and at least 4 on the Social subscale, with a combined score of 7 or greater. For a diagnosis of autism, participants needed a combined score of 10 or greater. While subjects came to us with a diagnosis on the spectrum as assessed by their personal healthcare providers, we also sent the video of their ADOS to our own psychiatrist for final confirmation of the diagnosis. Eighteen participants met ADOS criteria for ASD (autism or spectrum) and participated in the functional MRI experiment. Two were excluded due to an inability to perform the task and three due to excessive motion during the scan (see below for criteria). Of the 13 (10 males) remaining ASD participants, 5 met criteria for autism and 8 for spectrum. All participants will be referred to as ASD for the remainder of the paper. Participants were between 18 and 39 years of age (28.3 ± 6.9) and had average to very high intelligence as assessed using the Kaufman Brief Intelligence Test. IQ data from three participants were not obtained due to experimenter error (Table 1).
Table 1.
Participant information
| ID | Age | Sex | Verbal IQ | Nonverbal IQ | Composite IQ | ADOS Social | ADOS Comm |
|---|---|---|---|---|---|---|---|
| ASD1 | 25 | F | 122 | 103 | 115 | 5 | 5 |
| ASD2 | 27 | F | 128 | 120 | 128 | 8 | 3 |
| ASD3 | 34 | F | 120 | 123 | 126 | 4 | 3 |
| ASD4 | 18 | M | 116 | 125 | 124 | 6 | 2 |
| ASD5 | 19 | M | 135 | 92 | 116 | 6 | 3 |
| ASD6 | 20 | M | 142 | 130 | 141 | 5 | 2 |
| ASD7 | 27 | M | 106 | 120 | 116 | 5 | 3 |
| ASD8 | 27 | M | 122 | 125 | 127 | 9 | 5 |
| ASD9 | 29 | M | 87 | 103 | 94 | 7 | 4 |
| ASDIO | 30 | M | 120 | 120 | 123 | 10 | 3 |
| ASD11 | 37 | M | 145 | 125 | 139 | 5 | 2 |
| ASD21 | 38 | M | 113 | 112 | 115 | 4 | 3 |
| ASD13 | 39 | M | — | — | — | 5 | 3 |
| Mean | 28 | 3F | 121 | 117 | 122 | 6 | 3 |
| NT1 | 25 | F | — | — | — | — | — |
| NT2 | 26 | F | 135 | 109 | 126 | — | — |
| NT3 | 33 | F | 102 | 90 | 96 | — | — |
| NT4 | 20 | M | 120 | 120 | 123 | — | — |
| NT5 | 20 | M | 123 | 90 | 108 | — | — |
| NT6 | 20 | M | 131 | 111 | 125 | — | — |
| NT7 | 21 | M | — | — | — | — | — |
| NT8 | 24 | M | 130 | 96 | 116 | — | — |
| NT9 | 25 | M | 125 | 100 | 115 | — | — |
| NT10 | 26 | M | 125 | 130 | 132 | — | — |
| NTH | 30 | M | 120 | 109 | 118 | — | — |
| NT12 | 33 | M | 127 | 109 | 121 | — | — |
| NT13 | 35 | M | 96 | 96 | 96 | — | — |
| NT14 | 35 | M | 127 | 130 | 133 | — | — |
| Mean | 27 | 3F | 122 | 108 | 117 | — | — |
Social and Communication scores are reported from the ADOS for ASD individuals only as NT participants did not receive the ADOS.
NVIQ, nonverbal IQ; VIQ, verbal IQ; CIQ, composite IQ measure.
Neurotypical participants
Functional neuroimaging data were collected from two groups of NT participants: an ASD‐matched control group and a region‐of‐interest (ROI) control group. Both groups were recruited through a voluntary study participant list serve sampling the broader Boston community. The ASD‐matched control group included 13 healthy, typical adults who were matched to the ASD group on gender and mean age (Table 1). For the ROI control group, functional neuroimaging data from a separate group of 22 typically developing (TD) participants (22.1 years ± 2.5, 7 males) were collected. Data from these 22 participants were used in order to identify independent functional ROI for comparison of the ASD and ASD‐matched control groups. This independent ROI analysis allowed for comparison of response profiles between ASD and typical groups and provides greater information than whole‐brain analyses alone. All TD adults were screened for a family history of autism. Data from seven of the NT (1 from the ASD‐matched control group and 6 from the ROI control group) were published previously [Redcay et al., 2010].
Functional MRI Set‐Up
The experimental set‐up allowed for a real‐time face‐to‐face interaction while the participant was in the fMRI scanner. Extensive details on this method are provided by Redcay et al. 2008. Briefly, while the participant was in the fMRI scanner, an experimenter was seated in the control room in front of a laptop. A small camera was positioned between her and the laptop screen and was used to present a live video feed of her face to the participant via a projection screen. Similarly the experimenter was able to view a live video feed of the subject. Specifically, a camera in the back of the scanner bore captured video of the participant's eye which was displayed on the laptop screen in front of the experimenter (in the control room). The video feeds (of the experimenter and participant) were presented inside of a computer‐based image (described below). This allowed participant and experimenter to feel as though they were viewing two different sides of the same screen. Thus, with this set‐up both participant and experimenter were able to view each other in real‐time allowing for an interactive, face‐to‐face game. In the current study, participants engaged in a joint attention game, described below.
Joint Attention Task
The joint attention task was programmed in Matlab 7.8 using the Psychophysics Toolbox Extensions (PTB‐3) [Brainard, 1997; Pelli, 1997]. Participants were told that they would be playing a game with a live experimenter called “Catch the Mouse.” Both the participant and experimenter screen showed a “mouse house” in each of the four corners. Pipes connected these cheese houses. Participants were told that the goal of this game is to look at the mouse house in the corner of the screen where he or she thought the mouse was hiding. During joint attention trials, the mouse image would only appear when both participant and experimenter were looking at the correct corner. Two types of joint attention trials were possible: IJA and RJA. During subject‐initiated joint attention trials (IJA), the participant would receive a clue (i.e., a picture of a mouse tail) in the corner where the mouse was “hiding.” The participant would then shift attention and direct the experimenter's attention to the corner. When both experimenter and participant were looking at the same corner, the mouse would appear. During RJA trials, the experimenter received the clue on her screen, cued the subject to the appropriate corner through her gaze, and then when both subject and experimenter were looking at the house the mouse appeared. Participants were also given a SA control condition. In this case, participants were told that the experimenter was not playing during these trials. To emphasize this, and control for the presence of eye movement, the experimenter would close and open her eyes once during each trial. The participant was told to search for the clue and look at the house where he thought the mouse was hiding. Critically, the participant was told that the experimenter did not need to share attention on the house for the mouse to appear. For all conditions, the appearance of the mouse occurred when the experimenter clicked a button. A second experimenter, not visible to the participant, assisted with informing the experimenter when the subject was fixating on the correct location. Experimenter error was examined by comparing joint attention measures based on the appearance of the mouse (i.e., button click by the experimenter) and joint attention based on post‐hoc video coding.
In order to examine differences in functional brain organization underlying joint attention performance, this task design was intended to elicit joint attention behaviors on each trial for both typical and ASD participants. A design that allowed for spontaneous joint attention, and thus, may have revealed performance differences between groups could lead to differences due to performance alone (e.g., time spent in joint attention, engagement in task) rather than underlying differences in brain organization.
The joint attention trials were presented in blocked order of five trials per block. Each block was preceded by a 4 s cue as to the block type (e.g., “Help Lee find the mouse” for IJA blocks). Trials were presented in blocks to minimize task switching confounds. Each trial lasted an average of 6 s. Variability in trial length was due to jitter (0–1 s) in the appearance of the mouse tail at the start of the trial. The beginning, middle, and end of each run contained a 20‐s resting baseline during which time only a fixation cross was presented on the screen. Each run also contained two blocks of each condition type (IJA, RJA, SA) in semi‐counterbalanced order. Four functional runs were collected.
MRI Data Acquisition
Data were collected on a 3 Tesla Siemens Tim Trio scanner using a 12‐channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Center for Brain Research at the Massachusetts Institute of Technology. A structural T1‐weighted MPRAGE scan was collected at the beginning of each scan session (128 slices in the sagittal plane, slice thickness 1.3 mm, repetition time (TR) = 2530 ms, echo time (TE) = 3.39 ms). Whole brain, T2*‐weighted gradient echo‐planar images (EPI) were collected during the joint attention task at a resolution of 3.1 × 3.1 × 4.0 mm voxels (TR = 2 s, TE = 30 ms, 30 slices). Sequences used Siemens PACE online motion correction, which corrected for movement less than 8 mm per volume acquisition.
fMRI Data Preprocessing and Statistical Analyses
Data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) and in‐house matlab scripts. Several preprocessing steps were performed on the data. First, functional volumes were realigned based on the first image from the first functional run using a 6‐degree rigid spatial transformation. Images were then normalized to an EPI template in standard MNI space by applying linear and nonlinear transformations. Data were resampled to 2 mm isotropic voxels and smoothed using a smoothing kernel of 5 mm full‐width half‐maximum (fwhm). Data were low‐pass filtered at a rate of one cycle per run (or 1/264 Hz).
Data were inspected for artifacts using the artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect) that identifies outliers due to both global signal and motion. Outliers in global signal were noted if a volume exceeded 3 Z‐scores from the mean global signal. Outliers in motion were noted if a difference between two volumes exceeded 1 mm (measured through a composite of translational and rotational motion). Functional runs with outliers in more than 15% of the volumes were excluded. Participants with two runs or more that had greater than 15% of volumes that were outliers were excluded from the experiment (see above, Participants). No significant group differences were found in the mean degree of rotational (roll, pitch, yaw) or translational (x,y,z) movement between volumes as determined by a between group t‐test on the motion parameters from realignment [translational (ASD: mean −0.00003 ± 0.0001; NT: 0.00008 ± 0.0003, t(25) = 1.3); rotational (ASD: mean .018 ± 0.005, NT: mean 0.013 ± 0.005, t(25) = −0.87)]. ASD and NT groups did differ on the total number of outlier timepoints with the NT group having more outliers than the ASD group (ASD: mean 0.04% ± 0.5%; NT: mean 2% ± 1.8%, t(25) = 3.1, P < 0.004). Finally, to ensure quality of data was not different between groups, mean values from the residual means square map from within each ROI were extracted. No significant differences were found between groups in any ROI (P's > 0.2).
First level analyses were conducted within subject by performing a general linear model within each voxel. The general linear model included a regressor for each condition (i.e., IJA, RJA, and SA) as well as one for the instruction period (which was of no interest). Outlier timepoints were included in the model as covariates (one covariate per timepoint). Contrasts were performed within subject for each condition as compared to baseline, each joint attention condition compared to SA (i.e., IJA vs. SA and RJA vs. SA), and both joint attention conditions (JA) compared to SA (i.e., (RJA + IJA)‐SA). Contrasts were also performed to compare activation between initiating and responding joint attention conditions (IJA vs. RJA and RJA vs. IJA). Whole brain random effects analyses were run using two‐tailed t‐tests for each contrast of interest within each group (i.e., ASD, matched controls, and ROI controls). Additionally, between group t‐tests were conducted for the contrasts of RJA vs. SA, IJA vs. SA, JA vs. SA, RJA vs. IJA, and IJA vs. RJA between the ASD and matched control groups. Data were thresholded at P < 0.001 and corrected to P < 0.05 at the cluster level (k = 384 mm3) using a Monte Carlo simulation method with 10,000 iterations (AFNI's AlphaSim [Cox, 1996]).
Region of Interest Analyses
ROI were created from the map of the contrast of joint attention vs. SA (JA vs. SA) for the 22 NT participants in the ROI control group. The map was first threshold‐corrected at a voxel‐ and cluster‐wise threshold of P < 0.05 using nonparametric permutations (statistical non‐parametric mapping toolbox, snpm5b). This threshold resulted in six functionally defined regions (Fig. 1) including dorsal medial prefrontal cortex (dMPFC), right and left posterior STS (RpSTS and LpSTS), left inferior parietal lobe (LIPL), right inferior frontal gyrus (RIFG), and right anterior insula (RaIns). ROIs were created by identifying the peak voxels and all significant voxels surrounding the peaks within a 9 mm radius sphere from the group map of the 22 NT participants for each of the six clusters identified. These six ROI were used to extract percent blood oxygenated level dependent (BOLD) change data from each participant in the ASD and matched control groups. Percent signal change data were averaged spatially across all voxels within the ROI and temporally across the 6–30 s time window of each condition. This time window was chosen to capture the peak signal within each block, accounting for the hemodynamic lag.
Figure 1.
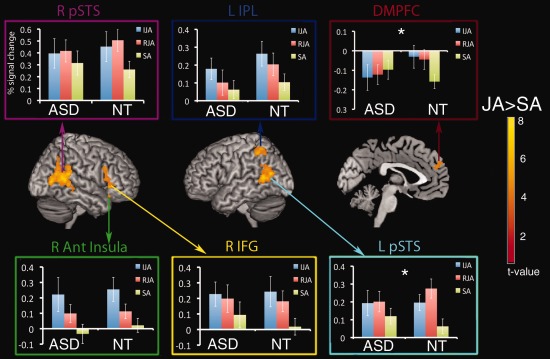
ROI analysis of BOLD response to joint attention conditions by group. Regions of interest from the region‐of‐interest group of controls are displayed on a template image (P < 0.05, voxel‐ and cluster‐corrected). Percent signal change data were extracted from the peak 6–30 s across the block and averaged across condition within each region within each group. Data are plotted with percent signal change on the y‐axis and condition on the x‐axis separated by ASD and NT groups. Standard error of the mean is also plotted. The DMPFC and LpSTS show a significant group × condition interaction (indicated by a “*”) such that differences between joint attention (IJA and RJA) and SA are larger in the NT group than ASD.
Region of Interest Statistical Analyses
Statistical analyses were conducted using JMP statistical software. Repeated measures ANOVAs were run with condition (IJA, RJA, and SA) as the within‐group variable and group (NT, ASD) as the between‐group measure. Subjects and condition nested within group were added to the ANOVA as random effects. Follow‐up contrasts using Tukey's test were conducted for regions showing a significant group × condition interaction.
Given the heterogeneity in the autism sample, exploratory correlation analyses were also conducted between % BOLD signal differences (i.e., RJA vs. SA and IJA vs. SA) in each ROI and several behavioral variables including IQ (Composite IQ, Verbal IQ, Nonverbal IQ), autism quotient (AQ) and ADOS scores. The ADOS scores investigated included a composite of the full score from the Language and Communication scales and from the Reciprocal Social Interaction Scales as behaviors in each of these domains were predicted to be related to joint attention abilities. Because ADOS scores are not linear, nonparametric rank order correlations (Spearman's rho) were computed between the two ADOS composite scores and BOLD signal differences between conditions within the six ROI.
Behavioral Data
Videos of both participant and experimenter were recorded during the joint attention task. These videos were coded offline using Vcode (http://social.cs.uiuc.edu/projects/vcode.html). Videos from four ASD participants and four NT participants were not recorded due to technical problems at the time of scanning or the loss of the videos before back‐up. The onset and duration of each eye movement made by the participant during an experimental condition was marked. Additionally, the onset and duration of experimenter gaze shifts towards the target (i.e., mouse house) were coded. These data allowed for measurement of several behavioral variables: (1) Accuracy, which was defined as the percent of trials in which the mouse appeared due to correct fixation(s) to the target location, (2) Total number of directed eye movements per block (including searching for target and shifts to target), (3) Latency to joint attention, which was defined by the time between appearance of the tail cue and fixation on the correct target location by both subject and experimenter in the RJA and IJA conditions, (4) Length of fixation on the target object (i.e., length of joint attention in the RJA and IJA conditions), and (5) Time between the start of the trial and the first eye movement. A separate repeated measures ANOVA was conducted for each of the five behavioral measures. Significant contrasts were followed up with Tukey's test (α = 0.05). Contrasts are reported as t‐tests but all reported were also significant with the Tukey post‐hoc test.
RESULTS
Behavioral Data
Accuracy (successfully fixating the target) was high for both groups across all conditions (see Fig. 2). The three conditions elicited different patterns of behavior, in both groups. Both groups took longer to achieve joint attention when the participant initiated, versus responded to, joint attention (ASD, t = 8.2; NT, t = 9.3). Both groups also spent more time looking at the mouse in the SA condition [(ASD: IJA vs. SA, t = −7.3; RJA vs. SA, t = −6.2) (NT: IJA vs. SA, t = −7.6, RJA vs. SA, t = −5.8)]. Finally, the latency to the participant's first eye movement in a trial was longest in the RJA condition [(ASD: IJA vs. RJA, t = −10.5; RJA vs. SA, t = 11.7) (NT: IJA vs. RJA, t = −14.2; RJA vs. SA, t = 16.8)].
Figure 2.
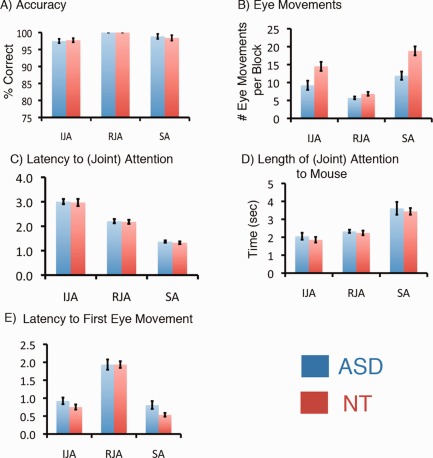
Behavioral data from ASD (blue) and NT (red) groups are shown for five behavioral measures. Accuracy (A) is defined as the percent of trials in which the mouse appeared due to a correct fixation to the target location. In the joint attention conditions, both experimenter and subject were required to make a correct fixation to the target location. Eye movements (B) are the averages of the total number of eye movements that occurred within each block. Latency to mouse appearance (C) is the latency to the joint (or solo for SA) attention event and (D) is the length of that joint (or solo for SA) attention event. The latency to first eye movement (E) is defined as the latency between the start of the trial and the subject's first eye movement.
Importantly, NT and ASD groups had matched performance on all measures except one: there was a group by condition interaction in the number of eye movements per block (F(2,38) = 9.46, P < 0.00005). Follow‐up Tukey tests (α = 0.05) revealed that both groups showed significantly fewer eye movements in the RJA condition than in the initiating or SA conditions, but the NT group showed significantly more eye movements in the SA condition than ASD participants. Post‐hoc correlation analyses examined whether total number of eye movements during joint attention conditions was correlated with % BOLD signal change in the six ROI. No relationship between eye movements and BOLD signal was found for the neurotypical group. For the ASD group, a significant negative correlation was seen between total number of eye movements in the SA condition and BOLD signal in the left pSTS (r = −0.72, n = 9, P < 0.017). A similar negative relationship reached trend level significance within the left pSTS for both IJA and RJA conditions [IJA, r = −0.64, n = 9, P < 0.06; RJA, r = −0.64, n = 9, P < 0.06]. A negative correlation between number of eye movements and BOLD signal during the SA condition in the dMPFC was significant in ASD (r = −0.68, n = 9, P < 0.043).
Experimenter error, as measured by the percentage of trials in which the mouse appeared but joint attention did not occur or in which joint attention occurred but the mouse did not appear, was minimal and not significantly different across all conditions for both groups (ASD: mean 1.66% ± 0.43% of trials; NT mean 2.8% ± 0.85% of trials).
Responding to Joint Attention
Neurotypicals
In the contrast of RJA as compared to SA, the neurotypical matched‐control group recruited regions primarily associated with social‐cognitive abilities (Table 2, Fig. 3A), including the bilateral pSTS [R: (46,−42,6), t = 5.5; L: (−62,−32,−8), t = 6.4)], the dMPFC [(2,58,32), t = 5.3], and the posterior cingulate [(0,−54,24), t = 9.9]. Additional regions recruited included LIPL [(−42,−66,48), t = 6.7], left middle occipital gyrus [(−54,−72,6), t = 9.5], and right middle temporal gyri [(44,−64,14), t = 5.7].
Table 2.
Regions showing within‐group differences for joint attention and solo attention conditions
| Region | Side | x | y | z | T |
|---|---|---|---|---|---|
| NT | |||||
| RJA>SA | |||||
| Posterior cingulate | 0 | –54 | 24 | 9.88 | |
| Middle occipital gyrus | L | –54 | –72 | 6 | 9.48 |
| Cuneus | L | –10 | –100 | 6 | 7.31 |
| Inferior parietal lobule | L | –42 | –66 | 48 | 6.69 |
| pSuperior temoral suclus | L | –62 | –32 | –8 | 6.35 |
| Middle temporal gyrus | R | 44 | –64 | 14 | 5.67 |
| pSuperior temoral sulcus | R | 46 | –42 | 6 | 5.51 |
| Dorsal medial prefrontal cortex | R | 2 | 58 | 32 | 5.34 |
| Lingual gyrus | L | 6 | –86 | –12 | 5.00 |
| SA>RJA | |||||
| Lingual gyrus | L | –12 | –82 | 16 | 13.10 |
| Anterior cingulate cortex | L | –10 | 20 | 28 | 7.24 |
| Precuneus | R | 28 | –52 | 52 | 5.23 |
| Putamen | R | 28 | 8 | 8 | 5.07 |
| IJA>SAL | |||||
| Inferior parietal lobe | R | 58 | –50 | 50 | 8.37 |
| Medial superior fontal gyrus | R | 2 | 34 | 36 | 7.12 |
| Frontal operculum/insula | R | 60 | 16 | 4 | 6.82 |
| Middle frontal gyrus | L | –28 | 48 | 24 | 6.34 |
| Inferior parietal lobe | L | –46 | –48 | 42 | 6.25 |
| Precuneus | L | –4 | –42 | 48 | 5.71 |
| Supramarginal gyrus | R | 64 | –30 | 26 | 5.67 |
| Superior temporal sulcus | R | 46 | –46 | 14 | 5.66 |
| Precuneus | R | 2 | –74 | 42 | 5.51 |
| Precentral gyrus | R | 48 | 12 | 46 | 5.44 |
| Inferior fontal gyrus/insula | L | –48 | 8 | 0 | 5.12 |
| Supplementary motor area | R | 2 | 8 | 56 | 5.01 |
| Inferior frontal gyrus | R | 50 | 26 | –10 | 4.94 |
| Middle temporal gyrus | R | 50 | –64 | 12 | 4.91 |
| SA>IJA | |||||
| Hippocampus | L | –30 | –28 | –14 | 9.23 |
| Insula | R | 40 | –16 | 12 | 8.26 |
| Paracentral lobule | R | 2 | –28 | 68 | 8.19 |
| Middle occipital gyrus | R | 42 | –80 | –4 | 7.79 |
| Parahippocampal gyrus | R | 34 | –10 | –30 | 7.57 |
| Precentral gyeus | L | –30 | –26 | 52 | 6.90 |
| Middle occipital gyrus | L | –26 | –96 | 4 | 6.83 |
| Fusiform gyrus | R | 22 | –34 | –16 | 6.26 |
| Postcentral gyrus | L | –60 | –14 | 42 | 6.17 |
| Lingual gyrus | L | –20 | –84 | –4 | 5.80 |
| Supplementary motor area | L | –8 | –14 | 54 | 5.68 |
| Cuneus | R | –26 | –96 | –8 | 5.39 |
| Inferior frontal gyrus | L | –34 | 32 | –16 | 5.27 |
| Paracentral lobule | R | 10 | –14 | 48 | 5.12 |
| ASD | |||||
| RJA>SA | |||||
| Posterior inferior frontal gyrus | R | 42 | 14 | 28 | 7.54 |
| SA>RJA | |||||
| Cerebellum, posterior lobe | L | –20 | –60 | –24 | 6.41 |
| Cuneus | L | –18 | –90 | 28 | 6.36 |
| Lingual gyrus | L | –14 | –74 | –12 | 6.32 |
| Middle temporal gyrus | R | 34 | –74 | 20 | 5.31 |
| IJA>SA | |||||
| Precentral gyrus | L | –48 | 8 | 32 | 6.66 |
| Supramarginal gyrus | R | 58 | –38 | 18 | 6.25 |
| Precuneus | R | 6 | –76 | 38 | 5.52 |
| Precentral gyrus | R | 54 | 6 | 44 | 5.40 |
| SA>IJA | |||||
| Precentral gyrus | R | 30 | –20 | 44 | 8.36 |
| Postcentral gyrus | L | –58 | –8 | 18 | 7.67 |
| Posterior cingulate | R | 14 | –50 | 10 | 7.53 |
| Putamen | L | –28 | –18 | 12 | 5.86 |
| Parahippocampal gyrus | L | –22 | –32 | –16 | 5.30 |
| Anterior cingulate | R | 10 | 30 | –10 | 4.95 |
Coordinates are in Montreal Neurological Institute space. T value represents value within peak voxel.
RJA, responding to joint attention; IJA, initiating joint attention; SA, solo attention; p, posterior.
Figure 3.
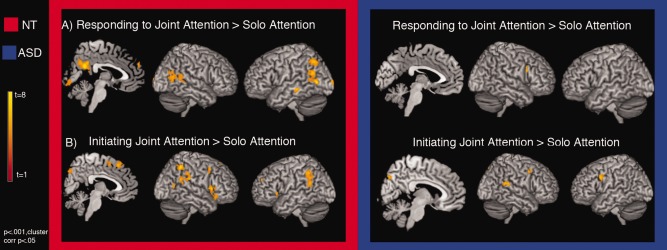
Within‐group comparisons of RJA (A) and IJA (B) in NT (red) and ASD (blue) groups. Group t‐maps are displayed on a template image. Maps are set at a threshold of P < 0.001, voxel‐corrected at P < 0.05.
ASD
In contrast to the controls, the ASD group did not show a greater response to RJA as compared to SA in regions typically associated with social‐cognitive processing (Table 2, Fig. 3A). Instead, the ASD group recruited a region within right posterior inferior frontal gyrus [(42,14,28), t = 7.5]. No other significant clusters were identified.
Neurotypicals vs. ASD
Direct statistical comparison of the NT group to the ASD group revealed greater recruitment of MPFC [(6,62,18), t = 4.6], and right pSTS [(46,−42,6), t = 4.9] in the NT group (Table 3).
Table 3.
Regions showing between–group differences for responding to joint attention and solo Attention conditions
| Region | Side | x | y | z | T |
|---|---|---|---|---|---|
| NT>ASD | |||||
| RJA>SA | |||||
| pSuperior temporal sulcus | R | 46 | –42 | 6 | 4.90 |
| Dorsal medial prefrontal cortex | R | 6 | 62 | 18 | 4.58 |
| IJA>SA | |||||
| None | |||||
| ASD>NT | |||||
| RJA>SA | |||||
| Putamen | R | 28 | 8 | 4 | 5.07 |
| Fusiform gyrus | R | 32 | –46 | –6 | 4.45 |
| Middle occiptal gyrus | R | 30 | –94 | 14 | 4.44 |
| IJA>SA | |||||
| None |
RJA, responding to joint attention; IJA, initiating joint attention; SA,solo attention; p, posterior.
Coordinates are in Montreal Neurological Institute space. T value is from the peak voxel within the cluster.
No regions showed significant between–group differences for intiating joint attention and solo attention.
ASD vs. Neurotypicals
Compared to the NT group, the ASD group showed greater recruitment of right putamen [(28,8,4), t = 5.1], right fusiform gyrus [(32,−46,−6), t = 4.5], and right middle occipital gyrus [(30,−94,14), t = 4.4]. The fusiform and middle occipital gyrus differences are driven by a greater response to SA than RJA in the NT group but no difference between conditions in the ASD group (Table 3).
Initiating Joint Attention
Neurotypicals
In the neurotypical matched control group, a contrast of IJA versus SA revealed frontal/insular regions, including bilateral anterior insula/frontal operculum [R: (60,16,4), t = 6.8; L: (−48,20,0), t = 5.1], medial superior frontal gyrus [(2,34,56), t = 7.1], left middle frontal gyrus (LMFG) [(−28,48,24), t = 6.3], supplementary motor area (SMA) [(2,8,56), t = 5.0], and right precentral gyrus (RPreG) [(48,12,46), t = 5.4]. Additionally, bilateral activation of inferior parietal lobe [R: (58,−50,50), t = 8.4; L: (−46,−48,42), t = 6.3], and right STS [(46,−46,14),t = 5.7] was found (Table 2, Fig. 3B).
ASD
Similar to controls, the ASD group showed recruitment of RPreG [(54,6,44), t = 5.40] in response to IJA as compared to SA. Additional activation was seen in left precentral gyrus [(−48,8,32), t = 6.66], right supramarginal gyrus [(58,−38,18), t = 6.35], and precuneus [(6,−76,38), t = 5.52] (Table 2, Fig. 3B).
Neurotypicals vs. ASD
Direct statistical comparison of the NT group to the ASD group revealed no regions showing greater activation to IJA than SA.
ASD vs. Neurotypicals
Comparison of the ASD group to the NT group revealed no regions showing greater activation to IJA than SA.
Initiating Joint Attention vs. Responding to Joint Attention
No region showed a significant group differences in the comparison of responding to (RJA) and initiating (IJA) joint attention conditions. For a full list of differences between conditions within each group see Supporting Information Table S1.
Region‐of‐Interest Analyses for Joint Attention
Random effects analyses with the 22 controls in the ROI group identified six regions that showed a greater response across both joint attention conditions combined as compared to SA (Fig. 1). These regions were the right posterior superior temporal sulcus (RSTS) [(62,−48,10), t = 6.9], extending into the middle temporal gyrus [(50,−68,6), t = 6.9], the left superior temporal sulcus (LSTS) [(−50,−56,12), t = 7.8], the LIPL [(−42, −52,58), t = 5.0], the RIFG [(56,24,−2), t = 5.3], the right anterior insula (RaIns) [(40,22,−20), t = 4.63], and the dMPFC [(2,58,34), t = 4.5] (Fig. 2).
Repeated measures ANOVA revealed a condition by group interaction in the left posterior STS (F(2,50) = 4.51, P < 0.016) and dMPFC (F(2,50) = 5.34, P < 0.008). No main effect of group was seen in any region. Follow‐up contrasts within groups (corrected using Tukey's test to control for multiple contrasts) showed that in left pSTS, NTs had a significantly greater response to IJA and RJA than SA. No significant differences between conditions were seen in ASD. Similarly in the dMPFC ROI, a greater response was seen to both IJA and RJA than SA in NTs but no difference between conditions was identified in ASD.
Within these six regions, no region showed a significant difference between IJA and RJA conditions in either the ASD or NT group.
Exploratory Correlation Analyses
AQ scores did not correlate significantly with BOLD signal in the joint as compared to SA conditions. Only nonverbal IQ (NVIQ) showed a significant relationship with brain activation; specifically, increasing NVIQ was related to greater activation during IJA than during SA conditions within the dMPFC (r = 0.64, n = 12, P < 0.025). No relationship was found between language and communication scores and response to joint attention within the six ROI. A significant negative relationship between Reciprocal Social Interaction (higher scores = greater social impairments) and BOLD signal during responding to joint (RJA) as compared to SA was found in the dMPFC (Spearman's rho = −0.62, n = 13, P < 0.023).
DISCUSSION
In this study examining joint attention in autism, we found an atypical pattern of brain activation in ASD individuals as compared to NT controls. This atypical pattern was characterized by a lack of differentiation between joint and solo interactions in autism, particularly in the left posterior STS, and the dMPFC for any condition (joint or solo). The greatest between group differences were seen in the RJA condition, not in IJA. NT individuals showed a greater recruitment of posterior STS and MPFC during RJA conditions than ASD individuals. Importantly, this pattern was not driven by group differences in task performance as equivalent behavioral performance was seen between groups. This pattern of results suggests that differences in the specialization of social‐cognitive brain areas underlie the difficulties with joint attention in ASD.
Reduced Differentiation Between Joint and Solo Conditions in the dMPFC in ASD
The dMPFC showed a reduced difference between joint attention conditions in the ASD, compared to the NT, group. Importantly, both groups showed deactivation in this region from the resting baseline in the SA condition. However, while the NT group showed relatively greater activation during the joint attention (as compared to solo) conditions, the ASD group did not. Deactivation during nonsocial tasks, and relative activation during social tasks, has been consistently reported in studies of typical adults in this region [Gusnard and Raichle, 2001]. The dMPFC is a key component of the social‐cognitive network and is involved in making judgments of another person [Mitchell et al., 2006; Moran et al., 2011], observing a social interaction [Centelles et al., 2011; Pierno et al., 2008], or reasoning about another person's mental state [Saxe and Kanwisher, 2003]. Previous research has identified that this region also appears to be recruited when detecting communicative intentions of another person towards oneself [Kampe et al., 2003; Schilbach et al., 2006] or when detecting one's own attention and another person's on the same object (i.e., shared attention) [Williams et al., 2005]. Thus the dMPFC may play a role in both mutual engagement with a social partner (or dyadic attention) as well as sharing attention with another on an object or event (or triadic attention), both of which are critical to establishing joint attention. DMPFC activity during social interactions and joint attention is seen early, within the first few months of life in typically developing children [Grossmann and Johnson, 2010; Grossmann et al., 2008]. Given this region's role in dyadic and triadic interactions, early atypical response profiles could set an infant on an altered developmental trajectory characterized by reduced engagement in critical social interactions. Reduced experience with social interactions could have a cascading effect on behavioral development and brain organization [Pelphrey et al., 2011]. Thus, a critical question for future research is whether this pattern of a reduced differentiation between social and nonsocial contexts in the dMPFC is present in infants who later develop autism.
Reduced Specialization of STS in ASD
In the current study, the posterior STS showed increased activation for the joint attention versus SA conditions in the NT group, but not in the ASD group. This group difference was significant in the left hemisphere. Interestingly, the group difference occurred because of both hypo‐activation during joint attention, and hyper‐activation during SA, in the ASD group, relative to the NT group. Both joint and solo conditions contained a live video feed of experimenter; however, only in the joint conditions were experimenter and participant engaged in a cooperative game together.
Reduced differentiation between social experimental and non‐social “control” conditions, and specifically hyper‐activation during control conditions, has been observed in a previous studies of ASD, particularly within posterior brain regions [Mason et al., 2008; Pelphrey et al., 2005; Lombardo et al., 2011]. For example, in a study of theory of mind processing, individuals with ASD show recruitment of the right TPJ and STS during both social and non‐social control inferences, while NT individuals recruited these regions only for the social inferences [Mason et al., 2008]. Similarly, in a study of gaze perception, individuals with ASD recruited the pSTS to the same extent during both congruent and incongruent contexts, while NT individuals had more activity in pSTS for incongruent gaze [Pelphrey et al., 2005]. Other studies may have also found hyper‐activation for control stimuli rather than hypo‐activation for the social stimuli, but failed to report it because whole‐brain contrast maps cannot differentiate between these two patterns.
Hyper‐activation of social brain regions for non‐social control stimuli may reflect atypical development of these brain regions. One possibility is that these brain regions initially have broad response profiles to a wide range of stimuli, and slowly become more selective in their responses by decreasing response to non‐social stimuli. There is some evidence that selectivity of the response to social stimuli emerges slowly in lateral posterior regions [e.g., pSTS and TPJ; Carter and Pelphrey, 2006; Saxe et al., 2009]. The posterior STS, given its involvement in multiple seemingly diverse tasks such as biological motion perception, intention understanding, and language comprehension [Hein and Knight, 2008; Redcay, 2008], may show broader response profiles to multiple diverse social and non‐social stimuli early on in development but become more selective over time [review, Redcay, 2008]. Patterns of functional correlations in the pSTS slowly differentiate into segregated regions during adolescence in typical development but not in autism [Shih et al., 2011]. In individuals with ASD, functional specialization of the pSTS may be delayed or disrupted, resulting in reduced differentiation between stimuli.
A study of gene expression in post‐mortem brain tissue suggests genetic factors could play a role in this reduced specialization [Voineagu et al., 2011]. Specifically, in neurotypical cases, a subset of genes were identified that showed differential gene expression between regions of frontal and temporal cortex. This differential gene expression was not seen in ASD cases suggesting that molecular markers regulating cortical specialization are altered.
Minimal Differences Seen in IJA
This novel set‐up allowed for the first time the study of subject‐initiated joint attention during fMRI data acquisition in ASD individuals. Based on the behavioral literature [Mundy and Newell, 2007; Mundy et al., 2009], we predicted that neural differences between groups would be the greatest when participants were required to initiate joint attention. However, between‐group comparisons did not reveal robust differences between ASD and NT groups in IJA. The absence of this difference may reflect the choice of task. Evidence suggests that individuals with autism show typical levels of IJA for imperative (or requesting) contexts. The distinction between groups is maximal in cases of declarative contexts or, sharing to share [Baron‐Cohen, 1989; Kasari et al., 1990]. The desire to engage in a cooperative activity for fun and share for sharing sake is at the heart of joint attention [Tomasello et al., 2005] Our current task was designed as a game with a concrete goal in order to ensure comparable engagement and behavioral performance between groups, and so may not have tapped into the motivation to share attention. However, without instructions to initiate joint attention in each trial, group differences in the number of joint attention initiations likely would have emerged, which would make it difficult to disentangle brain activation differences as due to underlying abnormalities in brain function or due to differences in statistical power (i.e., reduced number of trials in one group). A challenge for future studies will be to investigate spontaneous joint attention, potentially through the use of less goal‐directed designs [e.g., Schilbach et al., 2010], while controlling for statistical power between groups.
Level of Functioning Correlates With Differential Activation in the dMPFC
An additional factor that may have reduced differences between groups is the relatively high level of functioning in the autism sample. For many in the sample, social and communication scores on the ADOS were near the cut‐off values and the IQs were relatively high. Interestingly, a significant relationship between nonverbal IQ and reciprocal social interaction was found such that lower IQ and greater social impairments were related to a reduced difference in the dMFPC between joint as compared to SA conditions. Thus, these findings suggest atypical responses in the dMPFC may be even more pronounced in a lower‐functioning autism sample and that differences in the dMPFC in the current study were not driven by differences in nonverbal IQ (as we found reduced dMPFC differentiation in our ASD sample, who had a higher average NVIQ). It is possible, however, that differences (or a lack of difference) between groups in other regions were affected by the relatively high‐level of functioning (particularly higher NVIQ) and heterogeneity in diagnosis of the ASD sample. For example, several studies have reported abnormalities of the right posterior STS in autism in tasks of social perception and social cognition [reviews: Pelphrey et al., 2011; Redcay, 2008; Zilbovicius et al., 2006]. This high‐functioning sample may make the current results less generalizable to other individuals with autism or Asperger's. Future studies should use similar online measures of joint attention during fMRI data collection in a lower‐functioning and more homogenous sample with greater clinical severity.
Importance of a Live Interaction
In a previous paper [Redcay et al., 2010], we identified a number of brain regions that showed a greater response during an interaction with a real‐time video and audio feed of an experimenter than with a video recording of a previous similar interaction. Several of these regions were identified in the current study as involved in a joint attention game, including the bilateral pSTS, suggesting this region may play a key role in both engaging in a social interaction and joint attention. The regions engaged during the current study, however, are also reported in studies that simulate a social interaction. For example, the right posterior STS is recruited during responding to a gaze shift of a virtual character, picture of a face, or even cartoon faces [review, Nummenmaa and Calder, 2009]. A critical question that has not been systematically explored is the extent to which the same response pattern identified in the RJA condition of the current study would be seen in an offline joint attention task. And, perhaps more importantly, would similar atypical patterns be found in autism? Accumulating research suggests the brain is sensitive to social context, and real‐world stimuli may reveal differing patterns of brain activation [e.g., Redcay et al., 2010; Sebanz et al., 2006; Zaki and Ochsner, 2009;]. Studying social interaction in isolation may fail to capture the complexity and unpredictability that is exactly what can be so challenging for individuals with autism. We believe current endeavors to bring the “social” and “interaction” back to the study of the neuroscientific underpinnings of social interaction will bring greater insights into real‐world social impairments that characterize individuals with autism spectrum disorder.
Supporting information
Supporting Information Table 1
ACKNOWLEDGMENTS
The authors thank the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT, including Dr. Christina Triantafyllou, Steven Shannon and Sheeba Arnold Anteraper. They also thank Dr. Karen Shedlack for clinical judgment of the ADOS videos. Authors also thank Jasmine Wang, Jack Keller, Meghan Healey, Jacqueline Pigeon, Nina Lichtenberg, Ruth Ludlum, Daniel O'Young, and Nicolas Dufour for assistance with behavioral coding, scan acquisition, and/or data analyses. The authors report no conflicts of interest or financial disclosures.
These data were presented as a talk at the International Meeting for Autism Research on May 14, 2011 in San Diego, CA.
Footnotes
The psychiatrist assessment was used for clinical confirmation of an ASD diagnosis; no ADOS scores were modified based on her assessment.
REFERENCES
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Welch K, Pickles A (2007): Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol 75:594–604. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Moses LJ (2001): Links between social understanding and early word learning: Challenges to current accounts. Soc Dev 10:309–329. [Google Scholar]
- Baron‐Cohen S (1989): Perceptual role taking and protodeclarative pointing in autism. Br J Dev Psychol 7:113–127. [Google Scholar]
- Brainard DH (1997): The psychophysics toolbox. Spatial Vision 10:433–436. [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN (2002): The importance of eyes: How infants interpret adult looking behavior. Dev Psychol 38:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel RL, Koegel LK (2004): Joint attention and children with autism: A review of the literature. Ment Retard Dev Disabil Res Rev 10:169–175. [DOI] [PubMed] [Google Scholar]
- Carter EJ, Pelphrey KA (2006): School‐aged children exhibit domain‐specific responses to biological motoin. Soc Neurosci 1:396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centelles L, Assaiante C, Nazarian B, Anton J‐L, Schmitz C (2011): Recruitment of both the mirror and the mentalizing networks when observing social interactions depicted by point‐lights: A neuroimaging study. PloS One 6:e15749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T (2003): Why is joint attention a pivotal skill in autism? Philos Trans R Soc Lond B Biol Sci 358:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Delinicolas EK, Young RL (2007): Joint attention, language, social relating, and stereotypical behaviours in children with autistic disorder. Autism 11:425–436. [DOI] [PubMed] [Google Scholar]
- Farroni T, Massaccesi S, Menon E, Johnson MH (2007): Direct gaze modulates face recognition in young infants. Cognition 102:396–404. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M (2011): Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage 56:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH (2010): Selective prefrontal cortex responses to joint attention in early infancy. Biol Lett 6:540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Lloyd‐Fox S, Blasi A, Deligianni F, Elwell C, Csibra G (2008): Early cortical specialization for face‐to‐face communication in human infants. Proc R Soc B Biol Sci 275:2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:684–695. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT (2008): Superior temporal sulcus—It's my area: Or is it? J Cogn Neurosci 20:2125–2136. [DOI] [PubMed] [Google Scholar]
- Hood BM, Willen JD, Driver J (1998): Adult's eyes trigger shifts of visual attention in human infants. Psychol Sci 9:131–134. [Google Scholar]
- Kampe KKW, Frith CD, Frith U (2003): “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci 23:5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, Yirmiya N (1990): Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. J Autism Dev Disord 20:87–100. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, MRC AIMS Consortium ,Baron‐Cohen S (2011): Specialization of right temporo‐parietal junction for mentalizing and its relationship to social impairments in autism. Neuroimage 56:1832–1838. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutters M (2000).The autism diagnostic observation schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. [PubMed] [Google Scholar]
- Mason R, Williams D, Kana R, Minshew N (2008): Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 46:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materna S, Dicke PW, Thier P (2008): Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: A functional magnetic resonance imaging study. J Cogn Neurosci 20:108–119. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR (2006): Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50:655–663. [DOI] [PubMed] [Google Scholar]
- Morales M (1998): Following the direction of gaze and language development in 6‐month‐olds. Infant Behav Dev 21:373–377. [Google Scholar]
- Morales M (2000): Responding to joint attention across the 6‐ through 24‐month age period and early language acquisition. J Appl Dev Psychol 21:283–298. [Google Scholar]
- Moran JM, Lee SM, Gabrieli JDE (2011): Dissociable neural systems supporting knowledge about human character and appearance in ourselves and others. J Cogn Neurosci 23:2222–2230. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Cody‐Hazlett H, Poe MD, Gerig G, Gimpel‐Smith R, Piven J (2009): Longitudinal study of amygdala volume and joint attention in 2‐ to 4‐year‐old children with autism. Arch Gen Psychiatry 66:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Crowson M (1997): Joint attention and early social communication. J Autism Dev Disord 27:653–676. [DOI] [PubMed] [Google Scholar]
- Mundy P, Newell L (2007): Attention, joint attention, and social cognition. Curr Dir Psychol Sci 16:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C (1990): A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord 20:115–128. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV (2007): Individual differences and the development of joint attention in infancy. Child Dev 78:938–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM (2009): A parallel and distributed‐processing model of joint attention, social cognition and autism. Autism Res 2:2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PB, Adamson LB, Bakeman R (2008): Toddlers' joint engagement experience facilitates preschoolers' acquisition of theory of mind. Dev Sci 11:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ (2009): Neural mechanisms of social attention. Trends Cogn Sci 13:135–143. [DOI] [PubMed] [Google Scholar]
- Pelli D (1997): The video toolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision 10:437–442. [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G (2005): Neural basis of eye gaze processing deficits in autism. Brain 128( Part 5):1038–1048. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk, BC (2011): Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry 52:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno AC, Becchio C, Turella L, Tubaldi F, Castiello U (2008): Observing social interactions: The effect of gaze. Soc Neurosci 3:51–59. [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Hudac CM, Lantz SD, Minshew NJ, Vander Wyk BC, Pelphrey KA (2011): Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Dev Disord 41:1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E (2008): The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neurosci Biobehav Rev 32:123–142. [DOI] [PubMed] [Google Scholar]
- Redcay E, Dodell‐Feder D, Pearrow MJ, Mavros PL, Kleiner M, Gabrieli JDE, Saxe R (2010): Live face‐to‐face interaction during fMRI: A new tool for social cognitive neuroscience. NeuroImage 50:1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking peopleThe role of the temporo‐parietal junction in “theory of mind.” NeuroImage,19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe RR, Whitfield‐Gabrieli S, Scholz J, Pelphrey KA (2009): Brain regions for perceiving and reasoning about other people in school‐aged children. Child Dev 80:1197–1209. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, Newen A, Shah N, Jon F, Gereon R, Vogeley K (2006): Being with virtual others: Neural correlates of social interaction. Neuropsychologia 44:718–730. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K (2010): Minds made for sharing: Initiating joint attention recruits reward‐related neurocircuitry. J Cogn Neurosci 22:2702–2715. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Bekkering H, Knoblich G (2006): Joint action: bodies and minds moving together. Trends in Cognitive Sciences 10:70–76 [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yaguchi K, Hasegawa T (2005): Deviant gaze processing in children with autism: An ERP study. Neuropsychologia 43:1297–1306. [DOI] [PubMed] [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller R‐A (2011): Functional differentiation of posterior superior temporal sulcus in autism: A functional connectivity magnetic resonance imaging study. Biol Psychiatry 70:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, McGovern CW (2005): Improvement in cognitive and language skills from preschool to adolescence in autism. J Autism Dev Disord 35:15–23. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H (2005): Understanding and sharing intentions: The origins of cultural cognition. Behav Brain Sci 28:675–691; discussion 691–735. [DOI] [PubMed] [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G (2006): Early predictors of communication development in young children with autism spectrum disorder: Joint attention, imitation, and toy play. J Autism Dev Disord 36:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Foss‐Feig J, Shook D, Kaplan L, Kenworthy L, Gaillard WD (2011): Controlling attention to gaze and arrows in childhood: An fMRI study of typical development and Autism Spectrum Disorders. Dev Sci 14:911–924. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH (2011): Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474:380–384. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Perra O, Perrett DI, Whiten A (2005): An fMRI study of joint attention experience. NeuroImage 25:133–140. [DOI] [PubMed] [Google Scholar]
- Woodbury‐Smith MR, Robinson J, Wheelwright S, Baron‐Cohen S (2005): Screening adults for asperger syndrome using the AQ: A preliminary study of its diagnostic validity in clinical practice. J Autism Dev Disord 35:331–335. [DOI] [PubMed] [Google Scholar]
- Woodward AL (2003): Infants' developing understanding of the link between looker and object. Dev Sci 6:297–311. [Google Scholar]
- Zaki J, Ochsner K (2009): The need for a cognitive neuroscience of naturalistic social cognition. Ann NY Acad Sci 1167:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthelemy C, Samson Y (2000): Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. American Journal of Psychiatry 157:1988–1993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1


