Graphical abstract
Keywords: Sensory features, Autism, Infants, Social, Longitudinal, Attention, Risk markers
Highlights
-
•
Infants from a community sample who will be diagnosed with ASD show high sensory seeking by 20–24 months.
-
•
Sensory seeking features at 20–24 months predict social symptom severity at 3–5 years of age.
-
•
The relation between sensory seeking and future social symptoms is mediated by social orienting.
-
•
Sensory seeking is emerging at 13–15 months and not yet predictive of preschool social symptoms.
-
•
Reduced attention disengagement in at-risk infants precedes and predicts elevated sensory seeking.
Abstract
Recent work suggests sensory seeking predicts later social symptomatology through reduced social orienting in infants who are at high-risk for autism spectrum disorder (ASD) based on their status as younger siblings of children diagnosed with ASD. We drew on extant longitudinal data from a community sample of at-risk infants who were identified at 12 months using the First Year Inventory, and followed to 3–5 years. We replicate findings of Damiano et al. (in this issue) that a) high-risk infants who go on to be diagnosed with ASD show heightened sensory seeking in the second year of life relative to those who do not receive a diagnosis, and b) increased sensory seeking indirectly relates to later social symptomatology via reduced social orienting. We extend previous findings to show that sensory seeking has more clinical utility later in the second year of life (20–24 months) than earlier (13–15 months). Further, this study suggests that diminished attention disengagement at 12–15 months may precede and predict increased sensory seeking at 20–24 months. Findings add support for the notion that sensory features produce cascading effects on social development in infants at risk for ASD, and suggest that reduced attention disengagement early in life may set off this cascade.
1. Introduction
1.1. Differences in sensory responsiveness in individuals with autism spectrum disorder
Children with autism spectrum disorder (ASD) experience pervasive social deficits affecting their ability to fully participate in a range of activities across the lifespan (World Health Organization, 1993). Unusual responses to sensory stimuli are also highly prevalent and persistent in individuals with ASD (Ausderau et al., 2014, Billstedt et al., 2007) and currently regarded as core symptoms of the disorder (American Psychiatric Association, 2013). Children with ASD show a broad range of atypical responses to sensory stimuli, from hyporesponsiveness (Baranek et al., 2013) to hyperresponsiveness (Baranek et al., 2007). Children with ASD may also show high levels of sensory seeking, which is defined as a pattern of behavior that serves to intensify, repeat, or reinforce sensory experiences (Ben-Sasson et al., 2009a, Kirby et al., 2015, Damiano et al., 2017). Examples of sensory seeking include licking, smelling or visually sighting objects, craving intense pressure or movement stimulation, or being fascinated with specific sounds. Such behaviors may co-occur with hypo- and hyper-responsiveness (Ausderau et al., 2016).
1.2. Theory that early sensory differences produce cascading effects on higher level social skill
It has been proposed that differences in sensory responsiveness, particularly in the earliest stages of development, may produce cascading effects on higher level function, such as social skill (Cascio et al., 2016, Watson et al., 2011, Brandwein et al., 2015, Talay-Ongan and Wood, 2000). If this is the case, then intervening upon early sensory responsiveness may translate to improved social outcomes in children with ASD. This theory is intuitively appealing, given the precedence and possible “foundational” nature of early sensory development relative to the emergence of “higher-level” social and communication abilities. For further information regarding the cascading effects theory, refer to recent reviews by Cascio et al. (2016) and/or Baranek et al. (2014).
1.3. Empirical support for cascading effects theory
Research suggests that differences in sensory responsiveness emerge early in development in children affected by ASD. Early precursors of sensory features have been identified at approximately 9–18 months of age through retrospective video analysis and case studies (Baranek, 1999a, Dawson et al., 2000), prospective studies of infant siblings of older children with ASD (Germani et al., 2014), and community-screened samples of infants later diagnosed with ASD (Turner-Brown et al., 2013). In a qualitative study, Freuler et al. (2012) found that early precursors of later fully established sensory symptoms primarily included hyporesponsiveness and sensory seeking features.
Past work has also established concurrent links between sensory responsiveness and higher level function in children diagnosed with ASD (Ausderau et al., 2016, Watson et al., 2011, Lane et al., 2010, Hilton et al., 2007). For example, hyporesponsiveness to both social and nonsocial sensory stimuli has been linked to joint attention and language impairments in young children with ASD (Baranek et al., 2013). Hyperresponsiveness has been linked with reduced social-emotional behavior (Ben-Sasson et al., 2009b) in elementary school-aged children with ASD, as well as theorized to result in increased attention to detail and hyper-systemizing talents (Baron-Cohen et al., 2009) in high functioning adults with ASD. Sensory seeking has been associated with impairments in social and communication skill (Watson et al., 2011, Hilton et al., 2007), arousal modulation (McDonnell et al., 2015), and attention (Liss et al., 2006, Sabatos-Devito et al., 2016). Moreover, both hyporesponsiveness and sensory seeking behaviors are significantly associated with slower attention disengagement in children with ASD ages 4–13 years (Sabatos-Devito et al., 2016). This finding is particularly interesting given that attention disengagement has been implicated as a behavioral risk marker for a later diagnosis of ASD in several studies (Zwaigenbaum et al., 2005, Bryson et al., 2007, Elsabbagh et al., 2013, Elsabbagh et al., 2009), but has not been tested systematically in relation to sensory seeking during early infant development.
In sum, there has been a relative lack of systematic prospective research to date on the development of early sensory features as they relate to later ASD symptomatology. Few investigations of specific developmental mechanisms help explain how early sensory features may have cascading effects on later social outcomes in this population. A primary challenge to establishing these links is that ASD cannot always be definitively diagnosed in infancy (Ozonoff et al., 2015). A potential solution is to prospectively follow infants at high-risk for ASD and other language and/or communication impairments (Costanzoa et al., 2015).
1.4. Recent support for the cascading effects framework in toddlers at risk for ASD
One recent study by Damiano et al. (2017) that took this prospective approach demonstrated that sensory seeking features are elevated by 18 months (±one month) and predictive of future social symptomatology through reduced social orienting in infants who are at high familial risk of ASD. Social orienting is defined here as a behavioral response to (i.e., turning towards the source of) a socially-relevant stimulus, such as one’s name being called or a tap on the shoulder. The results of the aforementioned study provided some preliminary support for the notion that differences in sensory seeking may produce cascading effects on social development in infants at risk for ASD, but were limited. First, the high-risk classification of all infants in Damiano et al. (2017) was based on participants’ status as younger siblings of children who were diagnosed with ASD. Thus, it was unclear whether findings for sensory seeking were specific to infants at familial risk for ASD or were applicable to infants at broader risk for the disorder. Second, Damiano et al. (2017) measured both sensory seeking and social orienting at a single time point (i.e., at 18 months). As a result, we cannot be entirely confident that heightened sensory seeking temporally precedes reduced social orienting or that sensory seeking as measured earlier in infancy would be clinically useful as a predictor of future social symptomatology.
1.5. Purpose and research questions
Accordingly, in the present study, we attempted to systematically replicate and extend the findings of Damiano et al. (2017). First, we evaluated whether their findings for sensory seeking later in the second year of life generalize to infants identified as being at-risk for ASD according to a broad-based community screening versus family history of ASD. Second, we tested whether the indirect effect of sensory seeking on future social symptomatology (mediated through social orienting) held in our at-risk community sample if we measured sensory seeking earlier in the second year of life. Our primary research questions at the outset of this investigation of were:
(1a) Is increased sensory seeking as measured later in the second year of life (i.e., 20–24 months) related to future social symptom severity (i.e., at 3–5 years of age), as mediated by reduced social orienting in a community sample of infants at heightened, non-familial risk for ASD? This research question extends findings from Damiano et al. (2017) to infants at broader risk for ASD.
(1b) Is increased sensory seeking as measured earlier in the second year of life (i.e., 13–15 months) similarly predictive of future social symptom severity as mediated by social orienting (at 20–24 months) in a community sample of infants at heightened, non-familial risk for ASD? This research question further extends findings from Damiano et al. (2017) to determine at what point in the second year of life sensory seeking is predictive of cascading effects on social development.
After pursuing the analyses related to question 1a/b, we further explored the extent to which impairment in attention disengagement may be an earlier precursor of our primary variables of interest in this same sample. Specifically, our secondary (post-hoc) research question asked:
(2) Is difficulty with attention disengagement (i.e., “sticky attention”) as measured earlier in the second year of life (i.e., 12–15 months) predictive of social orienting (at 20–24 months) as mediated by sensory seeking later in the second year of life (i.e., 20–24 months)?
2. Materials and methods
2.1. Overview of study design
To answer these research questions, we drew on extant data from a longitudinal investigation of a community sample of infants identified at one year of age as high-risk for a later diagnosis of ASD. Children’s risk status at 12 months of age was ascertained with the First Year Inventory (FYI) (Reznick et al., 2007, Baranek et al., 2003), a parent report screening tool showing a positive predictive value of 0.31 (Turner-Brown et al., 2013). Children were subsequently followed throughout early childhood with comprehensive developmental assessments conducted at three time points. Although the primary aim of the earlier study was to test the efficacy of a parent-mediated intervention (Watson et al., in press), and thus children were assigned to treatment (Adapted Responsive Teaching) versus control (Referral to Early Intervention and Monitoring) conditions, there were no main effects of treatment on any of the primary child outcomes of interest in the RCT or on any of the variables relevant to the present study. Additionally, we demonstrate that significant effects observed in this report do not vary according to children’s treatment group assignment (see Exploration of Alternative Explanations in Results).
2.2. Participants
Prospective participants from six counties in central North Carolina were identified through state birth records and mailed the FYI within two weeks of the infant’s first birthday. A total of 8429 FYIs (14.5%) were completed and returned, 280 (3%) of which met the criteria for high-risk status, based on a two-domain cut-off in both sensory-regulatory and social-communication domains (for FYI scoring information see Reznick et al., 2007). Of the 280 infants classified as high-risk for ASD on the FYI, 87 families (31%) consented to the RCT after confirmation that they met additional inclusion criteria: a) infant birth weight >5 pounds, b) a primary caregiver available to participate in 6 months of in-home intervention sessions, and c) English as the primary language spoken in the home.
Pretest assessments were conducted for 87 infants at 13–15 months of age (Time 1). Following the 6 month treatment phase, 84 children returned for a posttest assessment at 20–24 months of age (Time 2). A follow-up assessment was conducted at 3–5 years of age (Time 3) with families who were reachable, willing to be assessed, and still living in the catchment area. A few families (n = 8) who were no longer within driving distance additionally agreed to complete questionnaires and interviews. The 55 children (63% of the larger study sample) for whom follow-up data were available at Time 3 comprise the present study sample. See Table A1 for further information regarding sample characteristics at each time point. The present study sample was highly similar to the sample of children enrolled in the larger parent project on all variables, with the exception that white families were more likely to return for the follow-up assessment (see Table S1 in Supplemental materials). Seventeen of the high-risk children who were included in the present study (31%) received a diagnosis of ASD at the Time 3 follow-up assessment.
2.3. Measurement of sensory seeking
Sensory seeking was measured at both Time 1 and Time 2 (i.e., when participants were 13–15 months and 20–24 months, respectively) using the Sensory Processing Assessment (Baranek, 1999b). The SPA is a 15- to 20-min play-based observational measure intended to assess behavioral patterns of sensory responsiveness in children approximately 9 months–6 years of age. Prior research suggests that this measure is psychometrically sound, with strong inter-rater reliability (e.g., yielding ICCs ranging from 0.91–0.99 in a prior sample of 48 children ages 6–37 months), as well as good discriminative validity (e.g., discriminating children with ASD from children with other developmental delays and/or typically developing children on a number of indices, including social orienting), convergent validity (e.g., correlating with other measures of sensory responsiveness such as the Sensory Experiences Questionnaire), and predictive validity (e.g., predicting social communication skill and broader ASD and related symptomatology) (Baranek et al., 2013, Baranek et al., 2007, Watson et al., 2011, Baranek, 1999b). Coders were trained to 90% fidelity in administration and in scoring on all sections of this assessment.
In the SPA, children are presented with a series of novel toys (e.g., water log, musical dome, fan) that afford sensory experiences across several modalities (i.e., auditory, visual, tactile). Across novel toys, the presence or absence of seven types of sensory interests, repetitions, and seeking behaviors (i.e., “seeking”) was recorded. These sensory seeking features included both body and object focused behaviors, specifically: arm/hand flapping, finger mannerisms (e.g., unusual posturing or repetitive flicking), mouthing of non-food objects, smelling of non-food objects, other repetitive sensory-motor movements (e.g., rocking or spinning oneself in circles), other repetitive object manipulations across sensory modalities (e.g., rubbing, sighting, spinning), and any other unusual sensory behaviors (e.g., pressing objects especially hard). The sensory seeking score was quantified as a sum score—that is, the total number of the seven types of sensory seeking features observed by the examiner. A higher score is indicative of greater sensory seeking.
2.4. Measurement of social orienting
Social orienting was also measured using the SPA (Baranek, 1999b). Orienting was defined as turning eyes or head in the direction of the stimulus. Over the course of the sample, when children were engaged with the novel toys, social orienting items in each of three modalities (i.e., auditory – name call, visual – hand wave, tactile – shoulder tap) were presented. As per the standard SPA protocol, each item was presented three times or until the child showed a definitive orienting response, whichever came first. Responses for each item were assigned a score from 1 to 4, wherein 1 = oriented on the first trial, 2 = did not orient on the first trial but did orient on the second trial, 3 = did not orient on either the first or second trial but did orient on the third trial, or 4 = did not orient across the three trials for that item. Social orienting was quantified as the mean of scores (1–4) across social orienting items administered. As such, a higher score is indicative of repeated cueing to elicit social orienting—that is, reduced orienting.
2.5. Measurement of attention disengagement
Attention disengagement is defined here as the ability to flexibly shift the focus of visual attention from a central fixation point to another target presented in the periphery (Zwaigenbaum et al., 2005, Bryson et al., 2007, Elsabbagh et al., 2013, Elsabbagh et al., 2009). In the parent study from which extant data were drawn for this report, attention disengagement was measured early in the second year of life using an aggregate of the parent-report items from the FYI (Baranek et al., 2003), as well as examiner-elicited items from the Autism Observation Scale for Infants (Bryson et al., 2008), an observational measure that was developed to monitor the emergence of early signs of autism in infants identified as high-risk for ASD. As indicated above, parents completed FYI questionnaires when their infants were approximately 12 months old, as part of the broad-based community screening intended to identify infants at heightened risk for autism. AOSIs were collected at Time 1 (when infants were 13–15 months old), during the first formal assessment of the study sample.
On the FYI, attention disengagement was assessed by five items (i.e., FYI #14, 30, 37, 50, 52). For each of these items, the parent is asked to respond to a question (e.g., “What do you typically have to do to get your baby to look up from playing with a favorite toy?”) by selecting one of multiple response options (e.g., “Just show him or her a different toy; Move, shake, or make a noise with the different toy; or Take the favorite toy away and give your baby the different toy”). Items were scored such that the parent response for each item that reflected the most ready disengagement from the infant = 1, and that parent responses reflecting more delayed and/or difficult disengagement received progressively higher scores (e.g., 2, 3, or 4), dependent upon the number of response options from which the parent could select. Attention disengagement from the FYI was quantified as the sum of scores across attention disengagement items. Thus, a higher attention disengagement score for the FYI reflected “sticky attention” – that is, reduced disengagement and/or the need for additional time and/or repeated or enhanced cueing to elicit attention disengagement from the infant.
In the context of the AOSI, infants were presented with three attention disengagement items, in which the child’s gaze was fixated on a toy at mid-line and a second toy was then presented in the periphery. Infants’ responses for each item were assigned a score from 1 to 3, wherein 1 = infant disengaged readily and immediately shifted gaze upon presentation of the second toy, 2 = infant disengaged with a delay upon presentation of the second toy, 3 = infant got “stuck” and did not disengage upon presentation of the second toy. Attention disengagement from the AOSI was quantified as the sum of scores (1–3) across attention disengagement items presented. Thus, a higher attention disengagement score also reflected reduced disengagement and/or the need for additional time to elicit attention disengagement from the infant (i.e., “sticky attention”).
2.6. Measurement of social symptom severity and diagnostic outcomes
When children were 3–5 years old, the Autism Diagnostic Observation Schedule—Second Edition (Lord et al., 2012) was administered by either a research-reliable licensed speech-language pathologist or a clinical psychology intern, supervised by a licensed clinical psychologist experienced in diagnosis of children with ASD. Social symptom severity was quantified using the Social Affect calibrated severity scores (Hus et al., 2014). These scores were log-10 transformed to correct for a positive skew. Diagnostic status at follow-up was based on a comprehensive ASD diagnostic assessment that included the ADOS-2 scores and the clinicians’ judgment that children met criteria for ASD according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (American Psychiatric Association, 2013).
2.7. Analytic plan
The analysis method that we planned to use assumes multivariate normality, which is more likely when univariate distributions do not grossly depart from the normal distribution (Tabachnick and Fidell, 2001). Thus, all continuous variables were evaluated for normality. Those showing univariate skewness >|1.0| or kurtosis >|3.0| were transformed prior to imputation and analysis. Missing data points for continuous variables (ranging from 5 to 22%) were then multiply imputed (Enders, 2011).
We first sought to systematically replicate the findings of Damiano et al. (2017) as closely as possible using the present dataset involving a community sample of infants at heightened, non-familial risk for ASD. We note that participants in the present analysis were slightly older (20–24 months; M = 22 months) than in Damiano’s analysis (18 months ± 30 days). To replicate the finding that sensory seeking predicts future social symptomatology through reduced social orienting, we tested the statistical significance of the indirect effect of Time 2 sensory seeking on Time 3 social symptom severity via Time 2 social orienting (Hayes, 2009). Two paths comprise the indirect effect. The first path (i.e., the “a path”) is the relation between sensory seeking and social orienting. The second path (i.e., the “b path”) is the relation between social orienting and future social symptom severity, controlling for sensory seeking. An indirect effect of seeking on social symptom severity through social orienting is statistically significant when the confidence interval for the product of the unstandardized coefficients for these two paths (a*b) does not include zero. Unstandardized regression coefficients and standard errors for a and b paths were obtained from pooled results of multiple regression analyses for the relations of interest, using the multiply-imputed dataset. The confidence interval for the indirect effect was then generated, and the statistical significance of the indirect effect thus tested, using PRODCLIN (MacKinnon et al., 2007).
We subsequently sought to extend the findings of Damiano et al. (2017) to examine sensory seeking earlier in the second year of life in our at-risk community sample. To test whether sensory seeking is a useful predictor of future social symptom severity through reduced social orienting and to establish temporal precedence for increased sensory seeking relative to reduced social orienting, we tested the statistical significance of the indirect effect of Time 1 sensory seeking on Time 3 social symptom severity through Time 2 social orienting, using the same approach to modern mediation analysis detailed above (Hayes, 2009). Lastly, in our post-hoc analysis, we explored whether attention disengagement at Time 1 precedes and predicts sensory seeking, and translates to reduced social orienting at Time 2, using a mediation model similar to the one described above.
3. Results
3.1. Primary analyses for sensory seeking as measured at 20–24 months of age
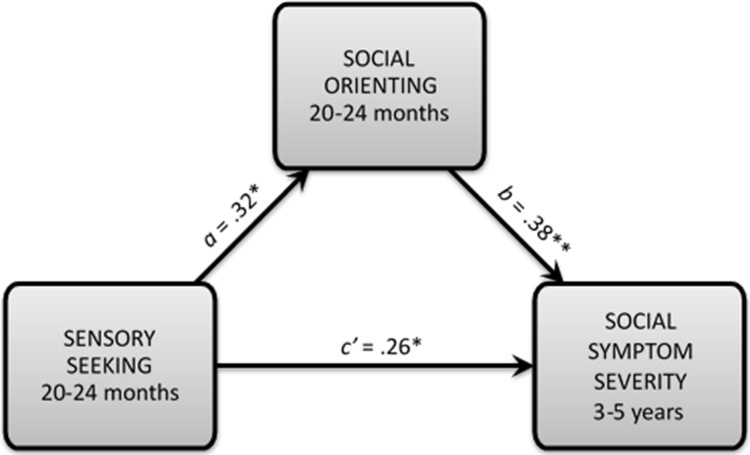
Sensory seeking as measured later in the second year of life (Time 2) was a significant predictor of social symptom severity at 3–5 years (Time 3) in our sample, not controlling for any other factors (c path; p value = 0.003). This total effect was moderate in magnitude (0.41). Higher seeking at 20–24 months predicted increased severity of social symptoms associated with ASD (i.e., reduced social affect) at 3–5 years of age in high-risk infants from our community sample. Sensory seeking as measured later in the second year of life (Time 2) additionally covaried with concurrent social orienting (Time 2; a path; p value = 0.013), and social orienting predicted future social symptom severity (Time 3) when controlling for sensory seeking at Time 2 (b path; p value = 0.002). Thus, both components of the indirect effect of interest were statistically significant.
The indirect effect (a*b) of sensory seeking as measured at Time 2 (20–24 months) on future social symptom severity through social orienting was statistically significant, 95% CI [0.005, 0.075]. This significant indirect effect confirms that social orienting mediates the relation between sensory seeking, as measured later in the second year of life, and future social symptom severity (i.e., that the association between sensory seeking at this time point and future social symptomatology is significantly reduced when accounting for social orienting). Thus, sensory seeking as measured later in the second year of life appears to indirectly impact future social symptom severity by reducing social orienting in this high-risk community sample.
However, the direct effect of sensory seeking at Time 2 on social symptom severity at Time 3 (i.e., the c’ path) remains statistically significant, even after controlling for Time 2 social orienting. Therefore, the association between sensory seeking later in the second year of life and future social symptom severity can be explained only in part by reduced social orienting in our sample. See Table A2 for the results of regression analyses relevant to this indirect effect and Fig. A1 for a depiction of this result.
Fig. A1.
Indirect effect of sensory seeking as measured later in the second year of life (i.e., 20–24 months) on future social symptom severity through social orienting in a community sample of infants at heightened, non-familial risk for autism spectrum disorder. a = the relation between sensory seeking and social orienting, not controlling for any other factors. b = the relation between social orienting and future social symptom severity, controlling for sensory seeking. c’ = the direct effect of sensory seeking on social symptom severity (i.e., the c’ path), controlling for social orienting. Values provided for a, b, and c’ paths are standardized coefficients. * p < 0.05. ** p < 0.01.
3.2. Primary analyses for sensory seeking as measured at 13–15 months of age
We subsequently tested whether seeking as measured earlier in the second year of life indirectly influenced future social symptom severity through reduced social orienting. Sensory seeking as measured at 13–15 months (Time 1) did not significantly predict social symptom severity at 3–5 years (Time 3; c path; p value = 0.65). The magnitude of the total effect of sensory seeking at Time 1 and social symptom severity at Time 3 was near zero (0.07). Sensory seeking as measured at this earlier time point (Time 1) furthermore failed to predict social orienting at Time 2 (a path; p value = 0.81). Social orienting at Time 2 does continue to predict future social symptom severity (Time 3), controlling for sensory seeking as measured at Time 1 (b path; p value < 0.001). However, the indirect effect (a*b) of sensory seeking as measured earlier in the second year (i.e., at Time 1; 13–15 months) on future social symptom severity through social orienting is not statistically significant, 95% CI [−0.027, 0.035]. See Table A3 for the results of regression analyses relevant to this non-significant indirect effect.
3.3. Secondary exploratory analyses
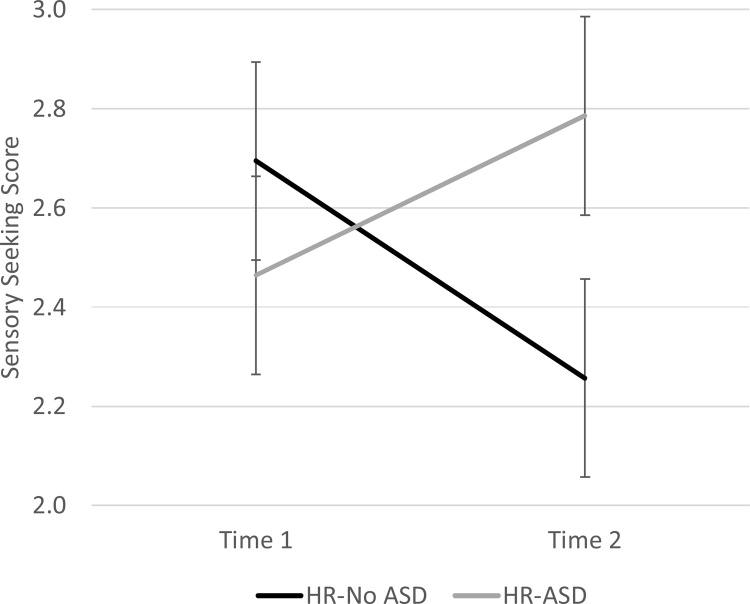
We then attempted to ascertain why sensory seeking as measured earlier in the second year of life was not useful as a predictor of future social orienting or social symptom severity. An intraclass correlation coefficient for Time 1 to Time 2 sensory seeking, derived using a two-way random effects model for absolute agreement, indicated that this construct was not stable across the second year of life, ICC = 0.098. A repeated measures Analysis of Variance with Time as a within-subjects factor and Outcome Group as a between-subjects factor further revealed that infants who did and did not go on to receive a diagnosis of ASD (HR-Dx ASD and HR-No ASD) were diverging in sensory seeking across the time points of interest, such that they differed more so in sensory seeking later in the second year of life than they did earlier in the second year of life, F(1,44) = 4.735, p = 0.035 for the Time * Outcome Group interaction (main effects were non-significant for this ANOVA). Follow-up comparisons confirmed that significant differences in seeking between outcome groups were detectable at Time 2, t(44) = −2.327, p = 0.02, but not at Time 1, t(44) = 0.83, p = 0.41. Thus, it appears that the heightened levels of seeking that seem to influence social outcomes of children on an atypical trajectory have not yet fully emerged by 13–15 months. Fig. A2 illustrates this result.
Fig. A2.
Differences in sensory seeking across the second year of life for high-risk infants according to diagnostic outcome. HR-No ASD = High-risk infants from a community sample who did not go on to receive a diagnosis of ASD (black line). HR-ASD = High-risk infants from a community sample who did go on to receive a diagnosis of ASD (gray line). Outcome group differences in sensory seeking were non-significant at Time 1 assessments, when infants were 13–15 months old, but significant by Time 2 assessments, when participants were 20–24 months old. Error bars represent standard error of the mean.
In post-hoc analyses, we further explored whether a factor that is closely related to sensory seeking—attention disengagement—may be helpful in predicting earlier in life which high-risk infants would go on to display high levels of seeking and subsequent social symptomatology. As described earlier, “sticky attention” was selected as a candidate predictor of future sensory seeking and reduced social orienting because deficits in attention disengagement had been previously a) identified as one of the earliest-emerging markers of ASD (Zwaigenbaum et al., 2005, Bryson et al., 2007, Elsabbagh et al., 2013, Elsabbagh et al., 2009) and b) associated with increased sensory seeking in children diagnosed with ASD (Sabatos-Devito et al., 2016).
Attention disengagement scores from the AOSI and the FYI were aggregated by averaging the z-scores for the two component variables to create a single metric of “sticky attention” to be used in analyses. We utilized an aggregate variable because doing so increases the stability and thus the potential construct validity of a metric, particularly in the earliest stages of development (Sandbank and Yoder, 2014, Rushton et al., 1983).
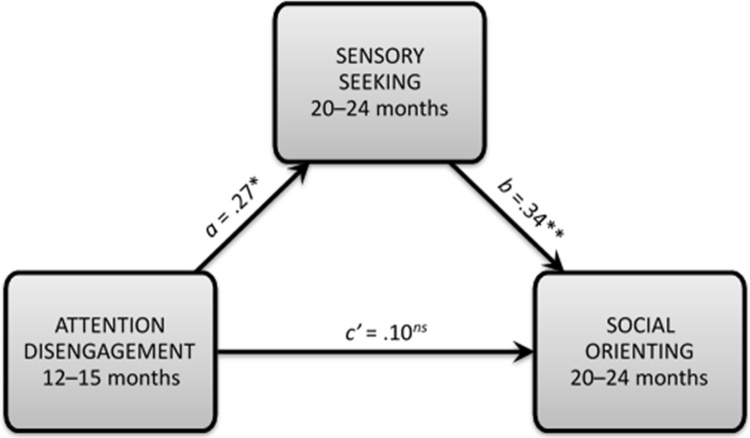
Results demonstrated that this aggregate index of sticky attention as measured at 12–15 months of age predicted future sensory seeking and translated to reduced social orienting at 20–24 months of age, 95% CI for the indirect effect of Time 1 attention disengagement on Time 2 social orienting through Time 2 sensory seeking [0.001, 0.297]. The effect of attention disengagement on social orienting is considered to be completely mediated by sensory seeking because the direct effect of earlier attention disengagement on future social orienting (i.e., the c’ path) is non-significant, controlling for sensory seeking. Table A4 presents the results of regression analyses relevant to this mediation relation. The indirect effect is depicted in Fig. A3.
Fig. A3.
Deficits in attention disengagement as measured earlier in the second year of life (i.e., 12–15 months) predict future sensory seeking and translate to reduced social orienting in a community sample of infants at heightened, non-familial risk for ASD. a = the relation between attention disengagement and sensory seeking, not controlling for any other factors. b = the relation between sensory seeking and social orienting, controlling for attention disengagement. c’ = the direct effect of attention disengagement on social orienting (i.e., the c’ path), controlling for sensory seeking. Values provided for a, b, and c’ paths are standardized coefficients. * p < 0.05. ** p < 0.01. ns = non-significant result.
3.4. Exploration of alternative explanations
As sensory responsiveness has previously been observed to vary according to mental age (e.g., Baranek et al., 2013, 2007), it is logical to question whether MA may account for the relations that we observed here. MA at entry to the study was not significantly associated with any of the predictors or putative mediators of interest (r values for Time 1 MA with Time 1 attention disengagement, Time 2 sensory seeking, and Time 2 social orienting were −0.12, −0.15, and −0.22 respectively; all p values > 0.05). The only variable with which Time 1 MA significantly correlated was Time 3 social symptom severity (r = −0.47, p = 0.002). Though this was unlikely to explain the mediation relations observed, we did re-run the one significant mediation model in which this was the outcome variable (i.e., the indirect effect of Time 2 sensory seeking on future social symptomatology through social orienting), controlling for MA. This indirect effect remained significant, 95% CI [0.001, 0.06].
Additionally, we considered whether chronological age (CA), on which infants varied to some extent (i.e., 3 month variation) at entry to the study, may account for the observed mediated relations. CA at entry to the study, however, was not associated with any of the predictors, putative mediators, or outcomes of interest in this study (r values for Time 1 CA with Time 1 attention disengagement, Time 2 sensory seeking, Time 2 social orienting, and Time 3 social symptom severity were −0.02, 0.07, 0.02, and 0.02 respectively; all p values > 0.05). Thus, CA could not account for any of the indirect effects that we observed.
Finally, it was possible that mediated effects could vary according to treatment group assignment. To evaluate this possibility, we re-ran each significant mediation relation with treatment group as a moderator term. Neither the indirect effect of Time 1 attention disengagement on future social orienting through sensory seeking, nor the indirect effect of Time 2 sensory seeking on future social symptom severity through social orienting significantly varied according to treatment group assignment, 95% CIs [−0.80, 0.06] and [−0.06, 0.14], respectively. Thus, none of the indirect effects that we observed were conditional on infants’ treatment group status in the larger study.
4. Discussion
The present study investigated the extent to which earlier sensory seeking features impact later social development in a community sample of infants at high-risk for a later diagnosis of ASD, ascertained at 12 months of age. Specifically, we tested whether the effect of sensory seeking at 20–24 months on future social symptomatology (at 3–5 years) was mediated primarily through social orienting, as proposed in the conceptual model by Damiano et al. (2017). The aforementioned study focused on a sample at high-risk for a future diagnosis of ASD based on their status as younger siblings of children with ASD, and demonstrated that elevated sensory seeking as measured at 18 months (±30 days) predicted future social deficits through reduced social orienting. Replication of findings with different samples is critical to building confidence in scientific conclusions and necessary for generalizability across populations (Asendorpf et al., 2013).
4.1. Sensory seeking features are generalizable clinical markers of risk for ASD
We found that high-risk infants who went on to receive an ASD diagnosis by 3–5 years of age were more likely to exhibit higher levels of sensory seeking features at 20–24 months of age than their counterparts who did not receive a later ASD diagnosis. The current study systematically replicated and extended Damiano et al.’s (2017) findings to a community sample of high-risk infants who were not selected on the basis of having familial risk of ASD. Taken together, these two studies suggest that by the latter half of the second year of life, sensory seeking features may be useful and generalizable clinical markers for ASD risk across populations, not unique to a genetically high-risk group.
Most studies investigating sensory features have been conducted with older children with ASD. Genetic influences on sensory features (Uljarević et al., 2014, Goldsmith et al., 2006) as well as social features of ASD (Constantino and Todd, 2000) are documented, but the large phenotypic heterogeneity in ASD points to additional factors likely impacting developmental trajectories of sensory and social features, as well as their transactions over time. Although some studies have suggested that sensory features may be precursors of social impairment in children at-risk for ASD, and that these features add to specificity of a later diagnosis (e.g., Turner-Brown et al., 2013), to our knowledge our study is the first to systematically test potential factors instigating downstream consequences in a community sample of infants at-risk for a later diagnosis of ASD.
4.2. Validation of the cascade model across populations of high-risk children
Our findings, in concert with those of Damiano et al. (2017) provide increased support for a theory of cascading effects—that is, specific sensory features (i.e., sensory seeking) manifesting in the latter half of the second year of life (i.e., at 18–24 months) may impact social symptomatology in the preschool years. Our study’s findings suggest that the developmental processes that may derail later social development appear to function similarly in both “infant sibs” and other at-risk infants in the general population. That is, the impact of sensory seeking features on social development over time appears to be at least partially mediated by social orienting abilities. We posit that susceptibilities in early sensory processing (e.g., sensory registration, multisensory integration) and/or “top-down” systems known to modulate sensory responses (e.g., attention mechanisms) may underlie the behavioral manifestation of sensory seeking behaviors evident by midway through the second year of life. Furthermore, these features may not only predispose children to challenges in engaging with their physical and social environments, but also alter their experiences and interactions, which are critical for learning and adaptive outcomes. Such “risk processes” may further amplify the neurological effects of early susceptibilities and shape the trajectory of social development in infants at-risk for ASD (Dawson, 2008). Understanding the timing of these derailments and specific mechanisms through which they occur is a critical need, as it has implications for intervention and prevention models.
Given the corroborating evidence that the effects of sensory seeking features on later social symptom severity are at least partially mediated by a child’s tendency to orient to social stimuli in his/her environment, we are becoming increasingly confident that reduced social orienting is a mechanism by which sensory seeking influences social outcomes in high-risk infants. The flip-side would suggest that improved social orienting ability may serve as a protective function for children with high levels of sensory seeking features. In slight contrast to Damiano et al. (2017), however, reduced social orienting did not fully account for the association between sensory seeking and future social impairment in our sample. We speculate that this partial versus complete mediation may be due to the longer period of time between assessments in the current study (i.e., from the second year of life through as far as five years of age). Over time, additional factors may contribute to important developmental transactions that effect the association of sensory seeking and social outcomes. For example, those children who tend to become overly engrossed in sensory experiences may not only be less likely to respond to bids from their social partners in the toddler years, but also less likely to initiate towards others or sustain social interactions with subsequent development.
4.3. Sensory seeking is still emerging and may be less useful as a predictor of social deficits at 13–15 months
In the current study, we extended the test of the cascade model to an earlier developmental time point, but found that sensory seeking features earlier in the second year of life were not predictive of future social symptomatology. This finding suggests that sensory seeking features at 13–15 months of age, as measured in this study, may be less sensitive and of limited clinical utility for long-range prediction of ASD-related symptoms. Exploratory analyses suggest one possible explanation for this null result is the instability of sensory seeking across the second year of life. Thus, sensory seeking features may be intensifying over the second year of life such that prognostic value of this construct reaches its peak and is clinically useful by 18–24 months of age, but not at 13–15 months of age. It is also possible that some sensory seeking features more common during infancy begin to diminish in the subgroup of infants that go on to have more typical developmental trajectories over the second year of life. Thus, some sensory seeking features may appear qualitatively easier to differentiate after 18 months of age for those infants who may later develop ASD or other developmental disorders.
4.4. Deficits in attention disengagement may precede and predict sensory seeking features
Studying the development of both sensory and social features early in life across different populations of children at high-risk for a later diagnosis of ASD is important to a) establish precedence of domain-specific deficits and b) unravel possible masking effects of neurological and behavioral compensations that may evolve later in life (Yirmiya et al., 2006). However, other more general developmental processes, supporting both sensory and social features, could be implicated even earlier in life. One such process is attention disengagement.
In addition to examining the predictive validity of early sensory seeking features for later social symptomatology, our post-hoc analyses explored the extent to which “sticky attention” may serve as a diathesis in the cascade theory presented earlier. Attention disengagement is a process that allows for flexibility of shifting attention to both social and non-social stimuli that are relevant for participation in everyday tasks and learning situations. It is considered to be an aspect of top-down attentional control that develops around 3–6 months of age and improves throughout early childhood, supported by cortical maturation and increasing functional connectivity across brain regions (Posner et al., 2014).
Past work had identified “sticky attention” as one of the earliest markers of ASD (Zwaigenbaum et al., 2005, Bryson et al., 2007, Elsabbagh et al., 2013, Elsabbagh et al., 2009), evident as early as 7–14 months in some studies. Problems with attention disengagement are hypothesized to lead to social impairment by interfering with social orienting (Mundy et al., 2009). Long latencies to disengage attention from central distractors have also been associated with higher sensory seeking features, particularly in multisensory conditions, in older children diagnosed with ASD (Sabatos-Devito et al., 2016).
Thus, we explored the extent to which an impairment in attention disengagement at 12–15 months could serve as a precursor of sensory seeking at 20–24 months, setting off a developmental cascade that ultimately leads to social symptomatology associated with ASD in the preschool period. The finding that reduced attention disengagement (measured at 12–15 months) predicted sensory seeking at 20–24 months, and translated to reduced social orienting, lends preliminary support to this notion. Neuroconstructivist accounts of development (Posner et al., 2014, Karmiloff-Smith, 2012) support the idea that the early microstructure of the brain is diffuse but becomes increasingly refined and specific over time through brain-behavior-environmental interactions. Thus, perturbations in basic neural processes (e.g., lack of synaptic pruning) early in development may differentially impact several developmental domains over time. For example, Karmiloff-Smith (2012) theorizes that some brain circuits may be “domain-relevant” by supporting more general functions such as visual pattern recognition early in infancy, but with repeated environmental interactions, these same circuits become more modular later in life, supporting specialized functions such as face perception. We reason that perhaps “domain-relevant” circuits supporting attention disengagement early in life are disrupted, which may later interfere with the specialization of functions for adaptive sensory responses or social interaction for children at risk for later ASD. Further research is needed to validate these hypotheses.
4.5. Clinical implications
Overall, these results point towards the potential clinical utility of different behavioral markers at different time points in children who are known to be at heightened risk for a future ASD diagnosis. Screening and intervention efforts that address attention disengagement more generally earlier in development, and that target sensory seeking features and/or deficits in social orienting more specifically relatively later in development, may facilitate children’s overall engagement with their physical and social environments and hold some promise in preventing or attenuating social impairment for those at especially high-risk for ASD. We recognize, though, that the application of these results in clinical practice is presently somewhat constrained by at least two factors. Additional research is needed, first to determine how we might best measure our constructs of interest in clinical practice, and second to determine how we might best intervene upon our constructs of interest in such young children. Theoretically, one may attempt to decrease the salience of (or perhaps even entirely remove, if necessary as a first step) the stimulus, object, or experience that is the central focus of a toddler’s “sticky attention” or sensory seeking features that may be precluding flexible social engagement. Alternatively, or perhaps simultaneously, one may attempt to increase the salience of peripheral targets or social bids and/or allow additional time to secure orienting from the toddler to ensure optimal engagement before increasing expectations for higher level learning. Recently, studies utilizing “parent responsiveness” strategies (e.g., Baranek et al., 2015) have begun to address a combination of sensory-regulatory functions as well as social-communication goals for infants at-risk for a later diagnosis of ASD, but efficacy studies are preliminary. Future research may examine the relative or combined effectiveness of different approaches.
4.6. Limitations and future directions
The current study has several limitations, including the use of extant data with a cohort that was enrolled in a treatment study. Although none of the effects that we reported significantly varied according to treatment group, it is nonetheless possible that participation in treatment could have influenced the relations of interest in this report in ways that we did not test or detect. Likewise, it is possible that shared method variance could have contributed to some of the associations we observed, as sensory seeking and social orienting were both measured using the SPA. Future studies may seek to tap these constructs using different measures (though we realize that psychometrically sound options for doing so at present are somewhat limited). Inclusion of a low-risk typically-developing control group would also be informative regarding for whom the observed relations hold.
Analyses conducted on associations with a key variable of interest—attention disengagement—will require replication in future studies. In particular, we note that these analyses were exploratory in nature. Furthermore, the AOSI and the FYI, although useful as composite measures of autism risk, were not specifically designed to tap individual differences in attention disengagement, as we have done in this study. We did aggregate scores from the AOSI and FYI in an attempt to increase the stability of the metric used in analyses (Sandbank and Yoder, 2014, Rushton et al., 1983). However, sampling of attention disengagement on each of these measure is somewhat limited. More extensive sampling may produce more stable, and thus potentially more construct valid, indices of sticky attention in future work.
Additionally, we were unable to establish temporal precedence for all variables in our mediation models, and thus are limited in our ability to draw conclusions regarding the likely direction of some associations. Moreover, correlational (albeit longitudinal) studies do not control for alternative explanations for associations that are observed. Future research is needed using well-controlled clinical trials to intervene directly on sensory seeking and/or difficulties with attention disengagement, while also measuring effects on future social orienting and future social symptomatology. Such designs would help to establish precedence of the constructs of interest and would increase our confidence that improvements in sensory or attention processes may ultimately translate to better social outcomes for infants at risk for a later diagnosis of ASD. The addition of biological markers and sensitive neurophysiological measures could provide more insights with respect to the mechanisms by which early differences in sensory responsiveness produce cascading developmental effects.
Conflict of Interest
None.
Acknowledgments
The research reported here was supported by a grant from the Institute of Education Sciences, U.S. Department of Education (R324A100305; PI: Watson) to the University of North Carolina at Chapel Hill. Data collection at follow-up was funded by a UNC-Chapel Hill Junior Faculty Development Award granted to L. Turner Brown and an Organization for Autism Research Graduate Student Research Grant awarded to S. Nowell. S. Nowell and M. DuBay are also supported by a doctoral training grant from the Department of Education, Office of Special Education Programs (H325D130041; PI: Crais). T. Woynaroski is additionally supported by CTSA award No. KL2TR000446 from the National Center for Advancing Translational Sciences and was provided with travel support for this collaboration through the Vanderbilt Kennedy Center (U54 HD083211; PI: Dykens). We thank The Ireland Family Foundation for their generous contribution to the PEARLS Advancement Fund. This study’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies. The authors declare no competing interests; there are no royalties obtained from any of the instruments used in this study. We also thank the participating families, as well as the staff and students who assisted with data collection.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.08.006.
Appendix A
Table A1.
Sample Characteristics (n = 55).
| Chronological Age in Months | |
| Mean (SD) age when FYI completed | 12 (0.19) |
| Range | 11–13 |
| Mean (SD) age at Time 1 | 13.69 (0.72) |
| Range | 13–15 |
| Mean (SD) age at Time 2 | 22.4 (0.81) |
| Range | 20–24 |
| Mean (SD) age at Time 3 | 53.7 (10.94) |
| Range | 35–70 |
| Mental Age in Months | |
| Mean (SD) mental age at Time 1 | 12.16 (2.18) |
| Range | 6.25–17.50 |
| DQ | |
| Mean (SD) DQ at Time 1 | 82.65 (14.52) |
| Range | 55–120 |
| Sex | |
| Boys | 36 (65%) |
| Race | |
| White | 44 (80%) |
| African-American | 6 (10.90%) |
| Mixed Race/Other | 5 (9.01%) |
Note: FYI = First Year Inventory (Baranek et al., 2003). DQ is the Early Learning Composite standard score from the Mullen Scales of Early Learning. Mental age is the average age equivalency from Visual Reception, Fine Motor, Receptive Language, and Expressive Language subscales of the Mullen Scales of Early Learning.
Table A2.
Regression Analyses for Indirect Effect of Sensory Seeking Measured Later in the Second Year of Life (i.e., 20–24 months) on Social Symptom Severity through Social Orienting.
| Model | Unstandardized Coefficients |
t | Significance | ||
|---|---|---|---|---|---|
| path | B | SE | |||
| Model 1 | Constant | 0.239 | 0.092 | 2.598 | 0.010 |
| c path | Seeking | 0.105 | 0.036 | 2.937 | 0.003** |
| Model 2 | Constant | 1.353 | 0.392 | 3.456 | 0.001 |
| a path | Seeking | 0.381 | 0.153 | 2.486 | 0.013* |
| Model 3 | Constant | 0.119 | 0.091 | 1.299 | 0.194 |
| b path | Orienting | 0.089 | 0.029 | 3.062 | 0.002** |
| c’ path | Seeking | 0.071 | 0.036 | 1.987 | 0.047* |
Note: Seeking = sensory seeking score, total number of the seven types of sensory seeking behaviors endorsed across the Sensory Processing Assessment (Baranek, 1999b) at the Time 2 (20–24 month) measurement period. Dependent variable for Models 1 and Model 3 is future social symptom severity (log10 transformed Autism Diagnostic Observation Schedule calibrated severity score for social affect). Dependent variable for Model 2 is social orienting (mean score across social orienting items) from the Sensory Processing Assessment (Baranek, 1999b) at Time 2 measurement period. Values reflect pooled results across multiply-imputed datasets. There was no evidence of undue influence on any analyses.
p value for effect of interest <0.05.
p value for effect of interest <0.005.
Table A3.
Regression Analyses for Indirect Effect of Sensory Seeking as Measured Earlier in the Second Year of Life (i.e., 13–15 months) on Social Symptom Severity through Social Orienting.
| Model | Unstandardized Coefficients |
T | Significance | ||
|---|---|---|---|---|---|
| path | B | SE | |||
| Model 1 | Constant | 0.532 | 0.085 | 6.266 | 0.000 |
| c path | Seeking | −0.014 | 0.031 | −0.456 | 0.648 |
| Model 2 | Constant | 2.199 | 0.376 | 5.847 | 0.000 |
| a path | Seeking | 0.033 | 0.137 | 0.241 | 0.810 |
| Model 3 | Constant | 0.292 | 0.097 | 3.019 | 0.003 |
| b path | Orienting | 0.109 | 0.028 | 3.885 | 0.000** |
| c’ path | Seeking | −0.018 | 0.027 | −0.651 | 0.515 |
Note: Seeking = sensory seeking score, total number of the seven types of sensory seeking behaviors endorsed across the Sensory Processing Assessment (Baranek, 1999b) at Time 1 (13–15 month) measurement period. Dependent variable for Models 1 and Model 3 is future social symptom severity (log10 transformed Autism Diagnostic Observation Schedule calibrated severity score for social affect). Dependent variable for Model 2 is social orienting (mean score across social orienting items) from the Sensory Processing Assessment (Baranek, 1999b) at Time 2 measurement period. Values reflect pooled results across multiply-imputed datasets. There was no evidence of undue influence on any analyses.
p value for effect of interest <0.005.
Table A4.
Regression Analyses for Indirect Effect of Attention Disengagement as Measured Earlier in the Second Year of Life (i.e., 12–15 months) on Social Orienting through Sensory Seeking.
| Model | Unstandardized Coefficients |
t | Significance | ||
|---|---|---|---|---|---|
| path | B | SE | |||
| Model 1 | Constant | 2.284 | 0.121 | 18.924 | 0.000 |
| c path | Disengagement | −0.013 | 0.183 | −0.071 | 0.943 |
| Model 2 | Constant | 2.445 | 0.099 | 24.720 | 0.000 |
| a path | Disengagement | 0.286 | 0.144 | 1.987 | 0.047* |
| Model 3 | Constant | 1.275 | 0.408 | 3.127 | 0.002 |
| b path | Seeking | 0.413 | 0.160 | 2.580 | 0.010* |
| c’ path | Disengagement | −0.131 | 0.180 | −0.728 | 0.467 |
Note: Disengagement = Attention disengagement aggregate score from Pretest/Time 1 (12–15 month) measurement period. Seeking = sensory seeking score, total number of the seven types of sensory seeking behaviors endorsed across the Sensory Processing Assessment (Baranek, 1999b) at Time 2 measurement period. Dependent variable for Models 1 and Model 3 is social orienting (mean score across social orienting items) from the Sensory Processing Assessment (Baranek, 1999b) at Time 2 measurement period. Dependent variable for Model 2 is sensory seeking score from Time 2 measurement period. Values reflect pooled results across multiply-imputed datasets. There was no evidence of undue influence on any analyses.
p value for effect of interest <0.05.
Appendix B. Supplementary data
The following are Supplementary data to this article:
References
- American Psychiatric Association . American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- Asendorpf J.B. Recommendations for increasing replicability in psychology. Eur. J. Pers. 2013;27(2):108–119. [Google Scholar]
- Ausderau K. National survey of sensory features in children with ASD: factor structure of the sensory experience questionnaire (3.0) J. Autism Dev. Disord. 2014;44(4):915–925. doi: 10.1007/s10803-013-1945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau K. Sensory subtypesand associated outcomes in children with autism spectrum disorders. Autism Res. 2016;9(12):1316–1327. doi: 10.1002/aur.1626. [DOI] [PubMed] [Google Scholar]
- Baranek G. University of North Carolina at Chapel Hill; Chapel Hill, NC: 2003. First Year Inventory (FYI) 2.0. [PubMed] [Google Scholar]
- Baranek G. Hyperresponsive sensory patterns in young children with autism: developmental delay, and typical development. Am. J. Ment. Retard. 2007;112(4):233–245. doi: 10.1352/0895-8017(2007)112[233:HSPIYC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baranek G. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism: children with developmental delays, and typically developing children. Dev. Psychopathol. 2013;25(2):307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek G. In: Sensory Features in Autism Spectrum Disorders, in Handbook of Autism and Pervasive Developmental Disorders. fourth edition. Volkmar F., editor. John Wiley & Sons, Inc.; Hoboken, JT, NJ: 2014. pp. 378–408. [Google Scholar]
- Baranek G. Preliminary efficacy of adapted responsive teaching for infants at risk of autism spectrum disorder in a community sample. Autism Res. Treat. 2015 doi: 10.1155/2015/386951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek G. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J. Autism Dev. Disord. 1999;29(3):213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baranek G. University of North Carolina at Chapel Hill; 1999. Sensory Processing Assessment for Young Children (SPA) (Unpublished manuscript) [Google Scholar]
- Baron-Cohen S. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Phil. Trans. R. Soc. B: Biol. Sci. 2009;364(1522):1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Carter A.S., Briggs-Gowan M.J. Sensory over-responsivity in elementary school: prevalence and social-emotional correlates. J. Abnorm. Child Psychol. 2009;37(5):705–716. doi: 10.1007/s10802-008-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstedt E., Carina Gillberg I., Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J. Child Psychol. Psychiatry. 2007;48(11):1102–1110. doi: 10.1111/j.1469-7610.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Brandwein A.B. Neurophysiological indices of atypical auditory processing and multisensory integration are associated with symptom severity in autism. J. Autism Dev. Disord. 2015;45(1):230–244. doi: 10.1007/s10803-014-2212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson S.E. A prospective case series of high-risk infants who developed autism. J. Autism Dev. Disord. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Bryson S.E. The autism observation scale for infants: scale development and reliability data. J. Autism Dev. Disord. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Cascio C.J. Toward an interdisciplinary approach to understanding sensory function in autism spectrum disorder. Autism Res. 2016;9(9):920–925. doi: 10.1002/aur.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J., Todd R. Genetic structure of reciprocal social behavior. Am. J. Psychiatry. 2000;157(12):2043–2045. doi: 10.1176/appi.ajp.157.12.2043. [DOI] [PubMed] [Google Scholar]
- Costanzoa V. Early detection of autism spectrum disorders: from retrospective home video studies to prospective ‘high risk’ sibling studies. Neurosci. Biobehav. Rev. 2015;55:627–635. doi: 10.1016/j.neubiorev.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Damiano-Goodwin C.R., Woynaroski T.G., Simon D.M., Ibañez L.V., Murias M., Kirby A., Newsom C.R., Wallace M.T., Stone W.L., Carissa J., Cascio C.J. Developmental sequelae and neurophysiologic substrates of sensory seeking in infant siblings of children with autism spectrum disorder. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.08.005. (same special issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. Case study of the development of an infant with autism from birth to two years of age. J. Appl. Dev. Psychol. 2000;21(3):299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention: brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 2008;20(3):775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M. Visual orienting in the early broader autism phenotype: disengagement and facilitation. J. Child Psychol. Psychiatry. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry. 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C. Analyzing longitudinal data with missing values. Rehabil. Psychol. 2011;56(4):267–288. doi: 10.1037/a0025579. [DOI] [PubMed] [Google Scholar]
- Freuler A. Precursors and trajectories of sensory features: qualitative analysis of infant home videos. Am. J. Occup. Ther. 2012;66(5):e81–e84. doi: 10.5014/ajot.2012.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani T. Brief report: assessment of early sensory processing in infants at high-risk of autism spectrum disorder. J. Autism Dev. Disord. 2014;44(12):3264–3270. doi: 10.1007/s10803-014-2175-x. [DOI] [PubMed] [Google Scholar]
- Goldsmith H.H. A population-based twin study of parentally reported tactile and auditory defensiveness in young children. J. Abnorm. Child Psychol. 2006;34(3):378–392. doi: 10.1007/s10802-006-9024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. Beyond Baron and Kenny statistical mediation analysis in the new millennium. Commun. Monogr. 2009;76(4):408–420. [Google Scholar]
- Hilton C., Graver K., LaVesser P. Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Res. Autism Spectr. Disord. 2007;1(2):164–173. [Google Scholar]
- Hus V., Gotham K., Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J. Autism Dev. Disord. 2014;44(10):2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Is development domain specific or domain general? A third alternative. In: Carver S., Shrager J., editors. The Journey from Child to Scientist: Integrating Cognitive Development and the Education Sciences. American Psychological Association; 2012. [Google Scholar]
- Kirby A.V. Observational characterization of sensory interests: repetitions, and seeking behaviors. Am. J. Occup. Ther. 2015;69(3) doi: 10.5014/ajot.2015.015081. 6903220010p1–6903220010p9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A.E. Sensory processing subtypes in autism: association with adaptive behavior. J. Autism Dev. Disord. 2010;40(1):112–122. doi: 10.1007/s10803-009-0840-2. [DOI] [PubMed] [Google Scholar]
- Liss M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lord C. 2nd edition (ADOS-2) Western Psychological Corporation; Los Angeles, CA: 2012. Autism Diagnostic Observation Schedule. [Google Scholar]
- MacKinnon D.P. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav. Res. Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell A. The role of physiological arousal in the management of challenging behaviours in individuals with autistic spectrum disorders. Res. Dev. Disabil. 2015;36:311–322. doi: 10.1016/j.ridd.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Mundy P., Sullivan L., Mastergeorge A.M. A parallel and distributed-processing model of joint attention: social cognition and autism. Autism Res. 2009;2(1):2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. J. Child Psychol. Psychiatry. 2015;56(9):988–998. doi: 10.1111/jcpp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Developing attention: behavioral and brain mechanisms. Adv. Neurosci. 2014;2014 doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick J.S. A parent-report instrument for identifying one-year-olds at risk for an eventual diagnosis of autism: the first year inventory. J. Autism Dev. Disord. 2007;37(9):1691–1710. doi: 10.1007/s10803-006-0303-y. [DOI] [PubMed] [Google Scholar]
- Rushton J., Brainerd C., Pressley M. Behavioral development and construct validity: the principle of aggregation. Psychol. Bull. 1983;94:18–38. [Google Scholar]
- Sabatos-Devito M. Eye tracking reveals impaired attentional disengagement associated with sensory response patterns in children with autism. J. Autism Dev. Disord. 2016;46(4):1319–1333. doi: 10.1007/s10803-015-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbank M., Yoder P.J. Topics in Early Childhood Special Education. 2014. Measuring representative communication in 3-year-olds with intellectual disabilities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B., Fidell L. 4th ed. Allyn and Bacon; Boston, MA: 2001. Using Multivariate Statistics. [Google Scholar]
- Talay-Ongan A., Wood K. Unusual sensory sensitivities in autism: a possible crossroads. Int. J. Disabil. Dev. Educ. 2000;47(2):201–212. [Google Scholar]
- Turner-Brown L.M. The first year inventory: a longitudinal follow-up of 12-month-old to 3-year-old children. Autism. 2013;17(5):527–540. doi: 10.1177/1362361312439633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarević M., Prior M.R., Leekam S.R. First evidence of sensory atypicality in mothers of children with Autism Spectrum Disorder (ASD) Mol. Autism. 2014;5(1):1–4. doi: 10.1186/2040-2392-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. J. Speech Lang. Hear. Res. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, L.R., Crais, E.R., Barnek, G.T., Turner-Brown, L., Sideris J., Wakeford, L., Kinard, J., Reznick, J.S., Martin, K.L., Nowell, S.W. Parent-mediated intervention for one-year-olds screen as at-risk for autism spectrum disorder: a randomized controlled trial. J. Autism Dev. Disord. (in press). [DOI] [PubMed]
- World Health Organization . 1993. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. [Google Scholar]
- Yirmiya N. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. J. Child Psychol. Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L. Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.