Abstract
Enantio- and diastereoselective conjunctive cross-coupling of β-substituted alkenylboron “ate” complexes is studied. While β substitution shifts the chemoselectivity of the catalytic reaction in favor of the Suzuki-Miyaura product, use of a boronic ester ligand derived from acenaphthoquinone allows the process to favor the conjunctive product, even with substituted substrates.
Graphical Abstract

Configurationally-defined benzhydryl stereocenters are important structural motifs that appear in a broad array of natural products and therapeutic agents.1 Accordingly, a variety of catalytic methods have been developed to target their construction.2 While recent advances in benzylic cross-coupling have provided important tools to target these features, an added synthetic challenge arises when benzylic stereocenters are sited adjacent to additional stereogenic centers. In these situations, multistep organic synthesis is often required for construction of the stereochemical dyad of interest.3 Our group has been developing a catalytic conjunctive cross-coupling reaction that converts vinylboron “ate” complexes and electrophiles to enantiomerically-enriched secondary or tertiary alkylboronic esters bearing a single stereocenter (eq. 1, Scheme 1).4 To address the problem of benzhydryl construction as outlined above, we questioned whether β-substituted alkenylboronic esters might engage in conjunctive cross-coupling and deliver compounds that bear vicinal stereogenic centers (eq. 2). In this report, we describe the development of this process and provide insight about how the structure of boron ligands can tip the reaction outcome in favor of the conjunctive coupling product or the classic Suzuki-Miyaura product.
Scheme 1.
Conjunctive Cross-Coupling of β-Substituted Alkenylboronates
Preliminary efforts to employ β-substituted alkenyl boronates in conjunctive cross-coupling reactions revealed a process that is dominated by direct Suzuki-Miyaura cross-coupling. For instance, in contrast to the conjunctive cross-coupling with the unsubstituted vinylB(neo)-derived “ate” complex 1a which occurs in 84% yield and 97:3 er (Scheme 1, eq. 3), when styrenylB(neo) was converted to the derived “ate” complex 1b and subjected to coupling with p-anisyltriflate, 1 mol% Pd(OAc)2 and 1.2 mol% Mandyphos (L), only 13% of the conjunctive coupling product 2b was obtained, with stilbene derivative 3b being the predominant product. The disparate reaction outcomes with 1a and 1b as substrate may be understood by consideration of competing catalytic reaction pathways. Mechanistic studies intimate that the metal-induced metallate rearrangement which underlies the conjunctive coupling occurs by metal-alkene binding (A, equation 1, box),4ffollowed by simultaneous RM migration and C-Pd bond formation at Cβ. In contrast, recent studies of transmetallation from organoboronic acid derivatives to bis(phosphine)Pd complexes suggests that the direct Suzuki-Miyaura reaction could arise by either (a) an open transition state originating from a palladium alkene complex where C-Pd bond formation occurs at Cα with concomitant rupture of the C-B bond (B, shaded box, Scheme 1)5, or (b) association of Pd with a boronic ester oxygen (C), which is then followed by transmetallation reaction through a closed four-centered transition state involving a five-coordinate Pd complex.6 For the reaction of substrate 1b, the added substitution at the β carbon of the alkene (R=H→Ph) would serve to inhibit conjunctive coupling as it requires Pd-C bond formation at a more hindered site. However, transmetallation through either B or C, ultimately leading to C-Pd bond formation at Cα, would be relatively unaltered by the addition of β-substituents on the alkenyl group. Thus, the net effect of alkene β-substitution is to disfavor the metallate shift relative to the direct transmetalation reaction and thereby alter the course of the reaction. Following this analysis, it was considered that alternate ligand sets on the boron atom that selectively interfere with either Pd(II)-oxygen binding or that hinder access to Cα might reestablish the metallate shift as a major reaction pathway.
To investigate the effect of boron ligands on the chemoselectivity of the coupling reaction in equation 2, we converted a series of styrenyl boronic esters to the derived “ate” complexes and subjected them to coupling with Pd(OAc)2 and MandyPhos ligand (Sp,Sp-L). As depicted in Table 1, with relatively unencumbered ligands on boron such as L1 (neopentyl glycol) or L2 (cis-1,2-cyclopentane diol) the Sukuzi-Miyaura product remains the predominant reaction product. However, when the boron center is more encumbered such as with L3 (pinacol) and L4, the proportion of conjunctive cross-coupling (C3) product was increased, consistent with the notion that hindered access to either the oxygen atom or Cα may retard the rate of the direct transmetalation reaction relative to metallate shift. It can also be noted that chemoselectivity with boron centers bearing even more highly encumbered ligands (L5, L6) begins to suffer, an outcome we consider might be due to hindered olefin binding. Lastly, support for the notion that the oxygen atom may be involved in the direct transmetalation process is provided by the reaction with hydrocarbon-based ligand L7 (9-BBN) wherein the metallate shift-based path operates to the near exclusion of the Suzuki-Miyaura reaction.
Table 1.
Effect of Boron Ligand on Chemoselectivity in Conjunctive Coupling Reactions.a
 | |||||
|---|---|---|---|---|---|
| entry | BL2 | C3:SM | C3 yield | dr | er |
| 1 | L1 (neo) | 1:5.8 | 13 (10) | >20:1 | 99:1 |
| 2 | L2 | 1:>20 | <5 | nd | nd |
| 3 | L3 (pin) | 1:2 | 35(30) | >20:1 | 98:2 |
| 4 | L4 | 1.7:1 | 56(46) | >20:1 | 99:1 |
| 5 | L5 | 1:2 | 30 | >20:1 | nd |
| 6 | L6 | 1:3 | 20 | >20:1 | nd |
| 7 | L7 (9-BBN) | >20:1 | 92(75) | >20:1 | 67:33 |
| 8 | L8 (mac) | 2.5:1 | 75(70) | >20:1 | 99:1 |
| 9b | L8 (mac) | 4.2:1 | 83(76) | >20:1 | 99:1 |

Yields are by 1H NMR versus an internal standard, yield in parentheses is after isolation. For entry 7, yield is of the derived alcohol; for the others, yield is of the boronic ester. Enantiomer ratio of derived alcohol was determined by SFC analysis on a chiral stationary phase and in comparison to authentic enantiomer mixture. Diastereomer ratio determined by analysis of the 1H NMR spectrum.
Reaction at 40 °C and with 1 equiv CsF.
Considering the observations in entries 1–6 of Table 1, we sought ligands that are both readily accessible and also effectively shield the oxygen atom and/or Cα without blocking access to the alkene Cβ. As shown in entry 8, ligand L8 (termed “mac”), readily prepared by methylation of acenaphthoquinone (vide infra), appears to satisfy these requirements, delivering the conjunctive product in good yield and enantioselectivity. Of note, the reaction chemoselectivity could be enhanced by conducting the reaction at 40 °C and in the presence of CsF; under these conditions the conjunctive coupling product is formed in 76% isolated yield, >20:1 dr, and with 99:1 enantioselectivity.
Diol 4 is readily available from acenaphthoquinone by a single-step diastereoselective (4.3:1 dr) carbonyl addition reaction employing trimethylaluminum in toluene,7 followed by crystallization of the diol from ethyl acetate solvent. Conversion of boronic acids to derived B(mac) esters is readily accomplished by esterification in the presence of catalytic FeCl3.8 In contrast to common boronic ester ligands (i.e. pinacol, neopentylglycol), when the B(mac) derivatives are converted to the four coordinate “ate” complexes, the issue of stereoisomerism arises and it was considered that this feature might pose a complication for selective and efficient catalytic coupling reactions. To study the properties of B(mac) “ate” complexes, the addition of phenyllithium to n-butylB(mac) in THF-d8 at room temperature was analyzed by 1H NMR analysis. As shown in Scheme 2b, the kinetic addition product appeared to arise from addition of the nucleophile cis relative to the vicinal methyl groups forming trans-6 in a 5:1 ratio.9 Of note, stirring the reaction for 30 minutes at 60 °C resulted in a 2:1 diastereomer ratio, and after 12 hours an equilibrium 1:1 ratio of the isomers was observed. Importantly, when reaction partners are reversed such that of n-BuLi was added to PhB(mac), an inverted initial kinetic ratio (ca. 1:12.5) results, but the same thermodynamic ratio is achieved upon heating (see Supporting Information for details). These experiments suggest that diastereomeric “ate” complexes should be interconverting during the course of reaction and that the configuration of these species will likely be inconsequential to conjunctive couplings.
Scheme 2.
Methylated Acenaphthoquinone (mac) as a Ligand for Boronic Esters.
With ready access to B(mac)-derived substrates and an understanding of their properties, these compounds were examined in conjunctive coupling reactions. As depicted in Table 2, the reaction can operate with both electron-rich and electron-poor electrophiles and, after oxidative work-up, provides the derived secondary alcohols with high diastereoselectivity (>20:1 dr) and enantioselectivity (generally above 98:2 er.). While substrates with heteroatom-containing functional groups do not pose an inherent problem, diminished yield was observed with electron-deficient electrophiles, an outcome that arises from competitive Suzuki-Miyaura coupling reactions (in general for Table 2, the remaining mass balance is comprised of Suzuki-Miyaura products). Fortunately, even with these substrates the stereoinduction remains high. It should also be noted that a substrate with a hindered migrating alkenyl group, reacted with anomalously low enantioselectivity, an observation that has been made in other systems.4a,c Examination of both aryl triflate and aryl bromide electrophiles showed that both electrophiles could be employed to furnish to conjunctive product; however, bromide electrophiles required the addition of KOTf to ameliorate the inhibitory activity of bromide salts4b and provided diminished product yields relative to their triflate counterparts. The reaction was also found to operate with several different migrating groups, with electron-rich migrating groups giving higher yields than those that are electron-poor. Surprisingly, under the current conditions, “ate” complexes containing alkyl migrating groups did not engage in the conjunctive coupling pathway and provided direct Suzuki-Miyaura coupling products instead. With respect to the substituent at the β-carbon of the alkenyl boronate, substrates containing electron-rich and electron-deficient arenes, as well as those containing alkyl groups were found to participate in the reaction. While an electron-rich β-substituent appeared to increase the conversion of the reaction, it also resulted in more competition from the Suzuki-Miyuara pathway (23; 1:1 conjunctive:Suzuki). Substrates with electron-deficient β-substituents furnished less Suzuki-Miyaura reaction, but required higher temperatures for the conjunctive process to proceed (24, 4:1 conjunctive:Suzuki). Lastly, while substrates with β-alkyl substitution (16-22) gave only modest yields, they reacted with outstanding selectivity and furnished products that would be otherwise difficult to access with single-step catalytic processes.
Table 2.
Pd/MandyPhos-Catalyzed Conjunctive Coupling of β-Substituted B(mac)-Derived “Ate” Complexes.a

|
Yields are isolated yields of purified material and represent and average yield of two different experiments. Enantiomer ratios was determined by SFC analysis on a chiral stationary phase and are in comparison to authentic racemic materials. Diastereoselectivity determined by analysis of the 1H NMR spectrum. For reactions of organobromide electrophiles, an equivalent of KOTf was added.
Reaction at 60 °C.
Reaction conducted with 2% Pd and 2.2% L1.
Although B(mac)-derived products are stable to column chromatography, they are poorly soluble in many organic solvents such that for the purposes of Table 2, it was most convenient to oxidize the coupling products and isolate the corresponding alcohols. To learn about other transformations of the B(mac) derivatives, a larger scale coupling reaction was undertaken and the reaction product examined. As shown in Scheme 3, a gram-scale coupling provided secondary boronic ester 26 with reaction efficiency and selectivity comparable to smaller scale reactions in Table 2. Consistent with the observations in Table 2, purified intermediate 26 underwent oxidation efficiently, providing alcohol 2b in outstanding yield and selectivity. Also noteworthy, is that direct amination10/Boc protection of 26 furnished carbamate 27 in excellent yield and as a single diastereomer. Lastly, it was found that B(mac)-derivate 26 underwent efficient modified Matteson homologation11 to furnish primary B(mac) derivative 28 as a single diastereomer.
Scheme 3.
Synthetic Transformations of AlkylB(mac) Derivatives.
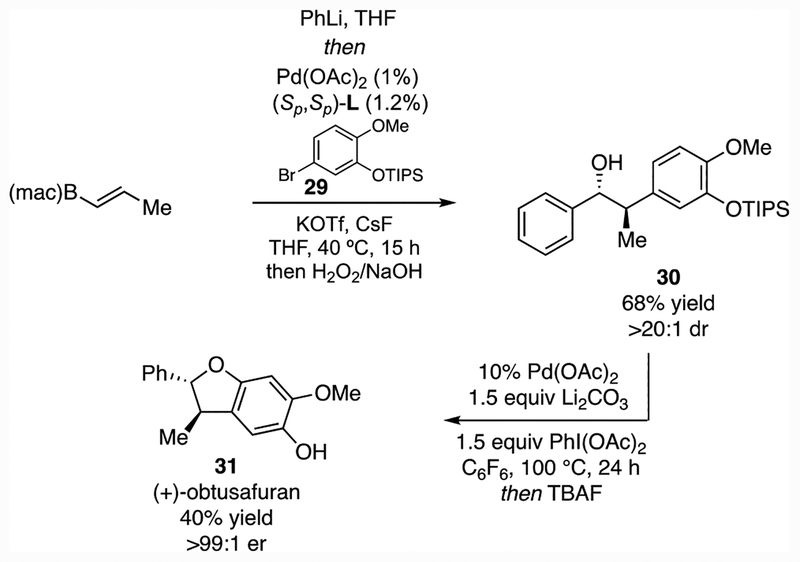
The diastereoselective conjunctive coupling establishes strategically-useful functional group arrays in ways that are not straightforward to access by contemporary asymmetric synthesis. This feature is illustrated by the application of the conjunctive coupling to the synthesis of obtusafuran12 (Scheme 4). While this target was previously prepared by a convenient enantioselective ketone reduction and cyclization, construction of the requisite ketone starting material required five steps of chemical synthesis.13 Employing trans-2-propenylB(mac) in a conjunctive coupling with PhLi and aryl bromide 29, furnished 30 in excellent yield and stereoselection. Subsequent oxidative cyclization14 and deprotection provided the target in just three synthesis steps (two reaction vessels) from simple starting materials.
Scheme 4.
Construction of (+)-Obtusafuran by Conjunctive Cross-Coupling.
In summary, we have established a catalytic, diastereo-, and enantioselective conjunctive coupling of β-substituted alkenylboronic esters. This process employs an encumbered diolato ligand to control the reaction of alkenylboron “ate” complexes, tipping the reaction in favor of a metallate shift-based pathway rather than direct transmetalation. Further studies on the mechanistic origin of chemoselectivity with B(mac) derived “ate” complexes will be reported in due course.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the NIH (R35-GM1217140). We thanks Solvias for a generous donation of MandyPhos ligands.
Footnotes
Supporting Information. Procedures, characterization and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Ameen D; Snape TJ, Chiral 1,1-Diaryl Compounds as Important Pharmacophores. Med. Chem. Commun 2013, 4, 893. [Google Scholar]
- (2).For a review: see:Mondal S; Roy D; Panda G, Overview on the Recent Strategies for the Enantioselective Synthesis of 1, 1‐Diarylalkanes, Triarylmethanes and Related Molecules Containing the Diarylmethine Stereocenter. ChemCatChem 2018, 10, 1941.For selected catalytic reactions, see:Imao D; Glasspoole BW; Laberge VS; Crudden CM, Cross Coupling Reactions of Chiral Secondary Organoboronic Esters With Retention of Configuration. J. Am. Chem. Soc 2009, 131, 5024.Zhou Q; Srinivas HD; Dasgupta S; Watson MP, Nickel-Catalyzed Cross-Couplings of Benzylic Pivalates with Arylboroxines: Stereospecific Formation of Diarylalkanes and Triarylmethanes. J. Am. Chem. Soc 2013, 135, 3307.Harris MR; Hanna LE; Greene MA; Moore CE; Jarvo ER, Retention or Inversion in Stereospecific Nickel-Catalyzed Cross-Coupling of Benzylic Carbamates with Arylboronic Esters: Control of Absolute Stereochemistry with an Achiral Catalyst. J. Am. Chem. Soc 2013, 135, 3303.Lewis CA; Gustafson JL; Chiu A; Balsells J; Pollard D; Murry J; Reamer RA; Hansen KB; Miller SJ, A Case of Remote Asymmetric Induction in the Peptide-Catalyzed Desymmetrization of a Bis(phenol). J. Am. Chem. Soc 2008, 130, 16358.Gao F; Lee Y; Mandai K; Hoveyda AH, Quaternary Carbon Stereogenic Centers through Copper‐Catalyzed Enantioselective Allylic Substitutions with Readily Accessible Aryl‐ or Heteroaryllithium Reagents and Aluminum Chlorides. Angew. Chem. Int. Ed 2010, 49, 8370.
- (3).Examples: Tapentadol:Zhang Q; Li J-F; Tian G-H; Zhang R-X; Sun J; Suo J; Feng X; Fang D; Jiang X-R; Shen J-S, A Practical and Enantioselective Synthesis of Tapentadol. Tetrahedron Asymm. 2012, 23, 577.Taranabant:Wallace DJ; Campos KR; Shultz CS; Klapars A; Zewge D; Crump BR; Phenix BD; McWilliams JC; Krska S; Sun Y; Chen CY; Spindler F, New Efficient Asymmetric Synthesis of Taranabant, a CB1R Inverse Agonist for the Treatment of Obesity. Org. Process Res. Dev 2009, 13, 84.Mixanpril:Turcaud S; Gonzalez W; Michel J; Roques BP; Fournie-Zaluski C, Diastereoselective Synthesis of Mixanpril, an Orally Active Dual Inhibitor of Neutral Endopeptidase and Angiotensin Converting Enzyme. Bioorg. Med. Chem. Lett 1995, 5, 1893.Paroxetine:Zhang Y; Liao YT; Liu XH; Yao Q; Zhou YH; Lin LL; Feng XM, Catalytic Michael/Ring‐Closure Reaction of α,β‐Unsaturated Pyrazoleamides with Amidomalonates: Asymmetric Synthesis of (−)‐Paroxetine. Chem. Eur. J 2016, 22, 15119.
- (4).Zhang L; Lovinger GJ; Edelstein EK; Szymaniak AA; Chierchia MP; Morken JP, Catalytic Conjunctive Cross-Coupling Enabled by Metal-Induced Metallate Rearrangement. Science, 2016, 351, 70.Lovinger GJ; Aparece MD; Morken JP, Pd-Catalyzed Conjunctive Cross-Coupling between Grignard-Derived Boron “Ate” Complexes and C(sp2) Halides or Triflates: NaOTf as a Grignard Activator and Halide Scavenger. J. Am. Chem. Soc 2017, 139, 3153.Edelstein EK; Namirembe S; Morken JP, Enantioselective Conjunctive Cross-Coupling of Bis(alkenyl)borates: A General Synthesis of Chiral Allylboron Reagents. J. Am. Chem. Soc 2017, 139, 5027.Chierchia MP; Law C; Morken JP, Nickel‐Catalyzed Enantioselective Conjunctive Cross‐Coupling of 9‐BBN Borates. Angew. Chem. Int. Ed 2017, 56, 11870.Lovinger GJ; Morken JP, Ni-Catalyzed Enantioselective Conjunctive Coupling with C(sp3) Electrophiles: A Radical-Ionic Mechanistic Dichotomy. J. Am. Chem. Soc 2017, 139, 17293.Myhill JA; Zhang L; Lovinger GA; Morken JP, Enantioselective Construction of Tertiary Boronic Esters by Conjunctive Cross‐Coupling. Angew. Chem. Int. Ed 2018, 57, 12799.For recent aligned studies on electrophile-induced metallate rearrangement, see the following citations and references cited therein:Wilson CM; Ganesh V; Noble A; Aggarwal VK, Enantiospecific sp2–sp3 Coupling of ortho- and para-Phenols with Secondaryand Tertiary Boronic Esters. Angew. Chem. Int. Ed 2017, 56, 16318.Ganesh V; Odachowski M; Aggarwal VK, Alkynyl Moiety for Triggering 1,2-Metallate Shifts: Enantiospecific sp2-sp3 Coupling of Boronic Esters with p-Arylacetylenes. Angew. Chem. Int. Ed, 2017, 56, 9752.Wang Y; Noble A; Sandford C; Aggarwal VK, Enantiospecific Trifluoromethyl-Radical-Induced Three-Component Coupling of Boronic Esters with Furans. Angew. Chem. Int. Ed, 2017, 56, 1810.Panda S; Ready JM, Tandem Allylation/1,2-Boronate Rearrangement for the Asymmetric Synthesis of Indolines with Adjacent Quaternary Stereocenters. J. Am. Chem. Soc, 2018, 140, 13242.
- (5).Ortuño MA; Lledós A; Maseras F; Ujaque G, The Transmetalation Process in Suzuki–Miyaura Reactions: Calculations Indicate Lower Barrier via Boronate Intermediate. ChemCatChem 2014, 6, 3132. [Google Scholar]
- (6).Huang Y-L, Weng C-M, and Hong F-E, Density Functional Studies on Palladium‐Catalyzed Suzuki–Miyaura Cross‐Coupling Reactions Assisted by N‐ or P‐Chelating Ligands. Chem. Eur. J 2008, 14, 4426.(b) Note that studies by Denmark suggest transmetalation of diphosphine complexes related to B may occur by dissociation of a phosphine in order to access a transition state involving a fourcoordinate Pd-complex, see:Thomas AA; Wang H; Zahrt AF; Denmark SE, Structural, Kinetic, and Computational Characterization of the Elusive Arylpalladium(II)boronate Complexes in the Suzuki–Miyaura Reaction. J. Am. Chem. Soc 2017, 139, 3805.With monophosphine Pd complexes, transmetalation through B-O-Pd bonded four-coordinate structures appears to predominate, see:Thomas AA; Zahrt AF; Delaney CP; Denmark SE, Elucidating the Role of the Boronic Esters in the Suzuki–Miyaura Reaction: Structural, Kinetic, and Computational Investigations. J. Am. Chem. Soc 2018, 140, 4401.Thomas AA; Denmark SE, Pre-transmetalation Intermediates in the Suzuki-Miyaura Reaction Revealed: The Missing Link. Science 2016, 352, 329.Carrow BP; Hartwig JF, Distinguishing Between Pathways for Transmetalation in Suzuki−Miyaura Reactions. J. Am. Chem. Soc 2011, 133, 2116.Amatore C; Jutand A; Le Duc G, Kinetic Data for the Transmetalation/Reductive Elimination in Palladium‐Catalyzed Suzuki–Miyaura Reactions: Unexpected Triple Role of Hydroxide Ions Used as Base. Chem. Eur. J 2011, 17, 2492.Matos K; Soderquist JA, Alkylboranes in the Suzuki−Miyaura Coupling: Stereochemical and Mechanistic Studies. J. Org. Chem 1998, 63, 461.
- (7).Baidossi W; Rosenfeld A; Wassermann BC; Schutte S; Schumann H; Blum J, [(3-Dimethylamino)propyl]dimethylaluminum: A Convenient Reagent for Methylation and Ethynylation of Carbonyl Compounds. Synthesis 1996, 9, 1127. [Google Scholar]
- (8).Wood JL; Marciasini LD; Vaultier M; Pucheault M, Iron Catalysis and Water: A Synergy for Refunctionalization of Boron. Synlett 2014, 25, 0551. [Google Scholar]
- (9).The configuration of the “ate” complexes was secured by both NOE and chemical shift analysis. See Supporting Information for details.
- (10).Mlynarski SN; Karns AS; Morken JP, Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates. J. Am. Chem. Soc 2012, 134, 16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sonawane RP; Jheengut V; Rabalakos C; Larouche-Gauthier R; Scott HK; Aggarwal VK, Enantioselective Construction of Quaternary Stereogenic Centers from Tertiary Boronic Esters: Methodology and Applications. Angew. Chem. Int. Ed 2011, 50, 3760. [DOI] [PubMed] [Google Scholar]
- (12).Gregson M; Ollis WD; Redman BT; Sutherland IO; Dietrichs HH; Gottlieb OR, Obtusastyrene and Obtustyrene, Cinnamylphenols from Dalbergia Retusa. Phytochemistry 1978, 17, 1395. [Google Scholar]
- (13).Chen C; Weisel M, Concise Asymmetric Synthesis of (+)-Conocarpan and Obtusafuran. Synlett 2013, 24, 189. [Google Scholar]
- (14).(a) Wang X; Lu Y; Dai H-X; Yu J-Q, Pd(II)-Catalyzed Hydroxyl-Directed C−H Activation/C−O Cyclization: Expedient Construction of Dihydrobenzofurans. J. Am. Chem. Soc, 2010, 132, 12203. [DOI] [PubMed] [Google Scholar]; (b) Wang H; Li G; Engle KM; Yu J-Q; Davies HML, Sequential C–H Functionalization Reactions for the Enantioselective Synthesis of Highly Functionalized 2,3-Dihydrobenzofurans. J. Am. Chem. Soc, 2013, 135, 6774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.