Abstract
We evaluated inosine monophosphate dehydrogenase (IMPDH) 1 and IMPDH2 pharmacogenetics in 247 recipient-donor pairs after nonmyeloablative hematopoietic cell transplant (HCT) recipients. Patients were conditioned with total body irradiation + fludarabine, received grafts from related or unrelated donors (10% HLA mismatch), with post-graft immunosuppression of mycophenolate mofetil (MMF) with a calcineurin inhibitor. Recipient and donor IMPDH genotype (rs11706052, rs2278294, rs2278293) were not associated with day 28 T-cell chimerism, acute graft versus host disease, disease relapse, cytomegalovirus reactivation, non-relapse mortality, or overall survival. Recipient IMPDH1 rs2278293 genotype was associated with a lower incidence of chronic graft-versus-host disease (GVHD) (hazard ratio of 0.72, p=0.008) in nonmyeloablative HCT recipients. Additional studies are needed to confirm these results with the goal of identifying predictive biomarkers to MMF that lower GVHD.
Keywords: hematopoietic cell transplant, pharmacogenomics, IMPDH, MPA, precision medicine, graft versus host disease
INTRODUCTION
Post-grafting immunosuppression for allogeneic hematopoietic cell transplant (HCT) recipients often consists of the combination of a calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF).1, 2 The development of lower-dose, nonmyeloablative conditioning increased the availability of this curative procedure to patients who could not tolerate the toxicity of high-dose conditioning regimens due to age or comorbidity.3 Nonmyeloablative HCT relies on achieving a delicate balance between recipient and donor cells, with the goal of ensuring sufficient immunosuppression of the recipient to maximize graft-versus-tumor effect but minimize graft-versus-host disease (GVHD).
MMF was administered every 12 hours (Q12h) in early studies in nonmyeloablative HCT recipients. Recipients of related donor grafts had acceptable engraftment rates with Q12h MMF. Patients receiving unrelated donor grafts experienced graft rejection, with improved engraftment rates when the daily MMF dose was increased by shortening the administration interval to every 8 hours (Q8h).1, 4 Since reliable engraftment was achieved, efforts have been ongoing to separate the graft-versus-tumor effect from GVHD. Examples of such efforts include examining the association of day +28 T-cell chimerism or neutrophil nadirs within the first three weeks post-HCT with relapse rates (i.e., graft-versus-tumor effect) and GVHD.5–7
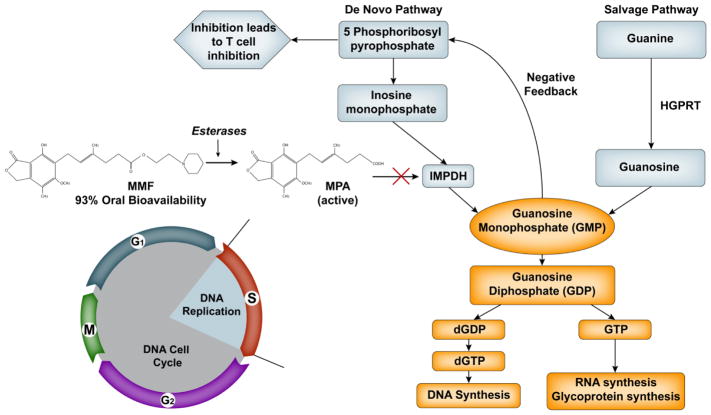
MMF is metabolized into mycophenolic acid (MPA) in the gastrointestinal tract. MPA inhibits both T and B lymphocytes by inhibiting inosine monophosphate dehydrogenase (IMPDH) type 1 and 2, which are important in de novo guanosine nucleotide synthesis (Figure 1). IMPDH catalyzes the oxidation of inosine 5′-monophosphate to xanthosine 5′-monophosphate (XMP) by a nicotinamide adenine dinucleotide positive-dependent reaction.8, 9 Decreased synthesis of guanosine monophosphate leads to a negative feedback inhibition of 5-phosphoribosyl-1-pyrophosphate and prevents T cell activation. Weight-based dosing of MMF is most commonly used; however this approach may result in substantive variability in the plasma exposure of its active metabolite MPA.10, 11 MPA inhibits both type 1 and 2 IMDPH, and polymorphisms in their genes might explain part of the inter-individual variability in clinical outcomes of HCT patients receiving MMF.12
Figure 1.
Schematic of mycophenolic acid’s inhibition of MPDH.
We13 and others14, 15 previously reported that low total MPA plasma exposure was associated with more severe acute GVHD in HCT recipients. In HCT patients receiving nonmyeloablative conditioning followed by an unrelated donor graft, we found that low total MPA exposure (i.e., area under the curve divided by the dosing frequency) was associated with increased grades III to IV acute graft-versus-host disease and increased nonrelapse mortality but not with day 28 T cell chimerism, chronic GVHD disease relapse, cytomegalovirus (CMV) reactivation, or overall survival (Table 4 of McDermott et al.13).
Seeking to further understand this variability in clinical outcomes, we anticipated taking a candidate gene approach towards those genes influencing protein expression relevant to MPA pharmacokinetics. However, when we built a population pharmacokinetic model of MPA after oral MMF administration, a high amount of interoccasion (i.e., within-patient) variability was observed in the absorption rate constant of MMF (CV% = 49.3%).16 This high within-patient variability suggested that non-genetic factors predominantly affect MPA absorption and plasma exposure. Thus, we chose not to evaluate pharmacokinetic-based candidate genes and instead evaluated genetic polymorphisms in IMPDH1 and IMPDH2, the genes that encode their corresponding proteins that are inhibited by MPA. We evaluated eight single nucleotide polymorphisms (SNPs) in both the recipient and donor. The IMPDH1 SNPs were rs2278293, rs2278294, a 9bp insertion in the P3 promotor in-93 to -83, -76T>C, -69A>G, -66A>G. The IMPDH2 SNPs were L263F (all were GG) and rs11706052. Recipients were in our previously published cohort of patients13 conditioned with total body irradiation and fludarabine, with grafts from human leukocyte antigens (HLA)-matched related or unrelated donors who received post-grafting immunosuppression with MMF and a CNI.
MATERIALS AND METHODS
Patient characteristics and treatment plan
We retrospectively evaluated IMPDH1 and IMPDH2 pharmacogenetics in 247 recipient-donor pairs of which the recipients (i.e. patients) received nonmyeloablative HCT with GCSF mobilized peripheral blood mononuclear cell (G-PBMC) grafts to treat a variety of hematological malignancies between March 1998 and July 2006 at the Fred Hutchinson Cancer Research Center, Seattle, WA. Given the greater risk of graft rejection among bone marrow recipients compared with G-PBMC recipients receiving Q12h MMF,1 patients in the original cohort who received bone marrow as the source of stem cells were excluded. Of the 308 patients in which we characterized the association of MPA plasma exposure with clinical outcomes,13 both donor and recipient DNA were available in 247 recipient-donor pairs. Written informed consent was obtained from all patients prior to participation in prospective treatment protocols. The Institutional Review Board at the Fred Hutchinson Cancer Research Center approved all study protocols, including this retrospective analysis, and an independent Data Safety Monitoring Board monitored safety in all prospective studies. Patient characteristics are summarized in Table 1.
Table 1.
Patients’ Characteristics a
| All patients | |
|---|---|
| Total number | 247 |
| Sex, female/male (% female) | 96/151 (39%) |
| Recipients’ ages yearb | 56 (9 – 74) |
| Recipients’ age < 21 year | 5 (2%) |
| CMV seropositive recipients | 156 (63%) |
| Female donor to male recipient | 61 (25%) |
| Donors’ age, year | 41 (17–76) |
| HLA-matched graft | 223 (90%) |
| HLA-mismatched graft | 24 (10%) |
| Year of transplant | |
| 1998 | 5 (2%) |
| 1999 | 15 (6%) |
| 2000 | 42 (17%) |
| 2001 | 31 (13%) |
| 2002 | 36 (15%) |
| 2003 | 23 (9%) |
| 2004 | 34 (14%) |
| 2005 | 34 (14%) |
| 2006 | 27 (11%) |
| Conditioning regimen | |
| 2 Gy TBI | 23 (9%) |
| 2 Gy TBI + auto | 16 (6%) |
| 2 Gy TBI + FLU 90mg/m2 | 183 (74%) |
| 2 Gy TBI + FLU 90mg/m2+ auto | 18 (7%) |
| 2 Gy TBI + FLU 90mg/m2+ imatinib | 3 (1%) |
| 3 Gy TBI + FLU 90mg/m2 | 4 (2%) |
| Post-grafting immunosuppressionb | |
| MMF Q8h | 117 (47%) |
| MMF Q12h | 130 (53%) |
| Cyclosporine + MMF | 197 (80%) |
| Tacrolimus + MMF | 50 (20%) |
number (percent) or median (range);
Abbreviations: autologous (auto); cytomegalovirus (CMV), fludarabine monophosphate (FLU), human leukocyte antigen (HLA), mycophenolate mofetil (MMF), every eight hour dosing (Q8h); every twelve hour dosing (Q12h); total body irradiation (TBI).
The conditioning regimen comprised a single fraction of 200 to 300 cGy total body irradiation (TBI) on day 0 with or without fludarabine (30 mg/m2/day intravenously) from day –4 to day –2 (cumulative dose 90 mg/m2).1 In general, the post-graft CNI was either cyclosporine or tacrolimus given through day +177. MMF was given at two different dose frequencies, either 15 mg/kg Q8h or Q12h. Adjusted ideal body weight16 was used to determine MMF dosing, and all doses were rounded to the nearest 250mg. MMF doses were not adjusted based IMPDH1 or IMPDH2 polymorphisms. Patients were asked to take MMF at the same time daily. MMF treatment started on day 0 and, in general, continued until day 27 (related donor) or day 40 (unrelated donor) at which time the MMF dose was reduced by 10% per week in the absence of GVHD. The majority of donor grafts were matched for HLA-A, -B, -C, DRB1 at high resolution DNA typing and DQB1 by intermediate-resolution techniques; twelve patients had a 2 allele or antigen mismatch. The median follow-up among patients at the time of last contact was 3.15 years (range, 0.05 – 18.08 years).
Pharmacogenetic analysis
Peripheral blood leukocytes were collected from freshly obtained samples and stored at the Fred Hutch DNA repository. Genomic DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) from peripheral blood leukocytes. The quality of DNA samples was tested by spectrophotometric analysis at 260 and 280 nm before genotyping.
IMPDH1 and IMPDH2 genotypes, chosen based on the candidate gene approach, were determined. Genotyping for the IMPDH1 variant IVS7+125G>A (rs2278293, Assay ID: C_15965182_10), IMPDH1 variant IVS8-106G>A (rs2278294, Assay ID: C_2830834_20) and IMPDH2 variant 3757T>C (rs11706052, Assay ID: C_1842928_10) was done using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA). The reaction mixture consisted of 4 ng of genomic DNA, 20 X Taqman SNP genotyping assay mix, and 2 X Genotyping mastermix in a total volume of 5 μL. Real-time PCR was done on the 7900HT system using standard Taqman cycling conditions. Allelic discrimination analysis was carried out using the Sequence Detection System, Version 2.3 (Applied Biosystems, Foster City, CA).
IMPDH1 P3 promoter variations as previously described17 were analyzed by sequencing. Briefly, the primary PCR reaction consisted of 40 ng genomic DNA, 2.0 mM MgCl2; 200 uM each of dATP, dCTP, dTTP; 100uM of dGTP; 100uM of 7-deaza-2′-dGTP; 200 nM of primers; 6% DMSO; 2% Formamide; and 1U of Amplitaq Gold DNA polymerase (Applied Biosystems, Foster City, CA) in a total volume of 10 μL. Touchdown PCR on a PE 9700 Thermocycler was as follows: one cycle for 15 min at 94°C; 15 cycles of 94°C for 30s, 78°C to 64 °C for 30s (decrease 1°C each cycle), 72°C for 45s; followed by 20 cycles of 94°C for 30s, 64°C for 30s, 72°C for 45s; and a final extension step of one cycle for 5 mins at 72°C. Following this, samples were cleaned up using ExoSAP-IT (Thermofisher Scientific) and sequencing reaction was done with standard Big Dye Ready Reaction (Applied Biosystems) prior to loading on an ABI 3130xl (Applied Biosystems) capillary electrophoresis system.
The IMPDH2 L264F variant as previously described18 was genotyped by RFLP analysis. The PCR reaction with 40ng genomic DNA, 5.0 mM MgCl2, 200uM nucleotides, 200nM primers (forward primer 5′ TCCAAAGATGCCAAGAAACA 3′ and reverse primer 5′ TCACCAAAACCACTACATCCA 3′), and 0.5U of Amplitaq Gold DNA polymerase was run on a PE 9700 Thermocycler as follows: one cycle for 5 mins at 94°C; 35 cycles of 94°C for 30s, 58°C for 45 s, 72°C for 45 s; and a final extension of one cycle for 5 mins at 72°C. The resulting 115 bp product was digested with Cac8I (New England BioLabs, Beverly, MA) and the fragments generated were analyzed by gel electrophoresis and ethidium bromide staining. The uncut 115 bp fragment was indicative of the variant ‘T’ allele while fragments of 80 bp and 35 bp indicated the presence of the wild type ‘C’ allele.
All methods were validated using a panel of 96 Utah Centre d’Étude du Polymorphisme Humain (Paris, France) DNA samples. The genotype and allele frequencies were consistent with reported results; all duplicates were concordant and all controls gave the expected genotype. In our samples, there was no significant deviation from the Hardy-Weinberg equilibrium for the genotyped SNPs.
Clinical outcomes
Day 28 donor T-cell chimerism was the primary endpoint of interest with the secondary endpoints being toxicity to MMF (i.e., neutropenia post-HCT, CMV reactivation), efficacy of MMF (i.e., chimerism and graft rejection), and HCT outcomes (i.e., acute GVHD, chronic GVHD, relapse). The assessment of clinical outcomes was described previously.13
In brief, neutropenia post-HCT was assessed only through day 28, because multiple potential confounding variables (e.g., viral infection or reactivation such as CMV requiring ganciclovir therapy, corticosteroid therapy) could affect the neutrophil count after day 28. Neutropenia was evaluated by examining daily complete blood counts with differential and assessment of absolute neutrophil count (ANC). CMV reactivation was also evaluated, as it represents a significant consequence of immunosuppressed status. CMV serological status was assessed in each patient and donor prior to HCT. All patients underwent weekly testing to detect the CMV pp65 antigen for the first three months following HCT.
The percentage of donor CD3+ T-cells present in the patients’ peripheral blood samples were assessed on days 28, 56, and 84 after HCT. Flow cytometry was used to sort CD3+ cells and chimerism was measured using fluorescence in situ hybridization and polymerase chain reaction of polymorphic microsatellite regions for sex-mismatched and sex-matched grafts, respectively.19 If donor CD3+ cells were less than or equal to 5% at any of the assessed time points after HCT, then the patient was noted to have graft rejection.
Acute GVHD and chronic GVHD were graded according to established criteria.20–22 We defined disease relapse or disease progression as disease recurrence following complete remission or progression of persistent disease.
Statistical analysis
Cox regression analysis was used to evaluate the association of patient and donor genotypes with time-to-event endpoints. Logistic regression was used to evaluate association with binary endpoints (day 28 donor T-cell chimerism < 50% and ANC nadir < median). All of the analyses used an additive (allelic) genetic association model. Death was treated as a competing risk for analysis of acute GVHD and chronic GVHD, non-relapse mortality (NRM), and CMV reactivation. Relapse was treated as a competing risk for the analysis of NRM. CMV reactivation was analyzed only among patients where either patient or donor was CMV seropositive at the time of transplant. Patients dying before day 35 were excluded from analysis of day 28 donor T-cell chimerism. Cumulative incidence of chronic GVHD was estimated by standard methods. All reported p-values are two-sided. Due to multiple comparisons, we report only the single association significant at the 0.007 level. All statistical analyses were performed using SAS version 9 (SAS, Inc., Cary, NC).
RESULTS
Pharmacogenetic results
The IMPDH1 and IMPDH2 genotypes evaluated were previously reported to be associated with clinical outcomes in either HCT patients or renal transplant recipients.23, 24 In our population, five of the variants were25, 26 all homozygous wild-type. Specifically, IMPDH1 -93 to -83 insertion (thus no patients had an insertion), IMPDH1-76T>C (all were TT), IMPDH1-69A>G (all were AA), IMPDH1-66A>G (all were AA), and IMPDH2 L263F (all were CC). Within the 247 recipient-donor pairs, IMPDH1 rs2278293 had 31.8% GG, 49.8% GA, 18.4% AA, IMPDH1 rs2278294 had 42.9% GG, 45.5% GA, 10.5% AA and IMPDH2 rs11706052 had 82.0% AA, 17.6% AG, 0.4% GG.
Association of recipient or donor genetic polymorphisms with clinical outcomes
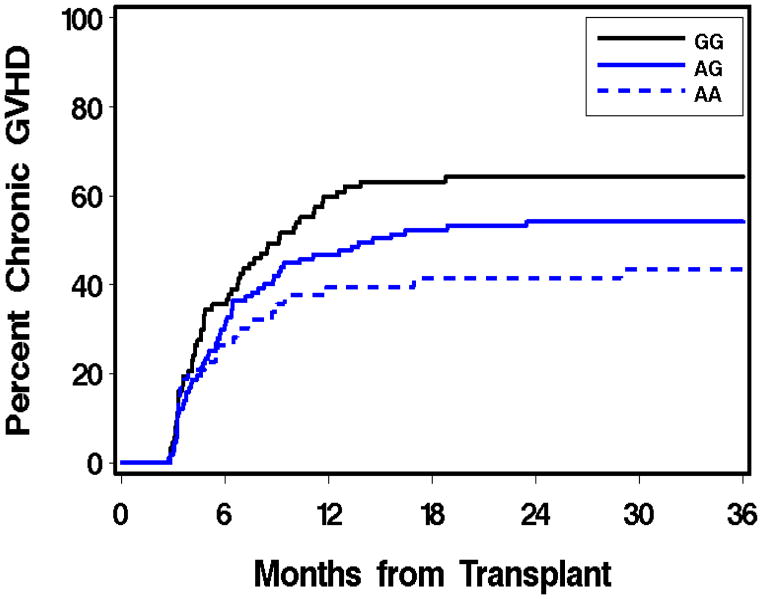
Table 2 summarizes the association of IMPDH1 or IMPDH2 genetic polymorphisms with clinical outcomes. Eight of the 247 patients experienced rejection, all of whom received MMF with cyclosporine. Two rejections occurred in related donor graft recipients, one of whom was conditioned with TBI only. The remaining six patients who rejected their grafts received HLA-matched unrelated grafts after fludarabine/TBI conditioning. Of these six patients, five received Q12h MMF and one received Q8h MMF. The association of rejection with IMPDH1 or IMPDH2 polymorphisms was not evaluated because of the low number (eight) of rejections. Other studies in the setting of nonmyeloablative HCT have found that low donor T-cell chimerism levels are predictive of graft rejection.1, 5, 25 Considering this, we evaluated whether IMPDH1 or IMPDH2 polymorphisms was associated with the subsequent degree of day 28 donor T-cell chimerism. In the 29 patients with day 28 donor T-cell chimerism of 50% or lower, it was not associated with IMPDH1 or IMPDH2 polymorphisms. Similarly, there was no statistically significant association between IMPDH genotype and grades 2–4 acute GVHD, nor between IMPDH genotype and grades 3–4 acute GVHD. However, IMPDH1 rs2278293 was associated with chronic GVHD (p=0.008), with heterozygous (GA) and homozygous (AA) variants having a lower risk of chronic GVHD (Figure 2). We found no association between the IMPDH polymorphisms evaluated and relapse, NRM or overall mortality.
Table 2.
Effect of IMPDH1 and IMPDH2 on clinical outcomes
| Clinical outcome | N events | Recipient IMPDH2 rs11706052 | Recipient IMPDH1 rs2278294 | Recipient IMPDH1 rs2278293 | Donor IMPDH2 rs11706052 | Donor IMPDH1 rs2278294 | Donor IMPDH1 rs2278293 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HRa 95% CI |
P | HR 95% CI |
P | HR 95% CI |
P | HR 95% CI |
P | HR 95% CI |
P | HR 95% CI |
P | ||
| Day 28 donor T-cell < 50%b | 29 | 1.99 0.9–4.3 |
0.08 | 0.92 0.5–1.6 |
0.77 | 1.05 0.6–1.8 |
0.86 | 0.80 0.3–2.4 |
0.70 | 1.14 0.6–2.1 |
0.67 | 0.89 0.5–1.6 |
0.70 |
| Acute GVHD 2–4c | 156 | 1.28 0.9–1.9 |
0.19 | 1.03 0.8–1.3 |
0.80 | 0.94 0.8–1.2 |
0.58 | 0.72 0.5–1.1 |
0.16 | 1.27 1.0–1.6 |
0.05 | 1.21 0.9–1.5 |
0.13 |
| Acute GVHD 3–4c | 42 | 1.97 1.1–3.7 |
0.03 | 0.75 0.5–1.2 |
0.22 | 0.81 0.5–1.2 |
0.31 | 0.94 0.4–2.1 |
0.87 | 1.54 1.0–2.4 |
0.07 | 1.46 0.9–2.3 |
0.11 |
| Chronic GVHD | 163 | 0.85 0.6–1.3 |
0.45 | 0.85 0.7–1.1 |
0.21 |
0.72 0.6–0.9 |
0.008 | 1.13 0.7–1.8 |
0.59 | 1.03 0.8–1.3 |
0.85 | 1.12 0.9–1.5 |
0.41 |
| Relapse/Progression | 106 | 1.18 0.8–1.9 |
0.47 | 1.01 0.8–1.3 |
0.94 | 1.14 0.9–1.5 |
0.33 | 0.73 0.4–1.3 |
0.29 | 0.99 0.7–1.4 |
0.93 | 1.02 0.8–1.4 |
0.89 |
| ANC nadir < 220/μl | 120 | 1.17 0.7–2.1 |
0.61 | 1.10 0.8–1.6 |
0.62 | 0.90 0.6–1.3 |
0.55 | 1.01 0.5–2.0 |
0.99 | 1.58 1.0–2.4 |
0.03 | 1.29 0.9–1.9 |
0.20 |
| CMV reactivationd | 121 | 0.96 0.7–1.4 |
0.83 | 0.97 0.8–1.2 |
0.83 | 0.83 0.7–1.0 |
0.08 | 0.80 0.5–1.2 |
0.28 | 1.21 0.9–1.5 |
0.12 | 1.03 0.8–1.3 |
0.84 |
| Non-relapse mortality | 73 | 1.12 0.7–1.9 |
0.67 | 0.87 0.6–1.2 |
0.40 | 0.95 0.7–1.3 |
0.72 | 1.06 0.6–1.9 |
0.85 | 1.35 0.9–1.9 |
0.09 | 1.19 0.8–1.7 |
0.33 |
| Overall mortality (OS) | 172 | 1.23 0.9–1.7 |
0.25 | 0.92 0.7–1.1 |
0.46 | 0.99 0.8–1.2 |
0.93 | 0.95 0.6–1.4 |
0.79 | 1.19 0.9–1.5 |
0.16 | 1.15 0.9–1.5 |
0.24 |
Hazard ratio or odds ratio is per copy of minor allele (additive model); Odds ratio is reported for the binary variables of day 28 donor T-cell chimerism <50% and ANC nadir <220/μL;
Day 28 donor T-cell analysis excludes patients dying before day 35;
More acute GVHD events were observed here than in our MPA pharmacokinetic/dynamic analysis13 because the latter dataset included GVHD events that occurred on or after day 25 which was after the MPA pharmacokinetic data was evaluated.
CMV reactivation excludes donor-negative/recipient-negative pairs.
Figure 2.
Recipient’s genotype of IMPDH1 rs2278293 by chronic GVHD (p=0.008).
DISCUSSION
In this analysis, we evaluated IMPDH1 and IMPDH2 pharmacogenetics in 247 recipient-donor pairs in consecutive HCT recipients who were given nonmyeloablative conditioning before receiving allogeneic grafts to treat hematological malignancies. Chronic GVHD was associated with the recipient’s IMPDH1 rs2278293 (HR=0.72; p=0.008), with recipients who are heterozygous (GA) and homozygous (AA) variants having a lower risk of chronic GVHD (Figure 2). To our knowledge, this is amongst the largest IMPDH pharmacogenetic analyses in HCT patients to date (Table 2). We have conducted various analyses seeking to identify risk factors for chronic GVHD in nonmyeloablative HCT patients. In this patient population, chronic GVHD was not associated donor age,28 cyclosporine duration,29 cyclosporine trough concentrations,30 mycophenolate dosing frequency (i.e., Q8h dosing did not have a different chronic GVHD rate than Q12h dosing4), total mycophenolic acid plasma exposure,12 free MPA plasma exposure,12 and recipient pretransplant IMPDH activity in peripheral blood mononuclear cells (PMNCs).31 Although donor relation, HLA-mismatch, and female donor/male recipient sex mismatch are associated with chronic GVHD, adjustment for these factors did not alter the association with rs2278293 genotype. Patient characteristics according to rs2278293 genotype are provided in Supplemental Table 1.
We13 and others14, 15 previously reported that low MPA plasma exposure was associated with more severe acute GVHD in HCT recipients. We also found that low total MPA plasma exposure also predicted high NRM, potentially due to a higher risk of severe acute GVHD, but not chronic GVHD. We initially planned to conduct a pharmacogenomic study of clinical outcomes with genes regulating that encode for drug metabolizing enzymes or transporters involved in MPA pharmacokinetics. However, our population pharmacokinetic analysis revealed a high amount of interoccasion (i.e., within-patient) variability was observed in the absorption rate constant of MMF (coefficient of variation % = 49.3%).16 Concomitant cyclosporine and serum albumin were significant covariates, with higher MPA clearance in patients receiving concomitant cyclosporine and having a low serum albumin. We subsequently conducted an in-depth evaluation of potential drug interactions (PDI) affecting MPA pharmacokinetics in nonmyeloablative HCT patients.26 Of the 187 concomitant medications, 11 (5.9%) had a PDI affecting MPA pharmacokinetics with the most common PDI, in descending order, being cyclosporine, omeprazole and pantoprazole. Cyclosporine is often used as post-graft immunosuppression with MPA. It inhibits ABCC2, thereby impairing enteroheptic recirculation by inhibiting MPA reabsorption.27 Proton pump inhibitors (PPIs) potently inhibit gastric acid secretion, subsequently increasing the gastric pH. Higher gastric pH is expected to decrease MPA absorption by decreasing the release and hydrolysis of MMF.28 The MPA – PPI drug interaction literature has provided mixed results (Supplemental Table 2). This high within-patient variability, revealed from our population pharmacokinetic modeling, suggested that non-genetic factors predominantly affect MPA plasma exposure. We therefore changed our approach to evaluate genetic polymorphisms in IMPDH1 and IMPDH2, which are the target enzymes of MPA.
We used the candidate gene approach to identify the IMPDH1 and IMPDH2 SNPs of interest. We found that IMPDH1 rs2278293 was associated with chronic GVHD; however, this is an intronic (noncoding) SNP so the putative mechanism of this pharmacogenomic association is not known. In kidney transplant recipients, rs2278293 was associated with the incidence of biopsy-proven acute rejection in the first year after transplantation23 and with subclinical acute rejection.29 IMPDH polymorphisms are associated with various clinical outcomes in renal transplant recipients treated with MMF. These cohorts were often larger (n=8229 to 96930) than our cohort of allogeneic HCT recipients (n=247). In 24031 consecutive pairs of allogeneic HCT recipients and their donors, Cao et al. evaluated the association of clinical outcomes with four IMPDH1 SNPs (i.e., IVS7 +125 G>A, IVS8-106 G>A, exon15 1572 G>A, and 5′ flanking intron-exon region C>T).31 They observed that a higher incidence of acute GVHD was associated with the presence of the IMPDH1 IVS8-106 G/G (rs2278294) genotype than other genotypes. Multivariate analysis confirmed that recipients with the IVS8-106 G/G genotype were at significantly higher risk of developing acute GVHD (relative risk [RR] = 2.018, P = .001) and grades 2–4 acute GVHD (RR = 2.232, P = .002). IVS8-106 (rs2278294) was not associated with acute GVHD in our cohort, potentially because of differences in the patient populations. Our cohort (n=247) received nonmyeloablative conditioning with related or unrelated donor grafts (10% HLA mismatch) with post-graft immunosuppression of MMF with a CNI. The cohort of Cao et al. (n=240) predominantly received myeloablative conditioning with related or unrelated donor grafts (17% HLA mismatch) and post-graft immunosuppression MMF (day 1–100) with cyclosporine and methotrexate.
Further IMPDH pharmacogenomic studies should be conducted in homogenous HCT populations to validate or refute these findings. Such studies should be paired with evaluating IMPDH activity before HCT and then during MMF administration. In a separate cohort of 86 nonmyeloablative HCT recipients, we found that low recipient pretransplant IMPDH activity was associated with increased day +28 T-cell chimerism, more acute GVHD, lower neutrophil nadirs, and more CMV reactivation but not with chronic GVHD, relapse, NRM, or overall mortality.32 With the weight-based dosing of MMF, we also observed substantial interpatient variability in the inhibition of IMPDH activity in pPMNCs. IMPDH decreased with increasing MPA plasma concentration, with maximum inhibition coinciding with maximum MPA concentration. The day +21 IMPDH area under the effect curve (AUEC) was associated with cytomegalovirus reactivation, NRM, and overall mortality.
In conclusion, we found that IMPDH1 rs2278293 genotype was associated with a lower incidence of chronic GVHD in nonmyeloablative HCT recipients. Additional studies are needed to confirm these results with the goal of identifying predictive biomarkers to MMF that lower GVHD, improve NRM, and increase overall survival.
Supplementary Material
Supplemental Table 1. Patient characteristics according to rs22780293 genotypea
Supplemental Table 2. Overview of potential drug interaction between proton pump inhibitors affecting MPA pharmacokinetics. Adapted from Jaklic et al.26
Highlights.
Mycophenolate mofetil (MMF) inhibits IMPDH 1 and 2
MMF response associated with IMPDH1 and 2 genotype in other patient populations
Recipient IMPDH1 rs2278293 genotype associated with chronic graft-vs-host-disease
No recipient or donor genotypes associated with other clinical outcomes
Acknowledgments
The authors are very grateful to the patients who participated in this study. In addition, the authors wish to thank the following staff for their invaluable help in making this work possible: Karen W. Maker, Gary H. Schoch, Meagan J. Bemer, and Isa Mambetsariev. The authors also wish to thank all physicians, nurses, and support personnel for their care of patients on this study.
Financial Support: Supported by grants from the National Institutes of Health (HL91744, HL036444, CA18029, CA 78902, AI33484 and HL087690).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 2.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Mineishi S. Overcoming the age barrier in hematopoietic stem cell transplantation: progress, but still a long way to go. Jama. 2011;306:1918–1920. doi: 10.1001/jama.2011.1612. [DOI] [PubMed] [Google Scholar]
- 4.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 6.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006 Oct;20:1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Gyurkocza B, Storer BE, et al. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biol Blood Marrow Transplant. 2013;19:792–798. doi: 10.1016/j.bbmt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laverdiére I, Caron P, Couture F, Guillemette C, Lévesque Er. Liquid Chromatography–Coupled Tandem Mass Spectrometry Based Assay to Evaluate Inosine-5′-monophosphate Dehydrogenase Activity in Peripheral Blood Mononuclear Cells from Stem Cell Transplant Recipients. Analytical chemistry. 2011;84:216–223. doi: 10.1021/ac202404y. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Porcher R, Fernandes J, et al. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia. 2009;23:545–556. doi: 10.1038/leu.2008.323. [DOI] [PubMed] [Google Scholar]
- 10.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet. 2007;46:13–58. doi: 10.2165/00003088-200746010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson A, Holler E. Polymorphisms of cytokine and innate immunity genes and GVHD. Best Practice & Research Clinical Haematology. 2008;21:149–164. doi: 10.1016/j.beha.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.van Gelder T, Hesselink DA, van Hest RM, Mathôt RA, van Schaik R. Pharmacogenetics in immunosuppressive therapy: the best thing since TDM? Therapeutic drug monitoring. 2004;26:343–346. doi: 10.1097/00007691-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 13.McDermott CL, Sandmaier BM, Storer B, et al. Nonrelapse Mortality and Mycophenolic Acid Exposure in Nonmyeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19:1159–1166. doi: 10.1016/j.bbmt.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenke A, Renner U, Richte M, et al. Pharmacokinetics of intravenous mycophenolate mofetil after allogeneic blood stem cell transplantation. Clin Transplant. 2001;15:176–184. doi: 10.1034/j.1399-0012.2001.150306.x. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia M, Militano O, Jin Z, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol. 2013;53:393–402. doi: 10.1002/jcph.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RL, Gearry RB, Barclay M, Kennedy M. IMPDH1 promoter mutations in a patient exhibiting azathioprine resistance. Pharmacogenomics J. 2007;7:312–317. doi: 10.1038/sj.tpj.6500421. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zeevi A, Webber S, et al. A novel variant L263F in human inosine 5′-monophosphate dehydrogenase 2 is associated with diminished enzyme activity. Pharmacogenetics and genomics. 2007;17:283–290. doi: 10.1097/FPC.0b013e328012b8cf. [DOI] [PubMed] [Google Scholar]
- 19.Bryant E, Martin PJ. Documentation of Engraftment and Characterization of Chimerism Following Hematopoietic Cell Transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. 2. Malden, MA: Blackwell Science, Inc; 1999. pp. 197–206. [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versushost disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Yang JW, Zeevi A, et al. IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther. 2008;83:711–717. doi: 10.1038/sj.clpt.6100347. [DOI] [PubMed] [Google Scholar]
- 24.Sombogaard F, van Schaik RH, Mathot RA, et al. Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenetics and genomics. 2009;19:626–634. doi: 10.1097/FPC.0b013e32832f5f1b. [DOI] [PubMed] [Google Scholar]
- 25.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 26.Jaklic A, Collins CJ, Mrhar A, et al. High prevalence of potential drug interactions affecting mycophenolic acid pharmacokinetics in nonmyeloablative hematopoietic stem cell transplant recipients. Int J Clin Pharmacol Ther. 2013;51:711–717. doi: 10.5414/CP201884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2010;16:421–429. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kofler S, Shvets N, Bigdeli AK, et al. Proton pump inhibitors reduce mycophenolate exposure in heart transplant recipients-a prospective case-controlled study. Am J Transplant. 2009;9:1650–1656. doi: 10.1111/j.1600-6143.2009.02682.x. [DOI] [PubMed] [Google Scholar]
- 29.Kagaya H, Miura M, Saito M, Habuchi T, Satoh S. Correlation of IMPDH1 gene polymorphisms with subclinical acute rejection and mycophenolic acid exposure parameters on day 28 after renal transplantation. Basic & clinical pharmacology & toxicology. 2010;107:631–636. doi: 10.1111/j.1742-7843.2010.00542.x. [DOI] [PubMed] [Google Scholar]
- 30.Oetting WS, Schladt DP, Leduc RE, et al. Validation of single nucleotide polymorphisms associated with acute rejection in kidney transplant recipients using a large multi-center cohort. Transpl Int. 2011;24:1231–1238. doi: 10.1111/j.1432-2277.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao W, Xiao H, Lai X, et al. Genetic variations in the mycophenolate mofetil target enzyme are associated with acute GVHD risk after related and unrelated hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:273–279. doi: 10.1016/j.bbmt.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Bemer MJ, Risler LJ, Phillips BR, et al. Recipient Pretransplant Inosine Monophosphate Dehydrogenase Activity in Nonmyeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2014;20:1544–1552. doi: 10.1016/j.bbmt.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hest RM, Mathot RA, Pescovitz MD, Gordon R, Mamelok RD, van Gelder T. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17:871–880. doi: 10.1681/ASN.2005101070. [DOI] [PubMed] [Google Scholar]
- 34.Kees MG, Steinke T, Moritz S, et al. Omeprazole impairs the absorption of mycophenolate mofetil but not of enteric-coated mycophenolate sodium in healthy volunteers. J Clin Pharmacol. 2012;52:1265–1272. doi: 10.1177/0091270011412968. [DOI] [PubMed] [Google Scholar]
- 35.Schaier M, Scholl C, Scharpf D, et al. Proton pump inhibitors interfere with the immunosuppressive potency of mycophenolate mofetil. Rheumatology. 2010;49:2061–2067. doi: 10.1093/rheumatology/keq238. [DOI] [PubMed] [Google Scholar]
- 36.Rissling O, Glander P, Hambach P, et al. No relevant pharmacokinetic interaction between pantoprazole and mycophenolate in renal transplant patients: a randomized crossover study. Br J Clin Pharmacol. 2015;80:1086–1096. doi: 10.1111/bcp.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura M, Inoue K, Kagaya H, et al. Influence of rabeprazole and lansoprazole on the pharmacokinetics of tacrolimus in relation to CYP2C19, CYP3A5 and MDR1 polymorphisms in renal transplant recipients. Biopharm Drug Dispos. 2007;28:167–175. doi: 10.1002/bdd.544. [DOI] [PubMed] [Google Scholar]
- 38.Ciftci HS, Karadeniz MS, Tefik T, et al. Influence of Proton Pump Inhibitors on Mycophenolic Acid Pharmacokinetics in Patients With Renal Transplantation and the Relationship With Cytochrome 2C19 Gene Polymorphism. Transplant Proc. 2017;49:490–496. doi: 10.1016/j.transproceed.2017.01.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Patient characteristics according to rs22780293 genotypea
Supplemental Table 2. Overview of potential drug interaction between proton pump inhibitors affecting MPA pharmacokinetics. Adapted from Jaklic et al.26