Abstract
Background:
Syphilis is resurgent in many developed countries and still prevalent in developing nations. Current and future control campaigns would benefit from the development of a vaccine, but although promising vaccine candidates were identified among the putative surface-exposed integral outer membrane proteins of the syphilis spirochete, immunization experiments in the rabbit model using recombinant antigens have failed to fully protect animals upon infectious challenge. We speculated that such recombinant immunogens, purified under denaturing conditions from Escherichia coli prior to immunization might not necessarily harbor their original structure, and hypothesized that enhanced protection would result from performing similar immunization/challenge experiments with native antigens.
Methods:
To test our hypothesis, we engineered non-infectious Borrelia burgdorferi strains to express the tp0897 (tprK) and tp0435 genes of Treponema pallidum subsp. pallidum and immunized two groups of rabbits by injecting recombinant strains intramuscularly with no adjuvant. TprK is a putative integral outer membrane protein of the syphilis agent, while tp0435 encodes the highly immunogenic T. pallidum 17-kDa lipoprotein, a periplasmic antigen that was also shown on the pathogen surface. Following development of a specific host immune response to these antigens as the result of immunization, animals were challenged by intradermal inoculation of T. pallidum. Cutaneous lesion development was monitored and treponemal burden within lesions were assessed by dark-field microscopy and RT-qPCR, in comparison to control rabbits.
Results:
Partial protection was observed in rabbits immunized with B. burgdorferi expressing TprK while immunity to Tp0435 was not protective. Analysis of the humoral response to TprK antigen suggested reactivity to conformational epitopes.
Conclusions:
Immunization with native antigens might not be sufficient to obtain complete protection to infection. Nonetheless we showed that non-infectious B. burgdorferi can be an effective carrier to deliver and elicit a specific host response to T. pallidum antigens to assess the efficacy of syphilis vaccine candidates.
INTRODUCTION
After years of steady decline, syphilis has become resurgent in many high income countries such as the United States, Europe, Canada, and China [1–6], and continues to be endemic in developing countries, where the majority of cases occur. WHO estimates suggest that the global syphilis burden might be as high as 36 million cases in adults, with an 0incidence of 11 million new cases occurring every year [7]. Globally, about 1.4 million pregnant women become infected every year and approximately 305,000 experience fetal loss or stillbirth due to vertical transmission of the syphilis spirochete, Treponema pallidum subsp. pallidum (T. pallidum) [8], while 215,000 infants are born prematurely and/or with clinical signs of infection [9, 10]. Evidence that symptomatic syphilis increases 2–5 times the likelihood of HIV transmission or acquisition further makes this infection a serious global health problem [11]. The development of an effective syphilis vaccine could aid public health control initiatives to stem syphilis spread [10, 12].
During infection, T. pallidum clearance from early cutaneous lesions is mediated by phagocytosis of opsonized treponemes by macrophages [13]. Hence, T. pallidum surface-exposed antigens are regarded as the most promising vaccine candidates. Because of the inherent fragility of T. pallidum outer membrane (OM), and the paucity of surface-exposed integral proteins this compartment, the experimental identification of these rare outer membrane proteins (OMPs) has been difficult [14, 15]. Progress towards this goal has been also hindered by the inability, until very recently, to culture this pathogen in vitro [16]. Nonetheless, over the past few years, several vaccine candidates have been proposed thanks to advances in computational methods to predict T. pallidum OMPs and the application of functional/biophysical assays to confirm such predictions [17, 18].
Among these putative OMPs, members of the T. pallidum repeat (Tpr) family of paralogs, such as TprK (encoded by the tp0897 gene in the reference Nichols strain genome), have emerged as promising candidates in vaccine development studies [19–21]. Although TprK is antigenically variable and known to play a role in immune evasion [22–24], the structural model of the protein [18] predicts three conserved surface-exposed loops in the TprK NH2-terminus [18, 25]. In response to immunization of rabbits with a recombinant NH2-terminal fragment of TprK purified under denaturing conditions together with the Ribi adjuvant, cutaneous lesion development and progression to ulceration was attenuated, and treponemal burden at challenge sites was significantly reduced compared to controls [19, 21]. Given the elevated degree of protection seen in these early experiments using a single immunogen that did not bear their native structure, we hypothesized that the use of properly folded antigens could induce antibodies to conformational epitopes absent in a denatured antigen and enhance protection.
Whole bacterial vaccine can be more effective than recombinant antigen-based vaccines [26]. In fact, only one study, performed by Miller et al. in the early 70’s [27] found that complete protection from T. pallidum infection could be achieved in rabbits by injecting them intravenously ~60 times with γ-irradiated T. pallidum over a 37-week period. This strategy is not tenable in humans but suggests the importance of maintaining vaccine candidates in a native conformation. Testing of live attenuated vaccines against various bacterial diseases has become common [28–30]. In addition, attenuated bacterial pathogens have been used for expression of heterologous antigens to induce protection against unrelated diseases [31, 32]. We decided to use a similar approach to present T. pallidum antigens in their native conformation within a spirochete membrane compartment similar to that of the syphilis pathogen. This heterologous antigen presentation system employs the B314/B31HP strains of Borrelia burgdorferi (B. burgdorferi) engineered to express T. pallidum genes. These strains have lost endogenous plasmids which encode virulence factors and are therefore non-infectious. Using this system, we reported that a limited amount of the T. pallidum Tp0435 lipoprotein, previously believed to be exclusively periplasmic, could also gain surface-exposure in both B. burgdorferi strains and then proceeded to confirm this finding in T. pallidum [33]. Such evidence led us to hypothesize that Tp0435 could also be a possible target for opsonic antibodies and that immunity to Tp0435 could improve treponemal clearance. Here, we evaluated live attenuated B. burgdorferi strains expressing Tp0435 or TprK as a novel syphilis vaccine design by; a) assessing whether immunity to these T. pallidum antigens could be induced in rabbits injected intramuscularly with such strains in absence of an adjuvant, and b) by assessing whether such immunity would protect against syphilis.
MATERIALS AND METHODS
Ethics statement
New Zealand White rabbits were used for treponemal propagation, immunizations, and immunization/challenge experiments. Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals and all experimental procedures were conducted under a protocol approved by the University of Washington IACUC (Protocol #4243.01; PI: Lorenzo Giacani). The designated members of Rutgers University New Jersey Medical School IACUC reviewed and approved the protocol under which mouse experiments were carried out (Protocol #D-14011; PI: Nikhat Parveen).
T. pallidum propagation for inoculum preparation and nucleic acid extraction
Only rabbits seronegative for both FTA-ABS and VDRL tests were used. The T. pallidum Nichols strain was propagated in New Zealand white rabbits by means of intratesticular (IT) inoculation as previously described [34]. Extraction of treponemal DNA was also performed as previously reported [22]. The Nichols strain was selected for this study because it possesses a nearly invariant tprK gene, unlike other isolates [35].
Generation of transgenic B. burgdorferi and strain propagation.
Two high passage non-infectious B. burgdorferi strains B314 and B31HP (provided to Dr. Parveen by Dr. John Leong, Tufts Medical Center) [33], derivatives of the B31 strain, were grown according to established protocols using BSKII medium containing 6% rabbit serum [36]. B. burgdorferi shuttle vector pJSB175 [37], used here to clone the tprK gene, was previously used to clone and induce T. pallidum tp0435 expression in both B. burgdorferi B314 and B31HP strains [33] that have lost several endogenous plasmids required for B31 strain infectivity [38–40]. This vector also contains a codon-optimized firefly luciferase which makes B. burgdorferi strains bioluminescent in the presence of D-luciferin. For tprK gene cloning, the sense primer 5’- ggggtacctcccccagttgcagcacta, and antisense 5’- acgcgtcgactcgcggtagtcaacaatacca, respectively, were used to amplify the tprK ORF plus 229 and 397 bp of the upstream and downstream flanking regions, respectively. Restriction enzyme cleavage sites for KpnI (sense primer) and SalI (antisense primer), respectively, are underlined in the sequences above. The 229 bp included upstream of the tprK start codon were experimentally shown to contain the gene transcriptional start site (TSS) using 5’-Rapid Amplification of cDNA Ends (5’-RACE) on T. pallidum (Nichols strain) mRNA (Giacani et al., unpublished). Based on the TSS position, the −10 and −35 consensus sequences for the tprK promoter are TATGGT and TTGACC, respectively. Amplification yielded a 2,080 bp amplicon as expected. Selection of recombinant pJSB175 carrying tprK was performed in TOP10 E. coli (ThermoFisher) on LB-spectinomycin (100 μg/ml) agar plates. Prior to transformation of B. burgdorferi B314 strain, the tprK sequence was confirmed by Sanger sequencing (see supplemental material and GenBank Accession number AF194369.1). Transformation and selection of B. burgdorferi B314 was performed as described [37].
Analysis of transcription of tprK gene in B. burgdorferi B314 by RT-PCR, and analysis TprK protein expression by mass-spectrometry and surface immunofluorescence.
B. burgdorferi B314 harboring the pJBS175-tprK vector was analyzed for its ability to express the tprK gene/protein using a) qualitative reverse transcription (RT)-PCR, b) liquid chromatography-mass spectrometry (LC-MS) and c) surface indirect immunofluorescence assay (IFA). Similar analyses were previously performed using the Tp0435-expressing strains [33]. For tprK message detection, 1.5 ml of culture containing 108 cells/ml were pelleted and resuspended in 400 μl of Ultraspec buffer (Biotex Laboratories, discontinued). Protocols for total RNA isolation, DNaseI treatment of purified RNA, cDNA synthesis, and tprK mRNA amplification were all previously reported [41]. For tprK amplification sense and antisense primers 5’- agtttgcgtctaacaccgactg and 5`- tcgcatggccatgttgagaaat (which yielded a 410-bp amplicon) were used. As an endogenous control, ospC mRNA was also targeted using the sense and antisense primers 5’-tgcggttttacttgctgtga and 5’-tccaagttcttcagcaccttt, respectively, that yielded a 364 bp amplicon. DNA from the control B314 strain (carrying the empty pJBS175 vector) and T. pallidum (Nichols strain) were used as controls.
TprK protein expression was assessed by LC-MS and IFA. For LC-MS, a total of 5×108 cells were harvested by centrifugation of B. burgdorferi B314 cells containing the empty vector or carrying the pJBS175-tprK vector, washed three times with Phosphate Buffer Saline (PBS, pH 7.5), pelleted again and resuspended in 200 μl of SDS-PAGE sample buffer. Proteins were separated in 10% polyacrylamide gels. Gels were stained using SimplyBlue SafeStain (ThermoFisher). Subsequently, a gel segment encompassing protein sizes between 40–60 kDa (and thus the predicted TprK size) was excised and bands were subjected to overnight in-gel trypsin digestion. Volume of digestion products was reduced to approximately 10 μl using a speed-Vac. Peptides were analyzed by LC-MS at the University of Washington MS facility of the Chemistry Department using a LTQ HP1100 mass spectrometer (ThermoFisher), results were analyzed using the Proteome Discoverer software.
IFA was performed as described previously [33] using both intact and methanol-treated cells. Antigen expression and localization was assessed using an anti-TprK polyclonal antiserum raised against a fragment of the TprK protein predicted to harbor conserved surface-exposed loops of the protein (aa 37–273) [19] (1:1,000 dilution). Control sera included a mouse monoclonal anti-FlaB (periplasmic target) serum diluted 1:50 and a mouse polyclonal anti-OspC (surface target) serum diluted 1:5,000. Anti-mouse or anti-rabbit secondary antibodies conjugated to Alexafluor 488 or TRITC (Life Technologies) were used at 1:2,000 dilution. Staining of DNA with 4’,6’-diamidino-2-phenylindole (DAPI) was used to detect all bacteria.
Mice Immunization with Tp0435-expressing B. burgdorferi strains.
We injected five Balb/c mice with Tp0435-expressing B. burgdorferi B314 and B31HP strains subcutaneously at a dose of 107 spirochetes in 100 μl medium mixed with equal volume of 30 mg/ml D-luciferin substrate. Mice were imaged using the In Vivo Imaging System 200 (Perkin Elmer) within 10 mins of injection to detect the presence of live spirochetes. All mice were imaged again the following day after the substrate was injected at the site of cell inoculation. Animals received two booster injections (107 spirochetes/injection) at two week intervals from the first immunization and mice were imaged. One week after the last immunization, mice were terminally bled and then euthanized. Sera from each group were pooled and titrated against recombinant Tp0435 (rTp0435). ELISA was conducted as previously described [42] after coating the 96-wells plate with 20 μg/ml of Tp0435 (50 μl/well). A previously generated antiserum against rTp0435 was included as a positive control [33].
Recombinant protein expression
Recombinant Tp0435 devoid of its putative signal peptide was expressed following ORF cloning into the pEXP5-NT TOPO vector (Life Technologies). Amplification of the tp0435 ORF from the Nichols strain DNA was performed using sense and antisense primers 5’-tgcacaaccgtgtgtccgca and 5’-tcatttctttgtttttttgagcac, respectively. Cloning into the expression vector was performed according to the manufacturer`s instructions. Cloned sequence is reported as supplementary material. rTp0435 was expressed and purified as a soluble protein using the protocol previously described [43]. Recombinant TprK protein (rTprK, aa 37–273) was already available from previous studies [19]. For rTprK renaturation, Profoldin M7 renaturing columns for membrane proteins were used according to the manufacturer’s protocol. Following renaturation, protein concentration was evaluated using a micro BCA assay (ThermoFisher). For ELISA using denatured versus renatured rTprK, denatured antigen was dialyzed against PBS in a Slide-A-Lyser dialysis cassette (ThermoFisher) with 10 kDa cut-off and stored at −20°C until use. A recombinant 6xHis-tagged OspC (rOspC, aa 19–209) was already available in the Parveen laboratory.
ELISA using synthetic and recombinant proteins to evaluate immunity to T. pallidum antigens in post-immunization rabbit sera.
Purified rTprK (aa 37–273), rTp0435 (aa 25–156), and rOspC (aa 19–209) in PBS-0.1% sodium azide were used to coat the wells of a 96-well ELISA EIA II Plus Microplate (ICN, Irvine, CA) as previously described [22]. Blood was drawn from rabbits prior to immunization and after each injection, and sera were extracted, heat-inactivated, and stored at −20°C until use. Sera were tested using a 1:20 dilution in 1% NFM-PBS to assess reactivity to rTprK, rTp0435, and at 1:200 dilution to assess reactivity to rOspC. Infected rabbit serum (IRS) was also used as positive control at 1:200 dilution. ELISA protocol was also previously described [22]. For each serum, the mean of background readings (from no antigen wells) was subtracted from the values of experimental wells for each antigen to obtain net absorbance.
Overlapping 20 aa-long synthetic peptides were synthesized based on the Nichols strain TprK sequence (GenBank accession number AF194369) as described elsewhere [44]. Forty peptides with 10 amino acids overlap, spanning the entire mature TprK sequence (aa 30–349, Table 1) [44], were used as antigens for testing sera from rabbits immunized with TprK-expressing B. burgdorferi B314 using a protocol already reported [44]. Results were analyzed using Student’s t-test with significance set at p<0.05.
Table 1.
TprK synthetic peptide sequences
| Peptide #a | Sequence |
|---|---|
| 1 | QVSFTPDIEGYAELAWGIAS |
| 2 | ELAWGIASDGGALKHGFKTT |
| 3 | LKHGFKTTTDFKIVFPIVAK |
| 4 | FKIVFPIVAKKDFKYRGEGN |
| 5 | KDFKYRGEGNVYAEINVKAL |
| 6 | VYAEINVKALKLSLESNGGA |
| 7 | KLSLESNGGAKFDTKGSAKT |
| 8 | KFDTKGSAKTIEATLHCYGA |
| 9 | IEATLHCYGAYLTIGKNPDF |
| 10 | YLTIGKNPDFKSTFAVLWEP |
| 11 | KSTFAVLWEPWTANGDYKSK |
| 12 | WTANGDYKSKGDKPVYEPGF |
| 13 | GDKPVYEPGFEGAGGKLGYK |
| 14 | EGAGGKLGYKQTDIAGTGLT |
| 15 | QTDIAGTGLTFDIAFKFASN |
| 16 | FDIAFKFASNTDWEGKDSKG |
| 17 | DSKGNVPAGVTPSKYGLGGD |
| 18 | TPSKYGLGGDILFGWERTRE |
| 19 | LGGDILFGWERTREDGVQEY |
| 20 | ILFGWERTREDGVQEYIKVE |
| 21 | IKVELTGNSTLSSDYAQARA |
| 22 | LTGNSTLSSDYAQARAPAAG |
| 23 | YAQARAPAAGAKVSMKLWGL |
| 24 | AKVSMKLWGLCALAATDVGH |
| 25 | CALAATDVGHKKNGAQGTVG |
| 26 | KKNGAQGTVGADALLTLGYR |
| 27 | ADALLTLGYRWFSAGGYFAS |
| 28 | WFSAGGYFASQASNVFGGVF |
| 29 | QASNVFGGVFLNMAMREHDC |
| 30 | LNMAMREHDCAAYIKLETKG |
| 31 | AAYIKLETKGSDPDTSFLEG |
| 32 | SDPDTSFLEGLDLGVDVRTY |
| 33 | LDLGVDVRTYMPVHYKVLKA |
| 34 | MPVHYKVLKALPRADIHFPV |
| 35 | LPRADIHFPVYGKVWGSYRH |
| 36 | YGKVWGSYRHDMGEYGWVKV |
| 37 | DMGEYGWVKVYANLYGGTNK |
| 38 | YANLYGGTNKKATPPAAPAT |
| 39 | KATPPAAPATKWSKEYCGYY |
| 40 | KATPPAAPATKWSKEYCGYYYCGYYECGVVVSPLEKVEIRLSWEQGKLQENSNVVIEKNVTERWQFVGACRLIW |
Sequences are listed from N to C terminus. The underlined amino acids fall within predicted external loops.
Rabbit immunization, T. pallidum challenge, and monitoring of cutaneous lesion development and treponemal burden within experimental lesions.
Rabbits were immunized by intramuscular (IM) injection of 1010 Borrelia cells resuspended in 1 ml of sterile saline. A total of five immunizations were performed over 10 weeks (one injection every 2 weeks). Each rabbit received 500 μl of cell suspension in each thigh muscle. Two groups of three rabbits each were immunized with B. burgdorferi B314 and B31HP expressing TprK and Tp0435, respectively. A third group of animals was immunized with the vector-control strain (B. burgdorferi B31HP carrying an empty pJSB175 vector). Briefly, on the day of each immunization, B. burgdorferi cells were enumerated by DFM, harvested by centrifugation and resuspended in saline to the desired concentration. Cells were injected within 30 mins of harvest. All animals were bled before the first immunization, and before each booster dose to assess development of humoral immunity against rTprK and rTp0435 proteins/peptides. No adjuvant was used. Two weeks following the last immunization, animals were bled and challenged by intradermal injection of T. pallidum (Nichols stain) on eight sites on their shaved backs. A total of 106 T. pallidum viable cells (assessed by motility count under dark-field microscopy) were inoculated at each challenge site. Three unimmunized control rabbits were also challenged. Following challenge, cutaneous lesion development was monitored by recording diameter of indurated lesions and progression (erythematous, indurating, ulcerating, and healing) every day for 30 days. Lesion aspirates were collected at day 16 post-challenge from each lesion and resuspended in 50 μl of sterile saline. Nineteen microliters of the suspensions were examined by DFM for presence of treponemes. A total of 200 fields per sample were examined blindly. At the end of the observation period, rabbits were bled to test sera by VDRL and FTA-ABS and then euthanized.
Two cutaneous lesion biopsies were obtained at day 16 (after obtaining needle aspirates) or 21 post-inoculation using a 4 mm biopsy punch from each immunized and control animals to evaluate treponemal burden by quantification of T. pallidum mRNA. In all cases, biopsy samples were minced with a sterile scalpel after collection and homogenized in 400 μl of Ultraspec buffer. Samples were then stored at −80°C until use. Extracted RNA was treated with DNaseI and reverse transcribed as reported [41]. Message quantification was performed using a qPCR approach that targets the message for the treponemal 47 kDa lipoprotein (encoded by the tp0574 gene) and normalizes the tp0574 signal to the message for the rabbit housekeeping gene HPRT message [41]. Data from message quantification and treponemal counts from aspirates were analyzed with Student’s unpaired two-tailed t-test and significance set at p≤0.05.
Rabbit immunization and lymphocyte proliferation assays
Three rabbits were immunized with B. burgdorferi B31HP expressing Tp0435 as described above but not challenged. Instead, animals were sacrificed and splenic lymphocytes were harvested and cultured as described [45]. Approximately 5 × 105 cells in 200 μl of culture medium were plated in quadruplicate for each animal and each antigen in flat-bottomed 96-well plates (Costar) and incubated at 37°C in a 5% CO2 atmosphere. Recombinant proteins (rTp0435, rOspC, and rTp0136) were added at 10 μg/ml final concentration. OspC was used as positive control, and Tp0136 (from the Nichols strain) was used as an unrelated T. pallidum protein. Expression of rTp0136 was previously described [46]. Ten microliters/well of a sonicated suspension of B. burgdorferi was used as positive controls and 0.5 μg/well Concanavalin A (ICN Pharmaceuticals) as positive T-cell control. Sonicated T. pallidum was also tested, and PBS alone was used to measure background reactivity. Three days after exposure to recombinant peptide or controls, cells were pulsed with 0.5 μCi 3H-thymidine per well and harvested after 24 hours to measure tritiated thymidine incorporation using a Wallac Microbeta Trilux (Perkin-Elmer) microplate scintillation analyzer. The geometric mean of quadruplicate wells with no antigens (determined as background value) was subtracted from the geometric means of the quadruplicate experimental wells. Data represent the geometric means (± standard error) of values for each antigen for three rabbits. Data were analyzed with Student’s unpaired two-tailed t-test and significance set at p≤0.05
RESULTS
Analysis of humoral response to recombinant Tp0435 in mice immunized with Tp0435-expressing B. burgdorferi B31HP and B314.
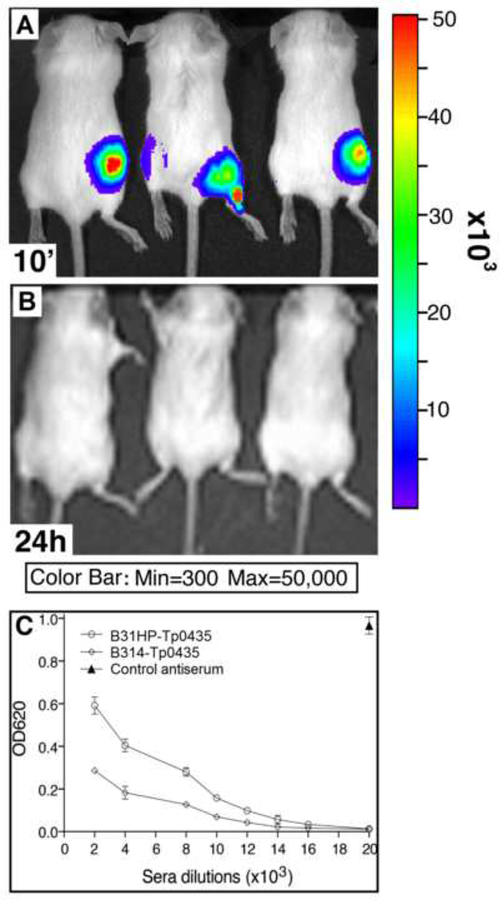
Light emitted by our non-pathogenic bioluminescent recombinant strains was measured by live imaging mice injected with D-luciferin following B. burgdorferi inoculation to assess strain viability. Although live imaging showed that B. burgdorferi B314 and B31HP strains expressing Tp0435 could be visualized in mice immediately after injection (Fig.1A shows three representative mice), no bioluminescence could be detected 24h post-injection after D-luciferin was injected again (Fig.1B). This result supports that spirochetes were unable to survive beyond a few hours of injection in mice, which is a critical feature of modified-live vaccines. After priming and booster injection of B. burgdorferi expressing Tp0435, high levels of antibody to rTp0435 were detected by antisera titration regardless of the B. burgdorferi parent strain (Fig.1C), showing that this heterologous system can be effectively used to induce a detectable humoral immune response against this antigen. Primary antibody control wells showed no reactivity of antigen with the secondary antibody conjugate (Average OD620 = 0.0003)
Figure 1: Generation of specific humoral immune response against Tp0435 protein using B. burgdorferi expressing this T. pallidum protein as immunogen in Balb/c mice.
Mice were immunized with 100 μl of non-infectious, bioluminescent B. burgdorferi strains B31HP- and B314-expressing Tp0435 mixed with an equal volume of 30 mg/ml D-luciferin substrate for B. burgdorferi codon-optimized luciferase encoded by the pJSB175-tp0435 plasmid. (A) Bioluminescence was observed within 10 mins of injection of the inoculum. (B) Loss of bioluminescence was detected within 24h, after delivering additional D-luciferin substrate at the cell injection site. (C) After a total of three immunizations, antibody titer against recombinant Tp0435 protein was determined by ELISA. B31HP-Tp0435: sera from mice immunized with Tp0435-expressing B31HP strain; B314-Tp0435: sera from mice immunized with Tp0435-expressing B314 strain. An antiserum against rTp0435 previously generated in mice using Freund’s adjuvant [33] was included as a control antiserum.
Analysis of humoral and cellular response to recombinant Tp0435 in rabbits immunized with Tp0435-expressing B. burgdorferi B31HP.
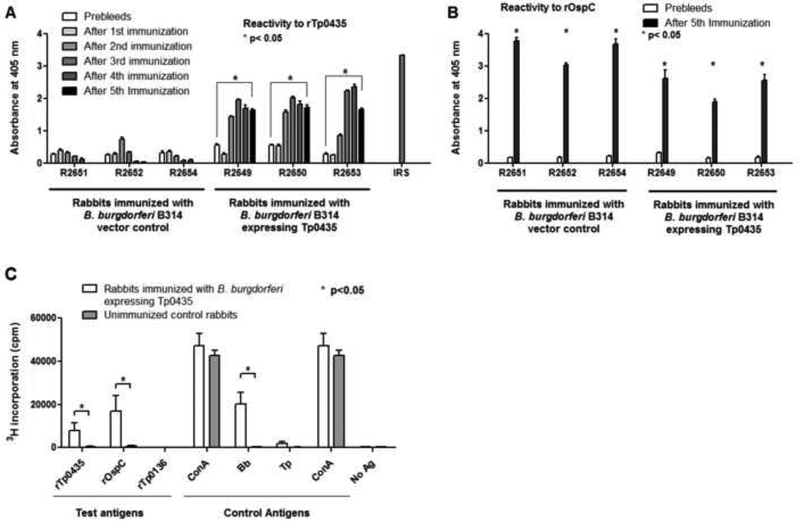
Following rabbit immunization with B. burgdorferi B31HP strain expressing T. pallidum antigens, a humoral response to rTp0435 (Fig.2A) was detected in each of the immunized animal groups. This response was significantly higher (p<0.05) than sera obtained from the same animals prior to immunization. In contrast, rabbits immunized with a B. burgdorferi control strain carrying an empty vector failed to mount a humoral response against rTp0435 compared to animals immunized with the Tp0435-expressing strains (Fig.2A). All rabbits in this study mounted a robust humoral response to B. burgdorferi rOspC (Fig.2B). Almost saturating antibody levels against rTp0435 were obtained after three immunizations with this B. burgdorferi strain.
Figure 2: Antibody reactivity, and cellular immunity determined by ex-vivo proliferation of T-cells from the immunized rabbits against recombinant Tp0435 and OspC proteins.
(A) Reactivity against recombinant full-length Tp0435 (rTp0435, w/o signal peptide sequence, aa 25–156) was tested with sera obtained from rabbits prior to immunization (Prebleeds) and two weeks after each boost immunized with B. burgdorferi B31HP with vector control (rabbits R2654, R2652, R2651), and from rabbits immunized with B31HP strain expressing Tp0435 (rabbits R2649, R2650, R2653). (B) Reactivity against recombinant OspC (rOspC, aa 19–209) was tested in sera from all rabbits immunized with B. burgdorferi B31HP strains used in this study prior to immunization and two weeks after the 5th (and last) injection. Asterisks (*) indicate whether reactivity values after the 5th immunization are significantly higher (p≤0.05) as compared to the pre-immunization sera. Serum form a rabbit infected long term (>90 days) with the Nichols strain (IRS) was used as a positive control for T. pallidum antigens. (C) T-cell proliferation assay with splenic lymphocytes from rabbits immunized with B. burgdorferi expressing Tp0435 and unimmunized control rabbits using test antigens (rTp0435, rOspC, and rTp0136) and control antigens ConcanavalinA (ConA), sonicated B. burgdorferi (Bb), sonicated T. pallidum (Tp), and No antigen (No Ag). Bars represent the geometric means ± standard error of mean in quadruplicate experimental values for each antigen for three rabbits. Significant differences (p≤0.05) are indicated by an asterisk (*).
We isolated lymphocytes from the spleens of rabbits immunized with Tp0435-expressing B. burgdorferi and from unimmunized rabbits. Compared to the control group, splenocytes from rabbits immunized with Tp0435-expressing B. burgdorferi displayed marked proliferation in response to both rTp0435 and rOspC incubation (Fig.2C). Only splenocytes from immunized rabbits proliferated in response to sonicated B. burgdorferi whereas both groups responded to Concavalin A (Fig.2C), a potent inducer of lymphocyte proliferation. No reactivity was seen against rTp0136, a T. pallidum adhesin underlated to Tp0435, and only a minimal response was seen against T. pallidum sonicate (Fig.2C). These results demonstrate that rabbit immunization with the Tp0435-expressing B31HP strain effectively stimulated both humoral and cellular immunity.
Analysis of tprK gene and protein expression RT-PCR, mass-spectrometry, and IFA.
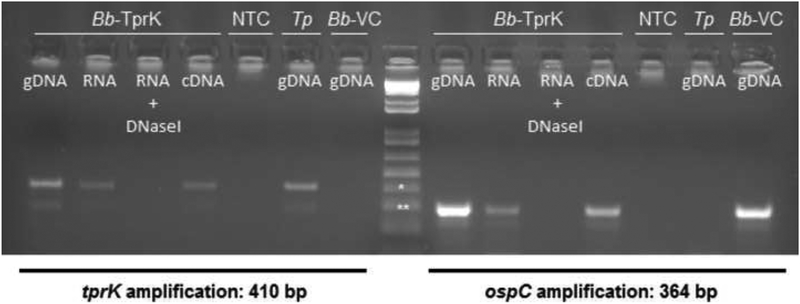
Analysis of tprK and ospC mRNA in B314 strains transformed with the pJBS175-tprK (Bb-TprK, in Fig.3), showed that specific message for tprK is detected upon amplification of cDNA from the TprK-expressing strain (Fig.3, left panel). As a positive control, specific message for ospC was detected using cDNA from the TprK-expressing strain. Amplification of tprK and ospC yielded a positive and negative result, respectively when T. pallidum DNA template was used (Tp, Fig.3). The DNaseI-treated RNA sample remained negative when used as template, indicating that amplification was not due to residual DNA carried over from the RNA isolation procedure. DNA extracted from the B. burgdorferi control strain (Bb-VC) only yielded positive amplification when the ospC gene was targeted (Fig.3, right panel).
Figure 3: Analysis of tprK and ospC gene expression by RT-PCR.
RT-PCR analysis of tprK (panel to the left of marker) and ospC (panel right of marker) cDNA. Bb-TprK: B. burgdorferi expressing TprK; Tp: T. pallidum; Bb-VC: B. burgdorferi empty-vector control strain; NTC: no template control. gDNA: genomic DNA; RNA: total RNA; RNA+DNaseI: DNaseI-treated RNA.
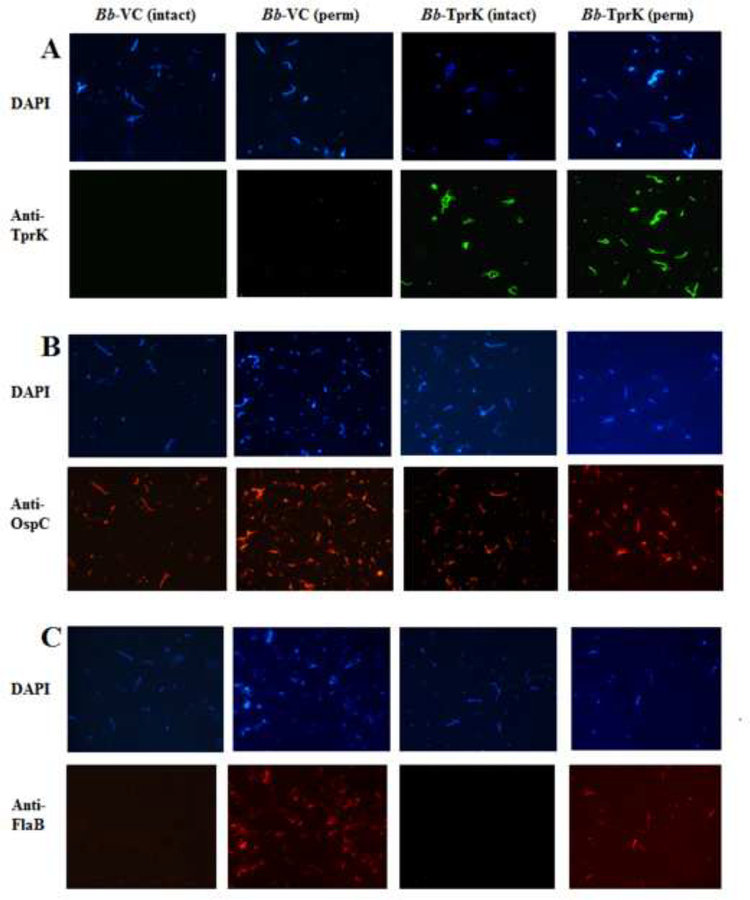
Mass spectrometry analysis of B. burgdorferi transformed with the pJSB175-tprK showed that TprK protein is indeed produced in these spirochetes. The TprK-specific peptides identified with high and low confidence by MS are listed in Table 2. No TprK peptides were recognized from in-gel digested bands isolated after PAGE from the empty vector control strain. The entire spectrum of peptides recognized in these experiments, based on our analysis using T. pallidum, B. burgdorferi, E. coli, and human proteomes as reference, are reported as supplementary file (Bb-TprK and Bb-VC MS results). IFA assays provided additional evidence that TprK is expressed and localized on the surface of intact B. burgdorferi carrying the pJBS175-tprK vector (Fig.4A). Reactivity to the anti-TprK antibody was absent in B. burgdorferi transformed with the empty pJBS175 with or without permeabilization (Fig.4A). All strains displayed OspC on the surface of B. burgdorferi irrespective of TprK expression, as expected (Fig.4B). Lack of labeling with the anti-flagellin antibody, unless cells were permeabilized by methanol treatment demonstrated maintenance of envelope integrity during the assay (Fig.4C).
Table 2.
TprK peptides identified by MS on B. burgdorferi B314 strain transformed with the pJB175-tprK vector
| SEQUENCE1 | PSMs2 | ΔCn3 | XC4 | MH+ (Da)5 | ΔM (ppm)5 | RT (min)6 |
|---|---|---|---|---|---|---|
| VELTGNSTLSSDYAQAR | 13 | 0.0000 | 4.08 | 1812.68974 | 448.33 | 29.46 |
| GEGNVYAEINVK | 12 | 0.0000 | 3.75 | 1293.25932 | 472.58 | 29.15 |
| SKGDKPVYEPGFEGAGGK | 8 | 0.0000 | 3.75 | 1824.12339 | 672.26 | 25.49 |
| QTDIAGTGLTFDIAFK | 11 | 0.0000 | 3.56 | 1698.62627 | 442.54 | 37.17 |
| YRGEGNVYAEINVK | 12 | 0.0000 | 3.50 | 1612.77536 | 596.96 | 28.26 |
| YGLGGDILFGWER | 9 | 0.0000 | 3.14 | 1483.07207 | 225.48 | 39.02 |
| GDKPVYEPGFEGAGGK | 6 | 0.0000 | 3.04 | 1607.96848 | 123.37 | 27.39 |
| FASNTDWEGK | 8 | 0.0000 | 2.96 | 1154.96318 | 391.23 | 26.47 |
| TREDGVQEYIK | 20 | 0.0000 | 2.95 | 1338.58362 | 682.81 | 25.70 |
| FASNTDWEGKDSK | 7 | 0.0000 | 1.93 | 1486.08442 | 954.95 | 24.68 |
| LSWEQGKLQENSNVVIEK | 1 | 0.0000 | 1.93 | 2102.10935 | 483.73 | 32.28 |
| ALAAGAKVSMKLWGLCALAATDVGHK | 2 | 0.0000 | 1.67 | 2581.49265 | −349.43 | 6.23 |
| STFAVLWEPWTANGDYK | 1 | 0.0000 | 1.29 | 1983.96709 | −492.42 | 37.73 |
| GDKPVYEPGFEGAGGKLGYK | 1 | 0.0000 | 1.25 | 2068.30376 | −353.03 | 28.44 |
| GSDPDTSFLEGLDLGVDVRTYMPVHYK | 1 | 0.0000 | 1.19 | 3011.89597 | 149.52 | 6.21 |
| VYANLYGGTNKKATPPAAPATK | 1 | 0.0556 | 1.19 | 2231.71726 | −663.33 | 38.01 |
| IVFPIVAK | 9 | 0.0000 | 1.12 | 887.20140 | 704.80 | 32.47 |
| TIEATLHCYGAYLTIGKNPDFK | 1 | 0.0000 | 1.12 | 2455.18503 | −19.41 | 31.18 |
| TYMPVHYKVLK | 1 | 0.0000 | 1.03 | 1378.07911 | −490.60 | 58.14 |
| ALPPAxBFPVYGK | 2 | 0.0000 | 0.99 | 1387.27629 | 4.48 | 47.21 |
| KVVYQRVGHR | 1 | 0.3595 | 0.98 | 1242.52641 | 646.93 | 34.26 |
| ALAAGAKVSMK | 1 | 0.5714 | 0.93 | 1045.86968 | −700.92 | 40.90 |
| VEIRLSWEQGK | 1 | 0.0000 | 0.89 | 1345.30881 | 432.41 | 26.28 |
| LWGLcALAATDVGHK | 7 | 0.0000 | 0.83 | 1611.63755 | −120.19 | 16.48 |
| DSSKYGLGGDILFGWER | 1 | 0.0930 | 0.78 | 2820.89939 | 165.81 | 57.92 |
| GNVPAGVTPSKYGLGGDILFGWERTR | 1 | 0.0000 | 0.76 | 2748.89170 | 533.01 | 9.48 |
| ATPPAAPATK | 2 | 0.0000 | 0.76 | 923.99565 | −562.02 | 47.41 |
| VEIRLSWEQGKLQENSNVVIEK | 1 | 0.2632 | 0.70 | 2597.28513 | −424.90 | 6.46 |
| LGYKQTDIAGTGLTFDIAFKFASNTDWEGK | 1 | 0.0000 | 0.61 | 3293.53891 | −331.85 | 52.05 |
| GNVPAGVTPSK | 2 | 0.0000 | 0.60 | 1027.41020 | 829.57 | 23.75 |
| YGSPRLVSNGFRHR | 1 | 0.0678 | 0.55 | 1646.51950 | 396.27 | 9.43 |
| WQFVGACR | 1 | 0.0179 | 0.55 | 966.72997 | 277.73 | 27.09 |
| LQENSNVVIEKNVTER | 1 | 0.3457 | 0.53 | 1871.87114 | −59.34 | 51.10 |
| KVVYQR | 1 | 0.0370 | 0.52 | 793.12248 | 819.31 | 50.89 |
| LWGLCALAATDVGHK | 1 | 0.1613 | 0.52 | 1556.23441 | 915.43 | 9.98 |
Shaded peptides were recognized with low confidence
Total number of identified peptide sequences (PSMs) for the protein
Normalized score difference between the currently selected PSM and the highest-scoring PSM for this spectrum
Score based on the number of fragment ions common to two different peptides with the same precursor mass and cross-correlation for all candidate peptides queried from the database by SEQUEST searches
Calculated mass of the peptide (Da) and mass measurement error (ΔM) in parts per million (ppm)
Peptide retention time during chromatographic separation
Figure 4: IFA of B. burgdorferi expressing TprK.
(A) Absence of surface labelling of unpermeabilized empty vector containing B314 (Bb-VC) control strain by anti-TprK primary antiserum serum followed by detection with anti-rabbit Alexa fluor 488-conjugated secondary antibodies in contrast to positive surface staining of B. burgdorferi B314 expressing TprK (Bb-TprK) with anti-TprK antiserum. All spirochetes in the respective fields were imaged after simultaneous staining with DAPI that stains DNA. (B) Staining of all permeabilized and intact B. burgdorferi B314 strains with anti-OspC antibody was detected using anti-mouse TRITC-conjugated antibodies. (C) Integrity of these bacteria during IFA was confirmed by lack of staining of periplasmic flagella with monoclonal antibodies to flagellin followed by detection with anti-mouse TRITC-conjugated antibodies, unless cells were permeabilized by methanol treatment.
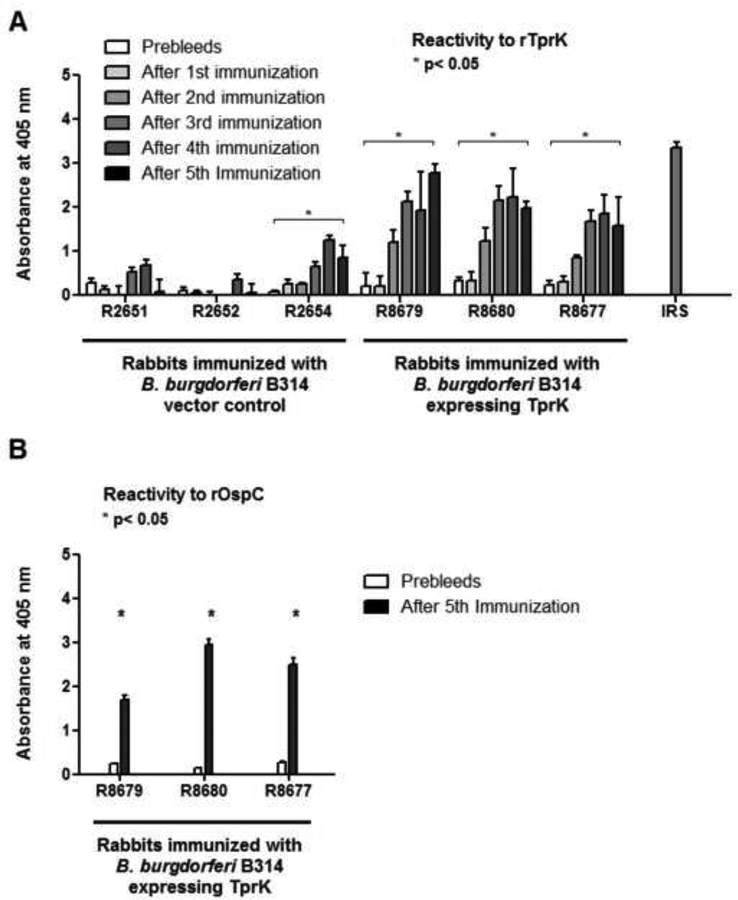
TprK-specific humoral immunity in immunized rabbits
Following rabbit immunization with B. burgdorferi B314 strain expressing T. pallidum TprK, a humoral response to rTprK (Fig.5A) was detected in each of the immunized animal groups. Sera from animals immunized with TprK-expressing B. burgdorferi had significantly higher reactivity to rTprK compared to the prebleed sera from the same animals (Fig.5A). Rabbits immunized with the control strain carrying the empty pJBS175 vector had no or weak reactivity to the rTprK antigen (Fig.5A), with the exception of one animal. Comparison of cumulative reactivity to rTprK in sera from immunized animals after the last immunization shows that the animals that received TprK-expressing B. burgdorferi had significantly higher reactivity than that observed in sera from animals immunized with the control strain. Sera from all rabbits injected with B. burgdorferi recognized rOspC (Fig.5B).
Figure 5: Antibody reactivity using sera from the immunized rabbits against recombinant TprK.
(A) Reactivity of antisera from immunized rabbits against rTprK (w/o signal peptide sequence, aa 37–273) was tested in sera collected prior to immunization and two weeks after each boost from rabbits immunized with B. burgdorferi B314 vector control (rabbits R2654, R2652, R2651) and with B. burgdorferi B314 expressing TprK (rabbits R8677, R8679, and R8680). (B) Reactivity of immunized rabbit sera against recombinant OspC (aa 19–209) was tested two weeks after the 5th (and last) injection and compared with the pre-immunization sera. Bars represent the mean OD readings after background (from no antigen wells) subtraction. Asterisks (*) indicate whether reactivity values after the 5th immunization are significantly higher (p≤0.05) than the pre-immunization rabbits sera.
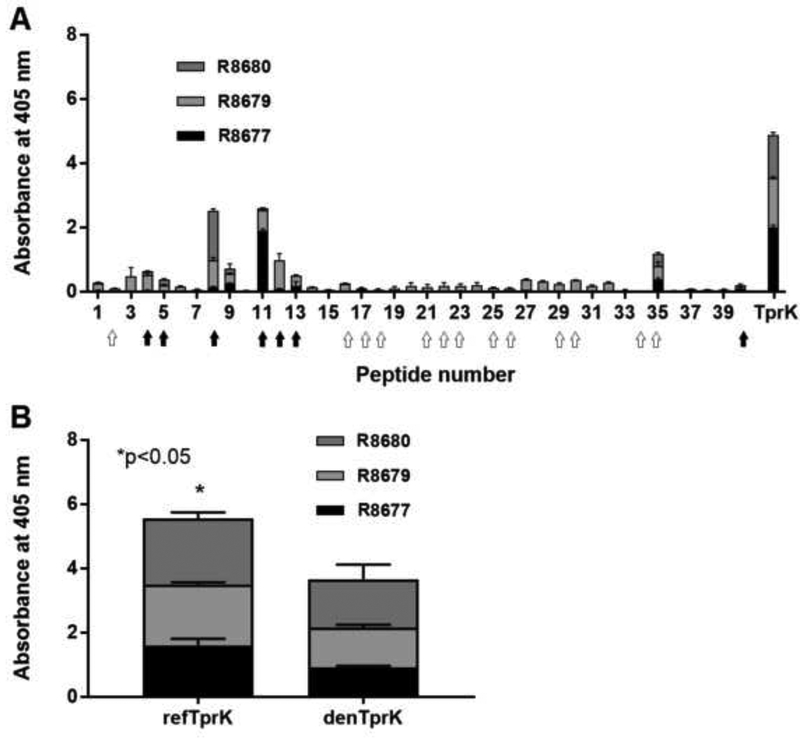
Results of epitope mapping using sera from animals immunized with TprK-expressing B. burgdorferi showed recognition of peptides mapping to the protein NH2- terminus, and relatively low recognition of peptides spanning the remainder of the TprK regions. Several of the recognized peptides (peptides # 8, 11, 12, 13, and 35) are predicted to harbor putative surface exposed sequences, based on the TprK structural model. Peptides 2, 5, 8, and 11–13 are predicted to harbor invariant surface loops (filled arrows in Fig.6A). Humoral reactivity to TprK refolded antigen was shown to be significantly higher (p≤0.05) than to the same recombinant antigen denatured with 6 M urea (Fig.6B) suggesting presence of antibodies to conformational epitopes.
Figure 6: Reactivity of sera from rabbits immunized with B. burgdorferi expressing TprK against TprK synthetic peptides and refolded or denatured TprK antigen.
(A) Sera collected from rabbits immunized with B. burgdorferi expressing TprK were used in ELISA to determine which sequences of TprK elicited a humoral response in each animal. Marked peptides contain conserved (filled arrows) and variable (open arrows) sequences predicted to be surface exposed. rTprK was used as a positive control. (B) Reactivity of sera from immunized animals (R8677, R8679, and R8680) to refolded TprK (refTprK) and TprK recombinant antigen denatured using 6M urea (denTprK). Bars represent the mean OD readings after background (from no antigen wells) subtraction. Asterisk (*) indicates significant increase in cumulative reactivity (p≤0.05).
Monitoring of cutaneous lesion development and treponemal burden within experimental lesions in challenged rabbits.
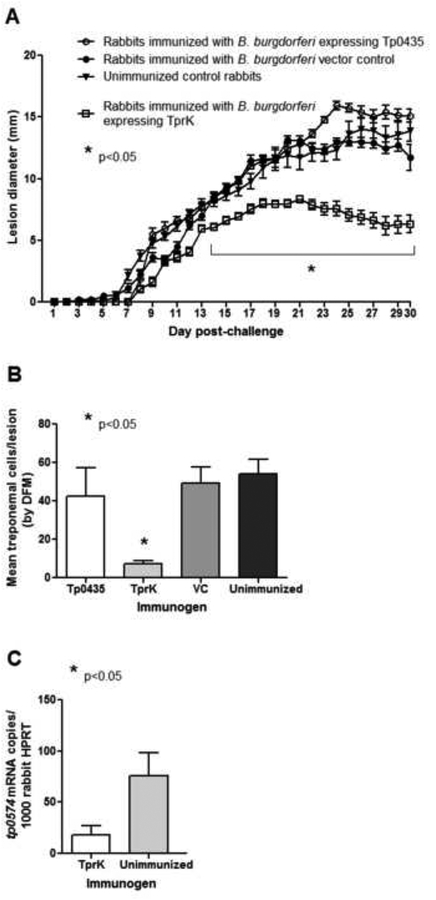
Following ID challenge with T. pallidum of animals immunized with Tp0435- or TprK-expressing B. burgdorferi, cutaneous lesion diameter was measured every day for 30 days in all animals. However, only animals immunized with TprK-expressing B. burgdorferi displayed reduced lesion size compared to all other groups (Fig.7A). Additionally, a lower percentage of lesions ulcerated in TprK-immune animals (44%) compared to Tp0435-immune animals (100%), unimmunized rabbits (100%) and rabbits that were injected with the B. burgdorferi empty-vector control strain (94%). Measurement of treponemal burden using dark-field microscopy (Fig.7B) showed a reduced number of cells in lesions from TprK-immunized animals compared to all the other groups. Diminished bacterial burden was also confirmed using RT-qPCR on cDNA from cutaneous lesion biopsies harvested from the animals (Fig.7C). At the end of the observation period, VDRL and FTA-ABS tests performed on individual serum samples collected from the challenged animals had all positive outcome, confirming active syphilis infection in all immunized and control groups.
Figure 7: Progression of cutaneous lesion development and assessment of treponemal burden within lesions by dark field microscopy of lesion aspirates and qPCR.
(A) Measurements of diameters (mm) of indurated/ulcerated lesions in all rabbits post-challenge. Animals were challenged by intradermal injection of T. pallidum (Nichols stain) on eight sites on their shaved backs. A total of 106 T. pallidum viable cells were inoculated at each challenge site. Treponemal burdens in lesions from immunized and control rabbits post-challenge were measured by dark-field microscopy (DFM) of lesion aspirates (B), and by qRT-PCR (C). Significant differences (p≤0.05) are indicated by asterisks (*).
DISCUSSION
Among the many approaches used in the past to attain an effective syphilis vaccine [47], only Miller’s one proved to be successful [27], although not practical for human use. Miller’s work, however, demonstrated that protective immunity to T. pallidum can be achieved, and still influences syphilis vaccine designs today. For example, the use of cells killed by ionizing radiation rather than chemical or physical treatments suggests that immunizing with unaltered antigens is pivotal for protection. The necessity to immunize weekly for over nine months suggests that the protective antigen(s) are scarce on the pathogen surface. Furthermore, the use of whole treponemal cells with their abundant periplasmic lipoproteins, whose acyl moieties are TLR2 ligands, could have induced a Th1-type response, known to be important to induce protective immunity [48]. Current syphilis vaccine designs have therefore embraced the concept of using recombinant antigens that are structurally similar to their native counterparts, even though the structures of some vaccine candidates, like the Tpr antigens, are still hypothetical.
Our surrogate Borrelia system offered the opportunity to reproduce some of the features of Miller’s experiment when used as an antigen carrier. These features include; a) immunization with an antigen localized in its natural cellular compartment, where it likely attained its native conformation, and b) providing an antigen surrounded by a lipoprotein-rich environment, which would work as a natural adjuvant. In addition, the use of a nonpathogenic B. burgdorferi strain allowed us to bypass any manipulation of the cells to impair their infectiousness. Confidence that this novel approach was indeed effective in inducing immunity to T. pallidum antigens came from preliminary immunization experiments in Balb/c mice injected with B. burgdorferi expressing Tp0435 (Fig.1). This early work led us to attempt the immunization process in rabbits, the model organism for syphilis. During natural and experimental syphilis, a very strong humoral response is generated to Tp0435. In addition to antibody production, T-cell epitopes have been identified within Tp0435 in past work [49]. Our immunization induced a cellular response against this antigen, supporting that our approach can produce immunity similar to that seen during experimental infection. The lack of protection seen in animals immunized with B. burgdorferi expressing Tp0435 was disappointing. Although we previously showed that a Tp0435 can become surface-exposed, T. pallidum cells appear to localize Tp0435 on their surface in a limited fashion and stochastically. This would make anti-Tp0435 antibodies unable to mediate opsonophagocytosis of T. pallidum cells not carrying this antigen on their surface [33], explaining lack of protection.
Our immunization approach showed important similarities to previous work where immunity to TprK was studied in experimentally infected or immunized rabbits. The results of past TprK epitope mapping experiments by Morgan et al. [44], for example, showed that we induced a response similar to that seen in rabbits experimentally infected with the Nichols strain at days 30 and 90 post-inoculation. In these rabbits, humoral reactivity to TprK was mostly directed against the protein NH2-terminus [44]. In our immunized animals, very limited reactivity was observed toward TprK peptides encompassing sequences in its central and COOH-terminus. This might reflect the immunodominant nature of the NH2- terminal TprK epitopes. Nonetheless, the immunity we generated was sufficient to partially protect animals, as shown by the reduced treponemal burden in the cutaneous lesions and the fact that significantly fewer lesions in immunized animals progressed to ulceration compared to controls. Such result is in accordance with previous work by Morgan et al. [19, 20].
Future vaccination attempts using our system will confirm the results reported here but also aim to improve the humoral reactivity to putative surface epitopes of TprK by inducing a reactivity profile that has been associated with protection in previous studies [19, 20]. However, a more widespread reactivity would probably not contribute to higher protection, as TprK predicted surface-exposed loops in the central region and COOH-terminus undergo antigenic variation during infection, and immunity to a sequence variant would be useless if the targeted variant is replaced with a different one. Nonetheless, an advantage of our approach over using recombinant antigens is suggested by the significantly higher reactivity to the refolded rTprK antigen compared to its denatured counterpart, which supports that our immunizations generated antibodies to conformational epitopes not present in the denatured protein. The structure of TprK is still hypothetical and mostly based on indirect experimental evidence [22, 24]; future studies using our surrogate system might also help understand which TprK sequences are actually surface exposed by performing IFA, immune-EM, or cryo-ET assays with antisera against various regions of TprK. Compared to previous vaccination trials using whole T. pallidum cells (reviewed and listed in Supplemental Table 1), our approach allows us to evaluate the protective ability of individual antigens, and to use a bacterial preparation that, unlike T. pallidum ones, is devoid of rabbit contaminants. In one instance, antisera raised in one animal against the B. burgdorferi vector-only control strain showed reactivity to the TprK recombinant antigen (Fig.5). This finding was surprising, as B. burgdorferi genome does not encode for a tprK orthologous gene, and homology search (performed by BLASTp) using the TprK sequence as query against the B. burgdorferi proteome did not show any hit. It is therefore unclear at the moment what could have generated such reactivity. We cannot rule out at the moment that a conformational, rather than a linear, homologous epitope to TprK in B. burgdorferi generated this cross-reactivity following immunizations.
Drawing further from the successful Miller study, it is plausible that multi-subunit vaccines are more likely to be effective than any vaccine design based on a single antigen, as they will provide a more extended arrays of targets for opsonic antibodies. A multivalent recombinant vaccine combining of the NH2-terminal fragment of TprK in combination with the Tp0751 antigen, and the NH2-terminus fragment shared by Subfamily I Tpr proteins TprC/D/D2/F/ and I has been proposed based on the result of past immunization/challenge experiments and is currently being tested [48]. Immunity to the Tpr-based antigens was shown to attenuate cutaneous lesion development in challenged animals, prevent ulceration, and decrease treponemal burden within lesions by enhancing opsonophagocytosis of T. pallidum. Immunity to Tp0751 was instead shown to inhibit treponemal dissemination to organs distant from the site of injection [48, 50]. Combining these antigens represents a two-pronged approach that aims at enhancing immune clearance while inhibiting pathogen dissemination to immune privileged sites such as the eyes, the central nervous system, the placenta, and the testes. Future studies will aim at using our surrogate system to deliver multiple antigens as well by injecting animals with a mixture of cells expressing different antigens. For such purpose, we have already engineered a TprD2-expressing B. burgdorferi strain. The inability of our surrogate system to replicate (and keep expressing the target antigen) in the host could be seen as a limitation, as the administration of an organism that can temporarily divide prior to immune clearance but that is however unable to cause disease, could overall decrease the number of necessary inoculation to obtain immunity to a desired antigen. Because our surrogate system does not grant us this option, alternatives are currently being explored to limit the number of immunization needed, such as enhancing expression of the target antigen using strong constitutive promoters of B. burgdorferi instead of the T. pallidum endogenous promoters. Experiments aimed at evaluating the protective ability of the Tp0954 antigen, for example, are being conducted in the laboratory using a tp0954 transgene under control of the OspC promoter.
To date only limited research has been conducted on syphilis vaccine development. The increasing application of modern research tools to syphilis research will improve our understanding of the pathogenesis of this serious disease and provide key insights for vaccine development. To this end, the establishment of cross-disciplinary collaborations is necessary to better understand both how this pathogen evades the host response and persists, as well as the host innate and adaptive responses to infection. In turn, such work will lead to a better definition of the correlates of protection from disease, particularly in human subjects. The availability of an excellent animal model that allows for pre-clinical studies to be performed will greatly facilitate the selection of vaccine designs to transition to clinical trials, and hopefully identify an effective syphilis vaccine.
Supplementary Material
Sfile1: Sequences of T. pallidum genes (Tp0435 and TprK) cloned into B. burgdorferi
Sfile2: Complete mass spectrometry results on Borrelia strains expressing TprK and Tp0435
Sfile3: Supplementary Table 1. List of past vaccine trials for syphilis using T. pallidum whole cell preparations.
ACKNOWLEDGMENTS
We thank student interns, Thayer Nasreddin, and Ghayoor Mir in the Parveen’s laboratory for cloning tprK and tp0435 genes in the pJSB175 vector. The authors wish to thank Barbara Molini for help with rabbit inoculation procedures, and Martin Sadilek for help with analysis of the MS data. This work was supported by the National Institute for Allergy and Infectious Diseases of the National Institutes of Health grant numbers U01AI115497 (to L.G, N.P, and A.C.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.WHO, Prevalence and incidence of selected sexually transmitted infections Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates World Health Organization, Geneva, 2011. [Google Scholar]
- 2.Herbert LJ and Middleton SI, An estimate of syphilis incidence in Eastern Europe. J Glob Health, 2012. 2(1): p. 010402.3484754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage EJ, et al. , Rapid increase in gonorrhoea and syphilis diagnoses in England in 2011. Euro Surveill, 2012. 17(29). [PubMed] [Google Scholar]
- 4.CDC, 2016 Sexually Transmitted Diseases Surveillance. https://www.cdc.gov/std/stats16/syphilis.htm, 2018.
- 5.Canadian sexually transmitted infections surveillance report. Can Commun Dis Rep, 33 (Suppl 1), 2007: p. 1–69. [PubMed] [Google Scholar]
- 6.Tucker JD and Cohen MS, China’s syphilis epidemic: epidemiology, proximate determinants of spread, and control responses. Curr Opin Infect Dis, 2011. 24(1): p. 50–5.3103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerbase AC, et al. , Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect, 1998. 74(Supplement 1): p. S12–6. [PubMed] [Google Scholar]
- 8.Goldenberg RL and Thompson C, The infectious origins of stillbirth. Am J Obstet Gynecol, 2003. 189(3): p. 861–73. [DOI] [PubMed] [Google Scholar]
- 9.Newman L, et al. , Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med, 2013. 10(2): p. e1001396.3582608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO, The global elimination of congenital syphilis: rationale and strategy for action. 2007.
- 11.White RG, et al. , Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. J Acquir Immune Defic Syndr, 2004. 37(4): p. 1500–13. [DOI] [PubMed] [Google Scholar]
- 12.CDC, The national plan to eliminate syphilis in the United States, 1999, Centers for Disease Control and Prevention: Atlanta, GA: U.S. Department of Health and Human Services. [Google Scholar]
- 13.Baker-Zander SA, Shaffer JM, and Lukehart SA, Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum ssp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol Med Microbiol, 1993. 6(4): p. 273–9. [DOI] [PubMed] [Google Scholar]
- 14.Penn CW, Cockayne A, and Bailey MJ, The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol, 1985. 131(Pt 9): p. 2349–57. [DOI] [PubMed] [Google Scholar]
- 15.Blanco DR, Miller JN, and Lovett MA, Surface antigens of the syphilis spirochete and their potential as virulence determinants. Emerg Infect Dis, 1997. 3(1): p. 11–20.2627599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmundson DG and Norris SJ, Long-Term In Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. pallidum. mBio, 2018. 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox DL, et al. , Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect Immun, 2010. 78(12): p. 5178–94.2981305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centurion-Lara A, et al. , Fine Analysis of Genetic Diversity of the tpr Gene Family among Treponemal Species, Subspecies and Strains. PLoS Negl Trop Dis., 2013. 16(7): p. e2222.3656149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan CA, Lukehart SA, and Van Voorhis WC, Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect Immun, 2002. 70(12): p. 6811–6.133068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan CA, Lukehart SA, and Van Voorhis WC, Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect Immun, 2003. 71(10): p. 5605–12.201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centurion-Lara A, et al. , Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J Exp Med, 1999. 189(4): p. 647–56.2192927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacani L, et al. , Antigenic variation in Treponema pallidum: TprK sequence diversity accumulates in response to immune pressure during experimental syphilis. J Immunol, 2010. 184(7): p. 3822–9.3042355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaFond RE, et al. , TprK sequence diversity accumulates during infection of rabbits with Treponema pallidum subsp. pallidum Nichols strain. Infect Immun, 2006. 74(3): p. 1896–906.1418662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centurion-Lara A, et al. , Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Molecular Microbiology, 2004. 52(6): p. 1579–96. [DOI] [PubMed] [Google Scholar]
- 25.LaFond RE, et al. , Sequence diversity of Treponema pallidum subsp. pallidum tprK in human syphilis lesions and rabbit-propagated isolates. J Bacteriol, 2003. 185(21): p. 6262–8.219401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheridan SL, et al. , Number and order of whole cell pertussis vaccines in infancy and disease protection. Jama, 2012. 308(5): p. 454–6. [DOI] [PubMed] [Google Scholar]
- 27.Miller JN, Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by γ - irradiation. J Immunol, 1973. 110(5): p. 1206–15. [PubMed] [Google Scholar]
- 28.Fletcher MA, Vaccine candidates in STD. Int J STD AIDS, 2002. 13 Suppl 2: p.38–41. [DOI] [PubMed] [Google Scholar]
- 29.Newcombe J, et al. , Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity. Infect Immun, 2004. 72(1): p. 338–44.343971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche AM, King SJ, and Weiser JN, Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun, 2007. 75(5): p. 2469–75.1865756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Duarte OG, Bumann D, and Meyer TF, The attenuated Salmonella vaccine approach for the control of Helicobacter pylori-related diseases. Vaccine, 1999. 17(13–14): p. 1667–73. [DOI] [PubMed] [Google Scholar]
- 32.Galen JE, et al. , A bivalent typhoid live vector vaccine expressing both chromosome- and plasmid-encoded Yersinia pestis antigens fully protects against murine lethal pulmonary plague infection. Infect Immun, 2015. 83(1): p. 161–72.4288866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan K, et al. , Treponema pallidum Lipoprotein TP0435 Expressed in Borrelia burgdorferi Produces Multiple Surface/Periplasmic Isoforms and mediates Adherence. Sci Rep, 2016. 6: p. 25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukehart SA and Marra CM, Isolation and laboratory maintenance of Treponema pallidum. Curr Protoc Microbiol, 2007. Chapter 12: p. 7:12A.1.1–12A.1.18. [DOI] [PubMed] [Google Scholar]
- 35.Giacani L, et al. , Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J Bacteriol, 2010. 192(10): p. 2645–6.2863575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuckert WR, Laboratory maintenance of Borrelia burgdorferi. Curr Protoc Microbiol, 2007. Chapter 12: p. Unit 12C 1. [DOI] [PubMed] [Google Scholar]
- 37.Blevins JS, et al. , Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi. Appl Environ Microbiol, 2007. 73(5): p. 1501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purser JE and Norris SJ, Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A, 2000. 97(25): p. 13865–70.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purser JE, et al. , A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol, 2003. 48(3): p. 753–64. [DOI] [PubMed] [Google Scholar]
- 40.Brisson D, et al. , Genetics of Borrelia burgdorferi. Annu Rev Genet, 2012. 46: p.515–36.3856702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacani L, et al. , Quantitative analysis of tpr gene expression in Treponema pallidum isolates: differences among isolates and correlation with T-cell responsiveness in experimental syphilis. Infect Immun, 2007. 75(1): p. 104–112.1828388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djokic V, et al. , Age-Related Differential Stimulation of Immune Response by Babesia microti and Borrelia burgdorferi During Acute Phase of Infection Affects Disease Severity. Frontiers in Immunology, 2018. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacani L, et al. , Identification of the Treponema pallidum subsp. pallidum TP0092 (σE) Regulon: implications for pathogen persistence in the host and syphilis pathogenesis J Bacteriol, 2013. 195(4): p. 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan CA, et al. , Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J Immunol, 2002. 169(2): p. 952–7. [DOI] [PubMed] [Google Scholar]
- 45.Lukehart SA, Baker-Zander SA, and Sell S, Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol, 1980. 124(1): p. 454–60. [PubMed] [Google Scholar]
- 46.Ke W, et al. , Treponema pallidum subsp. pallidum TP0136 protein is heterogeneous among isolates and binds cellular and plasma fibronectin via its NH2-terminal end. PLoS Negl Trop Dis, 2015. 9(3): p. e0003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giacani L, Lukehart S, and Centurion-Lara A, Syphilis, in Vaccines for Biodefense and Emerging and Neglected Diseases Barrett A and Stanberry L, Editors. 2008, Academic Press: London: p. 1194–1211. [Google Scholar]
- 48.Lithgow KV and Cameron CE, Vaccine development for syphilis. Expert Rev Vaccines, 2017. 16(1): p. 37–44.5513191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arroll TW, et al. , T-cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect Immun, 1999. 67(9): p. 4757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lithgow KV, et al. , A defined syphilis vaccine candidate inhibits dissemination of Treponema pallidum subspecies pallidum. Nat Commun, 2017. 8: p. 14273.5296639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sfile1: Sequences of T. pallidum genes (Tp0435 and TprK) cloned into B. burgdorferi
Sfile2: Complete mass spectrometry results on Borrelia strains expressing TprK and Tp0435
Sfile3: Supplementary Table 1. List of past vaccine trials for syphilis using T. pallidum whole cell preparations.